The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases
Abstract
1. Introduction to Polyphenols
2. Functions of Polyphenols in Drosophila Melanogaster
3. The Effects of Polyphenols on Neurodegenerative Diseases Using Drosophila Models
3.1. Alzheimer’s Disease (AD)
3.2. Parkinson’s Disease (PD)
3.3. Huntington’s Disease (HD)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Remesy, C.; Jimenez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Vallinas, M.; Gonzalez-Castejon, M.; Rodriguez-Casado, A.; Ramirez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stepkowski, T.M.; Brzoska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1–19. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef]
- Prasad, S.; Aggarwal, B.B. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Monroy, A.; Lithgow, G.J.; Alavez, S. Curcumin and neurodegenerative diseases. Biofactors 2013, 39, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Fremont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Kairisalo, M.; Bonomo, A.; Hyrskyluoto, A.; Mudo, G.; Belluardo, N.; Korhonen, L.; Lindholm, D. Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci. Lett. 2011, 488, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’aquila, C.; De Mari, M.; et al. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta 2014, 1842, 902–915. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Lall, R.K.; Syed, D.N.; Adhami, V.M.; Khan, M.I.; Mukhtar, H. Dietary polyphenols in prevention and treatment of prostate cancer. Int. J. Mol. Sci. 2015, 16, 3350–3376. [Google Scholar] [CrossRef]
- Owen, R.W.; Mier, W.; Giacosa, A.; Hull, W.E.; Spiegelhalder, B.; Bartsch, H. Identification of lignans as major components in the phenolic fraction of olive oil. Clin. Chem. 2000, 46, 976–988. [Google Scholar]
- Pohl, F.; Kong Thoo Lin, P. The Potential Use of Plant Natural Products and Plant Extracts with Antioxidant Properties for the Prevention/Treatment of Neurodegenerative Diseases: In Vitro, In Vivo and Clinical Trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef]
- Chandrashekara, K.T.; Shakarad, M.N. Aloe vera or resveratrol supplementation in larval diet delays adult aging in the fruit fly, Drosophila melanogaster. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 965–971. [Google Scholar] [CrossRef]
- Balasubramani, S.P.; Mohan, J.; Chatterjee, A.; Patnaik, E.; Kukkupuni, S.K.; Nongthomba, U.; Venkatasubramanian, P. Pomegranate Juice Enhances Healthy Lifespan in Drosophila melanogaster: An Exploratory Study. Front. Public Health 2014, 2, 245. [Google Scholar] [CrossRef]
- Peng, C.; Chan, H.Y.; Huang, Y.; Yu, H.; Chen, Z.Y. Apple polyphenols extend the mean lifespan of Drosophila melanogaster. J. Agric. Food Chem. 2011, 59, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, I.; Abraham, S.K. Coffee mitigates cyclophosphamide-induced genotoxic damage in Drosophila melanogaster germ cells. Drug Chem. Toxicol. 2019, 42, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Roehrs, T.; Roth, T. Caffeine: Sleep and daytime sleepiness. Sleep Med. Rev. 2008, 12, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.S.; Ahn, S.H.; Noh, D.O.; Hong, K.B.; Han, S.H.; Suh, H.J. Effect of explosion-puffed coffee on locomotor activity and behavioral patterns in Drosophila melanogaster. Food Res. Int. 2017, 100, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Lopez, T.E.; Pham, H.M.; Barbour, J.; Tran, P.; Van Nguyen, B.; Hogan, S.P.; Homo, R.L.; Coskun, V.; Schriner, S.E.; Jafari, M. The impact of green tea polyphenols on development and reproduction in Drosophila melanogaster. J. Funct. Foods 2016, 20, 556–566. [Google Scholar] [CrossRef]
