Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. Cultivation Conditions
2.3. Kinetic Parameters
2.4. Extraction of Photosynthetic Pigments
2.5. Identification of Photosynthetic Pigments by HPLC-ESI/APCI-HRTOF-MSn
2.6. Quantification of Photosynthetic Pigments by HPLC-UV-Visible Detection
2.7. Statistical Analysis
3. Results
3.1. Microalgae Growth/Kinetic Parameters
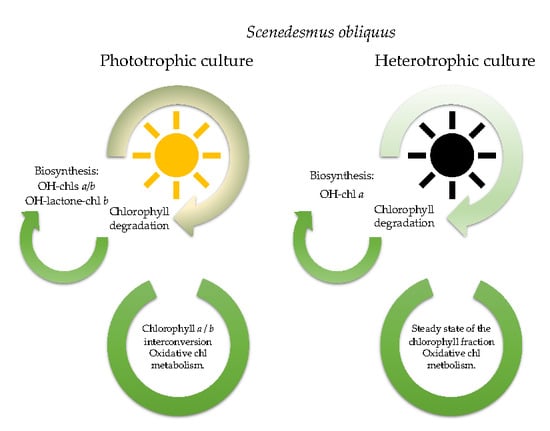
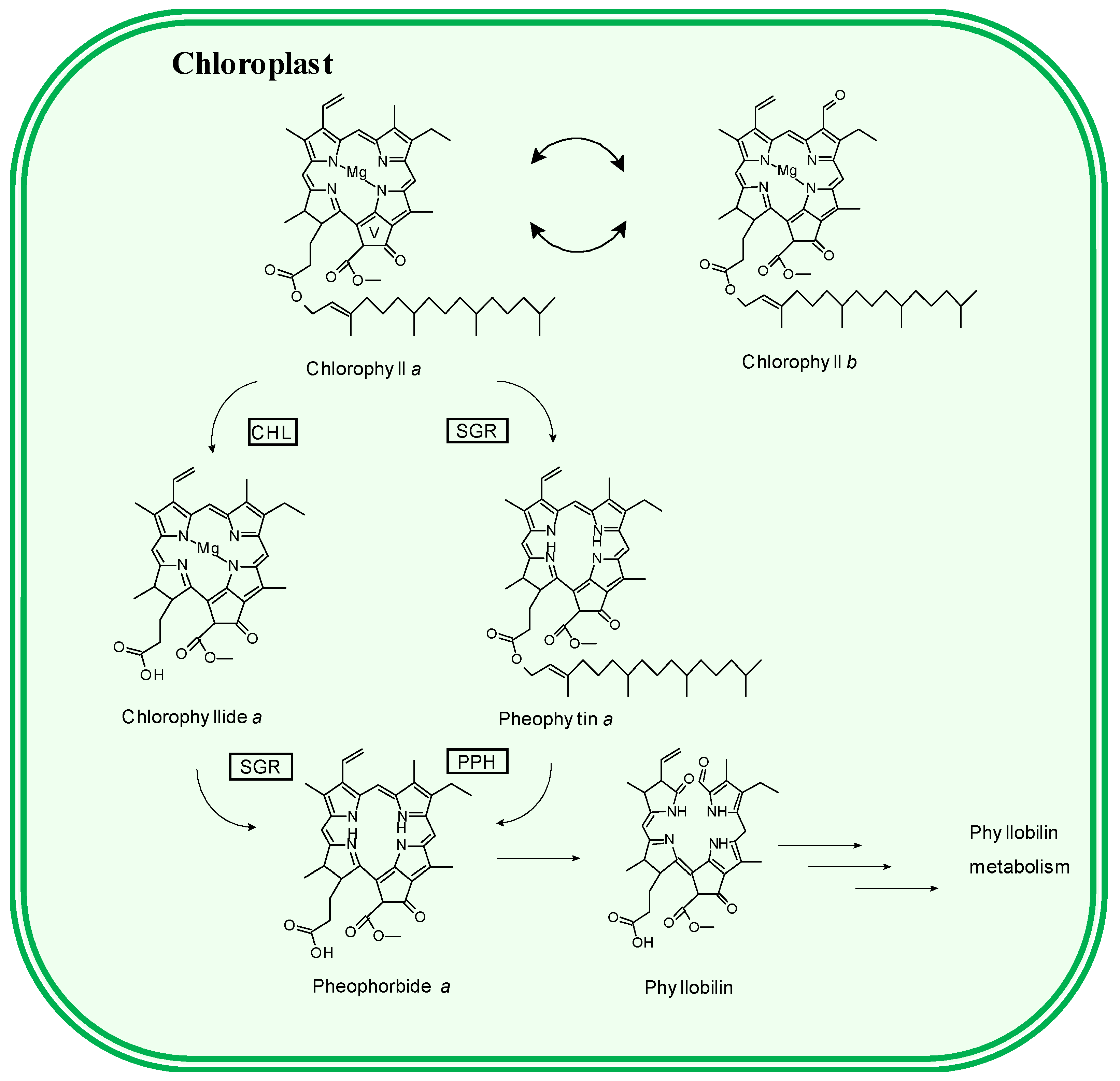
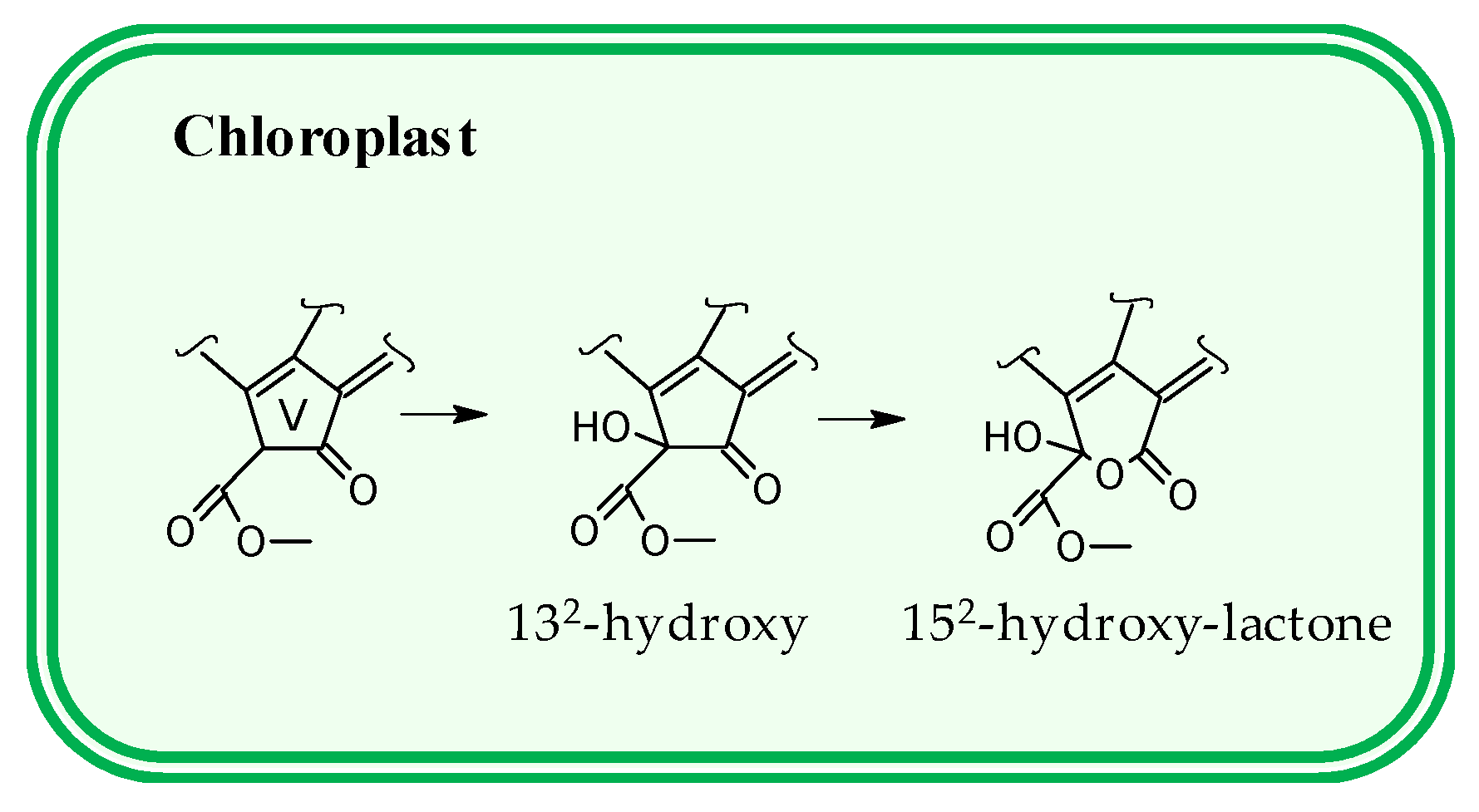
3.2. Pigment Profile During Phototrophic Growth
3.3. Pigment Evolution During Heterotrophic Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viera, I.; Roca, M.; Perez-Galvez, A. Mass Spectrometry of Non-allomerized Chlorophylls a and b Derivatives from Plants. Curr. Org. Chem. 2018, 22, 842–876. [Google Scholar] [CrossRef]
- Catarina, M.M.; Duarte, F.; Malcata, X. Supercritical fluid extraction of carotenoids and chlorophylls a, b and c, from a wild strain of Scenedesmus obliquus for use in food processing. J. Food Eng. 2013, 116, 478–482. [Google Scholar]
- Chacon-Lee, T.L.; González-Marino, G.E. Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Green Natural Colorants. Molecules 2019, 24, 154. [Google Scholar] [CrossRef]
- Queiroz Zepka, L.; Jacob-Lopes, E.; Roca, M. Catabolism and bioactive properties of chlorophylls. Curr. Opin. Food Sci. 2019, 26, 94–100. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Böhm, V.; Courtney, P.D.; Schwartz, S.J. Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. Food Chem. Toxicol. 2002, 67, 2589–2595. [Google Scholar] [CrossRef]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef]
- Hoshina, C.; Tomita, K.; Shioi, Y. Antioxidant Activity of Chlorophylls: Its Structure-Activity Relationship. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Springer: Berlin, Germany, 1998; pp. 3281–3284. [Google Scholar]
- Lanfer-Marquez, U.M.; Barros, R.M.C.; Sinnecker, P. Antioxidant activity of chlorophylls and their derivatives. Food Res. Int. 2005, 38, 885–891. [Google Scholar] [CrossRef]
