Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Protocol
2.2. Tomato-Oleoresin Preparation
2.3. Nutritional Evaluation
2.4. Metabolic and Hormonal Analysis
2.5. Systolic Blood Pressure (SBP)
2.6. Lycopene Bioavailability Evaluation
2.7. Cardiac Malondialdehyde (MDA) Levels
2.8. Circulating Advanced Oxidation Protein Products
2.9. Circulating Carboxymethyl Lysine
2.10. Echocardiographic Study
2.11. Myocardial Function by Isolated Papillary Muscle Study
2.12. β-Adrenergic System Study
2.13. Statistical Analysis
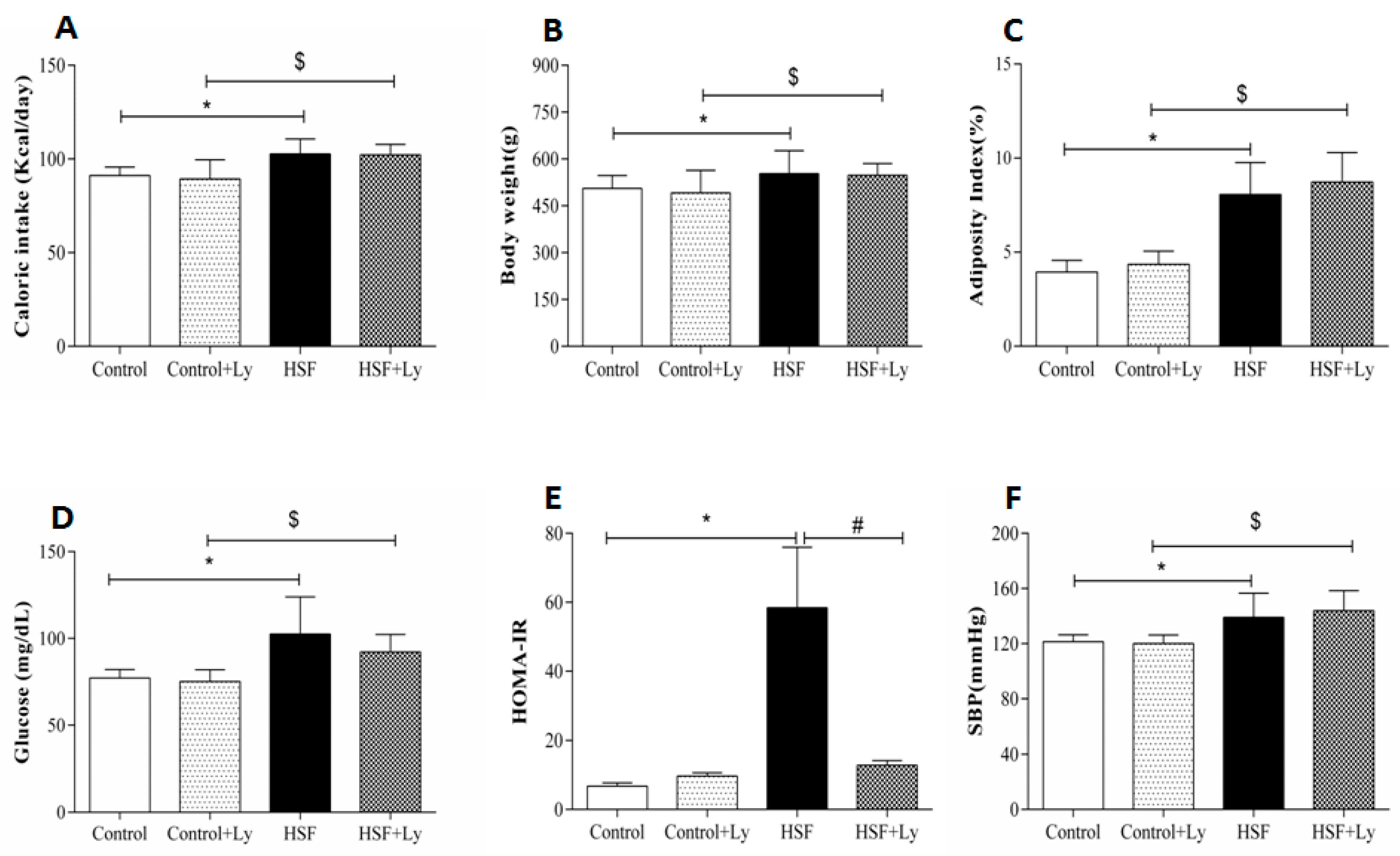
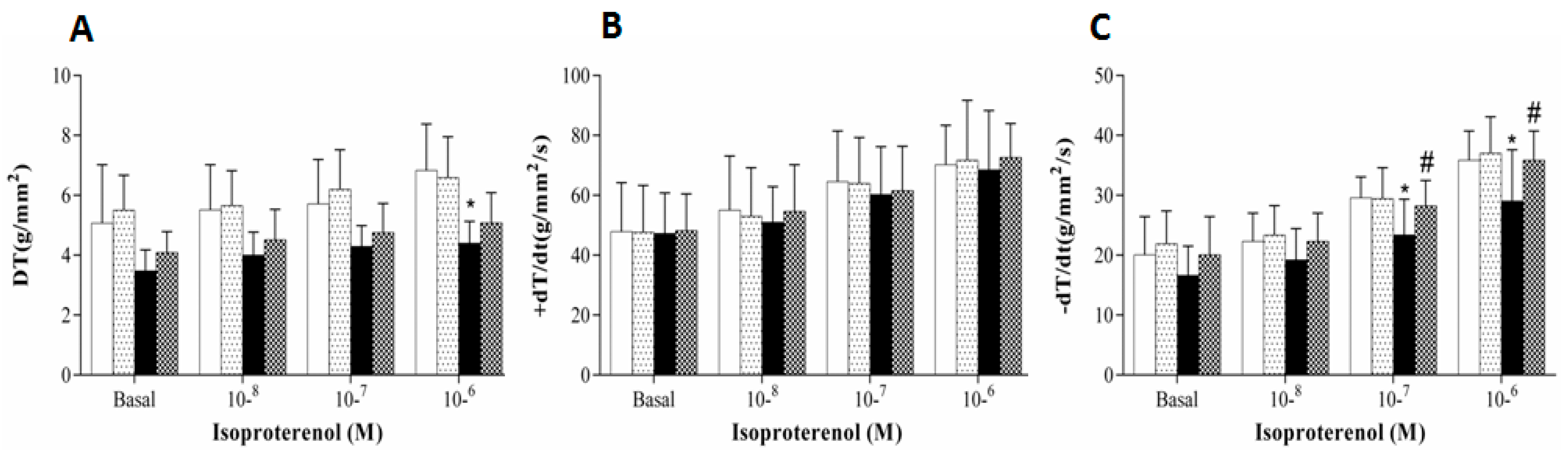
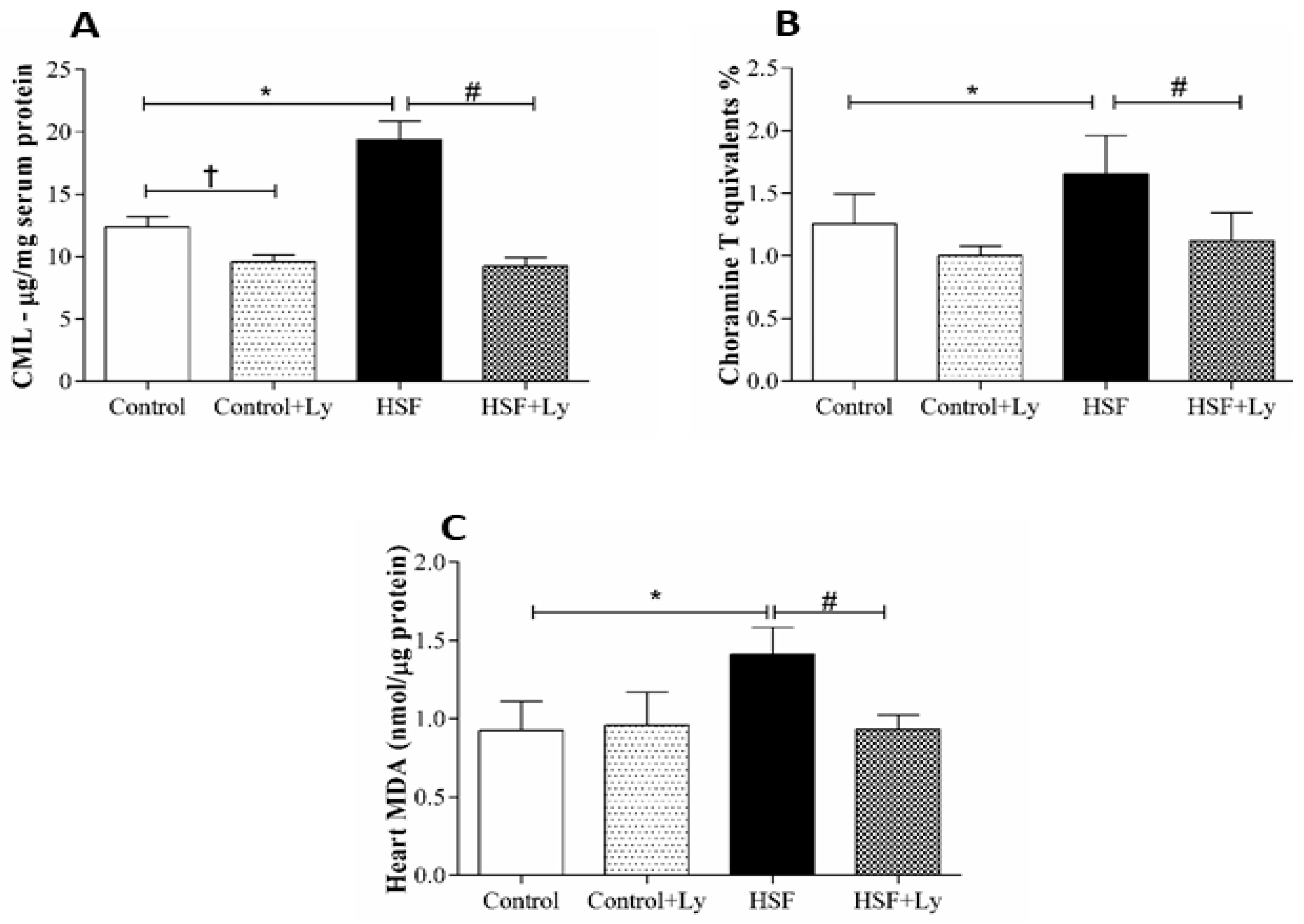
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alpert, M.A.; Lambert, C.R.; Panayiotou, H.; Terry, B.E.; Cohen, M.V.; Massey, C.V.; Hashimi, M.W.; Mukerji, V. Relation of duration of morbid obesity to left ventricular mass, systolic function, and diastolic filling, and effect of weight loss. Am. J. Cardiol. 1995, 76, 1194–1197. [Google Scholar] [CrossRef]
- Scaglione, R.; Dichiara, M.A.; Indovina, A.; Lipari, R.; Ganguzza, A.; Parrinello, G.; Capuana, G.; Merlino, G.; Licata, G. Left ventricular diastolic and systolic function in normotensive obese subjects: Influence of degree and duration of obesity. Eur. Heart J. 1992, 13, 738–742. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Relling, D.P.; Esberg, L.B.; Fang, C.X.; Johnson, W.T.; Murphy, E.J.; Carlson, E.C.; Saari, J.T.; Ren, J. High-fat diet-induced juvenile obesity leads to cardiomyocyte dysfunction and upregulation of Foxo3a transcription factor independent of lipotoxicity and apoptosis. J. Hypertens. 2006, 24, 549–561. [Google Scholar] [CrossRef] [PubMed]
- du Toit, E.F.; Nabben, M.; Lochner, A. A potential role for angiotensin II in obesity induced cardiac hypertrophy and ischaemic/reperfusion injury. Basic Res. Cardiol. 2005, 100, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhu, B.-H.; Relling, D.P.; Esberg, L.B.; Ceylan-Isik, A.F. High-fat diet-induced obesity leads to resistance to leptin-induced cardiomyocyte contractile response. Obesity 2008, 16, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.S.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; do Nascimento, A.F.; Luvizotto, R.D.A.M.; de Campos, D.H.S.; Okoshi, K.; Pai-Silva, M.D.; Padovani, C.R.; Cicogna, A.C. Cardiac remodeling in a rat model of diet-induced obesity. Can. J. Cardiol. 2010, 26, 423–429. [Google Scholar] [CrossRef]