- Li, Y.M.; Chan, H.Y.; Huang, Y.; Chen, Z.Y. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol. Nutr. Food Res. 2007, 51, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Massie, H.R.; Aiello, V.R.; Williams, T.R. Inhibition of iron absorption prolongs the life span of Drosophila. Mech. Ageing. Dev. 1993, 67, 227–237. [Google Scholar] [CrossRef]
- Nagpal, I.; Abraham, S.K. Ameliorative effects of gallic acid, quercetin and limonene on urethane-induced genotoxicity and oxidative stress in Drosophila melanogaster. Toxicol. Mech. Methods 2017, 27, 286–292. [Google Scholar] [CrossRef]
- Marianna Fantin, F.G. Barbara Napoli, Alessia Forgiarini, Sentiljana Gumeni, Sara De Martin, Monica Montopoli, Chiara Vantaggiato, Genny Orso, Flavonoids Regulate Lipid Droplets Biogenesis in Drosophila melanogaster. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar]
- Ataie, A.; Shadifar, M.; Ataee, R. Polyphenolic Antioxidants and Neuronal Regeneration. Basic Clin. Neurosci. 2016, 7, 81–90. [Google Scholar] [CrossRef]
- Cha, S.J.; Kim, H.; Choi, H.J.; Lee, S.; Kim, K. Protein Glutathionylation in the Pathogenesis of Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2017, 2017, 2818565. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, C.M.; Wang, J.; Friedman, L.; Vittorino, R.; Conley, L.M.; Ho, L.; Fivecoat, H.-J.C.; Pasinetti, G.M. Grape-seed polyphenolic extract improves the eye phenotype in a Drosophila model of tauopathy. Int. J. Alzheimers Dis. 2010, 2010, 576357. [Google Scholar] [PubMed]
- Miyazaki, H.; Okamoto, Y.; Motoi, A.; Watanabe, T.; Katayama, S.; Kawahara, S.I.; Makabe, H.; Fujii, H.; Yonekura, S. Adzuki bean (Vigna angularis) extract reduces amyloid-beta aggregation and delays cognitive impairment in Drosophila models of Alzheimer’s disease. Nutr. Res. Pract. 2019, 13, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, R.; Francioso, A.; d’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.; Andrews, L.; Krause, J.; Hanak, T.; Lee, D.; Gelb, M.; Pallanck, L. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J. Neurosci. 2010, 30, 5525–5532. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, S.R.; Haddadi, M.; Shivanandappa, T.; Ramesh, S.R. Attenuation of neuromotor deficits by natural antioxidants of Decalepis hamiltonii in transgenic Drosophila model of Parkinson’s disease. Neuroscience 2015, 293, 136–150. [Google Scholar] [CrossRef]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Neuroprotective Effects of Methanolic Extract of Avocado Persea americana (var. Colinred) Peel on Paraquat-Induced Locomotor Impairment, Lipid Peroxidation and Shortage of Life Span in Transgenic knockdown Parkin Drosophila melanogaster. Neurochem. Res. 2019, 44, 1986–1998. [Google Scholar] [CrossRef]
- Fatima, A.; Khanam, S.; Rahul, R.; Jyoti, S.; Naz, F.; Ali, F.; Siddique, Y.H. Protective effect of tangeritin in transgenic Drosophila model of Parkinson’s disease. Front. Biosci. 2017, 9, 44–53. [Google Scholar]
- Long, J.; Gao, H.; Sun, L.; Liu, J.; Zhao-Wilson, X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a Drosophila Parkinson’s disease model. Rejuvenation Res. 2009, 12, 321–331. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Ara, G.; Jyoti, S.; Afzal, M. Effect of Capsaicin on the Climbing Ability in Drosophila Model of Parkinson’s Disease. Am. J. Drug Dis. Dev. 2012, 2, 50–54. [Google Scholar] [CrossRef][Green Version]
- Siddique, Y.H. Protective Role of Black-Tea Extract in a Transgenic Drosophila Model of Parkinson’s Disease. Neuroprot. Eff. Phytochem. Neurolog. Disord. 2017, 15, 317–334. [Google Scholar]
