Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats
Abstract
1. Introduction
2. Materials and Methods
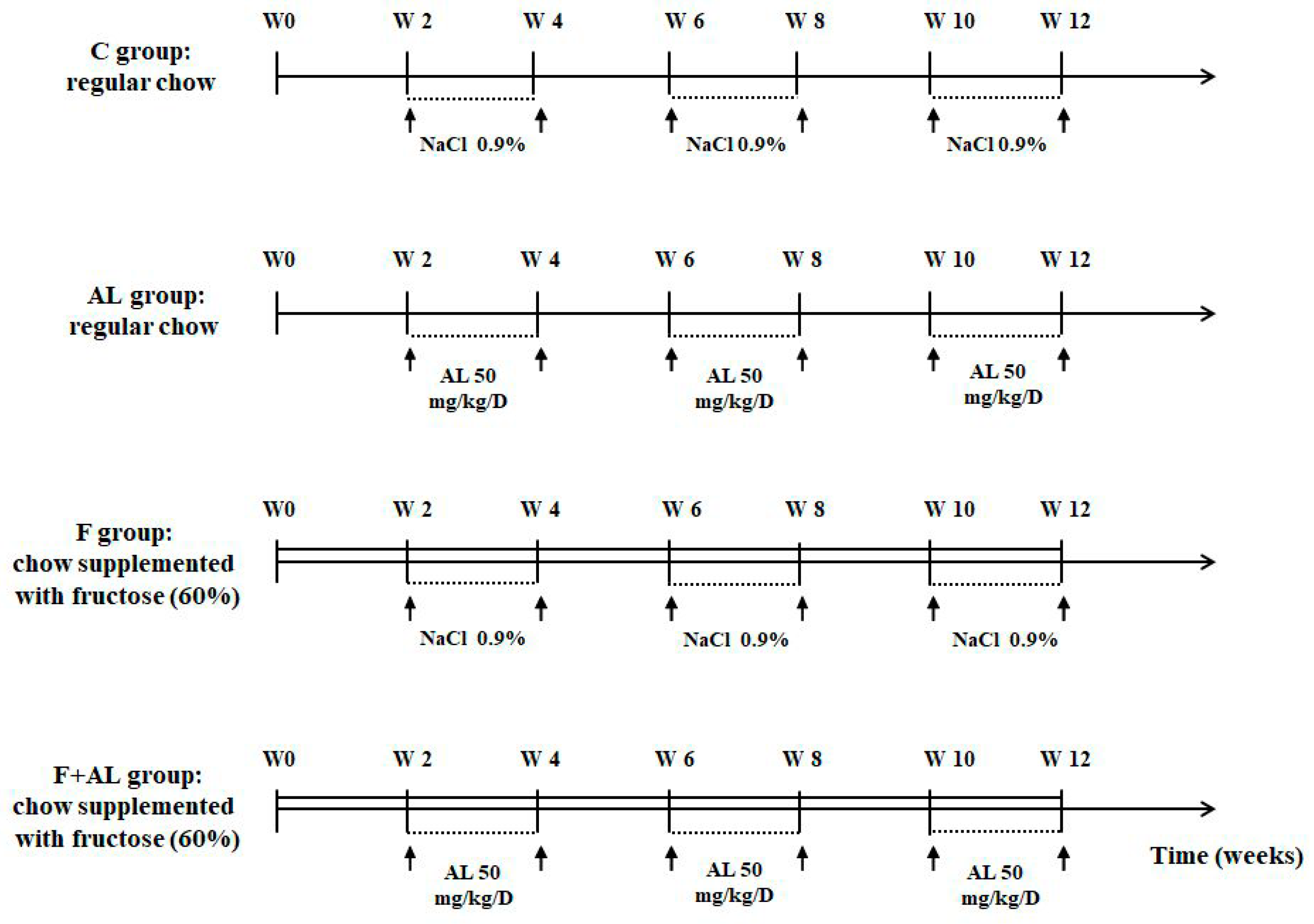
2.1. Animals and Experimental Protocol
- Control group (C, n = 12) received regular chow and the rats were injected i.p. with saline solution (NaCl 0.9%).
- Lipoic acid-treated group (AL, n = 12) received regular chow and the rats were injected i.p. with 50 mg/kg/day racemic alpha-lipoic acid (Thiogamma 600®, Wörwarg Pharma, Germany) for 14 days/month over 12 weeks.
- Fructose-fed group (F, n = 12) received chow supplemented with fructose (60%) and the rats were injected i.p. with saline solution.
- Fructose-fed group treated with alpha-lipoic acid (F + AL, n = 12) received chow supplemented with fructose (60%) and the rats were injected with the same dose of alpha-lipoic acid at the same intervals of times as the previous group (AL group).
