Use of Natural Antioxidants from Newfoundland Wild Berries to Improve the Shelf Life of Natural Herbal Soaps
Abstract
1. Introduction
2. Materials and Methods
2.1. Soap Production by Cold Saponification
2.2. Addition of Natural Additives to Herbal Soaps
2.3. Chemical Analysis of the Natural Herbal Soaps
2.3.1. Extraction of Samples
2.3.2. Total Phenolic Content (TPC) Analysis
2.3.3. Antioxidant Activity Analysis (FRAP Method)
2.3.4. Total Oxidant Status (TOS) Analysis
2.3.5. Soap Lipid Extraction
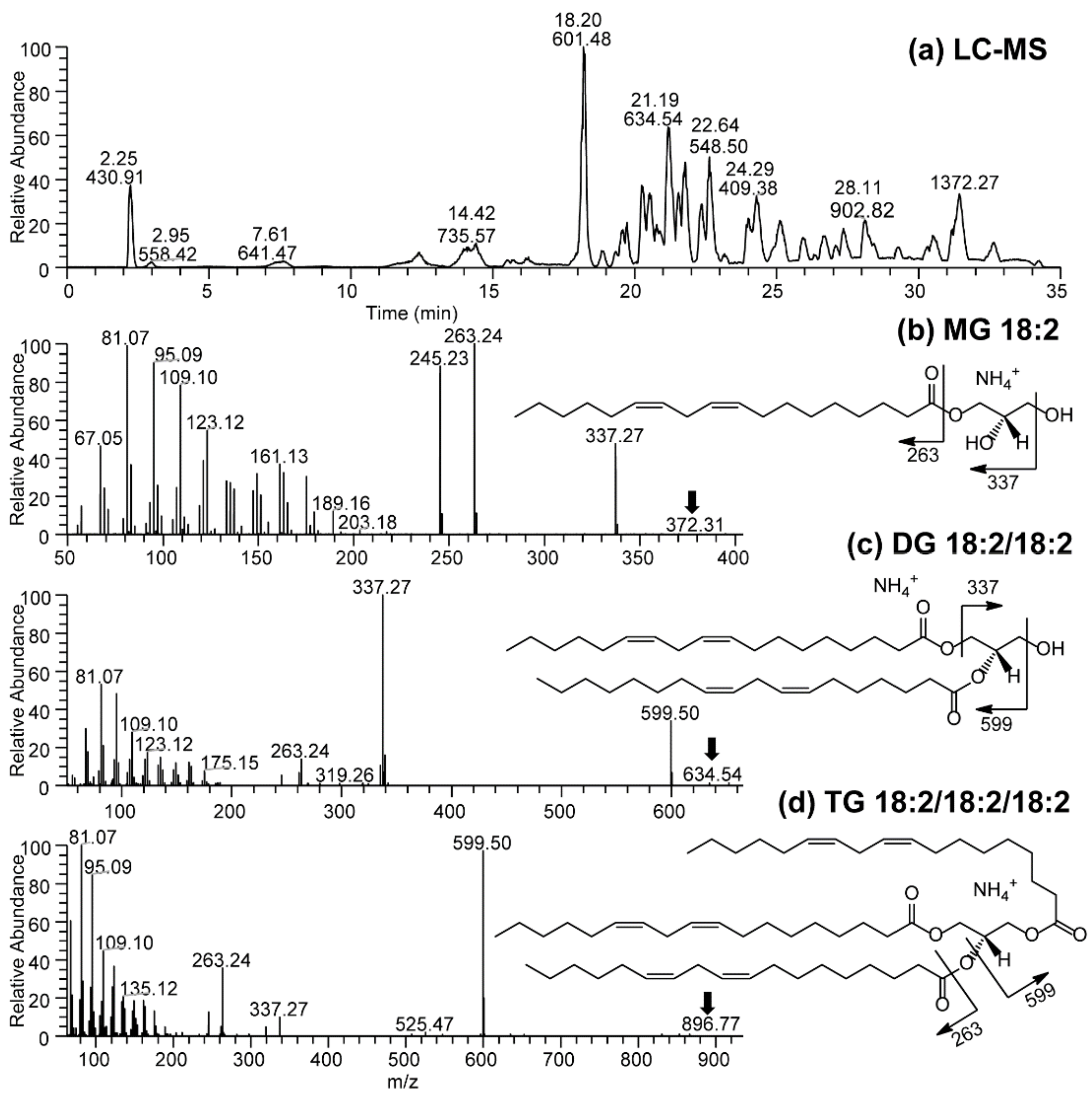
2.3.6. Lipid Analysis
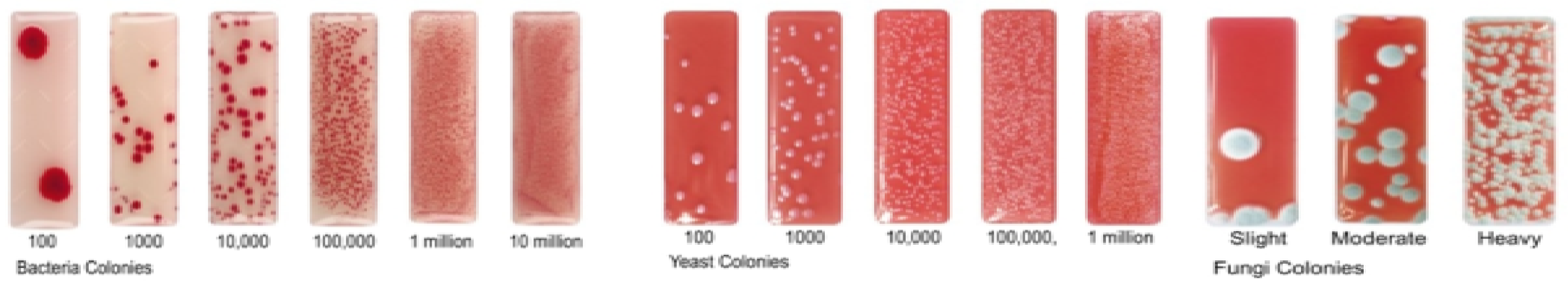
2.4. Microbial Tests
2.5. Statistical Analysis
3. Results and Discussion
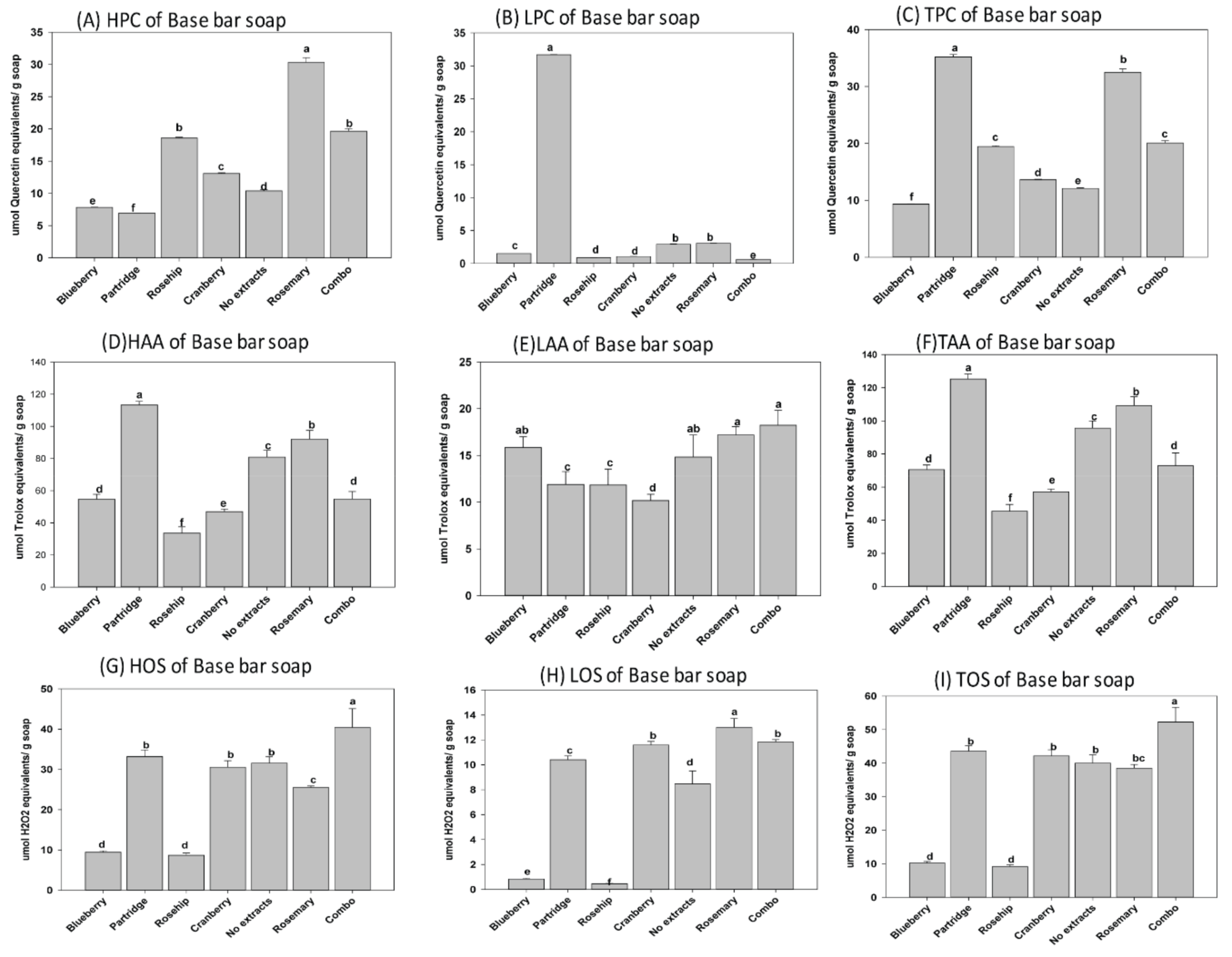
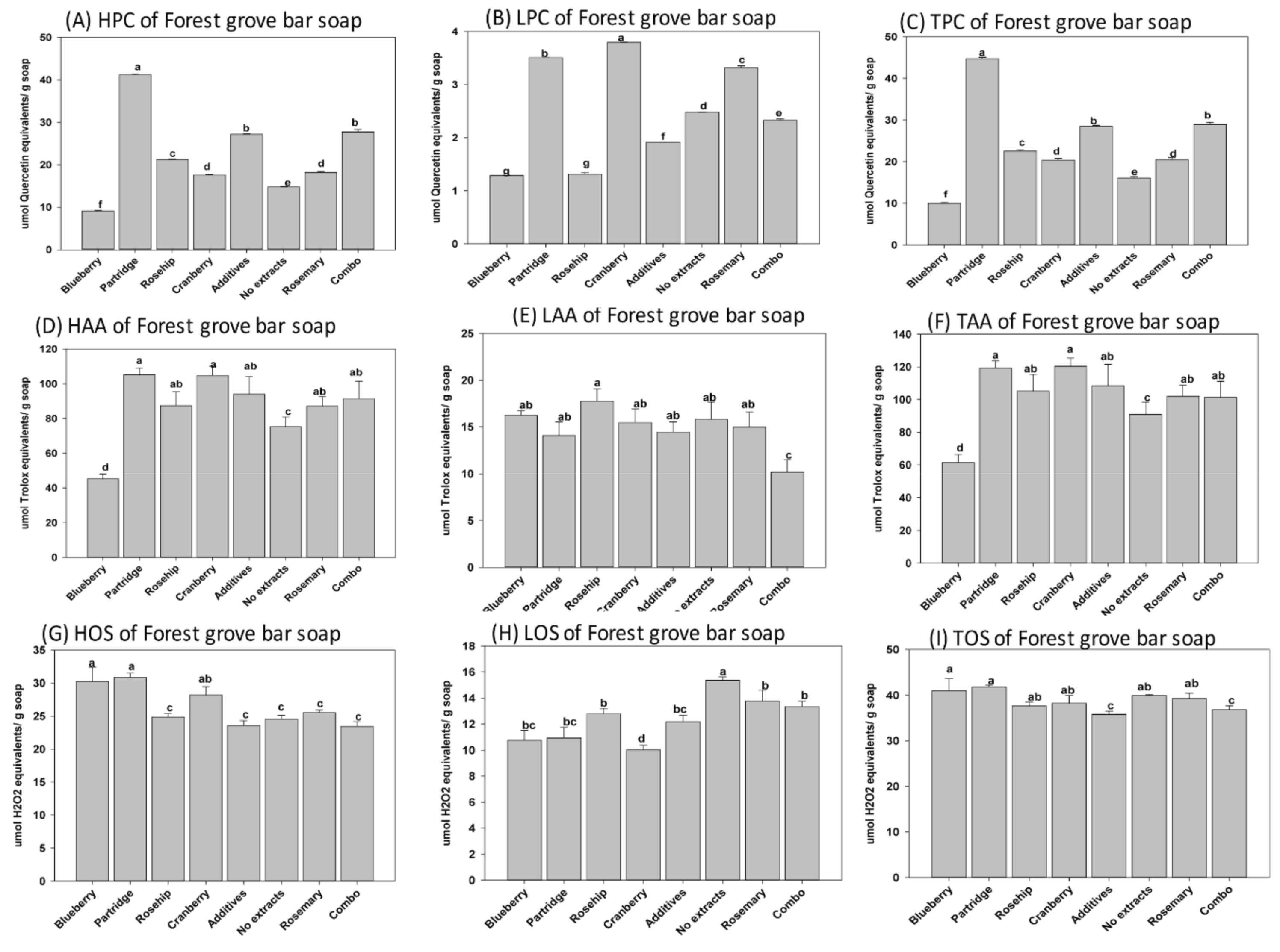
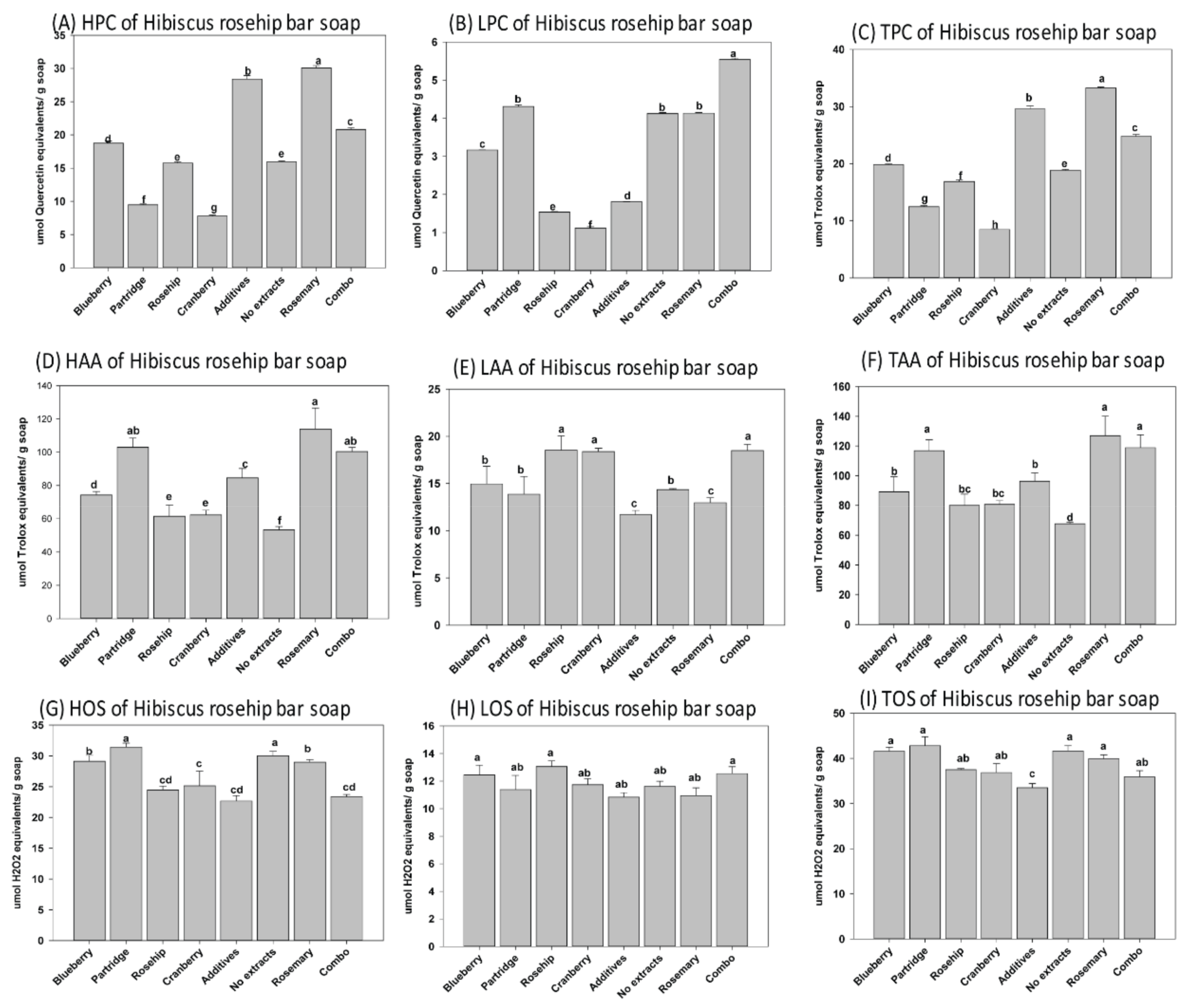
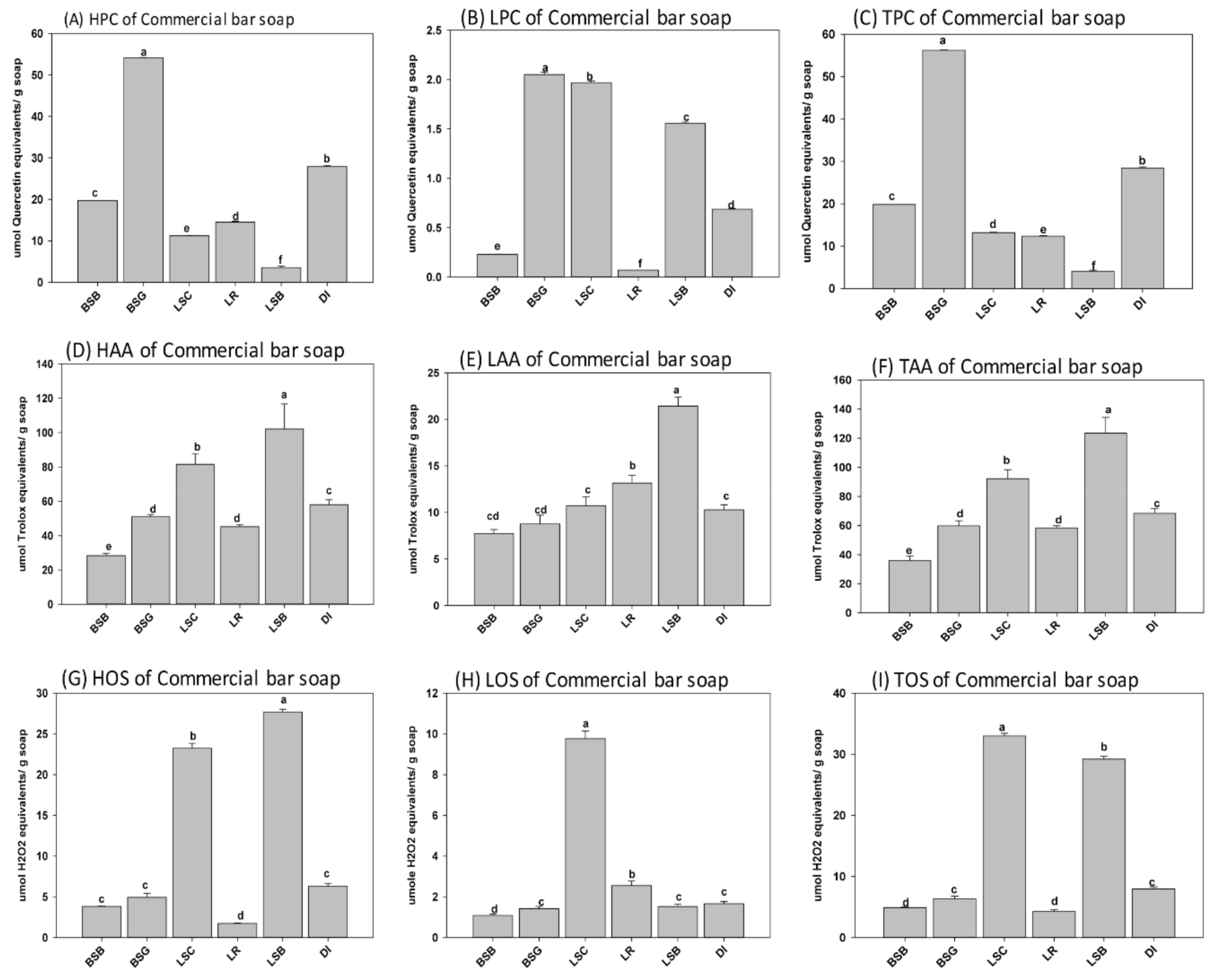
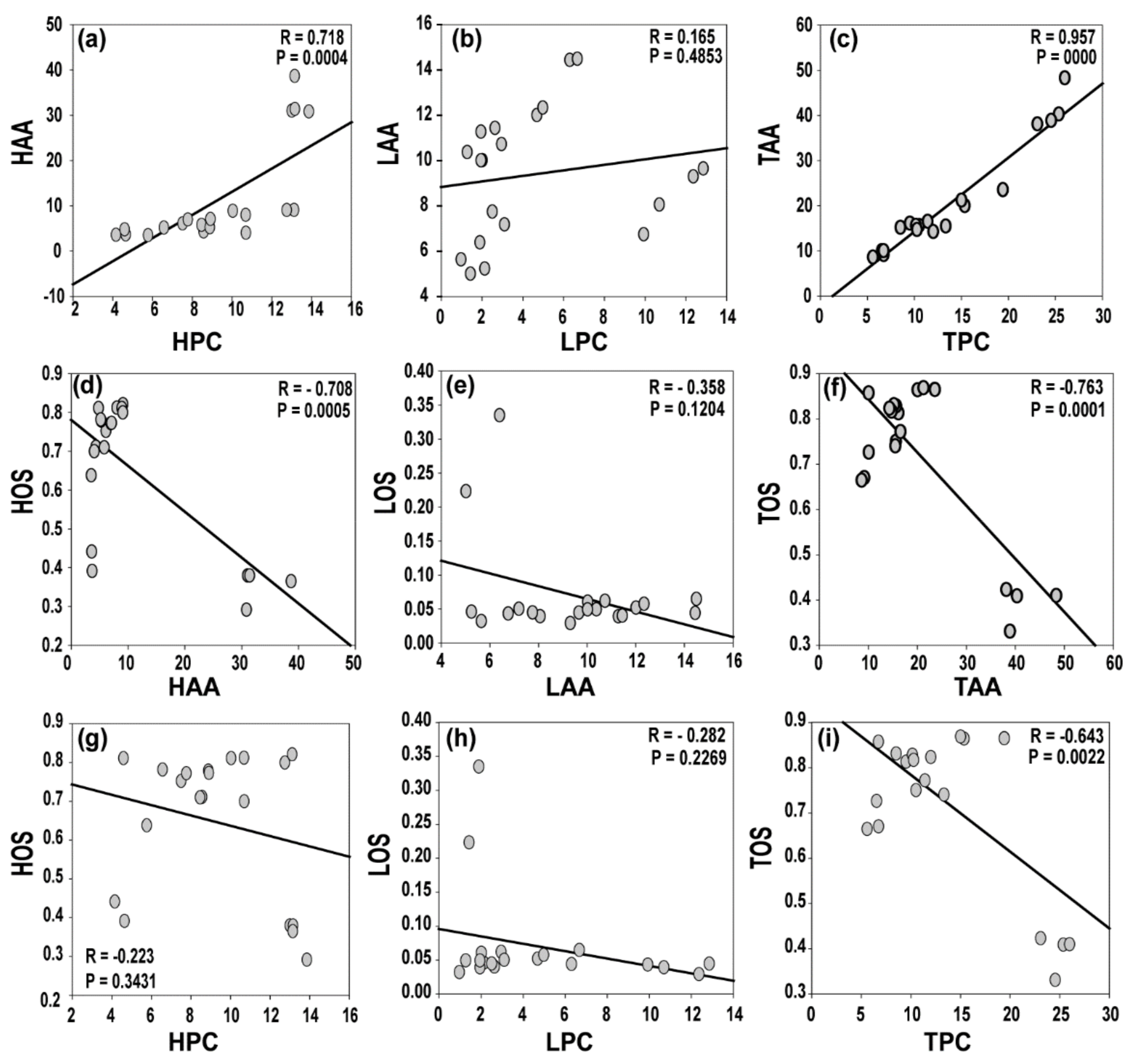
3.1. Phenolic Content, Oxidantion Status, and Antioxidant Activity of Natural Herbal Soaps
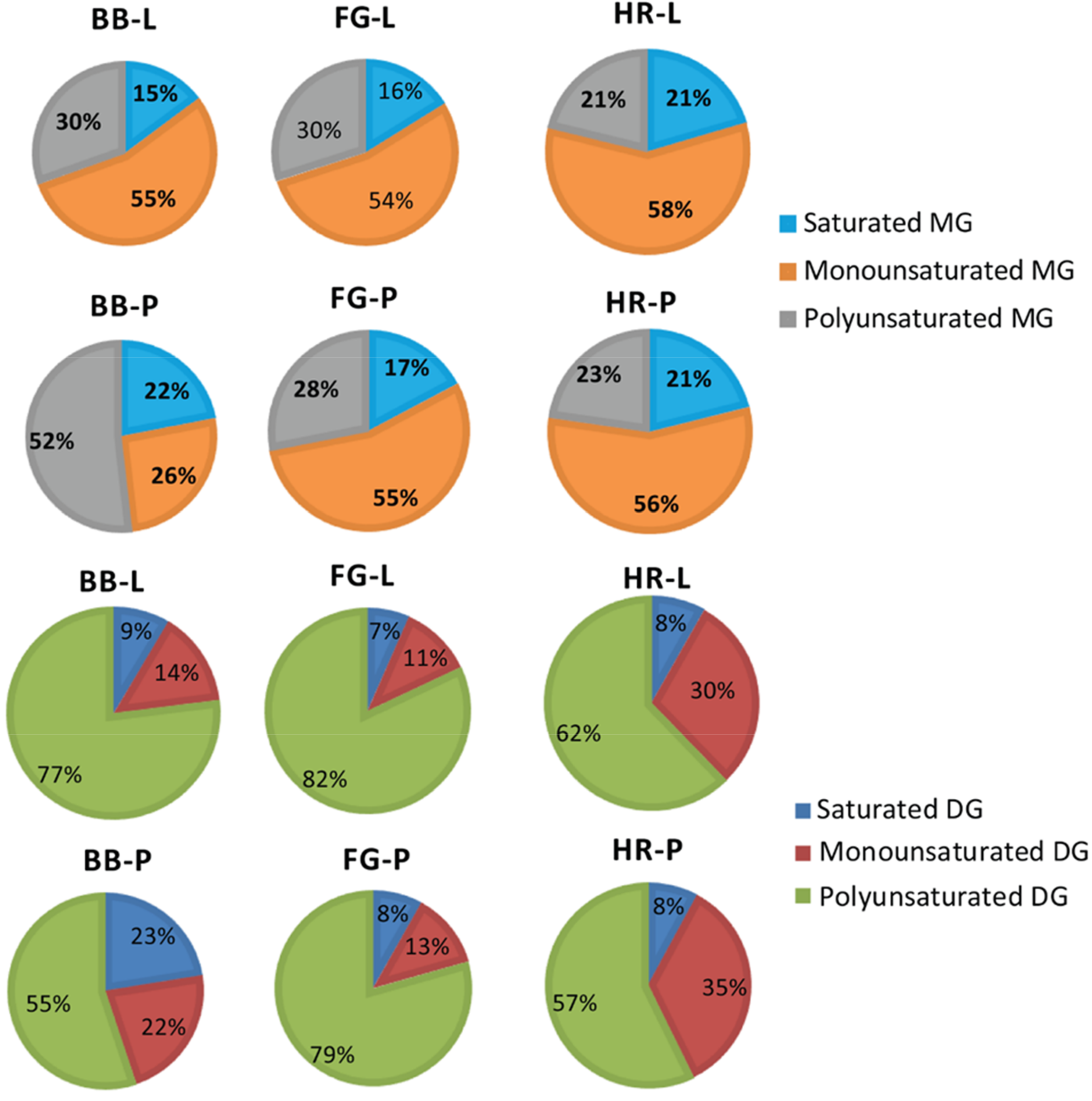
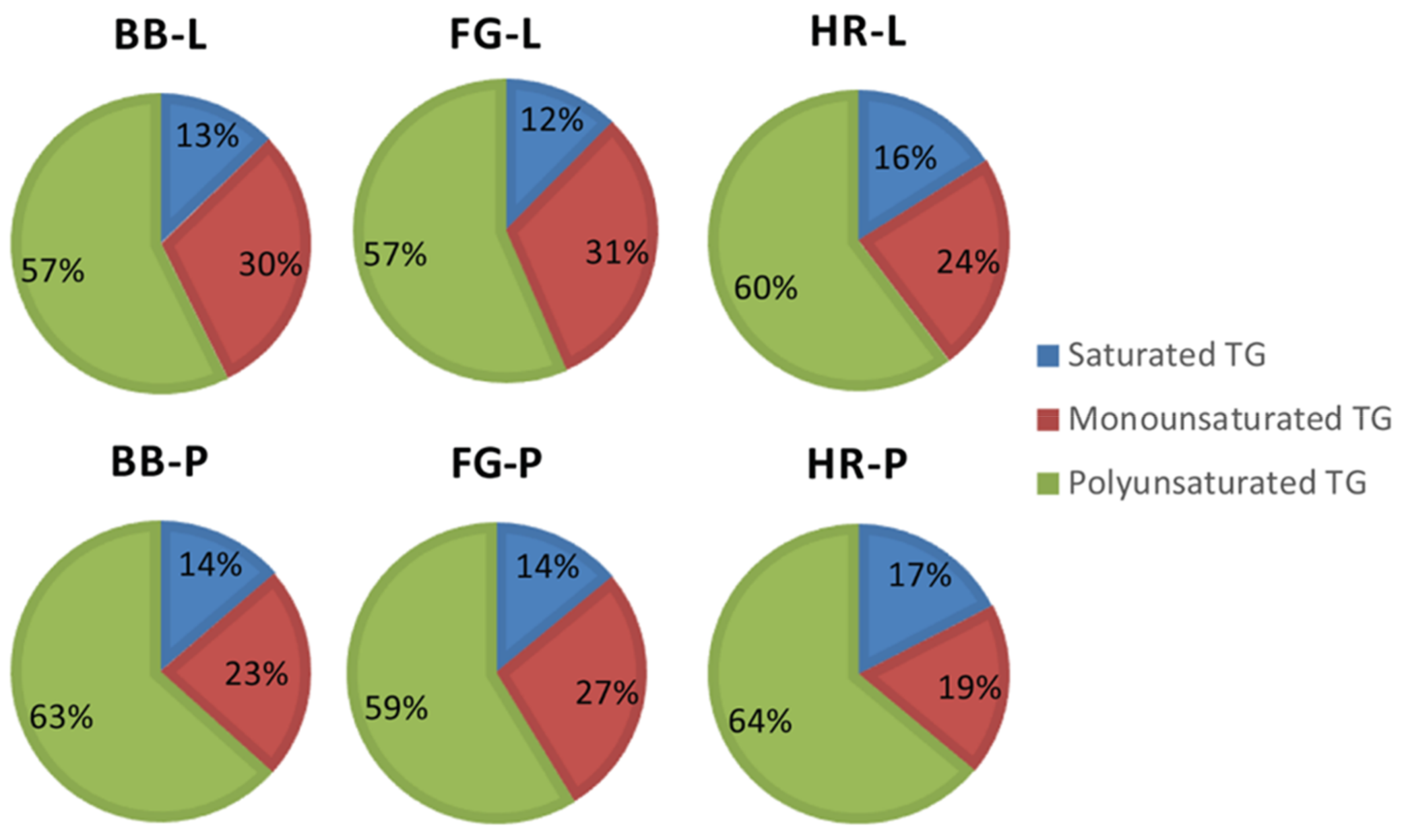
3.2. Effects of Cold Saponification and Wild Berry Extracts on the Unsaponified Neutral Lipids Composition of Natural Herbal Soaps
