The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations
Abstract
1. Introduction
2. Aim
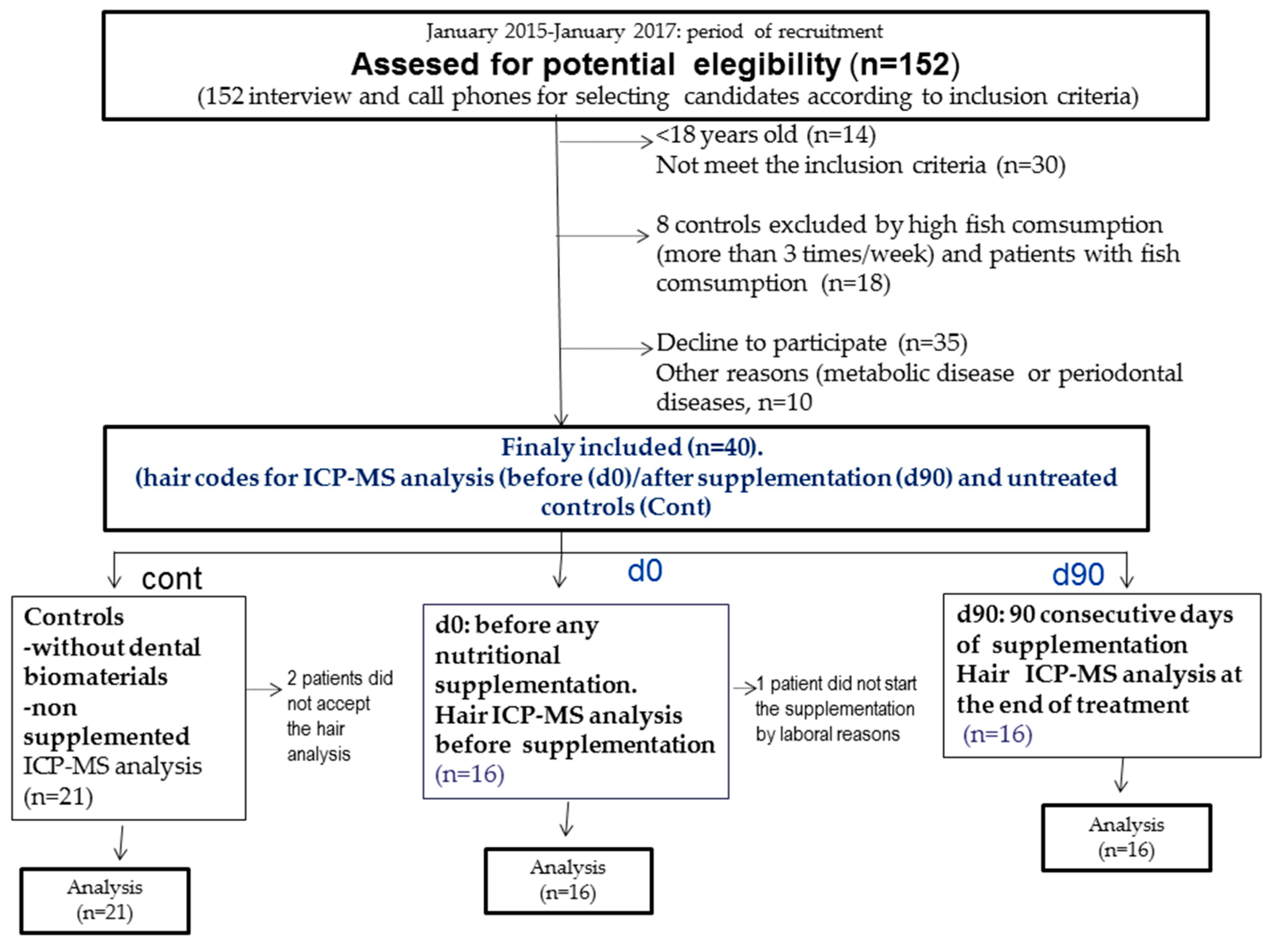
3. Materials and Methods
3.1. Patients
3.2. Inclusion Criteria
3.3. Exclusion Criteria
3.4. Composition of Nutritional Supplementation (Algae and Other Bioactive Phytomolecules)
3.5. Inductible Coupled Mass Spectromery Analysis (ICP-MS)
3.6. Super Oxide Dismutase-1 (SOD-1 Activity)
3.7. Statistical Analysis
4. Results
4.1. SOD-1 Activation Reflects Antioxidants Responses in Patients after Long-Term Supplementation with Algae Extract and Aminoazuphrates Compared with Untreated Controls
4.2. Reduced Mercury (Hg++) and Silver (Ag) Levels after 90 Days of Nutritional Supplementation (d90) as Compared with Their Baseline Levels (d0) as ell as Untreated Controls (Without Dental Materials and non Supplemented, cont)
4.3. Levels of Oligoelements Involved in Metabolic Functions (Se, Mn++, Li, Mg++, Ge, S, P, I, Ca2+, Sr, Na+, K+)
4.4. Metals of Environmental Exposure
4.5. Effects on Selenium (Se) Ratios and Heavy Metals after 90 Days of Nutritional Supplementation
4.6. Correlations between Selenium (Se) and Heavy Metals Ratios after 90 Days of Nutritional Supplementation
5. Discussion
5.1. Detoxification of Heavy Metals in Patients with Long-Term Amalgam Fillings and Titanium Dental Implants
5.2. SOD-1 Activity in Patients with Long-Term Dental Titanium Implants and Amalgams Restorations
5.3. Possible Role of Selenium (Se) in Detox after Long-Term Chlorella CV Supplementation in Patients
5.4. The Nutritional Supplementation after 90 Days Prevented Certain Oligoelements Deficit in Patients with Long-Term Titanium Implants and Dental Amalgam Restorations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dierickx, P.J. In vitro interaction of organic mercury compounds with soluble glutathione s-transferases from rat liver. Pharmacol. Res. Commun. 1985, 17, 489–500. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazúr, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Aschner, M.; Rocha, J.B.T. Oxidative stress in MeHg induced neurotoxicity. Toxicol. Appl. Pharmacol. 2011, 256, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, E.A.; Sokolova, T.V.; Emelyanova, L.V.; Zakharova, I.O. Mitochondrial Electron Transport Chain in Heavy Metal-Induced Neurotoxicity: Effects of Cadmium, Mercury, and Copper. Sci. J. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.F.; Eccles, K.M.; Chan, H.M. High selenium exposure lowers the odds ratios for hypertension, stroke, and myocardial infarction associated with mercury exposure among Inuit in Canada. Environ. Int. 2017, 102, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J., II; Ha, Y.-C.; Lee, Y.-K.; Koo, K.-H. High levels of heavy metals increases the prevalence of sarcopenia in the Ederly population. J. Bone Metab. 2016, 23, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Joska, L.; Fojt, J.; Cvrcek, L.; Březina, V. Properties of titanium-alloyed DLC layers for medical applications. Biomatter 2014, 4, e29505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Puchyr, R.F.; Bass, D.A.; Gajewski, R.; Calvin, M.; Marquardt, W.; Urek, K.; Druyan, M.E.; Quig, D. Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS). Biol. Elem. Res. 1998, 62, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Bravo-González, L.A.; Kyung, H.M.; Merino, J.J. Increased Zn/Glutathione Levels and Higher Superoxide Dismutase-1 Activity as Biomarkers of Oxidative Stress in Women with Long-Term Dental Amalgam Fillings: Correlation between Mercury/Aluminium Levels (in Hair) and Antioxidant Systems in Plasma. PLoS ONE 2015, 10, e0126339. [Google Scholar] [CrossRef]
