Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control
Abstract
1. Introduction
2. Breast Cancer
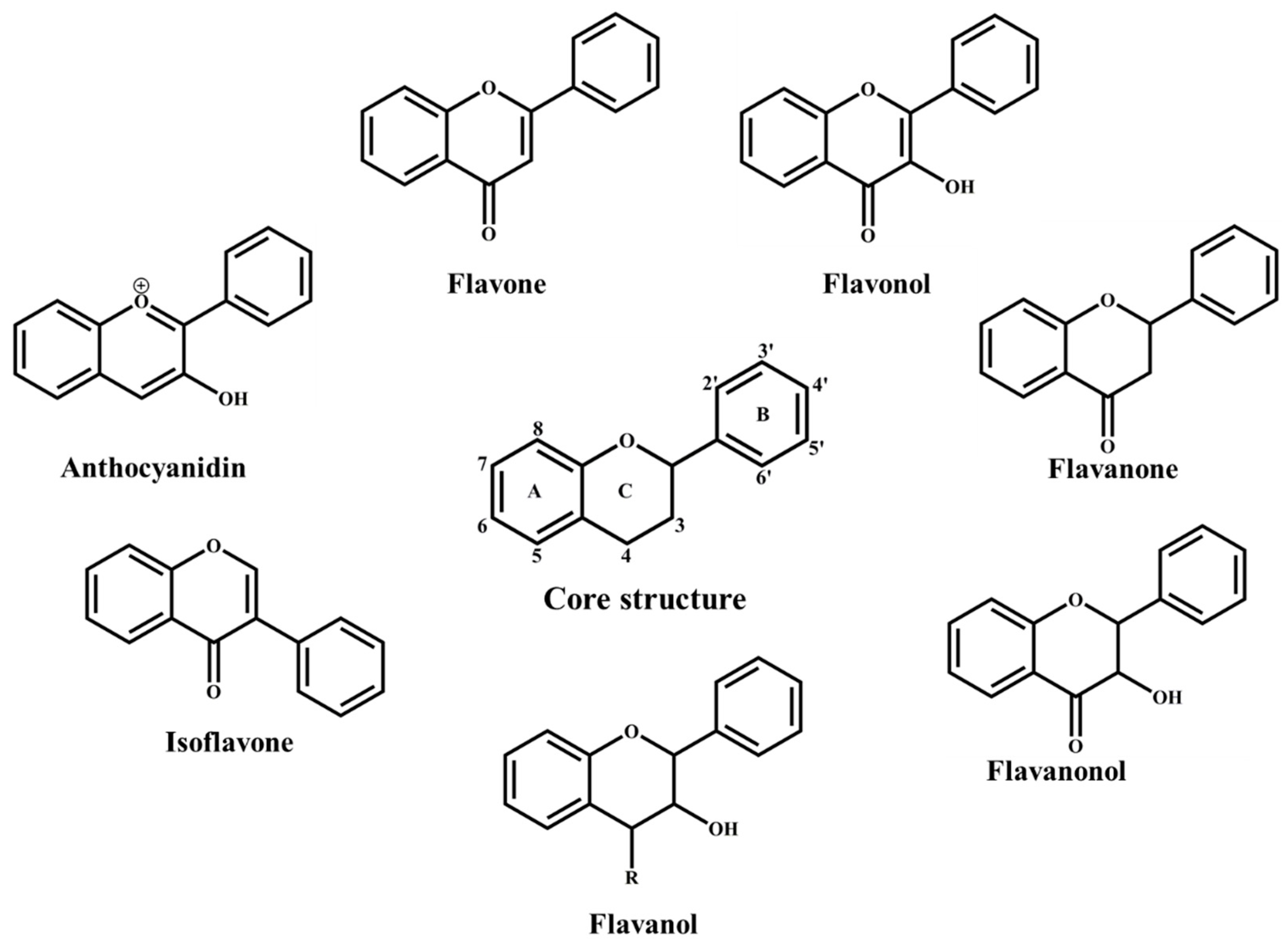
3. Flavonoids Classification and Distribution
4. Flavonoids: Molecular Mechanisms of Action
4.1. Antioxidant Effects of Flavonoids in Breast Cancer
4.2. Flavonoid-Protein Interactions as Regulators of Breast Cancer
5. Dietary Flavonoids and Breast Cancer
5.1. Potential Role of Flavonoids in Breast Cancer Therapeutics
5.2. Potential Role of Flavonoids in Breast Cancer Prevention
6. Clinical Applications of Flavonoids: Challenges and Opportunities
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Breast Cancer: Prevention and Control. Available online: www.who.int/cancer/detection/breastcancer/en/index1.htm (accessed on 5 February 2019).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Ferrini, K.; Ghelfi, F.; Mannucci, R.; Titta, L. Lifestyle, nutrition and breast cancer: Facts and presumptions for consideration. Ecancermedicalscience 2015, 9, 557. [Google Scholar] [CrossRef]
- Nattenmuller, C.J.; Kriegsmann, M.; Sookthai, D.; Fortner, R.T.; Steffen, A.; Walter, B.; Johnson, T.; Kneisel, J.; Katzke, V.; Bergmann, M.; et al. Obesity as risk factor for subtypes of breast cancer: Results from a prospective cohort study. BMC Cancer 2018, 18, 616. [Google Scholar] [CrossRef]
- Batra, P.; Sharma, A.K. Anti-cancer potential of flavonoids: Recent trends and future perspectives. 3 Biotech 2013, 3, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Vernarelli, J.A.; Lambert, J.D. Flavonoid intake is inversely associated with obesity and C-reactive protein, a marker for inflammation, in US adults. Nutr. Diabetes 2017, 7, e276. [Google Scholar] [CrossRef] [PubMed]
- Sak, K. Intake of individual flavonoids and risk of carcinogenesis: Overview of epidemiological evidence. Nutr. Cancer 2017, 69, 1119–1150. [Google Scholar] [CrossRef] [PubMed]
- Parihar, A.; Doseff, A. Emerging roles of flavonoids in brain health. In Phytopharmaceuticals for Brain Health; CRC Press: Florida, FL, USA, 2017; pp. 175–196. [Google Scholar]
- Beatrice Magne Nde, C.; Zingue, S.; Winter, E.; Beatriz Creczynski-Pasa, T.; Michel, T.; Fernandez, X.; Njamen, D.; Clyne, C. Flavonoids, breast cancer chemopreventive and/or chemotherapeutic agents. Curr. Med. Chem. 2015, 22, 3434–3446. [Google Scholar]
- Hou, D.-X.; Kumamoto, T. Flavonoids as Protein Kinase Inhibitors for Cancer Chemoprevention: Direct Binding and Molecular Modeling. Antioxid. Redox Signal. 2010, 13, 691–719. [Google Scholar] [CrossRef]
- Van’t Veer, L.J.; Dai, H.; Van De Vijver, M.J.; He, Y.D.; Hart, A.A.; Mao, M.; Peterse, H.L.; Van Der Kooy, K.; Marton, M.J.; Witteveen, A.T. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002, 415, 530. [Google Scholar] [CrossRef]
- Wirapati, P.; Sotiriou, C.; Kunkel, S.; Farmer, P.; Pradervand, S.; Haibe-Kains, B.; Desmedt, C.; Ignatiadis, M.; Sengstag, T.; Schütz, F. Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008, 10, R65. [Google Scholar] [CrossRef]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A. Molecular portraits of human breast tumours. Nature 2000, 406, 747. [Google Scholar] [CrossRef]
- Dai, X.; Li, T.; Bai, Z.; Yang, Y.; Liu, X.; Zhan, J.; Shi, B. Breast cancer intrinsic subtype classification, clinical use and future trends. Am. J. Cancer Res. 2015, 5, 2929–2943. [Google Scholar]
- Molecular Subtypes of Breast Cancer. Available online: https://www.breastcancer.org/symptoms/types/molecular-subtypes (accessed on 7 February 2019).