3.3. Effects of Antioxidants on Shelf Life of the Natural Herbal Soaps
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R. Biosurfactants in cosmetic formulations: Trends and challenges. Crit. Rev. Biotechnol. 2017, 37, 911–923. [Google Scholar] [CrossRef]
- Spitz, L. Soap Technology for the 1990’s; American Oil Chemists’ Society: Champaign, IL, USA, 1990. [Google Scholar]
- Vidal, N.P.; Adeseun, A.O.; Pham, T.H.; Mumtaz, A.; Manful, C.; Callahan, G.; Stewart, P.; Keough, D.; Thomas, R.H. The Effects of Cold Saponification on the Unsaponified Fatty Acid Composition and Sensory Perception of Commercial Natural Herbal Soaps. Molecules 2018, 23, 2356. [Google Scholar] [CrossRef]
- Zubair, M.F.; Atolani, O.; Ibrahim, S.O.; Oguntoye, O.S.; Abdulrahim, H.A.; Oyegoke, R.A.; Olatunji, G.A. Chemical and biological evaluations of potent antiseptic cosmetic products obtained from Momordica charantia seed oil. Sustain. Chem. Pharm. 2018, 9, 35–41. [Google Scholar] [CrossRef]
- Friedman, M.; Wolf, R. Chemistry of soaps and detergents: Various types of commercial products and their ingredients. Clin. Dermatol. 1996, 14, 7–13. [Google Scholar] [CrossRef]
- Berneckė, S.; Maruska, A. Analysis of free fatty acids in soap samples by means of gas chromatography-mass spectrometry. Chemija 2013, 24, 307–311. [Google Scholar]