- Siddique, Y.H.; Naz, F.; Jyoti, S. Effect of curcumin on lifespan, activity pattern, oxidative stress, and apoptosis in the brains of transgenic Drosophila model of Parkinson’s disease. Biomed. Res. Int. 2014, 2014, 606928. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Arellano, H.F.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Dmp53, basket and drICE gene knockdown and polyphenol gallic acid increase life span and locomotor activity in a Drosophila Parkinson’s disease model. Genet. Mol. Biol. 2013, 36, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pfleger, C.M.; Friedman, L.; Vittorino, R.; Zhao, W.; Qian, X.; Conley, L.; Ho, L.; Pasinetti, G.M. Potential Application of Grape Derived Polyphenols in Huntington’s Disease. Transl. Neurosci. 2010, 1, 95–100. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legleiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef]
- Varga, J.; Der, N.P.; Zsindely, N.; Bodai, L. Green tea infusion alleviates neurodegeneration induced by mutant Huntingtin in Drosophila. Nutr. Neurosci. 2018, 1–7. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Perry, G.; Zhu, X.; Smith, M.A.; Sorensen, A.; Avila, J. Alzheimer’s Disease: Advances for a New Century; IOS Press: Amsterdam, The Netherlands, 2013; Volume 3. [Google Scholar]
- Ittner, L.M.; Gotz, J. Amyloid-beta and tau—A toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef]
- Saragoni, L.; Hernandez, P.; Maccioni, R.B. Differential association of tau with subsets of microtubules containing posttranslationally-modified tubulin variants in neuroblastoma cells. Neurochem. Res. 2000, 25, 59–70. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Ono, K.; Condron, M.M.; Ho, L.; Wang, J.; Zhao, W.; Pasinetti, G.M.; Teplow, D.B. Effects of grape seed-derived polyphenols on amyloid beta-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008, 283, 32176–32187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Yemul, S.; Wang, J.; Pasinetti, G.M. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J. Alzheimers Dis. 2009, 16, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.W.; Wszolek, M.F.; Shulman, J.M.; Salvaterra, P.M.; Lewis, J.; Hutton, M.; Feany, M.B. Tauopathy in Drosophila: Neurodegeneration without neurofibrillary tangles. Science 2001, 293, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Sato, S. Polyphenol-containing azuki bean (Vigna angularis) extract attenuates blood pressure elevation and modulates nitric oxide synthase and caveolin-1 expressions in rats with hypertension. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 491–497. [Google Scholar] [CrossRef]
- Shoval, H.; Weiner, L.; Gazit, E.; Levy, M.; Pinchuk, I.; Lichtenberg, D. Polyphenol-induced dissociation of various amyloid fibrils results in a methionine-independent formation of ROS. Biochim. Biophys. Acta 2008, 1784, 1570–1577. [Google Scholar] [CrossRef]
- Quik, M.; O’Leary, K.; Tanner, C.M. Nicotine and Parkinson’s disease: Implications for therapy. Mov. Disord. 2008, 23, 1641–1652. [Google Scholar] [CrossRef]
- Schwarzschild, M.A.; Agnati, L.; Fuxe, K.; Chen, J.F.; Morelli, M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006, 29, 647–654. [Google Scholar] [CrossRef]
- Kalda, A.; Yu, L.; Oztas, E.; Chen, J.F. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson’s disease. J. Neurol. Sci. 2006, 248, 9–15. [Google Scholar] [CrossRef]
- Sveinbjornsdottir, S. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139, 318–324. [Google Scholar] [CrossRef]
- Hauser, D.N.; Hastings, T.G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiol. Dis. 2013, 51, 35–42. [Google Scholar] [CrossRef]