- Baselga, J.; Perez, E.A.; Pienkowski, T.; Bell, R. Adjuvant trastuzumab: A milestone in the treatment of HER-2-positive early breast cancer. Oncologist 2006, 11, 4–12. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231. [Google Scholar] [CrossRef]
- Shah, R.; O’Regan, R.M. Adjuvant endocrine therapy. Cancer Treat. Res. 2018, 173, 15–29. [Google Scholar] [CrossRef]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.K.; Speers, C.H.; D’Yachkova, Y.; Kang, A.; Malfair-Taylor, S.; Barnett, J.; Coldman, A.; Gelmon, K.A.; O’Reilly S, E.; Olivotto, I.A. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007, 110, 973–979. [Google Scholar] [CrossRef]
- Greenberg, P.A.; Hortobagyi, G.N.; Smith, T.L.; Ziegler, L.D.; Frye, D.K.; Buzdar, A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 2197–2205. [Google Scholar] [CrossRef]
- Dafni, U.; Grimani, I.; Xyrafas, A.; Eleftheraki, A.G.; Fountzilas, G. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res. Treat. 2010, 119, 621–631. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Nanda, R.; Chow, L.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D. Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Miles, D.; Roché, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef]
- Mittendorf, E.; Clifton, G.; Holmes, J.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G. Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann. Oncol. 2014, 25, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.; DeLord, J.; Im, S. Preliminary efficacy and safety of pembrolizumab (MK-3475) in patients with PD-L1-positive, ER-positive (ER+)/HER-2 negative breast cancer enrolled in Keynote 028. In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 8 December 2015. [Google Scholar]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.-A.; Shaw Wright, G. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H.; LoRusso, P.; Khalil, M.; Gartner, E.M.; Khaira, D.; Soulieres, D.; Dorazio, P.; Trosko, J.; Ruter, J.; Mariani, G.L. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of ICOS expression on patient T cells. Clin. Cancer Res. 2010, 16, 3485–3494. [Google Scholar] [CrossRef]
- Siegel, R.; DeSantis, C.; Virgo, K.; Stein, K.; Mariotto, A.; Smith, T.; Cooper, D.; Gansler, T.; Lerro, C.; Fedewa, S. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012, 62, 220–241. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S. Late effects of cancer treatment in breast cancer survivors. South Asian J. Cancer 2014, 3, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, W.; Zheng, W.; Gu, K.; Chen, Z.; Zheng, Y.; Shu, X.O. Obesity and weight change in relation to breast cancer survival. Breast Cancer Res. Treat. 2010, 122, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Majed, B.; Moreau, T.; Senouci, K.; Salmon, R.J.; Fourquet, A.; Asselain, B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res. Treat. 2008, 111, 329–342. [Google Scholar] [CrossRef]
- Saquib, N.; Flatt, S.W.; Natarajan, L.; Thomson, C.A.; Bardwell, W.A.; Caan, B.; Rock, C.L.; Pierce, J.P. Weight gain and recovery of pre-cancer weight after breast cancer treatments: Evidence from the women’s healthy eating and living (WHEL) study. Breast Cancer Res. Treat. 2007, 105, 177–186. [Google Scholar] [CrossRef]
- Shahrokni, A.; Wu, A.J.; Carter, J.; Lichtman, S.M. Long-term toxicity of cancer treatment in older patients. Clin. Geriatr. Med. 2016, 32, 63–80. [Google Scholar] [CrossRef]
- Hernandez, J.J.; Pryszlak, M.; Smith, L.; Yanchus, C.; Kurji, N.; Shahani, V.M.; Molinski, S.V. Giving drugs a second chance: Overcoming regulatory and financial hurdles in repurposing approved drugs as cancer therapeutics. Front. Oncol. 2017, 7, 273. [Google Scholar] [CrossRef]
- Schnipper, L.E.; Meropol, N. ASCO addresses the rising cost of cancer care. J. Oncol. Pract. 2009, 5, 214–215. [Google Scholar] [CrossRef]
- Jiang, N.; Doseff, A.I.; Grotewold, E. Flavones: From biosynthesis to health benefits. Plants 2016, 5, 27. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Baudry, A.; Lepiniec, L.; Grotewold, E. The Science of Flavonoids; Springer: New York, NY, USA, 2006; pp. 97–122. [Google Scholar]
- Hollman, P.C.H.; Bijsman, M.N.C.P.; van Gameren, Y.; Cnossen, E.P.J.; de Vries, J.H.M.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef]
- Hostetler, G.; Riedl, K.; Cardenas, H.; Diosa-Toro, M.; Arango, D.; Schwartz, S.; Doseff, A.I. Flavone deglycosylation increases their anti-inflammatory activity and absorption. Mol. Nutr. Food Res. 2012, 56, 558–569. [Google Scholar] [CrossRef]
- Wen, X.; Walle, T. Methylated flavonoids have greatly improved intestinal absorption and metabolic stability. Drug Metab. Dispos. 2006, 34, 1786–1792. [Google Scholar] [CrossRef]
- Surichan, S.; Androutsopoulos, V.P.; Sifakis, S.; Koutala, E.; Tsatsakis, A.; Arroo, R.R.; Boarder, M.R. Bioactivation of the citrus flavonoid nobiletin by CYP1 enzymes in MCF7 breast adenocarcinoma cells. Food Chem. Toxicol. 2012, 50, 3320–3328. [Google Scholar] [CrossRef]
- Wei, G.-J.; Hwang, L.; Tsai, C.-L. Absolute bioavailability, pharmacokinetics and excretion of 5,7,3′,4′-tetramethoxyflavone in rats. J. Funct. Food 2014, 7, 136–141. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Nicholas, C.; Batra, S.; Vargo, M.A.; Voss, O.H.; Gavrilin, M.A.; Wewers, M.D.; Guttridge, D.C.; Grotewold, E.; Doseff, A.I. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-κB through the suppression of p65 phosphorylation. J. Immunol. 2007, 179, 7121–7127. [Google Scholar] [CrossRef]
- Lin, H.Y.; Shen, S.C.; Lin, C.W.; Yang, L.Y.; Chen, Y.C. Baicalein inhibition of hydrogen peroxide-induced apoptosis via ROS-dependent heme oxygenase 1 gene expression. Biochim. Biophys. Acta 2007, 1773, 1073–1086. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J.; Castell, M. Flavonoids, inflammation and immune system. Nutrients 2016, 8, 659. [Google Scholar] [CrossRef]
- Peluso, I.; Miglio, C.; Morabito, G.; Ioannone, F.; Serafini, M. Flavonoids and immune function in human: A systematic review. Crit. Rev. Food Sci. Nutr. 2015, 55, 383–395. [Google Scholar] [CrossRef]
- Damianaki, A.; Bakogeorgou, E.; Kampa, M.; Notas, G.; Hatzoglou, A.; Panagiotou, S.; Gemetzi, C.; Kouroumalis, E.; Martin, P.M.; Castanas, E. Potent inhibitory action of red wine polyphenols on human breast cancer cells. J. Cell. Biochem. 2000, 78, 429–441. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, C.Y.; Lee, K.R.; Lin, H.J.; Chen, T.H.; Wan, L. Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. BMC Cancer 2015, 15, 958. [Google Scholar] [CrossRef]
- Cho, S.G.; Woo, S.M.; Ko, S.G. Butein suppresses breast cancer growth by reducing a production of intracellular reactive oxygen species. J. Exp. Clin. Cancer Res. 2014, 33, 51. [Google Scholar] [CrossRef] [PubMed]
- Taabodi, M.; May, E.B.; Mack, K.M.; Squibb, K.S.; Ishaque, A.B. Oxidative stress pathways of flavonoid toxicity in human breast tumor cells. Int. J. Clin. Exp. Pathol. 2017, 10, 2554–2567. [Google Scholar]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Chobot, V.; Hadacek, F. Exploration of pro-oxidant and antioxidant activities of the flavonoid myricetin. Redox Rep. 2011, 16, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Zhao, X.-C.; Tian, L.; Cao, J.-G.; Liu, F. Induction of apoptosis by 5,7-dihydroxy-8-nitrochrysin in breast cancer cells: The role of reactive oxygen species and Akt. Int. J. Oncol. 2010, 37, 1345–1352. [Google Scholar]
- Samarghandian, S.; Azimi-Nezhad, M.; Borji, A.; Hasanzadeh, M.; Jabbari, F.; Farkhondeh, T.; Samini, M. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn. Mag. 2016, 12, S436. [Google Scholar] [PubMed]
- Kim, T.H.; Woo, J.S.; Kim, Y.K.; Kim, K.H. Silibinin induces cell death through reactive oxygen species–dependent downregulation of notch-1/ERK/Akt signaling in human breast cancer cells. J. Pharmacol. Exp. Ther. 2014, 349, 268–278. [Google Scholar] [CrossRef]
