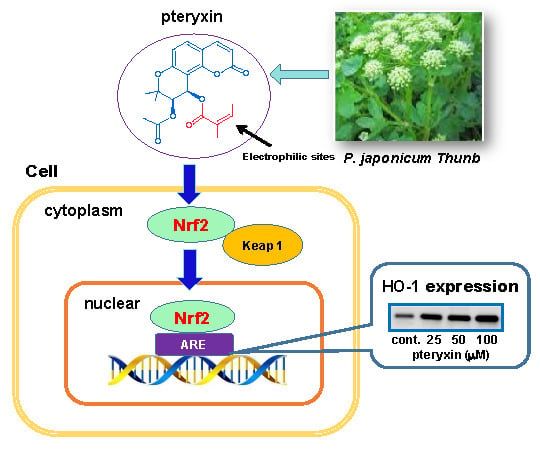
Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264.7 Macrophage Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
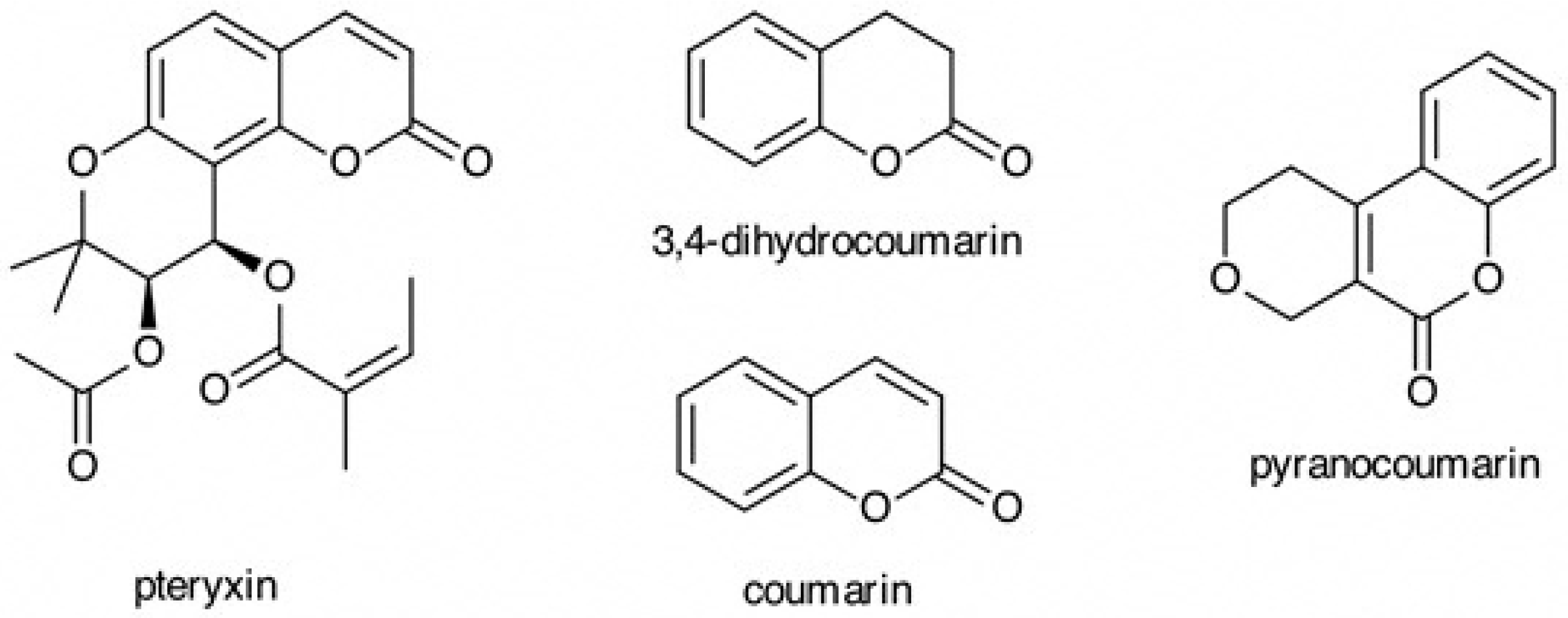
2.2. Isolation of Pteryxin
2.3. Analysis of Pteryxin
2.4. Cell Culture
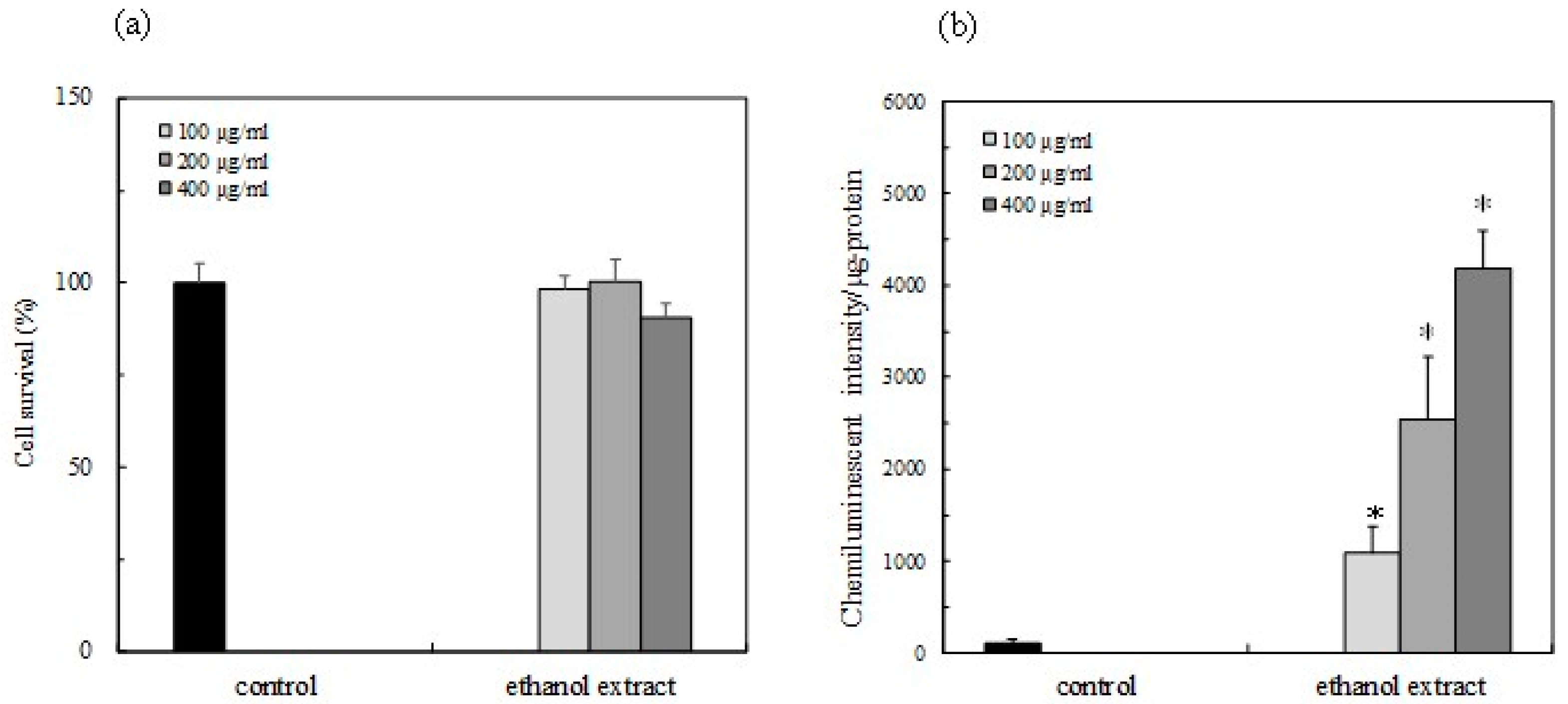
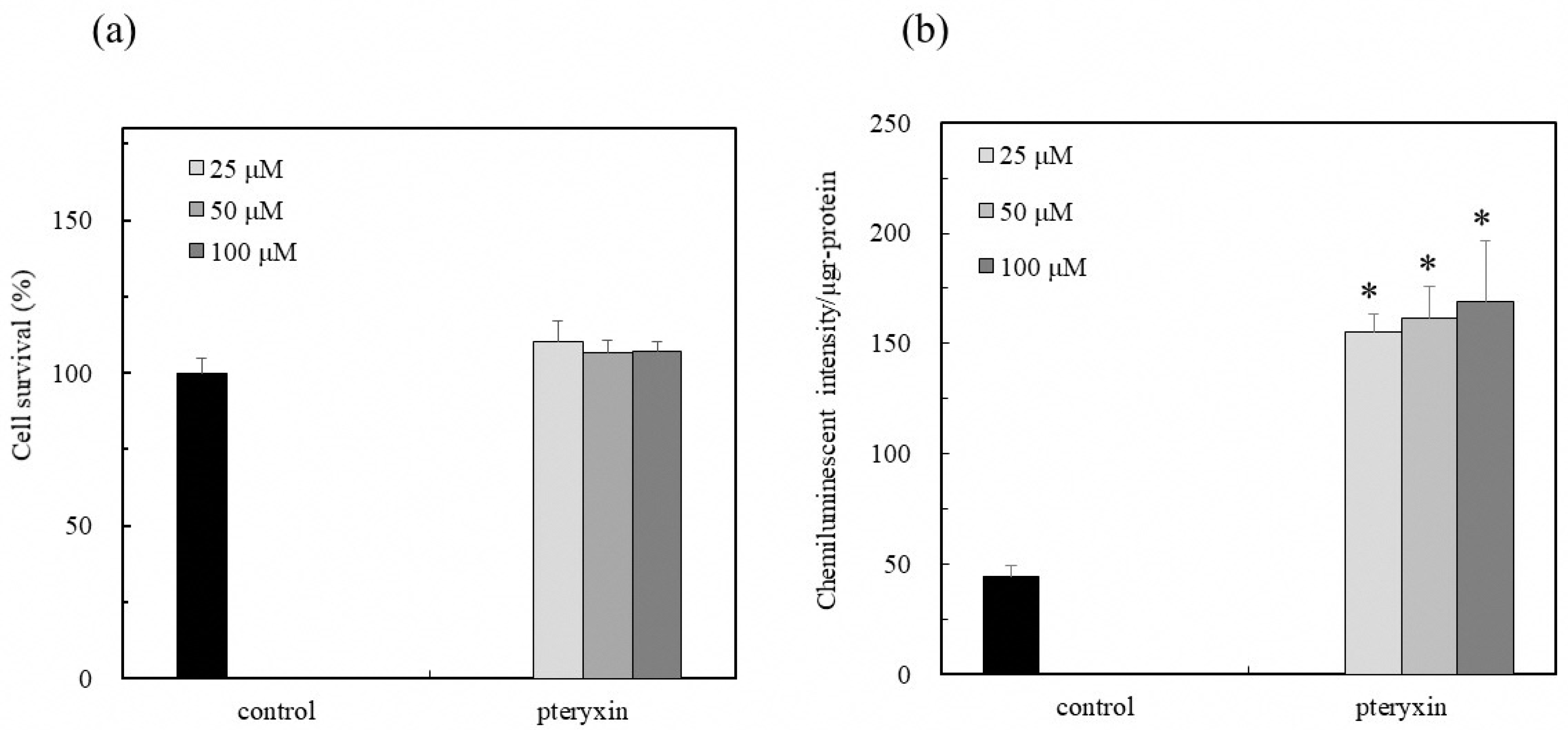
2.5. Cell Survaival
2.6. Activity of Nrf2-ARE Signaling
2.7. Nuclear Extraction and Determination of Nrf2
2.8. Protein Expression by Western Blot Analysis
3. Results
3.1. Determination of Pteryxin
3.2. Nrf2-ARE Signaling
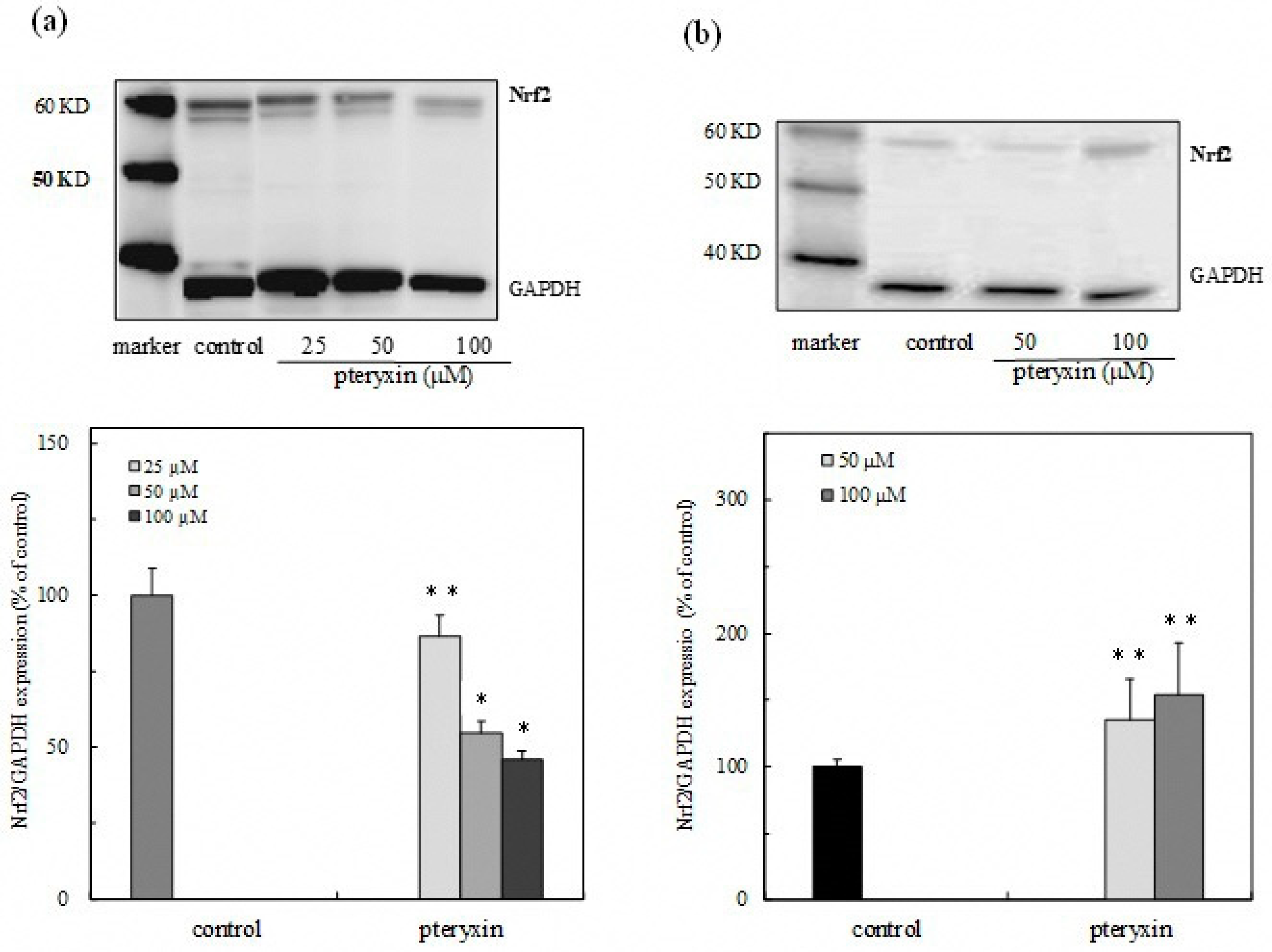
3.3. Nrf2 Expression in Cytoplasm and Nuclei
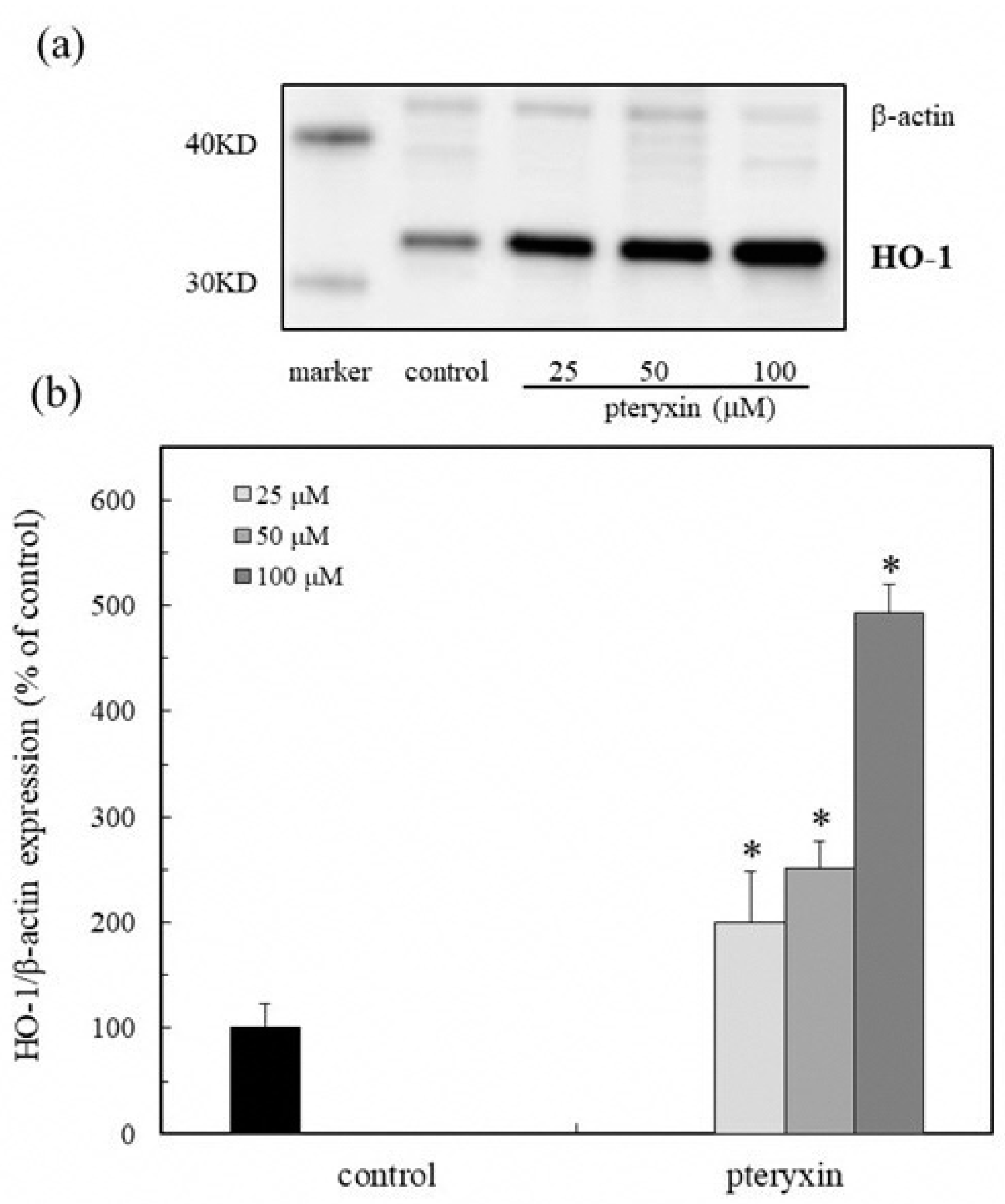
3.4. HO-1 Expression
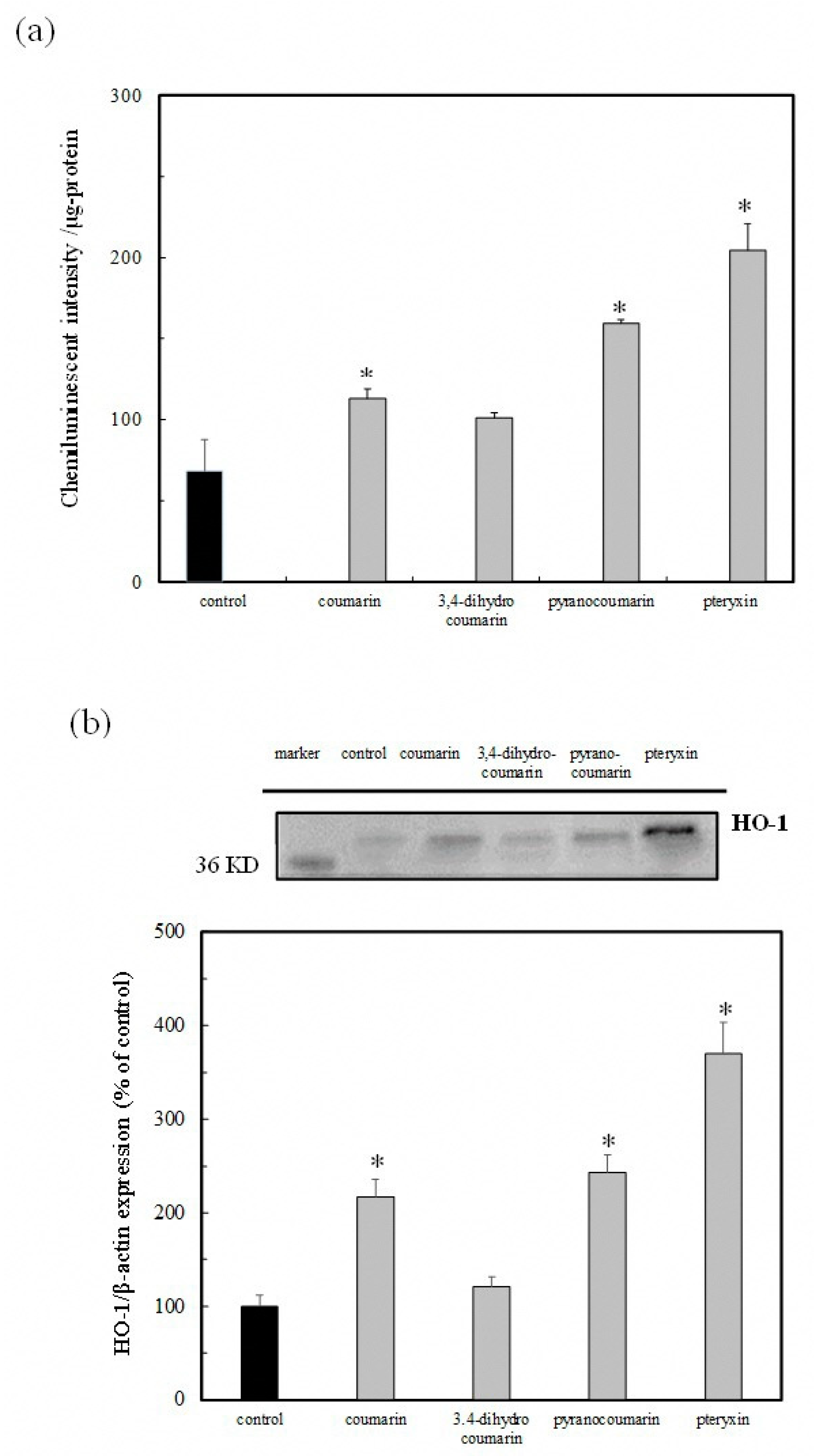