- Xavier, A.A.; Pérez-Gálvez, A. Carotenoids as a Source of Antioxidants in the Diet. Subcell. Biochem. 2016, 79, 359–375. [Google Scholar] [CrossRef]
- Chen, W.; Hsu, Y.; Chang, J.; Ho, S.; Wang, L.; We, Y. Enhancing production of lutein by a mixotrophic cultivation system using microalga Scenedesmus obliquus CWL-1. Bioresour. Technol. 2019, 291, 121891. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Sant’Anna, C. Impact of culture conditions on the chlorophyll content of microalgae for biotechnological applications. World J. Microbiol. Biotechnol. 2017, 33, 20. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nagarajan, D.; Zhanga, Q.; Chang, J.; Lee, D. Heterotrophic cultivation of microalgae for pigment production: A review. Biotechnol. Adv. 2018, 36, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, G.A. Greening in the dark: Light-independent chlorophyll biosynthesis from anoxygenic photosynthetic bacteria to gymnosperms. J. Photochem. Photobiol. B Biol. 1998, 43, 87–100. [Google Scholar] [CrossRef]
- Abeliovich, A.; Weisman, D. Role of heterotrophic nutrition in growth of the alga Scenedesmus obliquus in high-rate oxidation ponds. Appl. Environ. Microbiol. 1978, 35, 32–37. [Google Scholar] [PubMed]
- Van Wagenen, J.; De Francisci, D.; Angelidaki, I. Comparison of mixotrophic to cyclic autotrophic/heterotrophic growth strategies to optimize productivity of Chlorella sorokiniana. J. Appl. Phycol. 2015, 27, 1775–1782. [Google Scholar] [CrossRef]
- Sun, X.; Ren, L.; Zhao, Q.; Ji, X.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Flórez-Miranda, L.; Cañizares-Villanueva, O.; Melchy-Antonio, O.; Martínez-Jerónimo, F.; Mateo Flores-Ortíz, C. Two stage heterotrophy/photoinduction culture of Scenedesmus incrassatulus: Potential for lutein production. J. Biotechnol. 2017, 262, 67–74. [Google Scholar] [CrossRef]
- Ferreira, V.S.; Pinto, R.F.; Sant’Anna, C. Low light intensity and nitrogen starvation modulate the chlorophyll content of Scenedesmus dimorphus. J. Appl. Microbiol. 2015, 120, 661–670. [Google Scholar] [CrossRef]