- Arango, D.; Parihar, A.; Villamena, F.A.; Wang, L.; Freitas, M.A.; Grotewold, E.; Doseff, A.I. Apigenin induces DNA damage through the PKCδ-dependent activation of ATM and H2AX causing down-regulation of genes involved in cell cycle control and DNA repair. Biochem. Pharmacol. 2012, 84, 1571–1580. [Google Scholar] [CrossRef]
- Martínez-Pérez, C.; Ward, C.; Turnbull, A.K.; Mullen, P.; Cook, G.; Meehan, J.; Jarman, E.J.; Thomson, P.I.; Campbell, C.J.; McPhail, D. Antitumour activity of the novel flavonoid Oncamex in preclinical breast cancer models. Br. J. Cancer 2016, 114, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-X.; Dai, Y.-Y.; Pan, Y.-F.; Wu, X.-M.; Yang, Y.; Bian, K.; Zhang, D.-D. Total flavonoids from Radix Glycyrrhiza exert anti-inflammatory and antitumorigenic effects by inactivating iNOS signaling pathways. Evid. Based Complement. Alternat. Med. 2018, 2018, 6714282. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Fristiohady, A.; Nguyen, C.H.; Milovanovic, D.; Huttary, N.; Krieger, S.; Hong, J.; Geleff, S.; Birner, P.; Jäger, W. Apigenin and luteolin attenuate the breaching of MDA-MB231 breast cancer spheroids through the lymph endothelial barrier in vitro. Front. Pharmacol. 2018, 9, 220. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Larghi, P.; Mancino, A.; Rubino, L.; Porta, C.; Totaro, M.G.; Rimoldi, M.; Biswas, S.K.; Allavena, P.; Mantovani, A. Macrophage polarization in tumour progression. Semin. Cancer Biol. 2008, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Condeelis, J.; Pollard, J.W. Macrophages: Obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006, 124, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.W. Tumour-educated macrophages promote tumour progression and metastasis. Nat. Rev. Cancer 2004, 4, 71. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef]
- Medrek, C.; Pontén, F.; Jirström, K.; Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012, 12, 306. [Google Scholar] [CrossRef]
- De Palma, M.; Lewis, C.E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 2013, 23, 277–286. [Google Scholar] [CrossRef] [PubMed]
- DeNardo, D.G.; Brennan, D.J.; Rexhepaj, E.; Ruffell, B.; Shiao, S.L.; Madden, S.F.; Gallagher, W.M.; Wadhwani, N.; Keil, S.D.; Junaid, S.A. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011, 1, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, E.S.; Askautrud, H.A.; Kees, T.; Park, J.-H.; Plaks, V.; Ewald, A.J.; Fein, M.; Rasch, M.G.; Tan, Y.-X.; Qiu, J. Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 2012, 21, 488–503. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef]
- Ditsworth, D.; Zong, W.-X. NF-kappaB: Key mediator of inflammation-associated cancer. Cancer Biol. Ther. 2004, 3, 1214–1216. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R.J. NF-κB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Blonska, M.; Czuba, Z.; Krol, W. Effect of flavone derivatives on interleukin-1β (IL-1β) mRNA expression and IL-1β protein synthesis in stimulated RAW 264.7 macrophages. Scand. J. Immunol. 2003, 57, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-C.; Huang, Y.-T.; Tsai, S.-H.; Lin-Shiau, S.-Y.; Chen, C.-F.; Lin, J.-K. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis 1999, 20, 1945–1952. [Google Scholar] [CrossRef]
- Woo, H.-M.; Kang, J.-H.; Kawada, T.; Yoo, H.; Sung, M.-K.; Yu, R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007, 80, 926–931. [Google Scholar] [CrossRef]
- Cardenas, H.; Arango, D.; Nicholas, C.; Duarte, S.; Nuovo, G.J.; He, W.; Voss, O.H.; Gonzalez-Mejia, M.; Guttridge, D.C.; Grotewold, E. Dietary apigenin exerts immune-regulatory activity in vivo by reducing NF-κB activity, halting leukocyte infiltration and restoring normal metabolic function. Int. J. Mol. Sci. 2016, 17, 323. [Google Scholar] [CrossRef]
- Liao, Y.; Shen, W.; Kong, G.; Lv, H.; Tao, W.; Bo, P.J.P.o. Apigenin induces the apoptosis and regulates MAPK signaling pathways in mouse macrophage ANA-1 cells. PLoS ONE 2014, 9, e92007. [Google Scholar] [CrossRef]
- Nair, M.P.; Mahajan, S.; Reynolds, J.L.; Aalinkeel, R.; Nair, H.; Schwartz, S.A.; Kandaswami, C. The flavonoid quercetin inhibits proinflammatory cytokine (tumor necrosis factor alpha) gene expression in normal peripheral blood mononuclear cells via modulation of the NF-κβ system. Clin. Vaccine Immunol. 2006, 13, 319–328. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Gurley, E.C.; Zhou, H. Flavonoid apigenin inhibits lipopolysaccharide-induced inflammatory response through multiple mechanisms in macrophages. PLoS ONE 2014, 9, e107072. [Google Scholar] [CrossRef]
- Elyasinia, F.; Keramati, M.R.; Ahmadi, F.; Rezaei, S.; Ashouri, M.; Parsaei, R.; Yaghoubi, M.; Elyasinia, F.; Aboutorabi, A.; Kaviani, A. Neutrophil-lymphocyte ratio in different stages of breast cancer. Acta Med. Iran. 2017, 55, 228–232. [Google Scholar]
- Zielińska-Przyjemska, M.; Ignatowicz, E. Citrus fruit flavonoids influence on neutrophil apoptosis and oxidative metabolism. Phytother. Res. 2008, 22, 1557–1562. [Google Scholar] [CrossRef]
- Palucka, K.; Coussens, L.M.; O’shaughnessy, J. Dendritic cells, inflammation and breast cancer. Cancer J. 2013, 19, 511–516. [Google Scholar] [CrossRef]
- Yoneyama, S.; Kawai, K.; Tsuno, N.H.; Okaji, Y.; Asakage, M.; Tsuchiya, T.; Yamada, J.; Sunami, E.; Osada, T.; Kitayama, J.; et al. Epigallocatechin gallate affects human dendritic cell differentiation and maturation. J. Allergy Clin. Immunol. 2008, 121, 209–214. [Google Scholar] [CrossRef]
- Harris, G.K.; Qian, Y.; Leonard, S.S.; Sbarra, D.C.; Shi, X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J. Nutr. 2006, 136, 1517–1521. [Google Scholar] [CrossRef]
- Rathee, P.; Chaudhary, H.; Rathee, S.; Rathee, D.; Kumar, V.; Kohli, K. Mechanism of action of flavonoids as anti-inflammatory agents: A review. Inflamm. Allergy Drug Targets 2009, 8, 229–235. [Google Scholar] [CrossRef]
- Comalada, M.; Ballester, I.; Bailon, E.; Sierra, S.; Xaus, J.; Galvez, J.; de Medina, F.S.; Zarzuelo, A. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: Analysis of the structure-activity relationship. Biochem. Pharmacol. 2006, 72, 1010–1021. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Morris, M.E. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol. Pharmacol. 2004, 65, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Amin, H.; Nayak, D.; Bhatnagar, M.; Kacker, P.; Chakraborty, S.; Kitchlu, S.; Vishwakarma, R.; Goswami, A.; Ghosal, S. Assessment of microtubule depolymerization property of flavonoids isolated from Tanacetum gracile in breast cancer cells by biochemical and molecular docking approach. Chem. Biol. Interact. 2015, 239, 1–11. [Google Scholar] [CrossRef]
- Arango, D.; Morohashi, K.; Yilmaz, A.; Kuramochi, K.; Parihar, A.; Brahimaj, B.; Grotewold, E.; Doseff, A.I. Molecular basis for the action of a dietary flavonoid revealed by the comprehensive identification of apigenin human targets. Proc. Natl. Acad. Sci. USA 2013, 110, E2153–E2162. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Rajabi, H.; Kufe, D. Mucin 1 C-terminal subunit oncoprotein is a target for small-molecule inhibitors. Mol. Pharmacol. 2011, 79, 886–893. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, L.; Bertucci, A.M.; Pope, R.M.; Datta, S.K. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-κB activation pathway. Immunol. Lett. 2008, 121, 74–83. [Google Scholar] [CrossRef]
- Huang, R.-Y.; Yu, Y.-L.; Cheng, W.-C.; OuYang, C.-N.; Fu, E.; Chu, C.-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J. Immunol. 2010, 184, 6815–6821. [Google Scholar] [CrossRef]