3.5. Nrf2-ARE Signaling Activity and HO-1 Protein Expression by Coumarin Derivatives
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkhail, P. Traditional uses, phytochemistry and pharmacological properties of the genus. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Antioxidant compounds from the leaves of Peucedanum japonicum Thunb. J. Agric. Food Chem. 2003, 51, 5255–5261. [Google Scholar] [CrossRef] [PubMed]
- Hisamoto, M.; Kikuzaki, H.; Ohigashi, H.; Nakatani, N. Constituents of the leaves of Peucedanum japonicum Thunb. and their biological activity. J. Agric. Food Chem. 2004, 52, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Nukitrangsan, N.; Okabe, T.; Toda, T.; Inafuku, M.; Iwasaki, H.; Oku, H. Anti-obesity activity of Peucedanum japonicum Thunb extract in obese diabetic animal model C57BL/6JHam Slc-ob/ob Mice. Int. J. Life Sci. Med. Res. 2012, 2, 28–34. [Google Scholar] [CrossRef]
- Nugara, R.N.; Inafuku, M.; Iwasaki, H.; Oku, H. Partially purified Peucedanum japonicum Thunb extracts exert anti-obesity effects in vitro. Nutrition 2014, 30, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Nugara, R.N.; Inafuku, M.; Takara, K.; Iwasaki, H.; Oku, H. Pteryxin: A coumarin in Peucedanum japonicum Thunb leaves exerts antiobesity activity through modulation of adipogenic gene network. Nutrition 2014, 30, 1177–1184. [Google Scholar] [CrossRef]
- Okabe, T.; Toda, T.; Nukitrangsan, N.; Inafuku, M.; Iwasaki, H.; Oku, H. Peucedanum japonicum Thunb inhibits high-fat diet induced obesity in mice. Phytother. Res. 2011, 25, 870–877. [Google Scholar] [CrossRef]