- Ryer, F.V. Acid sodium stearates. Oil Soap 1946, 23, 310–313. [Google Scholar] [CrossRef]
- Lynch, M.L. Acid-soaps. Curr. Opin. Colloid Interface Sci. 1997, 2, 495–500. [Google Scholar] [CrossRef]
- Woolath, E. The Manufacture of Soaps, other Detergents and Glycerine; Ellis Horwood Ltd.: Chichester, UK, 1985; pp. 263–268. [Google Scholar]
- Kuntom, A.; Kifli, H.; Lim, P.-K. Chemical and physical characteristics of soap made from distilled fatty acids of palm oil and palm kernel oil. J. Am. Oil Chem. Soc. 1996, 73, 105–108. [Google Scholar] [CrossRef]
- George, E.D. Fatty acid distribution of fats, oils and soaps by high-performance liquid chromatography without derivatization. J. Am. Oil Chem. Soc. 1994, 71, 789–791. [Google Scholar] [CrossRef]
- Quintero-Flórez, A.; Nieva, L.S.; Sánchez-Ortíz, A.; Beltrán, G.; Perona, J.S. The Fatty Acid Composition of Virgin Olive Oil from Different Cultivars Is Determinant for Foam Cell Formation by Macrophages. J. Agric. Food Chem. 2015, 63, 6731–6738. [Google Scholar] [CrossRef]