- Seo, H.S.; Ku, J.M.; Choi, H.S.; Woo, J.K.; Lee, B.H.; Kim, D.S.; Song, H.J.; Jang, B.H.; Shin, Y.C.; Ko, S.G. Apigenin overcomes drug resistance by blocking the signal transducer and activator of transcription 3 signaling in breast cancer cells. Oncol. Rep. 2017, 38, 715–724. [Google Scholar] [CrossRef]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Mini-Rev. Med. Chem. 2017, 31, 1529–1538. [Google Scholar] [CrossRef]
- Chisholm, K.; Bray, B.J.; Rosengren, R.J. Tamoxifen and epigallocatechin gallate are synergistically cytotoxic to MDA-MB-231 human breast cancer cells. Anticancer Drugs 2004, 15, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Mishra, S.; Suman, S.; Shukla, Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Ji, P.; Liu, B.; Qiao, H.; Wang, X.; Zhou, L.; Deng, T.; Ba, Y. Apigenin enhances the cisplatin cytotoxic effect through p53-modulated apoptosis. Oncol. Lett. 2017, 13, 1024–1030. [Google Scholar] [CrossRef]
- Wu, K.; Wang, C.; D’Amico, M.; Lee, R.J.; Albanese, C.; Pestell, R.G.; Mani, S. Flavopiridol and trastuzumab synergistically inhibit proliferation of breast cancer cells: Association with selective cooperative inhibition of cyclin D1-dependent kinase and Akt signaling pathways. Mol. Cancer Ther. 2002, 1, 695–706. [Google Scholar]
- Nagaria, T.S.; Williams, J.L.; Leduc, C.; Squire, J.A.; Greer, P.A.; Sangrar, W. Flavopiridol synergizes with sorafenib to induce cytotoxicity and potentiate antitumorigenic activity in EGFR/HER-2 and mutant RAS/RAF breast cancer model systems. Neoplasia 2013, 15, 939–951. [Google Scholar] [CrossRef]
- Cao, X.; Liu, B.; Cao, W.; Zhang, W.; Zhang, F.; Zhao, H.; Meng, R.; Zhang, L.; Niu, R.; Hao, X.; et al. Autophagy inhibition enhances apigenin-induced apoptosis in human breast cancer cells. Chin. J. Cancer Res. 2013, 25, 212–222. [Google Scholar] [CrossRef]
- Ding, J.; Polier, G.; Kohler, R.; Giaisi, M.; Krammer, P.H.; Li-Weber, M. Wogonin and related natural flavones overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein resistance of tumors by down-regulation of c-FLIP protein and up-regulation of TRAIL receptor 2 expression. J. Biol. Chem. 2012, 287, 641–649. [Google Scholar] [CrossRef]
- Ando, C.; Takahashi, N.; Hirai, S.; Nishimura, K.; Lin, S.; Uemura, T.; Goto, T.; Yu, R.; Nakagami, J.; Murakami, S. Luteolin, a food-derived flavonoid, suppresses adipocyte-dependent activation of macrophages by inhibiting JNK activation. FEBS Lett. 2009, 583, 3649–3654. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.-J.; Zhang, X.; Wang, X.; Bao, B.; Qu, W.; Liu, J. Luteolin reduces obesity-associated insulin resistance in mice by activating AMPKα1 signalling in adipose tissue macrophages. Diabetologia 2016, 59, 2219–2228. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Zhang, L.; Bian, H.-X.; Xu, N.; Bao, B.; Liu, J. Quercetin reduces obesity-associated adipose tissue macrophage infiltration and inflammation in mice: A mechanism including AMPKα1/SIRT1. J. Lipid Res. 2014, 55, 363–374. [Google Scholar] [CrossRef]
- Du, G.; Lin, H.; Yang, Y.; Zhang, S.; Wu, X.; Wang, M.; Ji, L.; Lu, L.; Yu, L.; Han, G. Dietary quercetin combining intratumoral doxorubicin injection synergistically induces rejection of established breast cancer in mice. Int. Immunopharmacol. 2010, 10, 819–826. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Liang, Y.; Besch-Williford, C.; Zhang, X.; Hyder, S.M. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm. Cancer 2012, 3, 160–171. [Google Scholar] [CrossRef]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, R80. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.Y.; Banh, T.; Hsiao, Y.H.; Cole, R.M.; Straka, S.R.; Yee, L.D.; Belury, M.A.J. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. Food. Res. 2017, 61, 1600934. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Pang, Z.-R.; Pan, M.-R.; Zhang, W. Flavonoid-enriched extract from Hippophae rhamnoides seed reduces high fat diet induced obesity, hypertriglyceridemia, and hepatic triglyceride accumulation in C57BL/6 mice. Pharm. Biol. 2017, 55, 1207–1214. [Google Scholar] [CrossRef]
- Rossi, E.L.; Khatib, S.A.; Doerstling, S.S.; Bowers, L.W.; Pruski, M.; Ford, N.A.; Glickman, R.D.; Niu, M.; Yang, P.; Cui, Z. Resveratrol inhibits obesity-associated adipose tissue dysfunction and tumor growth in a mouse model of postmenopausal claudin-low breast cancer. Mol. Carcinog. 2018, 57, 393–407. [Google Scholar] [CrossRef]
- Huang, Z.; Fang, F.; Wang, J.; Wong, C.-W.J. Structural activity relationship of flavonoids with estrogen-related receptor gamma. FEBS Lett. 2010, 584, 22–26. [Google Scholar] [CrossRef]
- Pick, A.; Müller, H.; Mayer, R.; Haenisch, B.; Pajeva, I.K.; Weigt, M.; Bönisch, H.; Müller, C.E.; Wiese, M. Structure–activity relationships of flavonoids as inhibitors of breast cancer resistance protein (BCRP). Bioorg. Med. Chem. 2011, 19, 2090–2102. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Tsukahara, S.; Asada, S.; Sugimoto, Y. Phytoestrogens/flavonoids reverse breast cancer resistance protein/ABCG2-mediated multidrug resistance. Bioorg. Med. Chem. 2004, 64, 4346–4352. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Masuyama, K.; Yoshioka, S.; Hasegawa, H.; Mitsuhashi, J.; Sugimoto, Y. Flavonoids inhibit breast cancer resistance protein-mediated drug resistance: Transporter specificity and structure–activity relationship. Cancer Chemother. Pharmacol. 2007, 60, 789–797. [Google Scholar] [CrossRef]
- Juvale, K.; Stefan, K.; Wiese, M.J. Synthesis and biological evaluation of flavones and benzoflavones as inhibitors of BCRP/ABCG2. Eur. J. Med. Chem. 2013, 67, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, L.; Xu, Y.; Jiang, S.; Shao, Y. Deciphering the binding behavior of flavonoids to the cyclin dependent kinase 6/cyclin D complex. PLOS ONE 2018, 13, e0196651. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Giuliano, A.E.; Law, R.E.; Van, A.H. Apigenin inhibits growth and induces G2/M arrest by modulating cyclin-CDK regulators and ERK MAP kinase activation in breast carcinoma cells. Anticancer Res. 2001, 21, 413–420. [Google Scholar] [PubMed]
- Hermenean, A.; Ardelean, A. Targeting the cytoskeleton with plant-bioactive compounds in cancer therapy. In Cytoskeleton-Structure, Dynamics, Function and Disease; IntechOpen: London, UK, 2017. [Google Scholar]
- Xuan, Y.; Wang, J.; Ban, L.; Lu, J.-J.; Yi, C.; Li, Z.; Yu, W.; Li, M.; Xu, T.; Yang, W. hnRNPA2/B1 activates cyclooxygenase-2 and promotes tumor growth in human lung cancers. Mol. Oncol. 2016, 10, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Venables, J.P.; Klinck, R.; Koh, C.; Gervais-Bird, J.; Bramard, A.; Inkel, L.; Durand, M.; Couture, S.; Froehlich, U.; Lapointe, E.; et al. Cancer-associated regulation of alternative splicing. Nat. Struct. Mol. Biol. 2009, 16, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Srinivasan, S.; Guja, K.; Mejia, E.; Garcia-Diaz, M.; Johnson, F.B.; Ruthel, G.; Kaufman, B.A.; Rappaport, E.F.; Glineburg, M.R. HnRNPA2 is a novel histone acetyltransferase that mediates mitochondrial stress-induced nuclear gene expression. Cell Discov. 2016, 2, 16045. [Google Scholar] [CrossRef]
- Han, N.; Li, W.; Zhang, M.J. The function of the RNA-binding protein hnRNP in cancer metastasis. J. Cancer Res. Ther. 2013, 9, 129–134. [Google Scholar]
- Aslan, E.; Guler, C.; Adem, S.J. In vitro effects of some flavonoids and phenolic acids on human pyruvate kinase isoenzyme M2. J. Enzyme Inhib. Med. Chem. 2016, 31, 314–317. [Google Scholar] [CrossRef]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J.L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2010, 463, 364–368. [Google Scholar] [CrossRef]
- Lin, Y.; Lv, F.; Liu, F.; Guo, X.; Fan, Y.; Gu, F.; Gu, J.; Fu, L.J. High expression of pyruvate kinase M2 is associated with chemosensitivity to epirubicin and 5-fluorouracil in breast cancer. J. Cancer 2015, 6, 1130. [Google Scholar] [CrossRef]