- Leopoldo, A.S.; Lima-Leopoldo, A.P.; Sugizaki, M.M.; Nascimento, A.F.D.; de Campos, D.H.S.; Luvizotto, R.D.A.M.; Castardeli, E.; Alves, C.A.B.; Brum, P.C.; Cicogna, A.C. Involvement of L-type calcium channel and serca2a in myocardial dysfunction induced by obesity. J. Cell. Physiol. 2011, 226, 2934–2942. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative Stress and Heart Failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Opie, L. The Heart. Physiology from Cell to Circulation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998. [Google Scholar]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–415. [Google Scholar] [CrossRef]
- Carvajal, K.; Balderas-Villalobos, J.; Bello-Sanchez, M.D.; Phillips-Farfán, B.; Molina-Munoz, T.; Aldana-Quintero, H.; Gómez-Viquez, N.L. Ca2+ mishandling and cardiac dysfunction in obesity and insulin resistance: Role of oxidative stress. Cell Calcium 2014, 56, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.C.; Fauconnier, J.; Yamada, T.; Lacampagne, A.; Zhang, S.J.; Katz, A.; Westerblad, H. Mitochondrial production of reactive oxygen species contributes to the β-adrenergic stimulation of mouse cardiomycytes. J. Physiol. 2011, 589, 1791–1801. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.L.; Reis, P.P.; Severino, F.E.; Felix, T.F.; Braz, M.G.; Nogueira, F.R.; Silva, R.A.C.; Cardoso, A.C.; Lourenço, M.A.M.; Figueiredo, A.M.; et al. Tomato (Lycopersicon esculentum) or lycopene supplementation attenuates ventricular remodeling after myocardial infarction through different mechanistic pathways. J. Nutr. Biochem. 