- Chen, D.M.; Li, J.; Dai, X.; Sun, Y.; Chen, F. Effect of phosphorus and temperature on chlorophyll a contents and cell sizes of Scenedesmus obliquus and Microcystis aeruginosa. Limnology 2011, 12, 187–192. [Google Scholar] [CrossRef]
- Masojídek, J.; Torzillo, G.; Koblízek, M.; Kopecký, J.; Bernardini, P.; Sacchi, A.; Komenda, J. Photoadaptation of two members of the Chlorophyta (Scenedesmus and Chlorella) in laboratory and outdoor cultures: Changes in chlorophyll fluorescence quenching and the xanthophyll cycle. Planta 1999, 209, 126–135. [Google Scholar] [CrossRef]
- Kuai, B.; Chen, J.; Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. J. Exp. Bot. 2018, 69, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, Y.; Shen, Y.; Yan, D.; He, X.; Dai, J.; Wu, Q. Oil accumulation mechanisms of the oleaginous microalga Chlorella protothecoides revealed through its genome, transcriptomes, and proteomes. BMC Genom. 2014, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Shimoda, Y.; Tanaka, A.; Ito, H. Chlorophyll a is a favorable substrate for Chlamydomonas Mg-dechelatase encoded by STAY-GREEN. Plant Physiol. Biochem. 2016, 109, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Guyer, L.; Schelbert Hofstetter, S.; Christ, B.; Silvestre Lira, B.; Rossi, M.; Hörtensteiner, S. Different Mechanisms Are Responsible for Chlorophyll Dephytylation during Fruit Ripening and Leaf Senescence in Tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef]
- Engel, N.; Curty, C.; Gossauer, A. Chlorophyll catabolism in Chorella protothecoides. Part 8: Facts and artefacts. Plant Physiol. Biochem. 1996, 34, 77–83. [Google Scholar]
- Bale, N.J.; Llewellyn, C.A.; Airs, R.L. Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of type II chlorophyll—A transformation products: Diagnostic fragmentation patterns. Org. Geochem. 2010, 41, 473–481. [Google Scholar] [CrossRef]
- Grabski, K.; Baranowski, N.; Skórko-Glonek, J.; Tukaj, Z. Chlorophyll catabolites in conditioned media of green microalga Desmodesmus subspicatus. J. Appl. Phycol. 2016, 28, 889–896. [Google Scholar] [CrossRef]
- Hynninen, P.H.; Hyvärinen, K. Tracing the Allomerization Pathways of Chlorophylls by 18O-Labeling and Mass Spectrometry. J. Org. Chem. 2002, 6712, 4055–4061. [Google Scholar] [CrossRef]