- Chiavarina, B.; Whitaker-Menezes, D.; Martinez-Outschoorn, U.E.; Witkiewicz, A.K.; Birbe, R.; Howell, A.; Pestell, R.G.; Smith, J.; Daniel, R.; Sotgia, F.; et al. Pyruvate kinase expression (PKM1 and PKM2) in cancer-associated fibroblasts drives stromal nutrient production and tumor growth. Cancer Biol. Ther. 2011, 12, 1101–1113. [Google Scholar] [CrossRef]
- Wong, N.; De Melo, J.; Tang, D. PKM2, a central point of regulation in cancer metabolism. Int. J. Cell Biol. 2013, 2013. [Google Scholar] [CrossRef]
- Goode, G.; Gunda, V.; Chaika, N.V.; Purohit, V.; Yu, F.; Singh, P.K. MUC1 facilitates metabolomic reprogramming in triple-negative breast cancer. PLoS ONE 2017, 12, e0176820. [Google Scholar]
- Li, J.; Inoue, J.; Choi, J.-M.; Nakamura, S.; Yan, Z.; Fushinobu, S.; Kamada, H.; Kato, H.; Hashidume, T.; Shimizu, M.J. Identification of the flavonoid luteolin as a repressor of the transcription factor hepatocyte nuclear factor 4α. J. Biol. Chem. 2015, 290, 24021–24035. [Google Scholar] [CrossRef]
- Bosetti, C.; Spertini, L.; Parpinel, M.; Gnagnarella, P.; Lagiou, P.; Negri, E.; Franceschi, S.; Montella, M.; Peterson, J.; Dwyer, J.; et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol. Biomarkers Prev. 2005, 14, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Schroeder, J.C.; Teitelbaum, S.L.; Neugut, A.I.; Gammon, M.D. Dietary flavonoid intake and breast cancer risk among women on long island. Am. J. Epidemiol. 2007, 165, 514–523. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef] [PubMed]
- Peeters, P.; Keinan-Boker, L.; Van Der Schouw, Y.; Grobbee, D. Phytoestrogens and breast cancer risk. Breast Cancer Res. Treat. 2003, 77, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; Lagiou, P.; Samoli, E.; Lagiou, A.; Katsouyanni, K.; La Vecchia, C.; Dwyer, J.; Trichopoulos, D. Flavonoid intake and breast cancer risk: A case–control study in Greece. Br. J. Cancer 2003, 89, 1255–1259. [Google Scholar] [CrossRef]
- Parihar, A.; Grotewold, E.; Doseff, A.I. Flavonoid dietetics: Mechanisms and emerging roles of plant nutraceuticals. In Pigments in Fruits and Vegetables; Springer: New York, NY, USA, 2015; pp. 93–126. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Choi, J.-A.; Kim, J.-Y.; Lee, J.-Y.; Kang, C.-M.; Kwon, H.-J.; Yoo, Y.-D.; Kim, T.-W.; Lee, Y.-S.; Lee, S.-J. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int. J. Oncol. 2001, 19, 837–844. [Google Scholar] [CrossRef]
- Madunić, I.V.; Madunić, J.; Antunović, M.; Paradžik, M.; Garaj-Vrhovac, V.; Breljak, D.; Marijanović, I.; Gajski, G. Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 537–550. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef]
- Sun, D.-W.; Zhang, H.-D.; Mao, L.; Mao, C.-F.; Chen, W.; Cui, M.; Ma, R.; Cao, H.-X.; Jing, C.-W.; Wang, Z.; et al. Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating MiRNAs. Cell Physiol. Biochem. 2015, 37, 1693–1711. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Hu, J.-J.; Fu, R.-Q.; Liu, X.; Zhang, Y.-H.; Li, J.; Liu, L.; Li, Y.-N.; Deng, Q.; Luo, Q.-S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Capasso, M.; Hagemann, T. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Johann, D.J. Cancer and the tumor microenvironment: A review of an essential relationship. Cancer Chemother. Pharmacol. 2009, 63, 571–582. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.-J.; Guan, R.; Du, L.; Gao, J.; Fu, X.-L.J. Strategies to target glucose metabolism in tumor microenvironment on cancer by flavonoids. Nutr. Cancer 2017, 69, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Buache, E.; Chenard, M.-P.; Dali-Youcef, N.; Rio, M.-C. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int. J. Dev. Biol. 2011, 55, 851–859. [Google Scholar] [CrossRef]
- Hoy, A.J.; Balaban, S.; Saunders, D.N. Adipocyte–tumor cell metabolic crosstalk in breast cancer. Trends Mol. Med. 2017, 23, 381–392. [Google Scholar] [CrossRef]
- Nickel, A.; Blücher, C.; Al Kadri, O.; Schwagarus, N.; Müller, S.; Schaab, M.; Thiery, J.; Burkhardt, R.; Stadler, S.C. Adipocytes induce distinct gene expression profiles in mammary tumor cells and enhance inflammatory signaling in invasive breast cancer cells. Sci. Rep. 2018, 8, 9482. [Google Scholar] [CrossRef]
- Bougaret, L.; Delort, L.; Billard, H.; Le Huede, C.; Boby, C.; De la Foye, A.; Rossary, A.; Mojallal, A.; Damour, O.; Auxenfans, C. Adipocyte/breast cancer cell crosstalk in obesity interferes with the anti-proliferative efficacy of tamoxifen. PLoS ONE 2018, 13, e0191571. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-L.; Yen, G.-C. Effects of flavonoids and phenolic acids on the inhibition of adipogenesis in 3T3-L1 adipocytes. J. Agric. Food Chem. 2007, 55, 8404–8410. [Google Scholar] [CrossRef] [PubMed]
- Harmon, A.W.; Harp, J.B. Differential effects of flavonoids on 3T3-L1 adipogenesis and lipolysis. Am. J. Physiol. Cell Physiol. 2001, 280, C807–C813. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.H.; Corrêa, R.; Farinasso, C.M.; de Sant’Ana Dourado, L.P.; Magalhães, K.G. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: New implications in cancer progression. Front. Immunol. 2017, 8, 1129. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Li, J.; Zhang, H.; Kitamura, T.; Zhang, J.; Campion, L.R.; Kaiser, E.A.; Snyder, L.A.; Pollard, J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 2011, 475, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Redmon, N.; Mazzio, E.; Soliman, K.F. Apigenin inhibits TNFα/IL-1α-induced CCL2 release through IKBK-epsilon signaling in MDA-MB-231 human breast cancer cells. PLoS ONE 2017, 12, e0175558. [Google Scholar] [CrossRef]
- Zheng, X.; Turkowski, K.; Mora, J.; Brüne, B.; Seeger, W.; Weigert, A.; Savai, R. Redirecting tumor-associated macrophages to become tumoricidal effectors as a novel strategy for cancer. Oncotarget 2017, 8, 48436–48452. [Google Scholar] [CrossRef]
- Genard, G.; Lucas, S.; Michiels, C. Reprogramming of tumor-associated macrophages with anticancer therapy therapies: Radiotherapy versus chemo-and immunotherapies. Front. Immunol. 2017, 8, 828. [Google Scholar] [CrossRef]
- De Oliveira Júnior, R.G.; Ferraz, C.A.A.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef]
- Guestini, F.; McNamara, K.M.; Ishida, T.; Sasano, H. Triple negative breast cancer chemosensitivity and chemoresistance: Current advances in biomarkers indentification. Expert Opin. Ther. Targets 2016, 20, 705–720. [Google Scholar] [CrossRef]
- Di Pietro, A.; Conseil, G.; Perez-Victoria, J.; Dayan, G.; Baubichon-Cortay, H.; Trompier, D.; Steinfels, E.; Jault, J.-M.; De Wet, H.; Maitrejean, M.; et al. Modulation by flavonoids of cell multidrug resistance mediated by P-glycoprotein and related ABC transporters. Cell. Mol. Life Sci. 2002, 59, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.K.; Parra, I.; Lemieux, P.; Oesterreich, S.; Hilsenbeck, S.G.; Fuqua, S.A. Hsp27 overexpression inhibits doxorubicin–induced apoptosis in human breast cancer cells. Breast Cancer Res. Treat. 1999, 56, 185–194. [Google Scholar] [CrossRef]
- Díaz-Chávez, J.; Fonseca-Sánchez, M.A.; Arechaga-Ocampo, E.; Flores-Pérez, A.; Palacios-Rodríguez, Y.; Domínguez-Gómez, G.; Marchat, L.A.; Fuentes-Mera, L.; Mendoza-Hernández, G.; Gariglio, P. Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy. PLoS ONE 2013, 8, e64378. [Google Scholar] [CrossRef] [PubMed]
- Voss, O.H.; Batra, S.; Kolattukudy, S.J.; Gonzalez-Mejia, M.E.; Smith, J.B.; Doseff, A.I. Binding of caspase-3 prodomain to heat shock protein 27 regulates monocyte apoptosis by inhibiting caspase-3 proteolytic activation. J. Biol. Chem. 2007, 282, 25088–25099. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.-N.; Huang, J.-M.; Xiong, X.-K.; Chen, M.-F.; Ong, C.-N.; Shen, H.-M.; Yang, X.-F. Chrysin promotes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced apoptosis in human cancer cell lines. Oncogene 2004, 23, 7712. [Google Scholar] [CrossRef]