2017, 46, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Francisqueti, F.; Minatel, I.; Ferron, A.; Bazan, S.; Silva, V.; Garcia, J.; De Campos, D.; Ferreira, A.; Moreto, F.; Cicogna, A.; et al. Effect of Gamma-Oryzanol as Therapeutic Agent to Prevent Cardiorenal Metabolic Syndrome in Animals Submitted to High Sugar-Fat Diet. Nutrients 2017, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Story, E.N.; Kopec, R.E.; Schwartz, S.J.; Harris, G.K. An Update on the Health Effects of Tomato Lycopene. Annu. Rev. Food Sci. Technol. 2010, 1, 189–210. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P. What are Typical Lycopene Intakes? J. Nutr. 2005, 135, 2042–2045. [Google Scholar] [CrossRef]
- Araujo, F.B.; Barbosa, D.S.; Hsin, C.Y.; Maranhao, R.C.; Abdalla, D.S.P. Evaluation of oxidative stress in patients with hyperlipidemia. Atherosclerosis 1995, 117, 61–71. [Google Scholar] [CrossRef]
- Engelhard, Y.N.; Gazer, B.; Paran, E. Natural antioxidants from tomato extract reduce blood pressure in patients with grade-1 hypertension: A double-blind, placebo-controlled pilot study. Am. Heart J. 2006, 151. [Google Scholar] [CrossRef]
- Bansal, P.; Gupta, S.K.; Ojha, S.K.; Nandave, M.; Mittal, R.; Kumari, S.; Arya, D.S. Cardioprotective effect of lycopene in the experimental model of myocardial ischemia-reperfusion injury. Mol. Cell. Biochem. 2006, 289, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ferron, A.; Francisqueti, F.; Minatel, I.; Silva, C.; Bazan, S.; Kitawara, K.; Garcia, J.; Corrêa, C.; Moreto, F.; Ferreira, A. Association between Cardiac Remodeling and Metabolic Alteration in an Experimental Model of Obesity Induced by Western Diet. Nutrients 2018, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Luvizotto, R.D.A.M.; Nascimento, A.F.; Imaizumi, E.; Pierine, D.T.; Conde, S.J.; Correa, C.R.; Yeum, K.-J.; Ferreira, A.L.A. Lycopene supplementation modulates plasma concentrations and epididymal adipose tissue mRNA of leptin, resistin and IL-6 in diet-induced obese rats. Br. J. Nutr. 2013, 110, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Pierine, D.T.; Navarro, M.E.L.; Minatel, I.O.; Luvizotto, R.A.M.; Nascimento, A.F.; Ferreira, A.L.A.; Yeum, K.-J.; Corrêa, C.R. Lycopene supplementation reduces TNF-α via RAGE in the kidney of obese rats. Nutr. Diabetes 2014, 4, e142. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.V.; Shen, H. Effect of low dose lycopene intake on lycopene bioavailability and oxidative stress. Nutr. Res. 2002, 22, 1125–1131. [Google Scholar] [CrossRef]