2.2. Hemodynamic Parameters
2.3. Biochemical Investigations
2.4. Oxidative Stress Markers
2.4.1. Plasma Lipid and Protein Oxidation Products
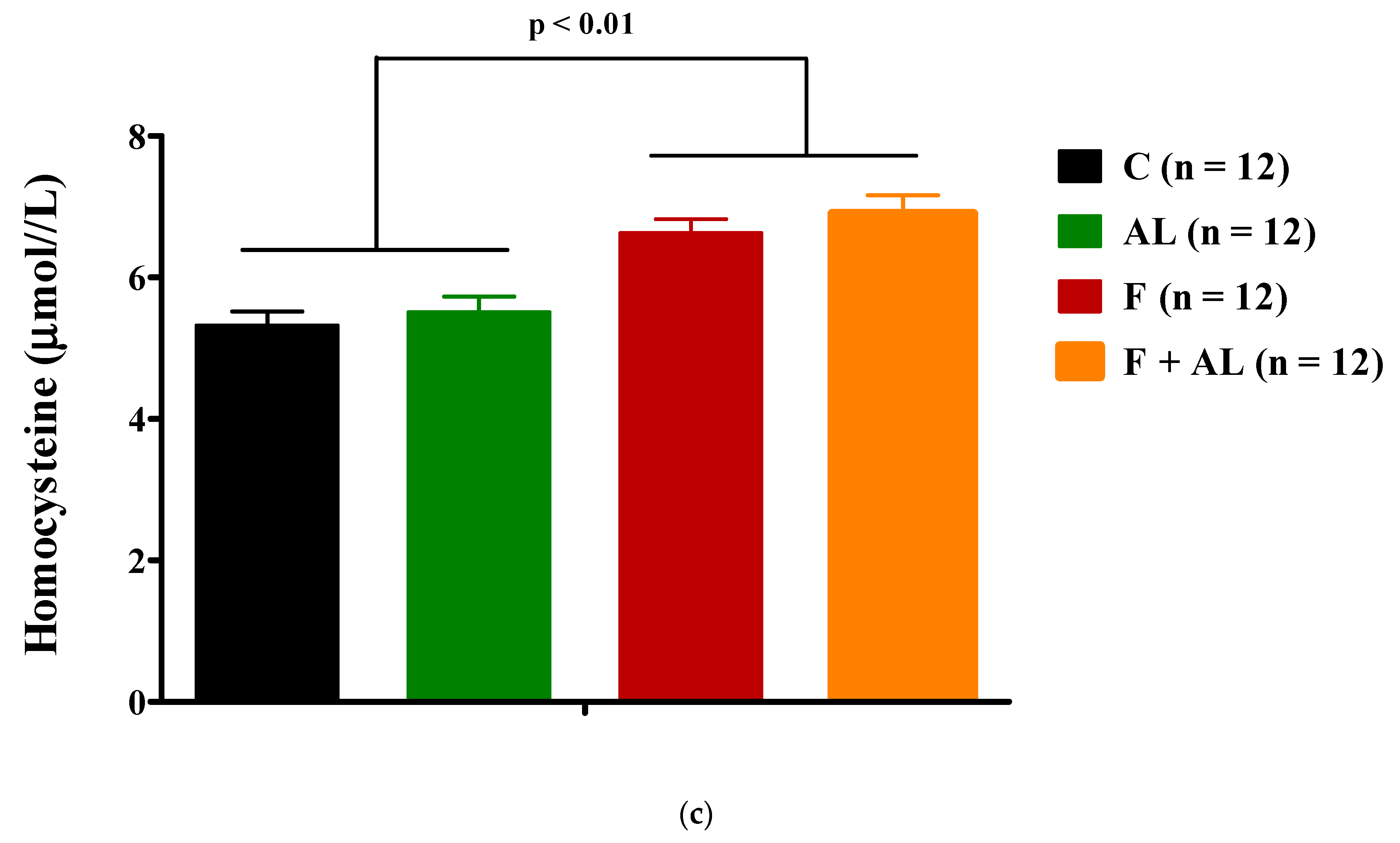
2.4.2. Homocysteine
2.4.3. Circulating Antioxidants Levels
2.5. Histopathological Analysis
2.6. Statistical Analysis
3. Results
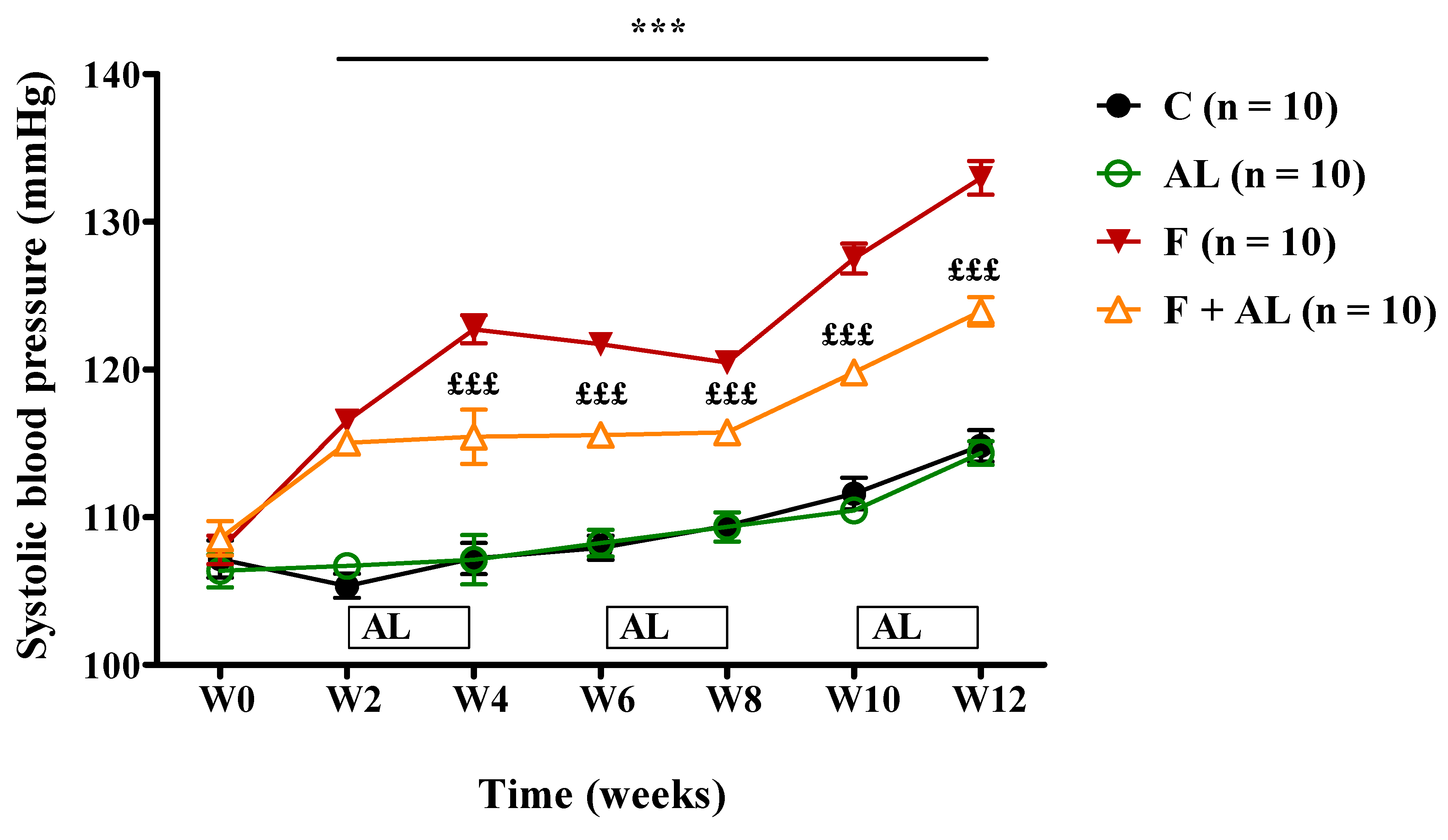
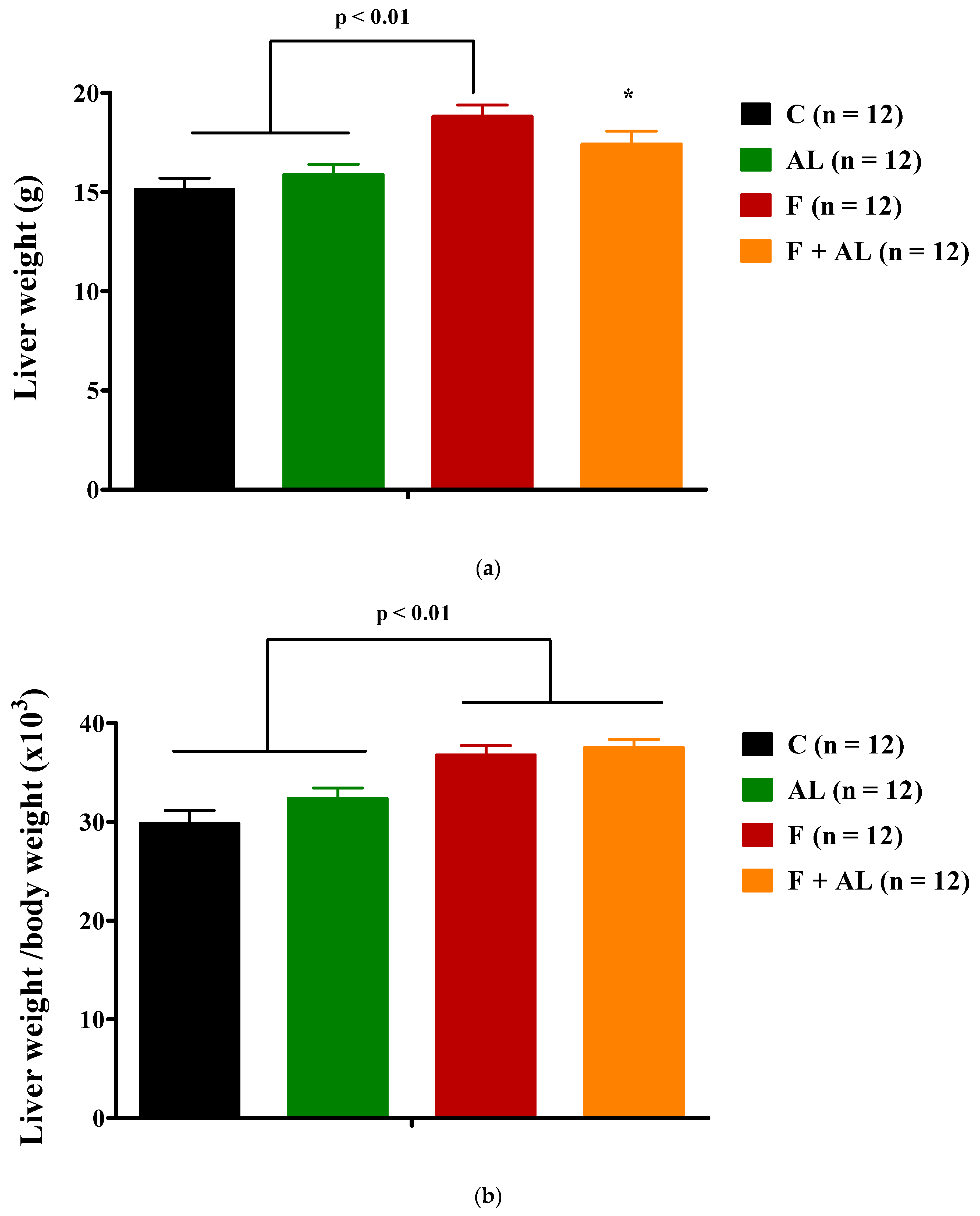
3.1. Body Weight and Hemodynamic Parameters
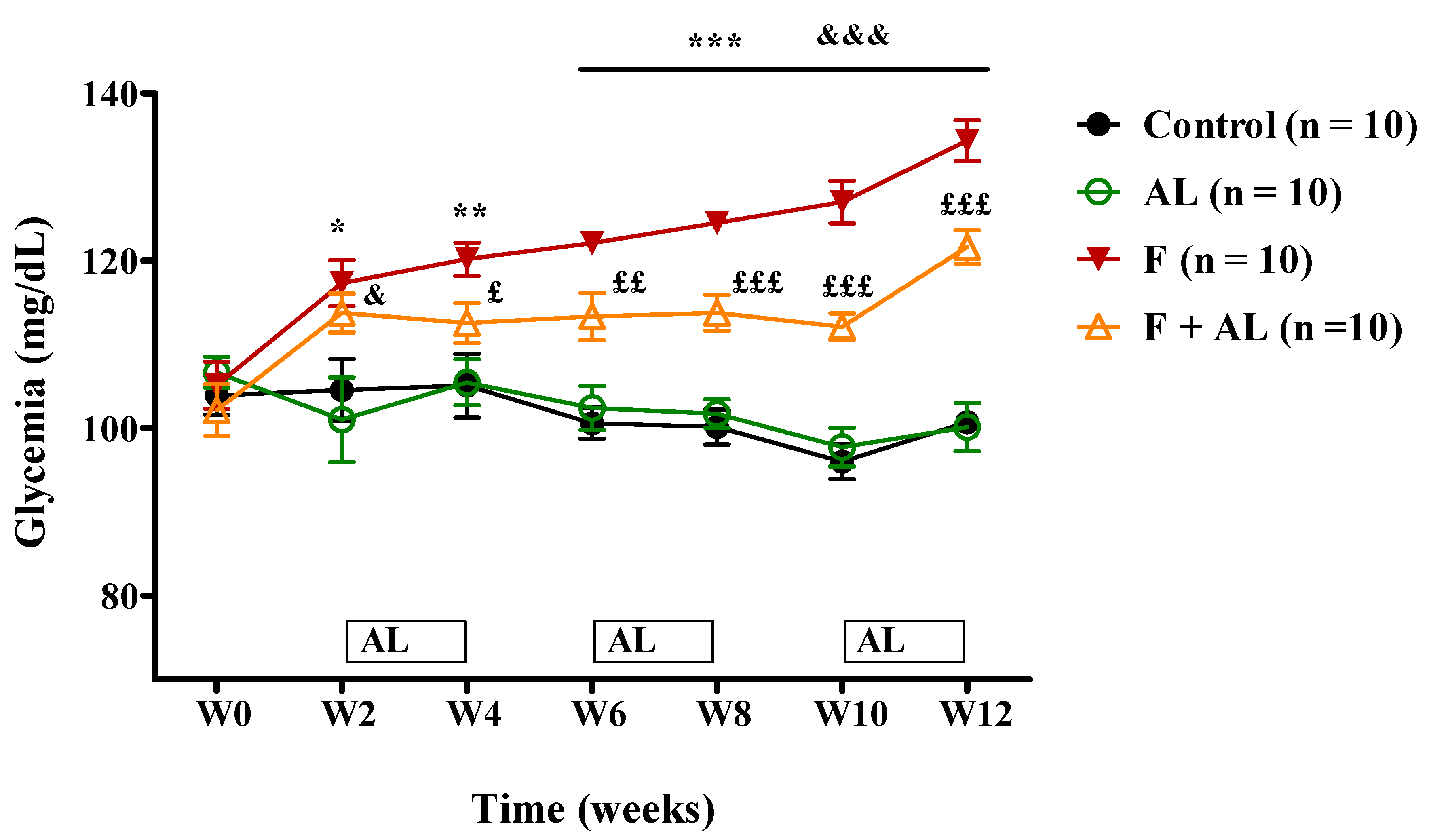
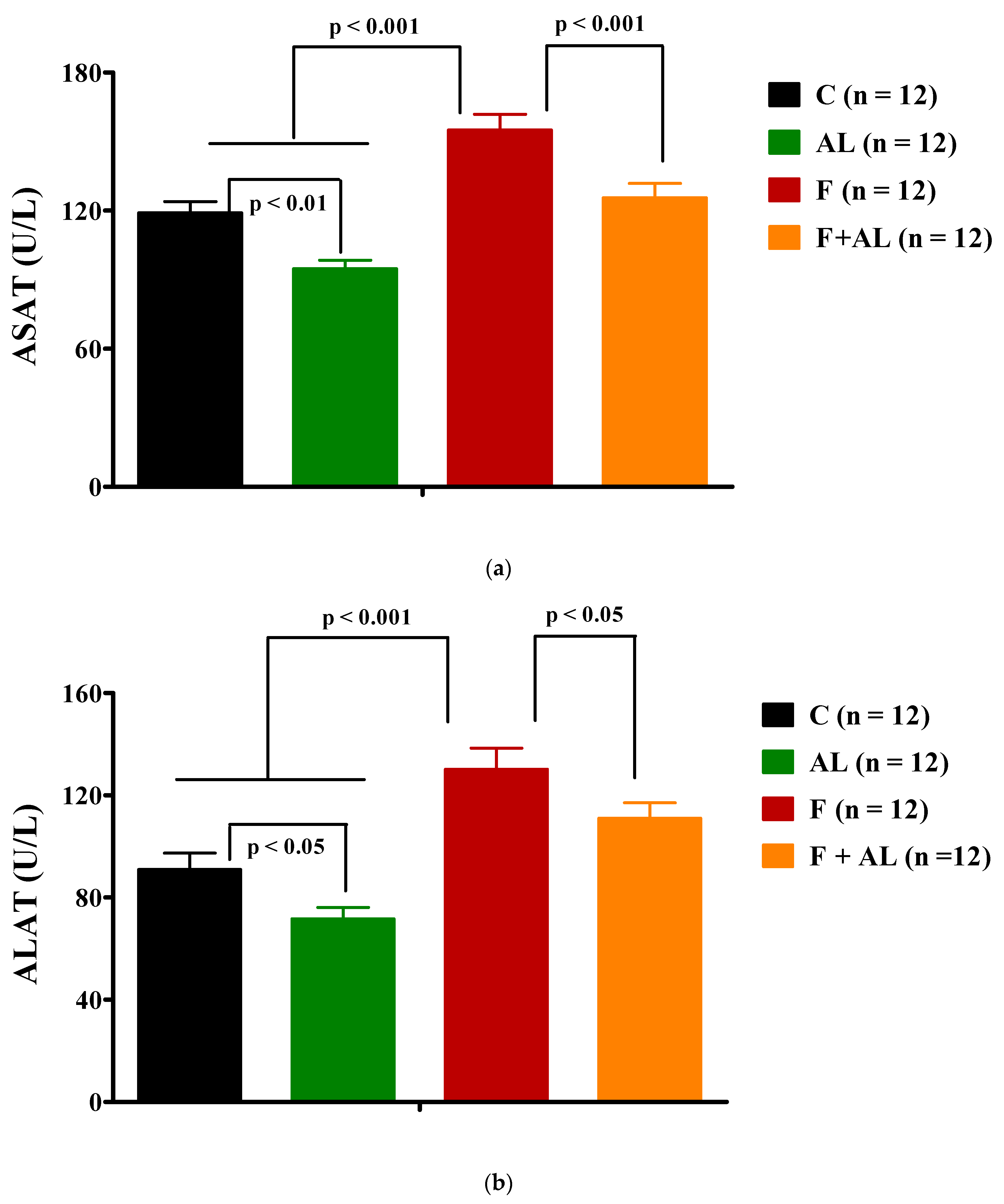
3.2. Biochemical Investigations
3.3. Oxidative Stress Markers
3.3.1. Plasma Lipid and Protein Oxidation Products
3.3.2. Homocysteine
3.3.3. Circulating Antioxidants Levels
Glutathione
Glutathione Peroxidase Activity
3.4. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wheatcroft, S.B.; Williams, I.L.; Shah, A.M.; Kearney, M.T. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet. Med. 2003, 20, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Styskal, J.; Van Remmen, H.; Richardson, A.; Salmon, A.B. Oxidative stress and diabetes: What can we learn about insulin resistance from antioxidant mutant mouse models? Free Radic. Biol. Med. 2012, 52, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Imrie, H.; Abbas, A.; Kearney, M. Insulin resistance, lipotoxicity and endothelial dysfunction. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2010, 1801, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Walker, M. Genetics of Insulin Resistance and the Metabolic Syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Houstis, N.; Rosen, E.D.; Lander, E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 2006, 440, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Hopps, E.; Noto, D.; Caimi, G.; Averna, M.R. A novel component of the metabolic syndrome: The oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 72–77. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Diabetes, oxidative stress and therapeutic strategies. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2709–2729. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martinez, P.; Garcia-Rios, A.; Delgado-Lista, J.; Perez-Jimenez, F.; Lopez-Miranda, J. Metabolic syndrome: Evidences for a personalized nutrition. Mol. Nutr. Food Res. 2012, 56, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Onat, A. Metabolic syndrome: Nature, therapeutic solutions and options. Expert Opin. Pharmacother. 2011, 12, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.Q.; Pei, J.J.; Xu, S.; Luo, D.Z.; Dong, S.Q.; Sun, M.H.; You, L.; Sun, Z.J.; Liu, X.P. Pioglitazone Improves Cognitive Function via Increasing Insulin Sensitivity and Strengthening Antioxidant Defense System in Fructose-Drinking Insulin Resistance Rats. PLoS ONE 2013, 8, e59313. [Google Scholar] [CrossRef]
- Otani, H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid. Redox Signal. 2011, 15, 1911–1926. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef]
- Gora̧ca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid—Biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- El Midaoui, A.; De Champlain, J. Prevention of hypertension, insulin resistance, and oxidative stress by α-lipoic acid. Hypertension 2002, 39, 303–307. [Google Scholar] [CrossRef]
- El Midaoui, A.; Lungu, C.; Wang, H.; Wu, L.; Robillard, C.; Deblois, D.; Couture, R. Impact of α-lipoic acid on liver peroxisome proliferator-activated receptor-α, vascular remodeling, and oxidative stress in insulin-resistant rats. Can. J. Physiol. Pharmacol. 2011, 89, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, E.J.; Kim, Y.-N.; Choi, C.; Lee, B.-H. Effects of α-lipoic acid and l-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr. Res. Pract. 2011, 5, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukkarasu, V.; Anitha Nandhini, A.T.; Anuradha, C.V. Effect of α-lipoic acid on lipid profile in rats fed a high-fructose diet. Exp. Diabesity Res. 2004, 5, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Ishizaka, N.; Ishizaka, Y.; Toda, E.-I.; Nagai, R.; Yamakado, M. Association Between Serum Uric Acid, Metabolic Syndrome, and Carotid Atherosclerosis in Japanese Individuals. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef]