- Shi, R.-X.; Ong, C.-N.; Shen, H.-M. Luteolin sensitizes tumor necrosis factor-α-induced apoptosis in human tumor cells. Oncogene 2004, 23, 7712. [Google Scholar] [CrossRef]
- Horinaka, M.; Yoshida, T.; Shiraishi, T.; Nakata, S.; Wakada, M.; Sakai, T. The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor–related apoptosis-inducing ligand. Mol. Cancer Ther. 2006, 5, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.; Barnes, S. Genistein inhibition of the growth of human breast cancer cells: Independence from estrogen receptors and the multi-drug resistance gene. Biochem. Biophys. Res. Commun. 1991, 179, 661–667. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, T.T.; Cunnick, J.E.; Murphy, P.A.; Hendrich, S. Daidzein and genistein glucuronides in vitro are weakly estrogenic and activate human natural killer cells at nutritionally relevant concentrations. J. Nutr. 1999, 129, 399–405. [Google Scholar] [CrossRef]
- Fenga, C.; Costa, C.; Caruso, E.; Raffa, L.; Alibrando, C.; Gangemi, S.; Docea, A.O.; Tsatsakis, A.M. Current evidence on the protective effect of dietary polyphenols on breast cancer. Farmacia 2016, 64, 1–12. [Google Scholar]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: A randomized clinical trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Turati, F.; Carioli, G.; Bravi, F.; Ferraroni, M.; Serraino, D.; Montella, M.; Giacosa, A.; Toffolutti, F.; Negri, E.; Levi, F.; et al. Mediterranean diet and breast cancer risk. J. Nutr. 1999, 129, 399–405. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar] [CrossRef]
- Yamamoto, S.; Sobue, T.; Kobayashi, M.; Sasaki, S.; Tsugane, S. Soy, isoflavones, and breast cancer risk in Japan. J. Natl. Cancer Inst. 2003, 95, 906–913. [Google Scholar] [CrossRef]
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar] [CrossRef]
- Cho, W. Cancer Chemoprevention and Treatment by Diet Therapy; Springer: New York, NY, USA, 2013. [Google Scholar]
- Caballero, B. The global epidemic of obesity: An overview. Epidemiol Rev. 2007, 29, 1–5. [Google Scholar] [CrossRef]
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. North Am. 2011, 34, 717–732. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Cecchini, R.S.; Costantino, J.P.; Cauley, J.A.; Cronin, W.M.; Wickerham, D.L.; Land, S.R.; Weissfeld, J.L.; Wolmark, N. Body mass index and the risk for developing invasive breast cancer among high-risk women in NSABP P-1 and STAR breast cancer prevention trials. Cancer Prev. Res. (Phila.) 2012, 5, 583–592. [Google Scholar] [CrossRef]
- Neuhouser, M.L.; Aragaki, A.K.; Prentice, R.L.; Manson, J.E.; Chlebowski, R.; Carty, C.L.; Ochs-Balcom, H.M.; Thomson, C.A.; Caan, B.J.; Tinker, L.F. Overweight, obesity, and postmenopausal invasive breast cancer risk: A secondary analysis of the women’s health initiative randomized clinical trials. JAMA Oncol. 2015, 1, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.M.; Press, M.F.; Haile, R.W.; Lynch, C.F.; Glaser, S.L.; Schildkraut, J.; Gammon, M.D.; Thompson, W.D.; Bernstein, J.L. Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast Cancer Res. Treat. 2011, 130, 587–597. [Google Scholar] [CrossRef]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a risk factor for triple-negative breast cancers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef]
- Yang, X.R.; Chang-Claude, J.; Goode, E.L.; Couch, F.J.; Nevanlinna, H.; Milne, R.L.; Gaudet, M.; Schmidt, M.K.; Broeks, A.; Cox, A. Associations of breast cancer risk factors with tumor subtypes: A pooled analysis from the Breast Cancer Association Consortium studies. J. Natl. Cancer Inst. 2010, 103, 250–263. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Chang, S.; Buzdar, A.U.; Hursting, S.D. Inflammatory breast cancer and body mass index. J. Clin. Oncol. 1998, 16, 3731–3735. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Hudis, C.A.; Dannenberg, A.J. Obesity and inflammation: New insights into breast cancer development and progression. Am. Soc. Clin. Oncol. Educ. Book. 2013, 33, 46–51. [Google Scholar] [CrossRef]
- Vaysse, C.; Lømo, J.; Garred, Ø.; Fjeldheim, F.; Lofteroed, T.; Schlichting, E.; McTiernan, A.; Frydenberg, H.; Husøy, A.; Lundgren, S. Inflammation of mammary adipose tissue occurs in overweight and obese patients exhibiting early-stage breast cancer. NPJ Breast Cancer 2017, 3, 19. [Google Scholar] [CrossRef]
- Sun, X.; Casbas-Hernandez, P.; Bigelow, C.; Makowski, L.; Jerry, D.J.; Schneider, S.S.; Troester, M.A. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Cancer Res. Treat. 2012, 131, 1003–1012. [Google Scholar] [CrossRef]
- Osman, M.A.; Hennessy, B.T. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin. Med. Insights Oncol. 2015, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Cowen, S.; McLaughlin, S.; Hobbs, G.; Coad, J.; Martin, K.; Olfert, I.; Vona-Davis, L. High-fat, high-calorie diet enhances mammary carcinogenesis and local inflammation in MMTV-PyMT mouse model of breast cancer. Cancers 2015, 7, 1125–1142. [Google Scholar] [CrossRef]
- Cranford, T.L.; Velázquez, K.T.; Enos, R.T.; Sougiannis, A.T.; Bader, J.E.; Carson, M.S.; Bellone, R.R.; Chatzistamou, I.; Nagarkatti, M.; Murphy, E.A.; et al. Effects of high fat diet-induced obesity on mammary tumorigenesis in the PyMT/MMTV murine model. Cancer Biol. Ther. 2019, 20, 487–496. [Google Scholar] [CrossRef]
- Wang, Y.; Gapstur, S.M.; Gaudet, M.M.; Peterson, J.J.; Dwyer, J.T.; McCullough, M.L. Evidence for an association of dietary flavonoid intake with breast cancer risk by estrogen receptor status is limited–3. J. Nutr. 2014, 144, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.J.; Caan, B.J.; Chen, W.Y.; Lu, W.; Chen, Z.; Kwan, M.L.; Flatt, S.W.; Zheng, Y.; Zheng, W.; Pierce, J.P. Soy food intake after diagnosis of breast cancer and survival: An in-depth analysis of combined evidence from cohort studies of US and Chinese women. Am. J. Clin. Nutr. 2012, 96, 123–132. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, J.; Park, K.; Lim, S.; Shin, A.; Sung, M.; Ro, J. Effect of dietary soy intake on breast cancer risk according to menopause and hormone receptor status. Eur. J. Clin. Nutr. 2010, 64, 924. [Google Scholar] [CrossRef][Green Version]
- Vitolins, M.Z.; Milliron, B.-J.; Hopkins, J.O.; Fulmer, A.; Lawrence, J.; Melin, S.; Case, D. Weight loss intervention in survivors of ER/PR-negative breast cancer. Clin. Med. Insights Women’s Health 2014, 7, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Chatterton, R.T.; Michel, N.; Bryk, M.; Lee, O.; Ivancic, D.; Heinz, R.; Zalles, C.M.; Helenowski, I.B.; Jovanovic, B.D. Soy isoflavone supplementation for breast cancer risk reduction: A randomized phase II trial. Cancer Prev. Res. 2012, 5, 309–319. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Gadgeel, S.; Parchment, R.; Lorusso, P.; Philip, P.A. A phase I study of flavopiridol and docetaxel. Investig. New Drugs 2006, 24, 305. [Google Scholar] [CrossRef]
- Maskarinec, G.; Suzuki, S.; Pagano, I.S.; Morimoto, Y.; Franke, A.A.; Ehya, H. Cytology in nipple aspirate fluid during a randomized soy food intervention among premenopausal women. Nutr. Cancer 2013, 65, 1116–1121. [Google Scholar] [CrossRef]
- Pop, E.A.; Fischer, L.M.; Coan, A.D.; Gitzinger, M.; Nakamura, J.; Zeisel, S.H. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis and estrogenic outcomes in healthy, postmenopausal women—A Phase I clinical trial. Menopause 2008, 15, 684. [Google Scholar] [CrossRef]
- Gemcitabine Hydrochloride and Genistein in Treating Women with Stage IV Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00244933?term=genistein&cond=breast+cancer&rank=1 (accessed on 24 January 2019).