- Thomas, R.H.; Bernards, M.A.; Drake, E.E.; Guglielmo, C.G. Changes in the antioxidant activities of seven herb- and spice-based marinating sauces after cooking. J. Food Compos. Anal. 2010, 23, 244–252. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Lobo, J.M.S. Main Benefits and Applicability of Plant Extracts in Skin Care Products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Villalobos-Delgado, L.H.; Mateo, J.; Caro, I.; Ramos, M.-Y.L.; Mendez, N.G.; Cansino, R.G.; Mondragón, E.G.G. Chapter 11-Natural Antioxidants in Fresh and Processed Meat. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Del Bo’, C.; Martini, D.; Porrini, M.; Klimis-Zacas, D.; Riso, P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015, 6, 2890–2917. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorders. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef]
- Hassan, L.E.A.; Ahamed, M.B.K.; Majid, A.S.A.; Baharetha, H.M.; Muslim, N.S.; Nassar, Z.D.; Majid, A.M.S.A. Correlation of antiangiogenic, antioxidant and cytotoxic activities of some Sudanese medicinal plants with phenolic and flavonoid contents. BMC Complement. Altern. Med. 2014, 14, 406. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Taghvaei, M.; Jafari, S.M. Application and stability of natural antioxidants in edible oils in order to substitute synthetic additives. J. Food Sci. Technol. 2015, 52, 1272–1282. [Google Scholar] [CrossRef]