- Tappy, L. Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J. Exp. Biol. 2018, 221, jeb164202. [Google Scholar] [CrossRef]
- Bray, G.A.; Nielsen, S.J.; Popkin, B.M. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am. J. Clin. Nutr. 2004, 79, 537–543. [Google Scholar] [CrossRef]
- Korandji, C.; Zeller, M.; Guilland, J.C.; Collin, B.; Lauzier, B.; Sicard, P.; Duvillard, L.; Goirand, F.; Moreau, D.; Cottin, Y.; et al. Time course of asymmetric dimethylarginine (ADMA) and oxidative stress in fructose-hypertensive rats: A model related to metabolic syndrome. Atherosclerosis 2011, 214, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Hontoria, P.L.; Pérez-Matute, P.; Fernández-Galilea, M.; Martínez, J.A.; Moreno-Aliaga, M.J. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. Eur. J. Nutr. 2013, 52, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.Y.; Ha, A.W.; Kim, W.K. α-lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet. Nutr. Res. Pract. 2012, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Cadenas, E. Lipoic acid: Energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 2011, 48, 26–32. [Google Scholar] [CrossRef]
- Mahmoud, M.F.; Hassan, N.A.; El Bassossy, H.M.; Fahmy, A. Quercetin Protects against Diabetes-Induced Exaggerated Vasoconstriction in Rats: Effect on Low Grade Inflammation. PLoS ONE 2013, 8, e63784. [Google Scholar] [CrossRef]
- Kearney, M.T.; Duncan, E.R.; Kahn, M.; Wheatcroft, S.B. Insulin resistance and endothelial cell dysfunction: Studies in mammalian models. Exp. Physiol. 2008, 93, 158–163. [Google Scholar] [CrossRef]