- Vergara-Domínguez, H.; Gandul-Rojas, B.; Roca, M. Formation of oxidised chlorophyll catabolites in olives. J. Food Compos. Anal. 2011, 24, 851–857. [Google Scholar] [CrossRef]
- Hynninen, P.H. Chemistry of chlorophylls: Modifications. In Chlorophylls; Scheer, H., Tsuchiya, T., Ohta, H., Okawa, K., Iwamatsu, A., Shimada, H., Masuda, T., Eds.; CRC Press: Boca Raton, FL, USA, 1991; pp. 145–209. [Google Scholar]
- Louda, W.; Mongkhonsri, P.; Baker, E.W. Chlorophyll degradation during senescence and death-III: 3–10 yr experiments, implications for ETIO series generation. Org. Geochem. 2011, 42, 688–699. [Google Scholar] [CrossRef]
- Walker, J.S.; Squier, A.H.; Hodgson, D.A.; Keely, B.J. Origin and significance of 132-hydroxychlorophyll derivatives in sediments. Org. Chem. 2002, 33, 1667–1674. [Google Scholar]
- Bale, N.J.; Airs, R.L.; Martin, P.; Lampitt, R.S.; Llewellyn, C.A. Chlorophyll-a transformations associated with sinking diatoms during termination of a North Atlantic spring bloom. Mar. Chem. 2015, 172, 23–33. [Google Scholar] [CrossRef]
- Steele, D.J.; Kimmance, S.A.; Franklin, D.J.; Airs, R.L. Occurrence of chlorophyll allomers during virus-induced mortality and population decline in the ubiquitous picoeukaryote Ostreococcus tauri. Environ. Microbiol. 2018, 20, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.J.; Tarran, G.A.; Widdicombe, C.E.; Woodward, E.M.S.; Kimmance, S.A.; Franklin, D.J.; Airs, R.L. Abundance of a chlorophyll a precursor and the oxidation product hydroxychlorophyll a during seasonal phytoplankton community progression in the Western English Channel. Prog. Oceanogr. 2015, 137, 434–445. [Google Scholar] [CrossRef]
- Walker, J.S.; Keely, B.J. Distribution and significance of chlorophyll derivatives and oxidation products during the spring phytoplankton bloom in the Celtic Sea April 2002. Org. Geochem. 2004, 35, 1289–1298. [Google Scholar] [CrossRef]