- Kitada, T.; Asakawa, S.; Hattori, N.; Matsumine, H.; Yamamura, Y.; Minoshima, S.; Yokochi, M.; Mizuno, Y.; Shimizu, N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998, 392, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.M.; Abou-Sleiman, P.M.; Caputo, V.; Muqit, M.M.; Harvey, K.; Gispert, S.; Ali, Z.; Del Turco, D.; Bentivoglio, A.R.; Healy, D.G.; et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science 2004, 304, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Meissner, W.G.; Frasier, M.; Gasser, T.; Goetz, C.G.; Lozano, A.; Piccini, P.; Obeso, J.A.; Rascol, O.; Schapira, A.; Voon, V.; et al. Priorities in Parkinson’s disease research. Nat. Rev. Drug Discov. 2011, 10, 377–393. [Google Scholar] [CrossRef]
- Xie, W.; Wan, O.W.; Chung, K.K. New insights into the role of mitochondrial dysfunction and protein aggregation in Parkinson’s disease. Biochim. Biophys. Acta 2010, 1802, 935–941. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Ballard, P.A.; Tetrud, J.W.; Langston, J.W. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): Seven cases. Neurology 1985, 35, 949–956. [Google Scholar] [CrossRef]
- Hertzman, C.; Wiens, M.; Bowering, D.; Snow, B.; Calne, D. Parkinson’s disease: A case-control study of occupational and environmental risk factors. Am. J. Ind. Med. 1990, 17, 349–355. [Google Scholar] [CrossRef]
- Liou, H.H.; Tsai, M.C.; Chen, C.J.; Jeng, J.S.; Chang, Y.C.; Chen, S.Y.; Chen, R.C. Environmental risk factors and Parkinson’s disease: A case-control study in Taiwan. Neurology 1997, 48, 1583–1588. [Google Scholar] [CrossRef]
- Bove, J.; Prou, D.; Perier, C.; Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRx 2005, 2, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hwang, M.S.; Park, H.J.; Shin, H.K.; Baek, J.U.; Choi, B.T. Herbal Prescriptions and Medicinal Herbs for Parkinson-Related Rigidity in Korean Medicine: Identification of Candidates Using Text Mining. J. Altern. Complement. Med. 2018, 24, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Bost, J.B.; S, N.J.H.; Crane, J.H. The avocado: Botany, production and uses. In History, Distribution and Uses [Online], 2nd ed.; Schaffer, B., Wolstenholme, B.N., Whiley, A.W., Eds.; CABI: Wallingford, UK, 2013; pp. 10–30. [Google Scholar]
- Chaumontet, C.; Bex, V.; Gaillard-Sanchez, I.; Seillan-Heberden, C.; Suschetet, M.; Martel, P. Apigenin and tangeretin enhance gap junctional intercellular communication in rat liver epithelial cells. Carcinogenesis 1994, 15, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Abe, K.; Gotoh, M.; Oka, K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br. J. Cancer 1995, 72, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.; Lee, H.S.; Ryoo, S.; Seo, J.H.; Min, B.S.; Lee, J.H. Tangeretin, a citrus flavonoid, inhibits PGDF-BB-induced proliferation and migration of aortic smooth muscle cells by blocking AKT activation. Eur. J. Pharmacol. 2011, 673, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Del-Rio, M.; Guzman-Martinez, C.; Velez-Pardo, C. The effects of polyphenols on survival and locomotor activity in Drosophila melanogaster exposed to iron and paraquat. Neurochem. Res. 2010, 35, 227–238. [Google Scholar] [CrossRef]
- Zuccato, C.; Valenza, M.; Cattaneo, E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol. Rev. 2010, 90, 905–981. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Leggo, J.; Coles, R.; Almqvist, E.; Biancalana, V.; Cassiman, J.J.; Chotai, K.; Connarty, M.; Crauford, D.; Curtis, A.; et al. Phenotypic characterization of individuals with 30-40 CAG repeats in the Huntington disease (HD) gene reveals HD cases with 36 repeats and apparently normal elderly individuals with 36-39 repeats. Am. J. Hum. Genet. 1996, 59, 16–22. [Google Scholar]