- Gagliardi, A.C.M.; Miname, M.H.; Santos, R.D. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis 2009, 202, 11–17. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Franchini, M.; Favaloro, E.J.; Targher, G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin. Chim. Acta 2008, 392, 1–7. [Google Scholar] [CrossRef]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Whaley-Connell, A.T.; Sowers, J.R. Fructose and uric acid: Is there a role in endothelial function? Curr. Hypertens. Rep. 2014, 16, 434. [Google Scholar] [CrossRef]
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of α-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Ghibu, S.; Richard, C.; Delemasure, S.; Vergely, C.; Mogosan, C.; Muresan, A. [An endogenous dithiol with antioxidant properties: Alpha-lipoic acid, potential uses in cardiovascular diseases]. Ann. Cardiol. Angeiol. (Paris) 2008, 57, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Ferder, M.D.; Inserra, F. The role of high-fructose corn syrup in metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Carbohydrate intake and nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Martins-Santos, M.E.S.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Konrad, D. Utilization of the insulin-signaling network in the metabolic actions of α-lipoic acid—Reduction or oxidation? Antioxid. Redox Signal. 2005, 7, 1032–1039. [Google Scholar] [CrossRef]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric acid-key ingredient in the recipe for cardiorenal metabolic syndrome. CardioRenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef]
- Caliceti, C.; Calabria, D.; Roda, A.; Cicero, A.F.G. Fructose intake, serum uric acid, and cardiometabolic disorders: A critical review. Nutrients 2017, 9, 395. [Google Scholar] [CrossRef]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Hu, H.; Zharikov, S.; Tuttle, K.R.; Short, R.A.; Glushakova, O.; Ouyang, X.; Feig, D.I.; Block, E.R.; Herrera-Acosta, J.; et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am. J. Physiol. Ren. Physiol. 2006, 290, F625–F631. [Google Scholar] [CrossRef] [PubMed]