- Anjos Ferreira, A.L.; Russell, R.M.; Rocha, N.; Placido Ladeira, M.S.; Favero Salvadori, D.M.; Oliveira Nascimento, M.C.M.; Matsui, M.; Carvalho, F.A.; Tang, G.; Matsubara, L.S.; et al. Effect of lycopene on doxorubicin-induced cardiotoxicity: An echocardiographic, histological and morphometrical assessment. Basic Clin. Pharmacol. Toxicol. 2007, 101, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Luvizotto, R.; Nascimento, A.; Miranda, N.; Wang, X.-D.; Ferreira, A. Lycopene-rich tomato oleoresin modulates plasma adiponectin concentration and mRNA levels of adiponectin, SIRT1, and FoxO1 in adipose tissue of obese rats. Hum. Exp. Toxicol. 2015, 34, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Ferron, A.J.T.; Jacobsen, B.B.; Grippa, P.; Ana, S. Cardiac Dysfunction Induced by Obesity Is Not Related to β -Adrenergic System Impairment at the Receptor-Signalling Pathway. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.L.A.; Salvadori, D.M.F.; Nascimento, M.C.M.O.; Rocha, N.S.; Correa, C.R.; Pereira, E.J.; Matsubara, L.S.; Matsubara, B.B.; Ladeira, M.S.P. Tomato-oleoresin supplement prevents doxorubicin-induced cardiac myocyte oxidative DNA damage in rats. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 631, 26–35. [Google Scholar] [CrossRef]
- Yagi, K. Simple Assay for the Level of Total Lipid Peroxides in Serum or Plasma. Free Radic. Antioxid. Protoc. 1998, 108, 101–106. [Google Scholar]
- Lee, R.; Margaritis, M.; Channon, K.M.; Antoniades, C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr. Med. Chem. 2012, 19, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhang, K.; Liu, Y.; Chen, J.; Cai, Q.; He, W.; Zhang, Y.; Wang, M.-H.; Wang, J.; Huang, H. Advanced oxidation protein products aggravate cardiac remodeling via cardiomyocyte apoptosis in chronic kidney disease. Am. J. Physiol. Circ. Physiol. 2017, 314, H475–H483. [Google Scholar] [CrossRef] [PubMed]