- Somers, E.C.; Ganser, M.A.; Warren, J.S.; Basu, N.; Wang, L.; Zick, S.M.; Park, S.K. Mercury Exposure and Antinuclear Antibodies among Females of Reproductive Age in the United States: NHANES. Environ. Health Perspect. 2015, 123, 792–798. [Google Scholar] [CrossRef]
- López, E.; Arce, C.; Oset-Gasque, M.; Cañadas, S.; González, M. Cadmium induces reactive oxygen species generation and lipid peroxidation in cortical neurons in culture. Free Radic. Biol. Med. 2006, 40, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, S.J.; Chen, Y.; Zhou, Z.; Wu, X.; Zhao, T.; Zhang, Z.; Feng, W.; Wang, W.; Li, Q.; Wu, X.; et al. Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. J. Hazard. Mater. 2015, 294, 109–120. [Google Scholar] [CrossRef]
- Soetan, K.O.; Olaiya, C.O.; Otewole, O.E. The importance of mineral elements for humans, domestic animals and plants. A review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Fattoretti, P.; Bertoni-Freddari, C.; Balietti, M.; Giorgetti, B.; Solazzi, M.; Zatta, P.; Bertoni-Freddari, C. Chronic Aluminum Administration to Old Rats Results in Increased Levels of Brain Metal Ions and Enlarged Hippocampal Mossy Fibers. Ann. N. Y. Acad. Sci. 2004, 1019, 44–47. [Google Scholar] [CrossRef]
- Vig, E.K.; Hu, H. Lead toxicity in older adults. J. Am. Geriatr. Soc. 2000, 48, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Travieso, L.; Cañizares, R.O.; Borja, R.; Benitez, F.; Domínguez, A.R.; Dupeyrón, R.; Valiente, V. Heavy metals removal by microalgae. Bull. Environ. Contam. Toxicol. 1999, 62, 144–151. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Pacyna, J.M.; Pacyna, E.G.; Aas, W. Changes of emissions and atmospheric deposition of mercury, lead, and cadmium. Atmos. Environ. 2009, 43, 117–127. [Google Scholar] [CrossRef]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and Tolerance of Humans to Heavy Metals through Microbial Processes: A Potential Role for Probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Minich, D.M. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J. Nutr. Metab. 2015, 2015, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Jervis, L.; Rees-Naesborg, R.; Brown, M. Biochemical responses of the marine macroalgae Ulva lactuca and Fucus vesiculosus to cadmium and copper-from sequestration to oxidative stress. Biochem. Soc. Trans. 1997, 25, 63. [Google Scholar] [CrossRef]
- Ben-Bassat, D.; Mayer, A.M. Volatilization of mercury by algae. Pshysiol. Plant. 1975, 33, 128–132. [Google Scholar] [CrossRef]
- Hamed, S.M.; Zinta, G.; Klöck, G.; Asard, H.; Selim, S.; AbdelGawad, H. Zinc-induced differential oxidative stress and antioxidant responses in Chlorella sorokiniana and Scenedesmus acuminatus. Ecotoxicol. Environ. Saf. 2017, 140, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Hwang, Y.K.; Lee, Y.W.; Yun, J.Y.; Hwang, J.M.; Yoo, J.D. Effect of Chlorella diet supplementation on blood and urine cadmium levels in cadmium poisoned rats. J. Biomed. Lab. Sci. 2003, 9, 133–137. [Google Scholar]
- Cantu, V.; Garza-González, M.T.; de la Rosa, J.R.; Loredo-Medrano, J.A. Biosorption of Pb2+ and Cd2+ in a fixed bed column with immobilised Chorella sp. biomass. J. Nutr. 1999, 129, 1731–1736. [Google Scholar]
- Castro, L.; Blázquez, M.L.; González, F.; Muñoz, J.A.; Ballester, A. Biosorption of Zn (II) from industrial effluents using sugar beet pulp and F. vesiculosus: From laboratory tests to a pilot approach. Sci. Total Environ. 2017, 598, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Isuru, W.; Se-Kwon, K. Angiotensin-I-Converting Enzyme (ACE) Inhibitors from Marine Resources: Prospects in the Pharmaceutical Industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef]