- Choi, R.Y.; Nam, S.J.; Ham, J.R.; Lee, H.I.; Yee, S.T.; Kang, K.Y.; Lee, M.K. Anti-adipogenic and anti-diabetic effects of cis-3, 4-diisovalerylkhellactone isolated from Peucedanum japonicum Thunb leaves in vitro. Bioorganic Med. Chem. Lett. 2016, 26, 4655–4660. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Nugara, R.N.; Inafuku, M.; Takara, K.; Ogi, T.; Ichiba, Y.; Iwasaki, H.; Okabe, T.; Oku, H.J. In vivo and in vitro anti-obesity activities of dihydropyranocoumarins derivatives from Peucedanum japonicum Thunb. J. Funct. Foods. 2017, 29, 19–28. [Google Scholar] [CrossRef]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-A.; Lee, I.-K. The Role of Nrf2: Adipocyte differentiation, obesity, and insulin resistance. Oxid. Med. Cell. Longev. 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kong, A.N. Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic. Biol. Med. 2004, 36, 1505–1516. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.-J.; Kundu, J.K.; Na, H.-K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Sonamoto, M.; Uehara, M. Dual biological functions of a cytoprotective effect and apoptosis induction by bioavailable marine carotenoid fucoxanthinol through modulation of the Nrf2 activation in RAW264.7 macrophage cells. Mar. Drugs 2017, 15, 305. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Miyazato, H.; Ueda, K. Marine peroxy sesquiterpenoids induce apoptosis by modulation of Nrf2-ARE signaling in HCT116 colon cancer cells. Mar. Drugs 2018, 16, 347. [Google Scholar] [CrossRef] [PubMed]