- Zuwala-Jagiello, J.; Murawska-Cialowicz, E.; Pazgan-Simon, M. Increased Circulating Advanced Oxidation Protein Products and High-Sensitive Troponin T in Cirrhotic Patients with Chronic Hepatitis C: A Preliminary Report. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kalousová, M.; Škrha, J.; Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with insulin dependent diabetes melli.us. Med. J. Bakirkoy 2011, 7, 130–135. [Google Scholar]
- Hegab, Z.; Gibbons, S.; Neyses, L.; Mamas, M.A. Role of advanced glycation end products in cardiovascular disease. World J. Cardiol. 2012, 4, 90. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Lima-Leopoldo, A.P.; Leopoldo, A.S.; Sugizaki, M.M.; Bruno, A.; Nascimento, A.F.; Luvizotto, R.A.; de Oliveira Júnior, S.A.; Castardeli, E.; Padovani, C.R.; Cicogna, A.C. Myocardial Dysfunction and Abnormalities in Intracellular Calcium Handling in Obese Rats. Arq. Bras. Cardiol. 2011, 97, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Maioli, T.U.; Gonçalves, J.L.; Miranda, M.C.G.; Martins, V.D.; Horta, L.S.; Moreira, T.G.; Godard, A.L.B.; Santiago, A.F.; Faria, A.M.C. High sugar and butter (HSB) diet induces obesity and metabolic syndrome with decrease in regulatory T cells in adipose tissue of mice. Inflamm. Res. 2016, 65, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Assis, R.; Arcaro, C.; Gutierres, V.; Oliveira, J.; Costa, P.; Baviera, A.; Brunetti, I. Combined effects of curcumin and lycopene or bixin in yoghurt on inhibition of LDL oxidation and increases in HDL and paraoxonase levels in streptozotocin-diabetic rats. Int. J. Mol. Sci. 2017, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene Improves Insulin Sensitivity through Inhibition of STAT3/Srebp-1c-Mediated Lipid Accumulation and Inflammation in Mice fed a High-Fat Diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610–617. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. In Vivo 2018, 32, 255–264. [Google Scholar] [PubMed]