- Docetaxel and Flavopiridol in Treating Patients with Locally Advanced or Metastatic Breast Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT00020332?term=flavopiridol&cond=cancer&rank=4 (accessed on 24 January 2019).
- Wu, A.; Koh, W.; Wang, R.; Lee, H.; Yu, M. Soy intake and breast cancer risk in Singapore Chinese Health Study. Br. J. Cancer 2008, 99, 196. [Google Scholar] [CrossRef]
- Wang, L.; Lee, I.M.; Zhang, S.M.; Blumberg, J.B.; Buring, J.E.; Sesso, H.D. Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 2009, 89, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Nakamura, K.; Tamai, Y.; Tsuji, M.; Kawachi, T.; Hori, A.; Takeyama, N.; Tanabashi, S.; Matsushita, S.; Tokimitsu, N. Soy isoflavone intake and breast cancer risk in Japan: From the Takayama study. Int. J. Cancer 2013, 133, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Torres-Sanchez, L.; Galvan-Portillo, M.; Wolff, M.S.; Lopez-Carrillo, L. Dietary consumption of phytochemicals and breast cancer risk in Mexican women. Public Health Nutr. 2009, 12, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Thanos, J.; Cotterchio, M.; Boucher, B.A.; Kreiger, N.; Thompson, L.U. Adolescent dietary phytoestrogen intake and breast cancer risk (Canada). Cancer Causes Control 2006, 17, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Ferrari, P.; González, C.A.; Tjønneland, A.; Olsen, A.; Bredsdorff, L.; Overvad, K.; Touillaud, M.; Perquier, F.; Fagherazzi, G.; et al. Dietary flavonoid and lignan intake and breast cancer risk according to menopause and hormone receptor status in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Breast Cancer Res. Treat. 2013, 139, 163–176. [Google Scholar] [CrossRef]
- Dos Santos Silva, I.; Mangtani, P.; McCormack, V.; Bhakta, D.; McMichael, A.J.; Sevak, L. Phyto-oestrogen intake and breast cancer risk in South Asian women in England: Findings from a population-based case-control study. Cancer Causes Control 2004, 15, 805–818. [Google Scholar] [CrossRef]
- Iwasaki, M.; Hamada, G.S.; Nishimoto, I.N.; Netto, M.M.; Motola, J.; Laginha, F.M.; Kasuga, Y.; Yokoyama, S.; Onuma, H.; Nishimura, H.; et al. Dietary isoflavone intake and breast cancer risk in case–control studies in Japanese, Japanese Brazilians, and non-Japanese Brazilians. Breast Cancer Res. Treat. 2009, 116, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ho, S.C.; Lin, F.; Cheng, S.; Fu, J.; Chen, Y. Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci. 2010, 101, 501–507. [Google Scholar] [CrossRef]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; de Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-de Jong, J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The R otterdam study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Maskarinec, G.; Park, S.-Y.; Ettienne, R.; Matsuno, R.K.; Long, C.; Steffen, A.D.; Henderson, B.E.; Kolonel, L.N.; Le Marchand, L. Dietary isoflavone intake is not statistically significantly associated with breast cancer risk in the Multiethnic Cohort. Br. J. Nutr. 2014, 112, 976–983. [Google Scholar] [CrossRef]
- Crew, K.D.; Brown, P.; Greenlee, H.; Bevers, T.B.; Arun, B.K.; Hudis, C.A.; McArthur, H.L.; Chang, J.; Rimawi, M.F.; Vornik, L.; et al. Phase IB randomized, double-blinded, placebo-controlled, dose escalation study of polyphenon E in women with hormone receptor-negative breast cancer. Cancer Prev. Res. 2012, 5, 1144–1154. [Google Scholar] [CrossRef]
- Dostal, A.M.; Samavat, H.; Bedell, S.; Torkelson, C.; Wang, R.; Swenson, K.; Le, C.; Wu, A.H.; Ursin, G.; Yuan, J.-M.J.F.; et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: Results of the Minnesota Green Tea Trial. Food Chem. Toxicol. 2015, 83, 26–35. [Google Scholar] [CrossRef]
- A Clinical Trial Comparing Gemcitabine and Carboplatin with and without P276-00 in Subjects with Metastatic Triple Negative Breast Cancer, with a Run-in of Escalating Dose of P276-00 Added to Gemcitabine and Carboplatin. Available online: https://clinicaltrials.gov/ct2/show/NCT01333137?term=flavone&cond=cancer&rank=1 (accessed on 24 January 2019).
- Effects of Soy Compounds on Breast Cancer, Prostate Cancer, and Bone Health. Available online: https://clinicaltrials.gov/ct2/show/NCT00200824?term=isoflavone&cond=cancer&rank=10 (accessed on 24 January 2019).
- Wu, A.H.; Spicer, D.; Garcia, A.; Tseng, C.-C.; Hovanessian-Larsen, L.; Sheth, P.; Martin, S.E.; Hawes, D.; MacDonald, H.; Tripathy, D. Double-blind randomized 12-month soy intervention had no effects on breast MRI fibroglandular tissue density or mammographic density. Cancer Prev. Res. 2015, 8, 942–951. [Google Scholar] [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Vitaglione, P.; Donnarumma, G.; Napolitano, A.; Galvano, F.; Gallo, A.; Scalfi, L.; Fogliano, V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007, 137, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Shukla, S.; Goel, A.; Sharma, P.; Khattri, S.; Kumar Pant, K. Nutrigenomics in Breast Cancer; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Steiner, C.; Arnould, S.; Scalbert, A.; Manach, C. Isoflavones and the prevention of breast and prostate cancer: New perspectives opened by nutrigenomics. Br. J. Nutr. 2008, 99, ES78–ES108. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Rimbach, G. Which sources of flavonoids: Complex diets or dietary supplements? Adv. Nutr. 2011, 2, 8–14. [Google Scholar] [CrossRef] [PubMed]
| Subtype | Biomarker Status | Prognosis |
|---|---|---|
| Luminal A | ER+ and/or PR+, HER2−, Ki67− | Good |
| Luminal B | ER+ and/or PR+, HER2−, Ki67+ ER+ and/or PR+, HER2+, Ki67+ | Medium |
| HER2 enriched | ER−, PR−, HER2+ | Poor |
| Basal-like | ER−, PR−, HER2−, basal markers (keratin 5, 6, 14, 17, EGFR etc.) | Poor |
| Normal-like | ER+ and/or PR+, HER2−, Ki67− | Medium |
| Study Type | Experimental Model | Treatments | Dose | Results | Reference |
|---|---|---|---|---|---|
| Cellular Studies | T47D and MCF-7 | Wine polyphenols | 1 pM–100 nM | Inhibited growth and antagonize H2O2 | [56] |
| HCC70, BT-474 and T47D | Butein | 0.001–100 μg/mL | Induced apoptosis through ROS reduction | [58] | |
| MDA-MB-468 | Naringenin | 2.5–50 μM | Oxidative stress induced apoptosis | [59] | |
| MDA-MB-231 and MDA-MB-468 | Myricetin | 20–100 μM | Oxidative stress induced apoptosis | [60] | |
| MDA-MB-453 | 5,7-dihydroxy, 8-nitrochrysin | 2–8 μM | Induced apoptosis by generation of ROS | [63] | |
| MDA-MB-231 | Silibinin | 30 μM | Induced apoptosis | [64] | |
| Human monocytes and RAW 264.7 | Apigenin | 0.1–25 μM | Reduced NFκB phosphorylation, TNF-α and IL-1β expression | [52] | |
| RAW 264.7 | Chrysin | 30 μM | Suppression IL-1β expression | [82] | |
| RAW 264.7 | Apigenin | 10–25 μM | Reduced NOS and COX expression | [83] | |
| ANA-1 | Apigenin | 12.5–200 μM | Induced apoptosis | [84] | |
| Human PBMC | Quercetin | 1–50 μM | Inhibited TNF-α expression | [87] | |
| Human Neutrophils | Hesperidin | 1–100 μM | Reduced ROS generation and induced apoptosis | [90] | |