- Simão, A.N.C.; Lozovoy, M.A.B.; Dichi, I. The uric acid metabolism pathway as a therapeutic target in hyperuricemia related to metabolic syndrome. Expert Opin. Ther. Targets 2012, 16, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Wiernsperger, N.; Geloen, A.; Rapin, J.R. Fructose and cardiometabolic disorders: The controversy will, and must, continue. Clinics 2010, 65, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Safety evaluation of α-lipoic acid (ALA). Regul. Toxicol. Pharmacol. 2006, 46, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.C.; Massa, M.L.; Schinella, G.; Gagliardino, J.J.; Francini, F. Lipoic acid prevents liver metabolic changes induced by administration of a fructose-rich diet. Biochim. Biophys. Acta 2013, 1830, 2226–2232. [Google Scholar] [CrossRef]
- Abdilla, N.; Tormo, M.C.; Fabia, M.J.; Chaves, F.J.; Saez, G.; Redon, J. Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. J. Hum. Hypertens. 2007, 21, 68–75. [Google Scholar] [CrossRef]
- Jensen, M.K.; Bertoia, M.L.; Cahill, L.E.; Agarwal, I.; Rimm, E.B.; Mukamal, K.J. Novel metabolic biomarkers of cardiovascular disease. Nat. Rev. Endocrinol. 2014, 10, 659–672. [Google Scholar] [CrossRef]
- Hoffman, M. Hypothesis: Hyperhomocysteinemia is an indicator of oxidant stress. Med. Hypotheses 2011, 77, 1088–1093. [Google Scholar] [CrossRef]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Stabler, S.P.; Sekhar, J.; Allen, R.H.; O’Neill, H.C.; White, C.W. Alpha-lipoic acid induces elevated S-adenosylhomocysteine and depletes S-adenosylmethionine. Free Radic. Biol. Med. 2009, 47, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Menè, P.; Punzo, G. Uric acid: Bystander or culprit in hypertension and progressive renal disease? J. Hypertens. 2008, 26, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Golbidi, S.; Badran, M.; Laher, I. Diabetes and alpha lipoic acid. Front. Pharmacol. 2011, 2, 69. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Ostadmohammadi, V.; Tabrizi, R.; Mobini, M.; Lankarani, K.B.; Moosazadeh, M.; Heydari, S.T.; Chamani, M.; Kolahdooz, F.; Asemi, Z. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Nutr. Metab. (Lond.) 2018, 15, 39. [Google Scholar] [CrossRef]