- Yang, P.M.; Chen, H.Z.; Huang, Y.T.; Hsieh, C.W.; Wung, B.S. Lycopene inhibits NF-κB activation and adhesion molecule expression through Nrf2-mediated heme oxygenase-1 in endothelial cells. Int. J. Mol. Med. 2017, 39, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.P.; Rodrigo, M.J.; Zacarias, L. Dietary Carotenoid Roles in Redox Homeostasis and Human Health. J. Agric. Food Chem. 2018, 66, 5733–5740. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Kyser, C.K.; Martin, M.M. beta-Adrenoceptor density and adenylyl cyclase activity in obese rabbit hearts. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.F.; Jones, A.E.; Hester, R.L.; Reinhart, G.A.; Cockrell, K.; Mizelle, H.L. Reduced cardiac contractile responsiveness to isoproterenol in obese rabbits. Hypertension 1997, 30, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Lima-Leopoldo, A.P.; Sugizaki, M.M.; Leopoldo, A.S.; Carvalho, R.F.; Nogueira, C.R.; Nascimento, A.F.; Martinez, P.F.; Luvizotto, R.A.M.; Padovani, C.R.; Cicogna, A.C. Obesity induces upregulation of genes involved in myocardial Ca2+ handling. Braz. J. Med. Biol. Res. 2008, 41. [Google Scholar] [CrossRef] [PubMed]
- Dincer, U.D. Cardiac β-adrenoceptor expression is markedly depressed in Ossabaw swine model of cardiometabolic risk. Int. J. Gen. Med. 2011, 4, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, M.F.; Silva, M.D.P.; Sugizaki, M.M.; Novelli, Y.S.D.; Sant’ana, L.S.; Aragon, F.F.; Padovani, C.R.; Novelli, E.L.B.; Cicogna, A.C. Artigo Original Influências de Dietas Ricas em Ácidos Graxos Saturados e Insaturados sobre o Miocárdio de Ratos. Arq. Bras. Cardiol. 2007, 88, 346–353. [Google Scholar] [CrossRef]
- de Lucia, C.; Eguchi, A.; Koch, W.J. New insights in cardiac β-Adrenergic signaling during heart failure and aging. Front. Pharmacol. 2018, 9, 904. [Google Scholar] [CrossRef]
- Wu, I.; Shiesh, Ã.S.; Kuo, P.; Lin, X. High Oxidative Stress Is Correlated with Frailty in Elderly Chinese. J. Am. Geriatr. Soc. 2009, 57, 1666–1671. [Google Scholar] [CrossRef]
- Zhang, G.X.; Kimura, S.; Nishiyama, A.; Shokoji, T.; Rahman, M.; Yao, L.; Nagai, Y.; Fujisawa, Y.; Miyatake, A.; Abe, Y. Cardiac oxidative stress in acute and chronic isoproterenol-infused rats. Cardiovasc. Res. 2005, 65, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Goyal, S.; Sharma, C.; Arora, S.; Kumari, S.; Arya, D.S. Cardioprotective effect of lycopene against isoproterenol-induced myocardial infarction in rats. Hum. Exp. Toxicol. 