- APA. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR; American Psychiatric Association: Washington, DC, USA, 2006. [Google Scholar]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Merino, J.J. Safe renoval of dental amalgams by using nasal filtres and phytoteraphy. IJSR Int. J. Sci. Res. 2015, 4, 2391–2395. [Google Scholar]
- Salvi, G.E.; Lindhe, J.; Lang, N.P. Examination of patients with periodontal disease. In Clinical Periodontology and Implants Dentistry, 5th ed.; Lindh, J., Lan, N.P., Karring, T., Eds.; Wiley: Oxford, UK, 2008; pp. 573–586. [Google Scholar]
- Saghiri, M.A.; Banava, S.; Sabzian, M.A.; Gutmann, J.L.; Asatourian, A.; Ramezani, G.H.; García-Godoy, F.; Sheibani, N. Correlation between long-term in vivo amalgam restorations and the presence of heavy elements in the dental pulp. J. Elem. Med. Biol. 2014, 28, 200–204. [Google Scholar] [CrossRef]
- Begerow, J.; Zander, D.; Freier, I.; Dunemann, L. Long-term mercury excretion in urine after removal of amalgam fillings. Int. Arch. Occup. Environ. Health 1994, 66, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Kremers, L.; Halbach, S.; Willruth, H.; Mehl, A.; Welzl, G.; Wack, F.-X.; Greim, H.; Wack, F.; Hickel, R. Effect of rubber dam on mercury exposure during amalgam removal. Eur. J. Oral Sci. 1999, 107, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Matsueda, T.; Iida, T.; Hasegawa, T. Chlorella Accelerates Dioxin Excretion in Rats. J. Nutr. 1999, 129, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Mahltig, B.; Soltmann, U.; Haase, H. Modification of algae with zinc, copper and silver ions for usage as natural composite for antibacterial applications. Mater. Sci. Eng. C 2013, 33, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.N.; Singh, N.K.; Upadhyay, A.K.; Verma, S. Chromate tolerance and accumulation in Chlorella vulgaris. A role of antioxidant enzymes and biochemical changes in detoxification of metals. Bioresour. Technol. 2013, 136, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Purchase, D.; Jones, H.; Garelick, H. Effects of arsenate (As5+) on growth and production of glutathione (GSH) and phytochelatins (PCS) in Chlorella vulgaris. Int. J. Phytoremediation 2011, 13, 834–844. [Google Scholar] [CrossRef]
- Lee, I.; Tran, M.; Evans-Nguyen, T.; Stickle, D.; Kim, S.; Han, J.; Park, J.Y.; Yang, M. Detoxification of chlorella supplement on heterocyclic amines in Korean young adults. Environ. Toxicol. Pharmacol. 2015, 39, 441–446. [Google Scholar] [CrossRef]
- Algaerve, T.D.; Barbisan, F.; Ribeiro, E.E.; Duarte, M.M.; Manica-Cattani, M.F.; Mostardeiro, C.P.; Lenz, A.F.; da Cruz, I.B. In vitro effects of Ala16Val manganese superoxide dismutase gene polymorphism on human white blood cells exposed to methylmercury. Genet. Mol. Res. 2014, 12, 5133–5144. [Google Scholar] [CrossRef]
- Mohseniazar, M.; Barin, M.; Zarredar, H.; Alizadeh, S.; Shanehbandi, D. Potential of Microalgae and Lactobacilli in Biosynthesis of Silver Nanoparticles. Bioimpacts 2011, 1, 149–152. [Google Scholar]
- Qian, H.; Zhu, K.; Lu, H.; Lavoie, M.; Chen, S.; Zhou, Z.; Deng, Z.; Chen, J.; Fu, Z. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses. Sci. Total Environ. 2016, 572, 1213–1221. [Google Scholar] [CrossRef]
- Hinson, J.A.; Forkert, P.G. Phase II enzymes and bioactivation. Can. J. Physiol. Pharmacol. 1995, 73, 1407–1413. [Google Scholar] [CrossRef]