- Naylor, C.C.; Keely, B.J. Sedimentary purpurins: Oxidative transformation products of chlorophylls. Org. Geochem. 1998, 28, 417–422. [Google Scholar] [CrossRef]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- Rippka, R.; Stanier, R.Y.; Deruelles, J.; Herdman, M.; Waterbury, J.B. Generic Assignments, Strain Histories and Properties of Pure Cultures of Cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Maroneze, M.M.; Siqueira, S.F.; Vendruscolo, R.G.; Wagner, R.; de Menezes, C.R.; Zepka, L.Q.; Jacob-Lopes, E. The role of photoperiods on photobioreactors—A potential strategy to reduce costs. Bioresour. Technol. 2016, 219, 493–499. [Google Scholar] [CrossRef]
- Francisco, É.C.; Franco, T.T.; Wagner, R.; Jacob-Lopes, E. Assessment of different carbohydrates as exogenous carbon source in cultivation of cyanobacteria. Bioprocess Biosyst. Eng. 2014, 37, 1497–1505. [Google Scholar] [CrossRef]
- Jeffrey, S.W.; Wright, S.W.; Zapata, M. Microalgal Classes and their Signature Pigments. In Phytoplankton pigments; Roy, S., Llewellyn, C.A., Egeland, E.S., Johnsen, G., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 3–77. [Google Scholar]
- Chen, K.; Ríos, J.J.; Pérez, A.; Roca, M. Development of an accurate and high-throughput methodology for structural comprehension of chlorophylls derivatives. (I) Phytylated derivatives. J. Chromatogr. A 2015, 1406, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Breithaupt, D.E.; Wirt, U.; Bamedi, A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta, L.) and several fruits by liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2002, 50, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.J.; Xavier, A.A.O.; Díaz-Salido, E.; Arenilla-Vélez, I.; Jarén-Galán, M.; Garrido-Fernández, J.; Pérez-Gálvez, A. Xanthophyll esters are found in human colostrum. Mol. Nutr. Food Res. 2017, 61, 1700296. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, C.; Ghosh, T.; George, B.; Pancha, Y.; Maurya, R.; Chokshi, K.; Ghosh, A.; Mishra, S. Microalgal carotenoids: Potential nutraceutical compounds with chemotaxonomic importance. Algal Res. 2016, 15, 24–31. [Google Scholar] [CrossRef]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef]