2013, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.; Atessahin, A.; Sahna, E.; Karahan, I.; Ozer, S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology 2006, 218, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Folden, D.V.; Gupta, A.; Sharma, A.C.; Li, S.Y.; Saari, J.T.; Ren, J. Malondialdehyde inhibits cardiac contractile function in ventricular myocytes via a p38 mitogen-activated protein kinase-dependent mechanism. Br. J. Pharmacol. 2003, 139, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
| Lycopene Concentration | Groups | |||
|---|---|---|---|---|
| Control | Control + Ly | HSF | HSF + Ly | |
| Plasma (µg/mL) | ND | 3.61 ± 0.68 | ND | 3.59 ± 2.31 |
| Heart (µg/g of tissue) | ND | 4.83 ± 2.37 | ND | 2.41 ± 0.36 |
| Variables | Groups | Effect | |||||
|---|---|---|---|---|---|---|---|
| Control | Control + Ly | HSF | HSF + Ly | Diet | Ly | Interaction | |
| LVDD (mm) | 7.15 ± 0.11 | 7.02 ± 0.11 | 6.70 ± 0.12 * | 6.92 ± 0.11 | 0.019 | 0.665 | 0.123 |
| LVDS (mm) | 2.83 ± 0.10 | 2.74 ± 0.10 | 3.17 ± 0.11 * | 2.91 ± 0.10 | 0.016 | 0.098 | 0.417 |
| LVPWD (mm) | 1.63 ± 0.04 | 1.53 ± 0.04 | 1.73 ± 0.04 * | 1.62 ± 0.04 # | 0.031 | 0.014 | 0.932 |
| AD (mm) | 3.91 ± 0.06 | 3.86 ± 0.06 | 3.89 ± 0.07 | 3.88 ± 0.06 | 0.999 | 0.682 | 0.740 |
| LA (mm) | 4.86 ± 0.11 | 4.85 ± 0.11 | 5.02 ± 0.11 | 4.88 ± 0.11 | 0.388 | 0.483 | 0.536 |
| HR (bpm) | 254 ± 14 | 265 ± 14 | 262 ± 15 | 262 ± 14 | 0.871 | 0.716 | 0.713 |
| E (cm/s) | 73.6 ± 2.1 | 73.2 ± 2.18 | 76.1 ± 2.1 | 75.1 ± 2.3 | 0.351 | 0.742 | 0.895 |
| PWSV (cm/s) | 58.6 ± 1.3 | 61.1 ± 1.3 | 56.1 ± 1.3 | 59.8 ± 1.4 | 0.181 | 0.028 | 0.622 |
| Dec. time (ms) | 47.2 ± 1.3 | 42.1 ± 1.3 | 50.6 ± 1.3 | 42.7 ± 1.4 | 0.128 | <0.001 | 0.322 |
| Tei-a (ms) | 116.1 ± 2.5 | 116.8 ± 2.5 | 99.1 ± 2.5 * | 111.7 ± 2.6 # | <0.001 | 0.012 | 0.024 |
| Tei-b (ms) | 86.6 ± 2.9 | 92.6 ± 2.9 | 77.7 ± 2.9 * | 85.5 ± 3.1 # | 0.012 | 0.028 | 0.761 |
| EF (%) | 0.93 ± 0.008 | 0.93 ± 0.008 | 0.88 ± 0.008 * | 0.93 ± 0.008 # | <0.001 | 0.006 | 0.008 |