- Ben-Bassat, D.; Mayer, A.M. Reduction of mercury chloride by Chlorella: Evidence for a reducing agent. Physiol. Plant. 1977, 40, 157–162. [Google Scholar] [CrossRef]
- Walker, A.F.; Middleton, R.W.; Petrowicz, O. Artichoke leaf extract reduces symptoms of irritable bowel síndrome in a post-marketing surveillance study. Phytother. Res. 2001, 15, 58–61. [Google Scholar] [CrossRef]
- N’jai, A.U.; Kemp, M.Q.; Metzger, B.T.; Hanlon, P.R.; Robbins, M.; Czuyprynski, C.; Barnes, D.M. Spanish Black Radish (Raphanus sativus L. var niger) diet enhances clearance of DMBA and diminishes toxic effects on bone marrow progenitor cells. Nutr. Cancer 2012, 64, 1038–1048. [Google Scholar] [CrossRef]
- Wei, S.; Wang, S.; Zhou, Q.; Zhan, J.; Ma, L.; Wu, Z.; Sun, T.; Prasad, M.N. Potential of Tarazacum mongolium hand-mazz for accelerating phytoextration of cadmium in combination with eco-friendly amendments. J. Hazard. Mater. 2010, 15, 480–484. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.Y.; Leung, G.P.H.; Yu, P.H.; Chan, S.W. A review of the pharmacological effects of Arctium lappa (burbock). Inflammapharmacology 2010, 19, 245–254. [Google Scholar] [CrossRef]
- Yan, X.; Murphy, B.T.; Hammond, G.B.; Vinson, J.A.; Neto, C.C. Antioxidant Activities and Antitumor Screening of Extracts from Cranberry Fruit (Vaccinium macrocarpon). J. Agric. Chem. 2002, 50, 5844–5849. [Google Scholar] [CrossRef]
- Wang, Z.; Kim, J.H.; Jang, Y.S.; Lee, J.Y.; Lim, S.S. Anti-obesity effect of Solidago virgaurea vs. Gigantea extract through regulation of adipogenesis and lipogenesis pathways in high-fat diet-induced obese mic (C57BL/6N). Food Nutr. Res. 2017, 13, 1273479. [Google Scholar] [CrossRef]
- Lara, M.S.; Gutierrez, J.I.; Timón, M.; Andrés, A.I. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L). as antioxidants in cooked pork patties packed in MPA. Meat Sci. 2011, 88, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Kirimer, N.; Tunalier, Z.; Başer, K.C.; Cingi, I. Antispasmodic and Spasmogenic Effects of Scolymus hispanicus and Taraxasteryl Acetate on Isolated Ileum Preparation. Planta Med. 1997, 63, 556–558. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Wesolowski, M. The phenolic contents and antioxidant activities of infussions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Lawal, A.F.; Ologundudu, A.; Adeniran, O.Y.; Omonkhua, A.; Obi, F. Antioxidant effects of heated garlic juice on cadmium-induced liver damage in rats as compared to ascorbic acid. J. Toxicol. Sci. 2011, 36, 549–557. [Google Scholar] [CrossRef]
- Yun, H.M.; Ban, J.O.; Park, K.R.; Lee, C.K.; Jeong, H.S.; Han, S.B.; Hong, J.T. Potential terapeutic effects of functional active compounts from garlic. Pharmacol. Ther. 2013, 142, 183–195. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Serguschenko, I.; Khotimchenko, Y. Lead Absorption and Excretion in Rats Given Insoluble Salts of Pectin and Alginate. Int. J. Toxicol. 2006, 25, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Uchida, E.; Aoki, H.; Hanamura, T.; Nagamine, K.; Kato, H.; Koizumi, T.; Ishigami, A. Acerola (Malpica emarginarta DC) juice intake supress UVB-induced skin pigmentation in SMP30/GNL knockout hairless mice. PLoS ONE 2017, 23, e0170438. [Google Scholar]
- Dean, C. The Magnesium Miracle; Ballantine books: New York, NY, USA, 2007. [Google Scholar]