- Mendoza-Núñez, V.M.; García-Martínez, B.I.; Rosado-Pérez, J.; Santiago-Osorio, E.; Pedraza-Chaverri, J.; Hernández-Abad, V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxid. Med. Cell. Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef]
- Uchida, R.; Okamoto, H.; Ikuta, N.; Terao, K.; Hirota, T. Enantioselective Pharmacokinetics of α-Lipoic Acid in Rats. Int. J. Mol. Sci. 2015, 16, 22781–22794. [Google Scholar] [CrossRef]
- Jones, W.; Li, X.; Qu, Z.; Perriott, L.; Whitesell, R.R.; May, J.M. Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002, 33, 83–93. [Google Scholar] [CrossRef]
- Handelman, G.J.; Han, D.; Tritschler, H.; Packer, L. Alpha-lipoic acid reduction by mammalian cells to the dithiol form, and release into the culture medium. Biochem. Pharmacol. 1994, 47, 1725–1730. [Google Scholar] [CrossRef]
- Mignini, F.; Capacchietti, M.; Napolioni, V.; Reggiardo, G.; Fasani, R.; Ferrari, P. Single dose bioavailability and pharmacokinetic study of a innovative formulation of α-lipoic acid (ALA600) in healthy volunteers. Minerva Med. 2011, 102, 475–482. [Google Scholar]


| Groups | Body Weight (g) | Glycemia (mg/dL) | Insulin (µU/mL) | HOMA-IR |
|---|---|---|---|---|
| C (n = 11) | 510.57 ± 10.50 | 100.82 ± 1.30 | 50.95 ± 6.24 | 12.74 ± 1.57 |
| AL (n = 10) | 480.08 ± 9.55 † | 97.20 ± 3.49 | 54.15 ± 5.66 | 12.84 ± 1.23 |
| F (n = 12) | 515.80 ± 15.95 | 133.23 ± 2.5 *** | 75.71 ± 3.74 *** | 25.00 ± 1.47 *** |
| F + AL (n = 10) | 465.54 ± 18.35 £ | 122.78 ± 1.97 ***££ | 62.73 ± 3.27 £ | 19.11 ± 1.26 ** £ |
| Groups | Total Cholesterol (mg/dL) | LDL-Cholesterol (mg/dL) | Triglyceride (mg/dL) | Uric Acid (mg/dL) | C-Reactive Protein (mg/dL) |
|---|---|---|---|---|---|
| C (n = 12) | 53.36 ± 2.87 | 8.08 ± 1.28 | 61.86 ± 4.86 | 1.10 ± 0.08 | 0.69 ± 0.05 |