- Moronkola, D.O.; Faruq, Z.U.; Adigun, O.A.; Ajiboye, C.O. Essential oil compositions of leaf, stem-bark, stem, root, flower, and fruit with seed of Blighia unijugata Baker (Sapindaceae). Afr. J. Pharm. Pharmacol. 2017, 11, 108–119. [Google Scholar]
- Cano, A.; Acosta, M.; Arnao, M.B. Hydrophilic and lipophilic antioxidant activity changes during on-vine ripening of tomatoes (Lycopersicon esculentum Mill.). Postharvest Biol. Technol. 2003, 28, 59–65. [Google Scholar] [CrossRef]
- Jimenez-Alvarez, D.; Giuffrida, F.; Vanrobaeys, F.; Golay, P.A.; Cotting, C.; Lardeau, A.; Keely, B.J. High-throughput methods to assess lipophilic and hydrophilic antioxidant capacity of food extracts in vitro. J. Agric. Food Chem. 2008, 56, 3470–3477. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-H.; Yeh, H.; Lin, S.-Y.; Yang, C.-M.; Tsai, H.-J.; Tsai, J.-J.; Chao, P.-Y. Antioxidant Activities of Methanol Extracts from Selected Taiwanese Herbaceous Plants. J. Food Nutr. Res. 2014, 2, 435–442. [Google Scholar] [CrossRef]