- El-Ansary, A.; Bjorklund, G.; Tinkow, A.A.; Skalny, A.V. Relationship between selenium, lead, and mercury in red blood cells of Saudi austistic children. Metab. Brain Dis. 2017, 32, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.; García-Barrera, T.; Gómez-Jacinto, V.; Gómez-Ariza, J.L.; Garbayo-Nores, I.; Vilchez-Lobato, C. Antagonistic interaction of selenomethionine enantiomers on methylmercury toxicity in the microalgae Chlorella sorokiniana. Metallomics 2014, 6, 347. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.K.; Mohanty, L.; Mohanty, R.C.; Mohapatra, P.K. Biotoxicity of mercury to Chlorella vulgaris as influenced by amino acids. Acta Biol. Hung. 1997, 48, 497–504. [Google Scholar]
- Ripps, H.; Shen, W. Review: Taurine: A “very essential” amino acid. Mol. Vis. 2012, 18, 2673–2686. [Google Scholar] [PubMed]
- Cabaña-Muñoz, M.E.; Parmigiani-Izquierdo, J.M.; Camacho-Alonso, F.; Merino, J.J. Increased Systemic Malondialdehyde Levels and Decreased Mo/Co, Co/Fe2+ Ratios in Patients with Long-Term Dental Titanium Implants and Amalgams. J. Clin. Med. 2019, 8, 86. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, X.; Zhang, J.; Wang, J.; Xie, H.; Wu, Z. Alpha lipoic acid protects against the cytotoxicity and oxidative stress induced by cadmum in HepG2 cells through regeneration of glutathione by glutathione reductase via Nrf-2/ARE signaling pathway. Environ. Toxicol. Pharmacol. 2016, 45, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Uchikawa, T.; Kumamoto, Y.; Maruyama, I.; Kumamoto, S.; Ando, Y.; Yasutake, A. The enhanced elimination of tissue methylmercury in Parachlorella beijerinckii-fed mice. J. Toxicol. Sci. 2011, 36, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Frankilin, J.; Raj, S.P. Accumulation of heavy metals (Cu, Cr, Pb and Cd) in freshwater micro algae (Chlorella sp.). J. Environ. Sci. Eng. 2013, 55, 371–376. [Google Scholar]
- Bai, Y.; Wang, D.; Cui, X.; Yang, Z.; Zhu, M.; Zhang, Z.; Xia, G.; Gong, Y. Preventive effects of selenium-enriched spiruline (SESP) on radiation pneumonitis. J. Environ. Pathol. Toxicol. Oncol. 1998, 17, 159–163. [Google Scholar] [PubMed]
- Evseeva, T.I.; Maĭstrenko, T.A.; Geras’kin, S.A. An assessment of relative contribution of DNA reparation and glutathione-dependent pathway of detoxification in response of Chlorella algae to uranium. Radiats Biol. Radioecol. 2013, 53, 236–245. [Google Scholar]
- Horikoshi, T.; Nakajima, A.; Sakaguchi, T. Update of uranium by various cell fractions of Chlorella vulgaris. Radioisotopes 1979, 28, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.B.D.; Hayward, A.R.; Hutchinson, T.C.; Emery, R.J.N. Identification and quantification of glutathione and phytochelatins from Chlorella vulgaris by RP-HPLC ESI-MS/MS and oxygen-free extraction. Anal. Bioanal. Chem. 2009, 395, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity of Fucus spiralis macroalgae and influence of the extracts storage temperature—A short report. J. Pharm. Biomed. Anal. 2016, 131, 503–507. [Google Scholar] [CrossRef]
- Lopes, G.; Andrade, P.B.; Valentão, P.; McPhee, D.J. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef]
- Turner, A.; Poon, H.; Taylor, A.; Brown, M.T. In situ determination of trace elements in Fucus sp by field-portabel-XRF. Sci. Total Environ. 2017, 593–594, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Lopes, C.B.; Figueira, P.; Rocha, L.S.; Duarte, A.C.; Vale, C.; Pardal, M.A.; Pereira, E. Bioaccumulation of Hg, Cd and Pb by Fucus vesiculosus in single and multi-metal contamination scenarios and its effect on growth rate. Chemosphere 2017, 171, 208–222. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Dall’Acqua, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; Giron, M.C.; et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs 2017, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 163, 258. [Google Scholar] [CrossRef]