- Yamamoto, A.; Lucas, J.J.; Hen, R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell 2000, 101, 57–66. [Google Scholar] [CrossRef]
- Browne, S.E.; Ferrante, R.J.; Beal, M.F. Oxidative stress in Huntington’s disease. Brain Pathol. 1999, 9, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Browne, S.E.; Bowling, A.C.; MacGarvey, U.; Baik, M.J.; Berger, S.J.C.; Muqit, M.M.; Bird, E.D.; Beal, M.F. Oxidative damage and metabolic dysfunction in Huntington’s disease: selective vulnerability of the basal ganglia. Ann. Neurol. 1997, 41, 646–653. [Google Scholar] [CrossRef] [PubMed]
| Categories of Polyphenol | Basic Chemical Structure |
|---|---|
| Flavonoid | 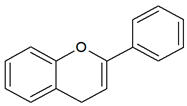 |
| Phenolic acid | 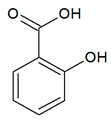 |
| Curcuminoid | 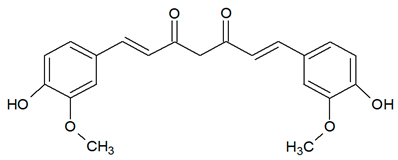 |
| Stilbene | 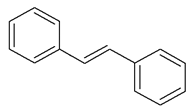 |
| Lignan | 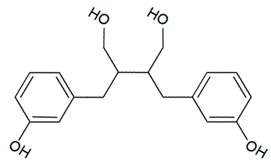 |
| Neurodegenerative Disease | Polyphenol Derived from Plants | Supplemental Amount | Effect | Reference |
|---|---|---|---|---|
| Alzheimer’s disease (AD) | Grape seed | 2.8 μg/mL GSPE in fly medium | Recover the defective Drosophila eye induced by tauopathy using R406W mutant tau fly | [33] |
| Adzuki bean | 1 mg/mL adzuki extract in fly medium | Restore abnormal memory, movement defects, and shortened lifespan in Aβ42-overexpressing fly | [34] | |
| Arabidopsis thaliana (At) | 40 μL/mL ethyl acetate extract of At in fly medium | Recover defective climbing capability in Aβ42-overexpressing fly | [35] | |
| Decaffeinated coffee and nicotine-free tobacco | 0.15% decaffeinated coffee and 0.03% nicotine-free tobacco in fly medium | Rescue damaged locomotive activity, shortened longevity, and reduced eclosion rate via the activation of Nrf2 in AD fly induced by Aβ42 overexpression | [36] | |
| Parkinson’s disease (PD) | Decalepis hamiltonii (Dh) root | 0.1 or 0.5% aqueous extract of Dh in fly medium | Restore the defective locomotive activity and circadian rhythm due to missense mutations (A30P and A35T) in an α-synuclein-overexpressing fly and protect against PQ sensitivity | [37] |
| Avocado Persea americana (Pa) peel | 1 or 5 mg/mL methanol extract of Pa in fly medium | Protect parkin knockdown fly against paraquat (PQ)- induced oxidative stress, mobility damage, shortened longevity, and lipid peroxidation | [38] | |
| Tangeritin | 5, 10, and 20 μM extract of tangeritin in fly medium | Rescue the reduction in locomotive activity and several oxidative stress markers and increase the dopamine content in PD fly model | [39] | |
| Grape | 0.16–0.64 mg extract in 100 g of fly medium | Protect against free radicals, free radical-induced lipid peroxidation, and DNA damages and life extension in an α-synuclein-overexpressing fly model | [40] | |
| Capsaicin | 0.1–1.0 μL/mL of capsaicin in fly medium | Delay the reduction in climbing activity in an α-synuclein-overexpressing fly model | [41] | |
| Black tea | 20, 40, and 60 μM extract of black tea in fly medium | Reduce lipid peroxidation, protein carbonyl content, and glutathione S-transferase activity, and increase glutathione and dopamine content in fly exposed to L-dopamine | [42] | |
| Decaffeinated coffee and nicotine-free tobacco | 0.15% decaffeinated coffee and 0.03% nicotine-free tobacco in fly medium | Restore the damaged climbing ability, shortened survival rate, and reduce dopaminergic neurons via Nrf2 activation in mutant α-synuclein-overexpressing fly, and loss-of-function mutant in parkin gene | [36] | |