- Pham, T.H.; Zaeem, M.; Fillier, T.A.; Nadeem, M.; Vidal, N.P.; Manful, C.; Cheema, S.; Cheema, M.; Thomas, R.H. Targeting modified lipids during routine lipidomics analysis using HILIC and C30 reverse phase liquid chromatography coupled to mass spectrometry. Sci. Rep. 2019, 9, 5048. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Saldanha, T.; Soares, R.A.M.; Torres, E.A.F.S. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012, 135, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry phenolic antioxidants - Implications for human health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Ayorinde, F.o.; Garvin, K.; Saeed, K. Determination of the fatty acid composition of saponified vegetable oils using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 608–615. [Google Scholar] [CrossRef]
- Tao, L. Oxidation of polyunsaturated fatty acids and its impact on food quality and human health. Adv. Food Technol. Nutr. Sci. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Hleba, L.; Vuković, N.; Horská, E.; Petrová, J.; Sukdolak, S.; Kačániová, M. Phenolic profile and antimicrobial activities to selected microorganisms of some wild medical plant from Slovakia. Asian Pac. J. Trop. Dis. 2014, 4, 269–274. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and Antioxidant Properties of Anthocyanin Rich Extracts from Blueberry and Blackcurrant Juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
| Sample | Bacteria | Yeast | Molds |
|---|---|---|---|
| Base Bar - L | − | − | + |
| Base Bar - P | − | − | − |
| Base Bar - S | − | − | − |
| Base Bar - C | + | − | − |
| Base Bar - No Extracts | − | − | − |
| Base Bar - M | + | − | − |
| Base Bar - Combo | − | − | − |
| Forest Grove - L | + | + | + |
| Forest Grove - P | + | − | + |
| Forest Grove - S | + | + | − |
| Forest Grove - C | + | + | + |
| Forest Grove - A | + | − | − |
| Forest Grove - No Extracts | + | − | − |
| Forest Grove - M | − | − | − |
| Forest Grove Combo | − | − | − |
| Hibiscus Rosehip - L | − | − | + |
| Hibiscus Rosehip - P | + | − | − |
| Hibiscus Rosehip - S | + | − | − |
| Hibiscus Rosehip - C | + | + | − |
| Hibiscus Rosehip - A | − | + | − |
| Hibiscus Rosehip -No Extracts | + | − | + |
| Hibiscus Rosehip - M | − | − | − |
| Hibiscus Rosehip - Combo | + | − | − |
| Commercial - BSB | + | + | + |
| Commercial - BSG | + | + | + |
| Commercial - LSC | + | − | + |
| Commercial - LR | + | + | + |
| Commercial - LSB | + | + | − |