- Simpore, J.; Kabore, F.; Zongo, F.; Dansou, D.; Bere, A.; Pignatelli, S.; Biondi, D.M.; Ruberto, G.; Musumeci, S. Nutrition rehabilitation of undernourished children utilizing Spiruline and Misola. Nutr. J. 2006, 5, 3. [Google Scholar] [CrossRef]
| GREEN-FLOR (Formulation-1) | |
| Nutritional supplementation during 90 consecutive days (4 capsules/day 2-0-2) | dosage (mg/day) |
| Chlorella: 80 mg/capsule | 320 mg/day |
| Spirulina: 80 mg/capsule | 320 mg/day |
| Kelp of Pacific: 60 mg/capsule | 240 mg/day |
| Fucus: 30 mg/capsule | 120 mg/day |
| Cardille: 25 mg/capsule | 100 mg/day |
| Pectine of apple: 60 mg/capsule | 240 mg/day |
| Acerole (rich in vitamin C): 50 mg/capsule | 200 mg/day |
| Fructooligosacarides: 280 mg | 1120 mg/day |
| Scolymus hispanicus: 60 mg/capsule | 240 mg/day |
| ERGYTAURINE (Formulation-2) | |
| Treatment: 90 consecutive days (2 capsules/day; 1-0-1) | dosage (mg or μg/day) |
| Selenium (Se): 25 μg/capsule | 50 μg/day |
| Vitamin B6: 0.8 μg/capsule | 1.6 μg/day |
| Folic Acid (B-9): 100 μg/capsule | 200 μg/day |
| Zinc (Zn++): 3.5 mg/capsule | 50 μg/day |
| Taurine: 120 mg/capsule | 240 mg/day |
| Extract of Raphanus niger L. 15 mg/capsule | 30 mg/day |
| ERGYLIXIR (Formulation-3) | |
| Extracts from: | dosage: mg/month |
| Cynara scolymus (artichoe) | 1440 mg |
| Raphanus niger | 900 mg |
| Taraxacum officinale | 400 mg |
| Arctium lappa | 320 mg |
| Vaccinium macrocarpo | 228 mg |
| Solidago virgaurea | 200 mg |
| Rosmarinus officinalis | 640 mg |
| Sambucus niger (elderberry: antocianines) | 200 mg |
| Sodium Moligdate | 50 μg |
| Selenium | 50 μg |
| Group | Median | 25% | 75% |
| SOD-1 activity (control) | 100 | 100 | 100 |
| SOD-1 activity (d0) | 143.5 | 139.500 * | 149 |
| SOD-1 activity (d90) | 121 | 119.000 *,# | 125 |
| SOD-1 Activity | Difference of Ranks | Q | p < 0.05? |
| Control vs. d0 (before treatment) | 34.000 | 6.7 | Yes |
| d0 vs. d90 (after 90 days) | 16.000 | 2.920 | Yes |
| d90 vs. Cont: controls | 18.500 | 3.600 | Yes |
| Heavy Metals from Dental Amalgams | ||||
| Hg++ | Median | 25% | 75% | H |
| Control | 1.6 | 1.25 | 2.3 | H = 13.85, p < 0.001. |
| d0 | 1.9 | 1.9 | 3.7 * | MW (* p < 0.005). |
| d90 | 1.15 | 0.34 | 2.1 *,# | MW (# d90 vs. d0, p = 0.049); * p < 0.05 d90 vs. Cont |
| Ag | Median | 25% | 75% | H |
| Control | 0.03 | 0.02 | 0.06 | H = 9.3, p = 0.01. |
| d0 | 0.1 | 0.03 | 0.155 # | MW (* p = 0.005 d0 vs. control). (# d90 vs. d0, p = 0.031) |
| d90 | 0.055 | 0.025 | 0.075 | |
| Sn | Median | 25% | 75% | H |
| Control | 0.045 | 0.02 | 0.095 | H = 6.27, p = 0.43. |
| d0 | 0.11 | 0.04 | 0.20 * | MW (* d0 vs. Cont, p = 0.023). |
| d90 | 0.03 | 0.02 | 0.105 # | MW (# d90 vs. d0, p = 0.047). |
| Zn++ | Median | 25% | 75% | H |
| Control | 195 | 180 | 230 | H = 5, p = 0.078 |
| d0 | 245 | 208 | 275 * | MW (* p < 0.05 d0 vs. control). |
| d90 | 210 | 180 | 242 | |
| Cu++ | Median | 25% | 75% | H |
| Control | 13.5 | 10.5 | 35.5 | H = 1.01, p = 0.6, n.s |
| d0 | 15 | 11 | 31 | |
| d90 | 13.5 | 10 | 19.5 | |
| Materials from Dental Titanium Alloys (Cr, Ni, Co) | ||||
| Al | Median | 25% | 75% | H |
| Control | 2.9 | 2.05 | 5.6 | H = 4.6, p = 0.1, n.s. |
| d0 | 3 | 1.6 | 4.6 | |
| d90 | 1.6 | 1.5 | 2.4 * | MW (* d90 vs. control, p = 0.029). |
| Cr | Median | 25% | 75% | H |
| Control | 0.35 | 0.31 | 0.39 | H=9.64, p = 0.008 |
| d0 | 0.35 | 0.35 | 0.39 | |
| d90 | 0.41 | 0.36 | 0.45 *,# | MW or Dunn’s.* p < 0.05 vs. cont, # p < 0.05 d90 vs. d0 |
| Co | Median | 25% | 75% | H |
| Control | 0.004 | 0.004 | 0.010 | H = 4.97, p = 0.083, n.s. |
| d0 | 0.017 | 0.04 | 0.035 * | MW * p < 0.05 d0 vs. control. |