- Telfer, A.; Pascal, A.; Gall, A. Natural functions. In Carotenoids; Britton, G., Liaanen-Jensen, S., Pfander, H., Eds.; Birkhauser: Basel, Switzerland, 2008; Volume 4, pp. 189–211. [Google Scholar]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta 2011, 1807, 968–976. [Google Scholar] [CrossRef]
- Richardson, K.; Beardall, J.; Raven, J.A. Adaptation of Unicellular Algae to Irradiance: An Analysis of Strategies. New Phytol. 1983, 93, 157–191. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Owens, T.G. Light-Shade Adaptation. Two strategies in marine phytoplankton. Plant Physiol. 1980, 66, 592–595. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Kana, T.M.; Anning, T.; Geider, R.J. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 2002, 38, 17–38. [Google Scholar] [CrossRef]
- Airs, R.L.; Keely, B.J. A high resolution study of the chlorophyll and bacteriochlorophyll pigment distributions in a calcite/gypsum microbial mat. Org. Geochem. 2003, 34, 539–551. [Google Scholar] [CrossRef]
- Cirulis, J.T.; Ashley Scott, J.; Ross, G.M. Management of oxidative stress by microalgae. Can. J. Physiol. Pharmacol. 2013, 91, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.R.; Hanumantha Rao, P.; Subramanian, V.V.; Sivasubramanian, V. Enzymatic and non-enzymatic antioxidant potentials of Chlorella vulgaris grown in effluent of a confectionery industry. J. Food Sci. Technol. 2014, 51, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Beisel, K.G.; Jahnke, S.; Hofmann, D.; Köppchen, S.; Schurr, U.; Matsubara, S. Continuous Turnover of Carotenes and Chlorophyll a in Mature Leaves of Arabidopsis Revealed by 14CO2 Pulse-Chase Labeling. Plant Physiol. 2010, 152, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Stadnichuk, I.N.; Rakhimberdieva, M.G.; Bolychevtseva, Y.V.; Yurina, N.P.; Karapetyan, N.V.; Selyakh, I.O. Inhibition by glucose of chlorophyll a and phycocyanobilin biosynthesis in the unicellular red alga Galdieria partita at the stage of coproporphyrinogen III formation. Plant Sci. 1998, 136, 11–23. [Google Scholar] [CrossRef]