| E/E’ | 13.3 ± 0.4 | 12.7 ± 0.4 | 15.3 ± 0.4 * | 13.9 ± 0.50 # | 0.002 | 0.049 | 0.439 |
| Variables | Groups | Effect | |||||
|---|---|---|---|---|---|---|---|
| Control | Control + Ly | HSF | HSF + Ly | Diet | Ly | Interaction | |
| DT(g/mm2) | 5.96 ± 1.25 | 6.29 ± 1.65 | 4.41 ± 1.11 * | 6.05 ± 1.19 # | 0.066 | 0.046 | 0.173 |
| RT(g/mm2) | 0.65 ± 0.11 | 0.61 ± 0.11 | 0.63 ± 0.08 | 0.57 ± 0.11 | 0.512 | 0.202 | 0.844 |
| +dT/dt(g/mm2/s) | 61.9 ± 10.1 | 63.5 ± 18.4 | 60.8 ± 11.7 | 65.5 ± 19.7 | 0.934 | 0.573 | 0.773 |
| −dT/dt(g/mm2/s) | 16.8 ± 2.4 | 17.5 ± 2.9 | 15.5 ± 3.3 | 16.1 ± 2.9 | 0.193 | 0.569 | 0.933 |
| CSA(mm2) | 1.11 ± 0.12 | 1.10 ± 0.23 | 1.25 ± 0.27 | 1.17 ± 0.3 | 0.181 | 0.801 | 0.912 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferron, A.J.T.; Aldini, G.; Francisqueti-Ferron, F.V.; Silva, C.C.V.d.A.; Bazan, S.G.Z.; Garcia, J.L.; Campos, D.H.S.d.; Ghiraldeli, L.; Kitawara, K.A.H.; Altomare, A.; et al. Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model. Antioxidants 2019, 8, 368. https://doi.org/10.3390/antiox8090368
Ferron AJT, Aldini G, Francisqueti-Ferron FV, Silva CCVdA, Bazan SGZ, Garcia JL, Campos DHSd, Ghiraldeli L, Kitawara KAH, Altomare A, et al. Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model. Antioxidants. 2019; 8(9):368. https://doi.org/10.3390/antiox8090368
Chicago/Turabian StyleFerron, Artur Junio Togneri, Giancarlo Aldini, Fabiane Valentini Francisqueti-Ferron, Carol Cristina Vágula de Almeida Silva, Silmeia Garcia Zanati Bazan, Jéssica Leite Garcia, Dijon Henrique Salomé de Campos, Luciana Ghiraldeli, Koody Andre Hassemi Kitawara, Alessandra Altomare, and et al. 2019. "Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model" Antioxidants 8, no. 9: 368. https://doi.org/10.3390/antiox8090368
APA StyleFerron, A. J. T., Aldini, G., Francisqueti-Ferron, F. V., Silva, C. C. V. d. A., Bazan, S. G. Z., Garcia, J. L., Campos, D. H. S. d., Ghiraldeli, L., Kitawara, K. A. H., Altomare, A., Correa, C. R., Moreto, F., & Ferreira, A. L. A. (2019). Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model. Antioxidants, 8(9), 368. https://doi.org/10.3390/antiox8090368