| d90 | 0.06 | 0.035 | 0.012 | |
| Ni | Median | 25% | 75% | H |
| Control | 0.055 | 0.04 | 0.10 | H = 3.07, p = 0.21, n.s. |
| d0 | 0.09 | 0.08 | 0.16 * | MW (* p < 0.05, d0 vs. Cont). |
| d90 | 0.11 | 0.04 | 0.16 | |
| V | Media | S.E.M | F | |
| Control | 0.04 | 0.003 | F (2.50) = 2.73, p = 0.07, n.s. | |
| d0 | 0.031 | 0.004 * | Bonferroni (p = 0.043, alpha (α) = 0.05, beta (β) = 0.42). * p < 0.05 vs. control | |
| d90 | 0.041 | 0.0035 # | (# p < 0.05, d90 vs. d0) | |
| * p < 0.05 vs. Cont | # p < 0.05 d90 vs. d0 | n.s: non significant effect (p > 0.05, n.s). | ||
| Se | Median | 25% | 75% | H |
| Control | 0.66 | 0.59 | 0.73 | H = 10.91, p = 0.004. |
| d0 | 0.6 | 0.47 | 0.67 * | MW (* d0 vs. control, p = 0.05). |
| d90 | 0.55 | 0.48 | 0.62 * # | MW (# d90 vs. d0, p = 0.039; * p < 0.05 vs. cont). |
| Mo | Median | 25% | 75% | H |
| Control | 0.034 | 0.0140 | 0.0032 | H = 14.5, p < 0.001. |
| d0 | 0.022 | 0.0079 | 0.023 * | MW or Dunn’s (* p < 0.05 d0 vs. control). |
| d90 | 0.020 | 0.0084 | 0.150 * | MW or Dunn’s: * p < 0.05 d90 vs. control |
| Mn++ | Median | 25% | 75% | H |
| Control | 0.075 | 0.06 | 0.10 | H = 5.42, p = 0.066, n.s. |
| d0 | 0.085 | 0.04 | 0.11 | |
| d90 | 0.115 | 0.07 | 0.18 * # | MW (* d90 vs. control, p = 0.05); MW (d90 vs. d0, p < 0.05; * p < 0.05 vs. cont) |
| Li | Median | 25% | 75% | H |
| Control | 0.008 | 0.006 | 0.012 | H = 1.45, p < 0.001. |
| d0 | 0.005 | 0.0045 | 0.0075 * | MW (* d0 vs. control, p = 0.03). |
| d90 | 0.023 | 0.010 | 0.010 * # | MW (* d90 vs. control, p < 0.05); MW (# d90 vs. d0, p = 0.05). |
| Ge | Median | 25% | 75% | H |
| Control | 0.031 | 0.024 | 0.033 | H = 13.1, p = 0.01. |
| d0 | 0.024 | 0.021 | 0.032 | MW (* d0 vs. control, p = 0.1, n.s). |
| d90 | 0.023 | 0.032 | 0.035 # | MW or Dunn’s (# p < 0.05 d90 vs. d0). |
| S | Median | 25% | 75% | H |
| Control | 47700 | 47200 | 49250 | H = 3.97, p = 0.13, n.s. |
| d0 | 46900 | 46350 | 47600 * | MW (* d0 vs. control, p < 0.05). |
| d90 | 46850 | 45600 | 49950 | |
| P | Median | 25% | 75% | H |
| Control | 185 | 155 | 197 | H = 8.88, p = 0.012. |
| d0 | 153 | 134 | 160 * | MW (* d0 vs. control, p < 0.05). |
| d90 | 170 | 154 | 179 # | MW (# d90 vs. d0, p = 0.004). |
| I | Median | 25% | 75% | H |
| Control | 0.58 | 0.37 | 2.05 | H=3.67, p = 0.15, n.s |
| d0 | 0.39 | 0.29 | 0.5 | |
| d90 | 0.47 | 0.31 | 0.77 | |
| Ca++ | Median | 25% | 75% | H |
| Control | 488 | 285 | 705 | H = 1.37, p = 0.5, n.s |
| d0 | 785 | 410 | 1262 | |
| d90 | 676 | 380 | 1060 | |
| Sr | Median | 25% | 75% | H |
| Control | 2.7 | 0.93 | 6.55 | H = 4.41, p = 0.11, n.s |
| d0 | 7 | 2.97 | 11.7 | d0 vs. control, p = 0.064, n.s. |
| d90 | 11.81 | 1.9 | 17.75 | d90 vs. control, p = 0.099, n.s |
| B | Median | 25% | 75% | H |
| Control | 0.5 | 0.41 | 0.87 | H = 1.5, p = 0.46, n.s. |
| d0 | 0.63 | 0.56 | 1.1 | |
| d90 | 0.71 | 0.52 | 0.8 | |
| Na+ | Median | 25% | 75% | H |
| Control | 35 | 14.5 | 73.25 | H = 6, p =0.05. |
| d0 | 62.5 | 52 | 140 * | MW or Dunn’s (* d0 vs. control, p = 0.046). |
| d90 | 48 | 33 | 75 | d90 vs. control, p = 0.1, n.s. |
| K+ | Median | 25% | 75% | H |
| Control | 13.5 | 4 | 31.5 | H = 1.3, p = 0.52, n.s |
| d0 | 5.5 | 3.5 | 44 | |
| d90 | 8.5 | 3 | 15 | |
| Mg++ | Median | 25% | 75% | H |
| Control | 50 | 32.5 | 94 | H = 3.63, p = 0.16, n.s. |
| d0 | 99 | 43.5 | 184.5 | |
| d90 | 137 | 57 | 345 | MW (d90 vs. control, p = 0.088, n.s). |
| Rb | Median | 25% | 75% | H |
| Control | 0.015 | 0.0045 | 0.031 | H = 2.72, p = 0.25, n.s. |
| d0 | 0.014 | 0.0040 | 0.019 | |
| d90 | 0.011 | 0.0030 | 0.013 | |
| Fe++ | Median | 25% | 75% | H |