- Kamalanathan, M.; Dao, T.L.; Panjhaphol, C.; Gleadow, R.; Beardall, J. Photosynthetic physiology of Scenedesmus sp. under photoautotrophic, and molasses-based heterotrophic and mixotrophic conditions. Phycologia 2017, 56, 666–674. [Google Scholar] [CrossRef]
- Guyer, L.; Salinger, K.; Krügel, U.; Hörtensteiner, S. Catalytic and structural properties of pheophytinase, the phytol esterase involved in chlorophyll breakdown. J. Exp. Bot. 2018, 69, 879–889. [Google Scholar] [CrossRef]
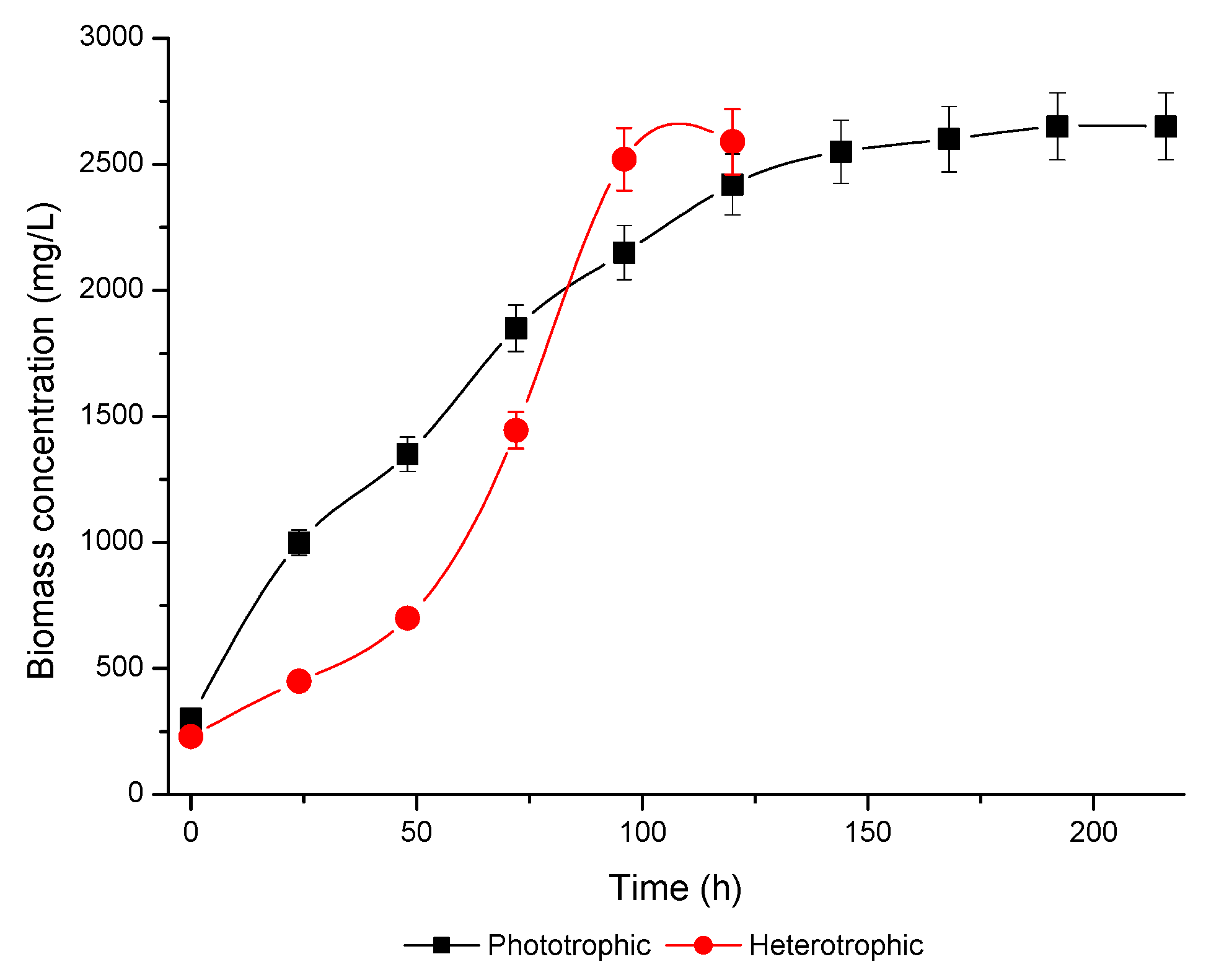
| Parameter | Phototrophic | Heterotrophic |
|---|---|---|
| Xmax (mg/L) | 2650 ± 111.8 | 2600 ± 97.5 |
| µmax (h−1) | 0.023 ± 0.00 | 0.024 ± 0.00 |
| RT (h) | 216 ± 0.00 | 120 ± 0.00 |
| GT (h) | 30.13 ± 0.60 | 28.8 ± 0.49 |
| PX (mg/L h) | 10.87 ± 0.43 | 19.75 ± 0.29 |
| Time (h) | Neox | Violax | Luteox | Antherax | Lutein | β-Carotene | Total |
|---|---|---|---|---|---|---|---|
| 0 | + | + | + | 0.0 | 703.3 | 30.0 | 703.3 |
| 24 | 127.0 | 11.0 | 20.0 | 0.0 | 689.5 | 30.1 | 877.2 |
| 48 | 142.0 | 10.4 | 26.3 | 0.0 | 860.7 | 38.9 | 1174.3 |
| 72 | 130.3 | 28.1 | 37.0 | 7.0 | 795.4 | 45.9 | 1044.0 |
| 96 | 83.7 | 25.8 | 40.7 | 15.6 | 931.2 | 53.2 | 1150.1 |
| 120 | 146.7 | 28.5 | 36.7 | 20.4 | 1238.3 | 176.4 | 1647.0 |
| 144 | 147.0 | 40.0 | 45.1 | 32.0 | 1443.9 | 224.7 | 1952.4 |
| 168 | 122.3 | 19.8 | 51.3 | 26.2 | 1125.0 | 220.0 | 1679.6 |
| 192 | 156.2 | 38.9 | 67.0 | 30.0 | 1313.2 | 227.1 | 1832.4 |
| 216 | 321.9 | 108.9 | 44.0 | 49.2 | 1408.7 | 212.6 | 2145.3 |
| Time (h) | Pheo a | OH-Pheo a | OH-Chl a | Chl a | Phy a |
|---|---|---|---|---|---|
| 0 | 0.0 | 0.0 | 0.0 | 2438.3 | 2787.7 |
| 24 | 0.0 | 0.0 | 85.0 | 2420.0 | 3350.0 |
| 48 | 20.4 | 0.0 | 111.1 | 3955.2 | 2950.7 |
| 72 | 33.0 | 0.0 | 148.6 | 3718.6 | 2375.9 |
| 96 | 155.6 | 0.0 | 114.0 | 4079.3 | 2413.7 |
| 120 | 210.0 | 22.9 | 133.3 | 3981.3 | 2590.0 |
| 144 | 262.7 | 13.7 | 127.5 | 7417.5 | 897.8 |
| 168 | 86.7 | 8.3 | 134.6 | 5935.8 | 878.5 |
| 192 | 31.7 | 11.3 | 0.0 | 6170.0 | 708.7 |
| 216 | 8.5 | 0.0 | 0.0 | 6878.1 | 192.1 |
| Time (h) | OH-Lact.-Chl b | OH-Chl b | Chl b | Series a/b |
|---|---|---|---|---|
| 0 | 100.0 | 116.7 | 1408.3 | 3.22 |