- Taira, J.; Nanbu, H.; Ueda, K. Nitric oxide-scavenging compounds in Agrimonia pilosa Ledeb on LPS-induced RAW264.7 macrophages. Food Chem. 2009, 115, 1221–1227. [Google Scholar] [CrossRef]
- Yagishita, Y.; Uruno, A.; Fukutomi, T.; Sugiyama, F.; Takahashi, S.; Yamamoto, M. Nrf2 improves leptin and insulin resistance provoked by hypothalamic oxidative stress. Cell Rep. 2017, 18, 2030–2044. [Google Scholar] [CrossRef] [PubMed]
- Takayama, N.; Iwamoto, N.; Sumi, D.; Shinkai, Y.; Tanaka-Kagawa, T.; Jinno, H.; Kumagai, Y. Peroxiredoxin 6 is a molecular target for 1, 2-naphthoquinone, an atmospheric electrophile in human pulmonary epithelial A549 cells. J. Toxicol. Sci. 2011, 36, 817–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taira, J.; Ogi, T. Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264.7 Macrophage Cells. Antioxidants 2019, 8, 621. https://doi.org/10.3390/antiox8120621
Taira J, Ogi T. Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264.7 Macrophage Cells. Antioxidants. 2019; 8(12):621. https://doi.org/10.3390/antiox8120621
Chicago/Turabian StyleTaira, Junsei, and Takayuki Ogi. 2019. "Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264.7 Macrophage Cells" Antioxidants 8, no. 12: 621. https://doi.org/10.3390/antiox8120621
APA StyleTaira, J., & Ogi, T. (2019). Induction of Antioxidant Protein HO-1 Through Nrf2-ARE Signaling Due to Pteryxin in Peucedanum Japonicum Thunb in RAW264.7 Macrophage Cells. Antioxidants, 8(12), 621. https://doi.org/10.3390/antiox8120621