| Curcumin | 25, 50, and 100 μM curcumin in fly medium | Increase the life span and reduce apoptosis and oxidative stress, such as lipid peroxidation and protein carbonyl content, in α-synuclein-overexpressing fly model | [43] | |
| Gallic acid | 0.1 mM gallic acid in fly medium | Rescue the loss of dopaminergic neurons and alleviate life span and locomotive activity with PQ treatment | [44] | |
| Huntington’s disease (HD) | Grape seed | 2.8 μg/mL GSPE in fly medium | Prolong the lifespan in Q93httexon1-overexpressing fly model | [45] |
| Green tea | 0.01–100 μM EGCG in fly medium and water extract of green tea in fly medium | Prolong the lifespan in Q93httexon1-overexpressing fly model | [46,47] |
| Neurodegenerative Disease | Type | Drosophila Model | Phenotype | Symptoms of Human Patient | Reference |
|---|---|---|---|---|---|
| Alzheimer’s disease (AD) | Familial | TauR406W-overexpression in the eye using an ey-Gal4 | Rough eye, reduced locomotion, and shortened lifespan | Impairment of learning and memory, sleep disorder, motor impairment, and decreased attention | [33,34,35,36] |
| Aβ42-overexpression in neurons using an elav-Gal4 | Reduced locomotion and shortened lifespan | ||||
| Aβ42-overexpression in glial cells using a repo-Gal4 | Reduced locomotion, shortened lifespan, and defect in memory and learning | ||||
| Parkinson’s disease (PD) | Familial | α-synucleinA30P- and α-synucleinA53P-overexpression in neurons using an elav-Gal4 | Reduced locomotion, shortened lifespan, increased lipid peroxidation, and reactive oxygen species | Tremor, rigidity, impaired balance, motor impairment, depression, and sleep disorder | [36,37,38,39,40,41,42,43,44] |
| Parkin RNAi-overexpression in dopaminergic neurons using a TH-Gal4 | Reduced locomotion and shortened lifespan | ||||
| α-synuclein-overexpression in neurons using an elav-Gal4 | Reduced locomotion, increased lipid peroxidation and reactive oxygen species, and decreased dopamine content | ||||
| α-synuclein-overexpression in dopaminergic neurons using a Ddc-Gal4 | Reduced locomotion and shortened lifespan | ||||
| α-synuclein-overexpression in dopaminergic neurons using a TH-Gal4 | Reduced locomotion and dopaminergic neuron loss | ||||
| parkin null mutant | Dopaminergic neuron loss | ||||
| Sporadic | Chronic exposure to paraquat | Reduced locomotion and shortened lifespan | |||
| Huntington’s disease (HD) | Familial | Q93httexon1-ovexpression in neurons using an elav-Gal4 | Reduced locomotion, shortened lifespan, and degeneration of photoreceptor neuron | Impairment of learning and memory, rigidity, and movement disorder | [45,46,47] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, S.J.; Do, H.-A.; Choi, H.-J.; Lee, M.; Kim, K. The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases. Antioxidants 2019, 8, 623. https://doi.org/10.3390/antiox8120623
Cha SJ, Do H-A, Choi H-J, Lee M, Kim K. The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases. Antioxidants. 2019; 8(12):623. https://doi.org/10.3390/antiox8120623
Chicago/Turabian StyleCha, Sun Joo, Hyeon-Ah Do, Hyun-Jun Choi, Mihye Lee, and Kiyoung Kim. 2019. "The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases" Antioxidants 8, no. 12: 623. https://doi.org/10.3390/antiox8120623
APA StyleCha, S. J., Do, H.-A., Choi, H.-J., Lee, M., & Kim, K. (2019). The Drosophila Model: Exploring Novel Therapeutic Compounds against Neurodegenerative Diseases. Antioxidants, 8(12), 623. https://doi.org/10.3390/antiox8120623