| 24 | 175.0 | 124.5 | 1472.5 | 3.30 |
| 48 | 179.3 | 418.1 | 2023.0 | 2.71 |
| 72 | 275.1 | 437.6 | 5557.0 | 1.02 |
| 96 | 122.6 | 308.6 | 1757.2 | 3.02 |
| 120 | 116.7 | 310.4 | 1724.0 | 3.13 |
| 144 | 132.0 | 231.8 | 2016.7 | 3.55 |
| 168 | 90.0 | 286.3 | 1802.7 | 3.19 |
| 192 | 56.8 | 31.1 | 1878.3 | 3.50 |
| 216 | 40.9 | 50.6 | 1747.2 | 3.85 |
| 240 | 51.0 | 63.5 | 2038.6 | 3.14 |
| Pigment | Residence Time (h) | |||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | 120 | |
| Neoxanthin | 86.4 | 76.9 | 80.0 | 43.5 | 36.5 | 13.3 |
| Violaxanthin | 42.2 | 18.7 | 18.4 | 10.4 | 8.5 | 1.8 |
| Lutein | 429.3 | 323.2 | 318.3 | 319.2 | 284.3 | 63.2 |
| β-carotene | 241.5 | 144.3 | 151.2 | 131.8 | 128.8 | 6.6 |
| Chld a | 3.4 | 3.5 | 3.5 | 3.5 | 8.5 | 108.0 |
| Pheo a | 78.3 | 157.7 | 145.0 | 67.2 | 37.2 | 299.9 |
| Chl b | 2284.0 | 2644.4 | 2778.2 | 1992.9 | 1164.0 | 895.4 |
| OH-chl a | 15.1 | 111.6 | 187.5 | 3.5 | 3.5 | 202.5 |
| Chl a | 4407.1 | 4343.0 | 4190.2 | 4636.3 | 2841.9 | 825.4 |
| Phy a | 1063.5 | 1102.6 | 766.1 | 326.4 | 262.0 | 207.0 |
| Tot. carot | 799.4 | 563.2 | 568.0 | 505.1 | 458.2 | 85.0 |
| Tot. chls | 7851.6 | 8362.9 | 8070.7 | 7030.1 | 4317.4 | 2538.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maroneze, M.M.; Zepka, L.Q.; Lopes, E.J.; Pérez-Gálvez, A.; Roca, M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants 2019, 8, 600. https://doi.org/10.3390/antiox8120600
Maroneze MM, Zepka LQ, Lopes EJ, Pérez-Gálvez A, Roca M. Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants. 2019; 8(12):600. https://doi.org/10.3390/antiox8120600
Chicago/Turabian StyleMaroneze, Mariana Manzoni, Leila Queiroz Zepka, Eduardo Jacob Lopes, Antonio Pérez-Gálvez, and María Roca. 2019. "Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus" Antioxidants 8, no. 12: 600. https://doi.org/10.3390/antiox8120600
APA StyleMaroneze, M. M., Zepka, L. Q., Lopes, E. J., Pérez-Gálvez, A., & Roca, M. (2019). Chlorophyll Oxidative Metabolism During the Phototrophic and Heterotrophic Growth of Scenedesmus obliquus. Antioxidants, 8(12), 600. https://doi.org/10.3390/antiox8120600