| AL (n = 12) | 53.08 ± 3.21 | 8.92 ± 1.18 | 67.50 ± 4.26 | 0.72 ± 0.04 †† | 0.70 ± 0.05 |
| F (n = 12) | 75.07 ± 3.51 *** | 56.64 ± 12.68 *** | 331.20 ± 23.15 *** | 2.65 ± 0.12 *** | 0.96 ± 0.05 ** |
| F + AL (n = 12) | 65.77 ± 3.20 * £ | 35.08 ± 4.7 ** £ | 151.73 ± 7.51 *** £££ | 0.84 ±0.08 £££ | 0.76 ±0.05 £ |
| Groups | Total Glutathione (μmol/L) | GSH (μmol/L) | GSSG (μmol/L) | GSH/GSSG Ratio | GPx Activity (U/g Hb) |
|---|---|---|---|---|---|
| C (n = 12) | 1304.56 ± 43.78 | 1238.18 ± 43.34 | 33.19 ± 3.08 | 41.11 ± 4.29 | 869.01 ± 28.78 |
| AL (n = 12) | 1673.43 ± 102.05 †† | 1566.93 ± 104.13 † | 53.25 ± 5.66 †† | 33.18 ± 4.05 | 875.29 ± 10.00 |
| F (n = 12) | 1015.82 ± 109.29 * &&& | 921.53 ± 110.44 ** &&& | 47.15 ± 3.66 ** | 21.33 ± 2.96 ** & | 790.13 ± 23.51 * |
| F + AL (n = 12) | 1392.23 ± 55.14 & ££ | 1338.13 ± 55.19 ££ | 27.05 ± 3.66 &&& £££ | 54.05 ± 5.76 £££ | 873.17 ± 21.41 £ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghibu, S.; Craciun, C.E.; Rusu, R.; Morgovan, C.; Mogosan, C.; Rochette, L.; Gal, A.F.; Dronca, M. Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats. Antioxidants 2019, 8, 636. https://doi.org/10.3390/antiox8120636
Ghibu S, Craciun CE, Rusu R, Morgovan C, Mogosan C, Rochette L, Gal AF, Dronca M. Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats. Antioxidants. 2019; 8(12):636. https://doi.org/10.3390/antiox8120636
Chicago/Turabian StyleGhibu, Steliana, Cristina Elena Craciun, Razvan Rusu, Claudiu Morgovan, Cristina Mogosan, Luc Rochette, Adrian Florin Gal, and Maria Dronca. 2019. "Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats" Antioxidants 8, no. 12: 636. https://doi.org/10.3390/antiox8120636
APA StyleGhibu, S., Craciun, C. E., Rusu, R., Morgovan, C., Mogosan, C., Rochette, L., Gal, A. F., & Dronca, M. (2019). Impact of Alpha-Lipoic Acid Chronic Discontinuous Treatment in Cardiometabolic Disorders and Oxidative Stress Induced by Fructose Intake in Rats. Antioxidants, 8(12), 636. https://doi.org/10.3390/antiox8120636