| Control | 6.7 | 6.2 | 7.7 | H = 3.7, p = 0.15, n.s. |
| d0 | 6.6 | 6.4 | 7.4 | |
| d90 | 7.7 | 6.5 | 8.4 # | MW (d90 vs. d0, # p = 0.022). |
| * p < 0.05 vs. control | # p < 0.05 d90 vs. d0 |
| Ba | Median | 25% | 75% | H |
| Control | 0.17 | 0.1 | 0.33 | H = 7.73, p = 0.021. |
| d0 | 0.54 | 0.25 | 0.84 * | MW (* d0 vs. control, p = 0.05). |
| d90 | 0.28 | 0.2 | 0.36 | MW (d90 vs. control, p = 0.11, n.s). |
| Pb | Median | 25% | 75% | H |
| Control | 0.11 | 0.07 | 0.3 | H = 3.41, p = 0.18, n.s. |
| d0 | 0.14 | 0.09 | 0.21 | |
| d90 | 0.085 | 0.05 | 0.14 # | MW (# d90 vs. d0, p = 0.047). |
| Cd | Median | 25% | 75% | H |
| Control | 0.009 | 0.009 | 0.010 | H = 4.73, p = 0.094, n.s. |
| d0 | 0.009 | 0.009 | 0.009 | |
| d90 | 0.009 | 0.009 | 0.009 | MW (d90 vs. control, p = 0.08, n.s). |
| Sb | Median | 25% | 75% | H |
| Control | 0.01 | 0.01 | 0.018 | H = 3.5, p = 0.16, n.s. |
| d0 | 0.01 | 0.01 | 0.01 | |
| d90 | 0.01 | 0.01 | 0.01 | |
| As | Median | 25% | 75% | H |
| Control | 0.038 | 0.01 | 0.018 | H = 0.9, p = 0.62, n.s. |
| d0 | 0.028 | 0.022 | 0.042 | |
| d90 | 0.028 | 0.023 | 0.052 | |
| * p < 0.05 vs. Cont | # p < 0.05 d90 vs. d0 |
| Se/Hg++ Ratio | Median | 25% | 75% | H |
| Control | 2.21 | 1.6 | 3.2 | H = 31.42, p < 0.001. |
| d0 | 0.23 | 0.17 | 0.31 * | MW (* d0 vs. control, p < 0.001). |
| d90 | 0.28 | 0.24 | 0.56 * # | MW (# d90 vs. d0, p = 0.05. * p < 0.05 vs. cont). |
| Se/Ag ratio | Median | 25% | 75% | H |
| Control | 22.3 | 12.26 | 37.7 | H = 6.25, p = 0.044 |
| d0 | 7.16 | 4.12 | 20.5 | MW (* d0 vs. control, p = 0.04). |
| d90 | 11 | 6.8 | 18.2 * | MW (* d90 vs. control, p = 0.032). |
| Se/Al ratio | Median | 25% | 75% | H |
| cont | 0.26 | 0.1 | 0.39 | H = 3.76, p = 0.15, n.s. |
| d0 | 0.14 | 0.07 | 0.29 | |
| d90 | 0.34 | 0.19 | 0.36 # | MW (# d90 vs. d0, p = 0.05). |
| Se/Pb | Median | 25% | 75% | H |
| Control | 4.57 | 1,79 | 9.1 | H = 1.12, p = 0.57, n.s |
| d0 | 3.81 | 2.2 | 6.9 | |
| d90 | 5.72 | 3.76 | 8.24 | |
| Mo/Hg++ | Median | 25% | 75% | H |
| Control | 0.018 | 0.011 | 0.03 | H = 13.51, p = 0.001 |
| d0 | 0.0089 | 0.0067 | 0.011 * | MW or Dunn’s, * d0 vs. Cont, p = 0.001 |
| d90 | 0.026 | 0.011 | 0.112 * # | MW or Dunn’s, # d90 vs. d0, p < 0.001, * p < 0.05 vs. control |
| Na+/K+ | Median | 25% | 75% | H |
| Control | 3.57 | 4.46 | 0.94 | H = 2.59, p = 0.2, n.s. |
| d0 | 7.74 | 16.3 | 0.82 * | MW (* p < 0.05, d0 vs. control). |
| d90 | 8 | 13.6 | 0.79 | MW (d90 vs. control, p = 0.065, n.s). |
| * p < 0.05 vs. control | # p < 0.05 d90 vs. d0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino, J.J.; Parmigiani-Izquierdo, J.M.; Toledano Gasca, A.; Cabaña-Muñoz, M.E. The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants 2019, 8, 101. https://doi.org/10.3390/antiox8040101
Merino JJ, Parmigiani-Izquierdo JM, Toledano Gasca A, Cabaña-Muñoz ME. The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants. 2019; 8(4):101. https://doi.org/10.3390/antiox8040101
Chicago/Turabian StyleMerino, José Joaquín, José María Parmigiani-Izquierdo, Adolfo Toledano Gasca, and María Eugenia Cabaña-Muñoz. 2019. "The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations" Antioxidants 8, no. 4: 101. https://doi.org/10.3390/antiox8040101
APA StyleMerino, J. J., Parmigiani-Izquierdo, J. M., Toledano Gasca, A., & Cabaña-Muñoz, M. E. (2019). The Long-Term Algae Extract (Chlorella and Fucus sp) and Aminosulphurate Supplementation Modulate SOD-1 Activity and Decrease Heavy Metals (Hg++, Sn) Levels in Patients with Long-Term Dental Titanium Implants and Amalgam Fillings Restorations. Antioxidants, 8(4), 101. https://doi.org/10.3390/antiox8040101