| Commercial - DI | + | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adigun, O.; Manful, C.; Prieto Vidal, N.; Mumtaz, A.; Pham, T.H.; Stewart, P.; Nadeem, M.; Keough, D.; Thomas, R. Use of Natural Antioxidants from Newfoundland Wild Berries to Improve the Shelf Life of Natural Herbal Soaps. Antioxidants 2019, 8, 536. https://doi.org/10.3390/antiox8110536
Adigun O, Manful C, Prieto Vidal N, Mumtaz A, Pham TH, Stewart P, Nadeem M, Keough D, Thomas R. Use of Natural Antioxidants from Newfoundland Wild Berries to Improve the Shelf Life of Natural Herbal Soaps. Antioxidants. 2019; 8(11):536. https://doi.org/10.3390/antiox8110536
Chicago/Turabian StyleAdigun, Oludoyin, Charles Manful, Natalia Prieto Vidal, Abira Mumtaz, Thu Huong Pham, Peter Stewart, Muhammad Nadeem, Dwayne Keough, and Raymond Thomas. 2019. "Use of Natural Antioxidants from Newfoundland Wild Berries to Improve the Shelf Life of Natural Herbal Soaps" Antioxidants 8, no. 11: 536. https://doi.org/10.3390/antiox8110536
APA StyleAdigun, O., Manful, C., Prieto Vidal, N., Mumtaz, A., Pham, T. H., Stewart, P., Nadeem, M., Keough, D., & Thomas, R. (2019). Use of Natural Antioxidants from Newfoundland Wild Berries to Improve the Shelf Life of Natural Herbal Soaps. Antioxidants, 8(11), 536. https://doi.org/10.3390/antiox8110536