| Human Dendritic cells | EGCG | 10–100 μM | Induced apoptosis | [92] | |
| RAW 264.7 | Luteolin | 25–100 μM | Inhibited COX-2 and xanthine oxidase expression | [93] | |
| Bone marrow derived mouse macrophages | Quercetin and Kaempferol | 25–50 μM | Inhibited TNF-α expression | [95] | |
| MCF-7 | Apigenin and Chrysin | 10–50 μM | BCRP inhibitors | [96] | |
| MCF-7 and T47D | Methoxyflavones from Tanacetum gracile | 1.5–5 μM | Induced cell cycle arrest through tubulin binding | [97] | |
| MDA-MB-231 | Apigenin | 25 μM | Inhibited hnRNPA2 dimerization affecting its splicing activity | [98] | |
| MCF-7 | Apigenin | 25–100 μM | Suppressed MUC-1 expression and induced apoptosis | [99] | |
| Cellular Study | CD4 T cells | Apigenin | 12.5–75 μM | Potentiated activation induced cell death by suppressing NFκB regulated anti-apoptotic pathways | [100] |
| Dendritic cells | Quercetin | 50 μM | Attenuated LPS induced DC activation | [101] | |
| MCF-7 | Apigenin | 20–80 μM | Reduced cell growth and expression of MDR1 and P-gp in MCF-7-doxorubicin resistant cells | [102] | |
| MCF-7, MDA-MB-231 and HMF | Rutin | 20 μM | Increased the cytotoxicity of cyclophosphamide and methotrexate | [103] | |
| MDA-MB-231 | EGCG | 10–25 μM | Synergistic enhancement of cytotoxicity with tamoxifen | [104] | |
| MCF-7 | Resveratrol | 50–250 μM | Increased sensitivity to doxorubicin | [79] | |
| MDA-MB-231 and MCF-7 | Resveratrol | 80–180 μM | Synergistic inhibition of growth with doxorubicin | [105] | |
| MCF-7 | Apigenin | 30 μM | Enhanced cisplatin cytotoxic activity | [106] | |
| BT-474 and SK-BR3 | Flavopiridol | 50–100 nM | Synergistic inhibition of cell proliferation with trastuzumab | [107] | |
| MDA-MB-231, MDA-MB-468 and SK-BR3 | Flavopiridol | 0.2 μM | Enhanced sorafenib induced cytotoxicity | [108] | |
| BT47D and MDA-MB-231 | Apigenin | 10–80 μM | Induced apoptosis and autophagy | [109] | |
| MDA-MB-231 | Wogonin | 50–100 μM | Sensitized TRAIL-induced apoptosis | [110] | |
| RAW 264.7 macrophages and 3T3-L1 adipocytes co-culture | Luteolin | 1–20 μM | Suppressed the adipocyte-dependent activation ofmacrophage | [111] | |
| 3D Study | MDA-MB-231 | Apigenin and Luteolin | 20 μM | Attenuate growth and intravasation through endothelial barrier | [69] |
| Animal Study | BALB/C-Tg (NFκB-RE-luc)-Xen mice | Apigenin | 50 mg/kg body weight | Reduced NFκB activity in lungs in vivo | [85] |
| CD-1 immunodeficient mice bearing MDA-MB-231 tumor | Oncamex | 25 mg/kg body weight | Inhibited tumor growth | [67] | |
| Athymic nu/nu nude mice bearing MDA-MB-231 tumors | Radix Glycyrrhiza extracts | 20–100 mg/kg body weight | Attenuated tumor growth through iNOS inhibition | [68] | |
| C57BL/6 mice | Luteolin | HFD with 0.01% luteolin | Inhibited inflammatory macrophage polarization in adipose tissue | [112] | |
| C57BL/6 mice | Quercetin | HFD with 0.1% luteolin | Attenuated macrophage recruitment and modulated M1/M2 macrophage ratio | [113] | |
| BALB/c mice bearing 4T1 tumors | Quercetin | 5mg/kg body weight | Synergistic inhibition of tumor growth with doxorubicin | [114] | |
| Athymic nu/nu nude mice bearing BT47D tumors | Apigenin | 50 mg/kg body weight | Inhibited the progression progestin dependent BT-474 xenograft tumors in nude mice through apoptosis | [115] | |
| Athymic nu/nu nude mice bearing MDA-MB-231 tumors | Apigenin | 25–50 mg/kg body weight | Inhibited tumor proliferation and proteasome activity | [116] | |
| Ovariectomized C57BL/6 mice injected with E0771 cells | Naringenin | HFD with 1–3% naringenin | Reduced adipose tissue mass and ameliorated adipose tissue inflammation | [117] | |
| C57BL/6 mice | Hippophae rhamnoides L. seeds extracts | 100–300 mg/kg body weight | Significant anti-obesity and anti-inflammatory effect | [118] | |
| Ovariectomized female C57BL/6 mice | Resveratrol | 300–600 mg/kg body weight | Inhibited obesity-associated increases in claudin-low mammary tumor growth and macrophage infiltration | [119] |
| Subclass of Flavonoid | Study Type | Region | Cases/Controls | Association with BC Risk | Reference |
|---|---|---|---|---|---|
| Flavones | Hospital based | Greece | 820/1548 | Inverse | [143] |
| Flavonols, Flavones | Hospital based | Italy | 2569/2588 | Inverse | [139] |
| Flavones | Population-based | New York | 1434/1440 | Inverse | [140] |
| Flavonoids | Cohort | America | 1351/38,408 | No effect | [213] |
| Isoflavones | Cohort | Singapore and China | 629/35,303 | Inverse | [214] |
| Isoflavones | Cohort | Japan | 134/15,264 | Inverse | [215] |
| Flavonols, Flavones | Hospital based | Mexico | 141/141 | Inverse | [216] |
| Isoflavones | Population-based | Canada | 3024/3420 | Inverse | [217] |
| Isoflavones | Hospital-based | Korea | 358/360 | Inverse | [205] |
| Flavonoids | Cohort | Europe | 11,576/334,850 | No effect | [218] |
| Flavone, Flavan-3-ol | Cohort | America | 56,630 | Inverse | [203] |
| Isoflavones | Population-based | England | 240/477 | Inverse | [219] |
| Isoflavones | Hospital-based | Japan, Brazil | 390/390 | Inverse | [220] |
| Isoflavones | Hospital-based | China | 438/438 | Inverse | [221] |
| Flavonoids | Cohort | Dutch | 199/3209 | Inverse | [222] |
| Isoflavones | Cohort | Multiethnic | 4769/84,450 | No effect | [223] |
| Flavonoid Subclass/Name | Study Type | Detail | Enrollment | Comments | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|
| Genistein (with Gemcitabine) | Phase II | Stage IV BC | 17 | No effect | NCT00244933 [211] |
| Genistein | Phase II | NA | 126 | No effect | NCT00290758 [207] |
| Genistein | Phase I | NA | 30 | Dose rangestudy | NCT00099008 [210] |
| Catechin | Phase I | HER2-, stage I-III BC | 40 | Dose range study | NCT00516243 [224] |
| Catechin | Phase II | NA | 1075 | No significant effect | NCT00917735 [225] |
| P276-00, flavone derived | Phase I | TNBC | 11 | NA | NCT01333137 [226] |
| Soy Isoflavone | Phase II | Post-menopausal | NA | NA | NCT00200824 [227] |
| Soy Isoflavone | Phase III | Pre-menopausal | 100 | No adverse effect | NCT00513916 [209] |
| Soy Isoflavone | NA | BRCA1 & 2 stage I-III BC | 110 | No effect | NCT01219075 [228] |
| Flavopiridol (with Trastuzumab) | Phase I | HER2+, stage IV BC | 50 | Dose range study | NCT00039455 [208] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudhakaran, M.; Sardesai, S.; Doseff, A.I. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants 2019, 8, 103. https://doi.org/10.3390/antiox8040103
Sudhakaran M, Sardesai S, Doseff AI. Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants. 2019; 8(4):103. https://doi.org/10.3390/antiox8040103
Chicago/Turabian StyleSudhakaran, Meenakshi, Sagar Sardesai, and Andrea I. Doseff. 2019. "Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control" Antioxidants 8, no. 4: 103. https://doi.org/10.3390/antiox8040103
APA StyleSudhakaran, M., Sardesai, S., & Doseff, A. I. (2019). Flavonoids: New Frontier for Immuno-Regulation and Breast Cancer Control. Antioxidants, 8(4), 103. https://doi.org/10.3390/antiox8040103





