Abstract
This review highlighted resistance training as an important training type for the brain. Most studies that use physical exercise for the prevention or treatment of neurodegenerative diseases have focused on aerobic physical exercise, revealing different behavioral, biochemical, and molecular effects. However, recent studies have shown that resistance training can also significantly contribute to the prevention of neurodegenerative diseases as well as to the maintenance, development, and recovery of brain activities through specific neurochemical adaptations induced by the training. In this scenario we observed the results of several studies published in different journals in the last 20 years, focusing on the effects of resistance training on three main neurological aspects: Neuroprotective mechanisms, oxidative stress, and cognition. Systematic database searches of PubMed, Web of Science, Scopus, and Medline were performed to identify peer-reviewed studies from the 2000s. Combinations of keywords related to brain disease, aerobic/resistance, or strength physical exercise were used. Other variables were not addressed in this review but should be considered for a complete understanding of the effects of training in the brain.
1. Introduction
Most studies suggest the use of physical exercise to decrease the risk of cardiovascular [1], pulmonary [2], immunological [3], metabolic [4], and neurodegenerative diseases [5]. Although the cellular mechanisms that are regulated by physical exercise and that reduce the risk of chronic diseases are not fully understood, it is widely accepted that, overall, regular physical exercise promotes synergistic effects in different organs and tissues, especially between muscle and brain, muscle and lungs, and muscle and heart. Furthermore, this synergy modulates gene expression and causes molecular, biochemical, and physiological changes. These changes promote interactions between bodily systems, improving physical fitness and cognitive performance and, consequently, decreases the risk of chronic diseases (Figure 1).
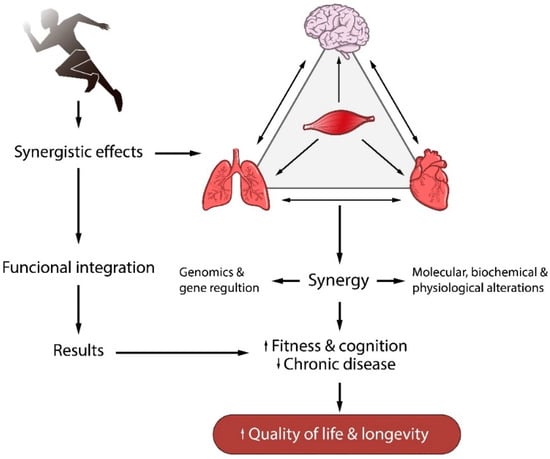
Figure 1.
Interactions between bodily systems from regular physical exercise. A synergistic effect between muscle, brain, and heart which modulates molecular, biochemical, and physiological changes, decreasing the risk of chronic diseases.
Despite aerobic exercise being more associated with neuroprotective mechanisms [6], recent studies have shown that resistance training also can significantly contribute to the prevention of neurodegenerative diseases [7,8,9,10] as well as to the maintenance, development, and recovery of brain activities through specific neurochemical adaptations induced by the training [11]. These biological changes induced by resistance training depend on the duration, intensity, frequency, and type of exercise which constitutes the basic parameters of an exercise training program for health [12]. Different from aerobic exercise in the form and intensity of execution and in the recruitment of energetic substrates, resistance training is performed against an external resistance to increase muscular strength and/or mass. This type of training depends primarily on anaerobic metabolism and promotes different stimuli depending on the intensity of muscular contraction, which affects muscle homeostasis. Resistance training induces muscle mechanical tension and increases intracellular calcium concentration. These changes activate different signaling pathways such as extracellular signal regulated kinase ERK/c-Jun N-terminal kinase (JNK), Ca2+/calmodulin-dependent protein kinase II (CaMKII), and fodfatidilinositol 3-quinase (PI3K)/protein kinase B (AKT)/ mammalian target of rapamycin (mTOR) which act upon specific targets and modulate gene expression through transcription and translation processes [13]. The effects of resistance exercise on skeletal muscle are well understood but effects on the brain have only been partially elaborated and are not always consistent.
The brain is susceptible to physical exercise by a change in the neuronal redox state. The acute response to physical exercise increases blood flow and enhances cell metabolism [14], adaptive changes include the positive regulation of antioxidants and the repair of enzymes, mitochondrial biogenesis, and redox regulation by different signaling pathways [6]. Such effects of physical training on the brain are derived from studies on aerobic training. However, in recent years, the number of experimental studies related to the effects of resistance (or strength) training on the brain have increased significantly, but the mechanisms are not yet fully understood. The aim of this review was to search recently published literature for studies investigating the effects of resistance training on three main neurological aspects: Neuroprotective mechanisms mediated by brain-derived neurotrophic factor (BDNF), oxidative stress, and cognition.
2. Mechanism of Resistance Exercise-Induced Neuroprotection: The Role of BDNF
According to Varendi and colleagues [15], the BDNF gene encoding human BDNF is located on chromosome 11 and eight different promoters result in varied mRNA transcripts. BDNF mRNA contains two alternative polyadenylated transcription stop sites providing binding sites for microRNAs in post-transcriptional regulation. More than 20 microRNAs are thought to control BDNF by its 3′UTR in vitro, but only one, miR-206, has been shown in vivo [15]. The BDNF protein is produced by many brain structures and other tissues such as the retina, motor neurons, kidneys, salivary, and prostate [16]. BDNF is also produced in skeletal muscle and could play an important role in the development of these muscles, as well as in degenerative muscular diseases [17]. After sciatic nerve damage, increases in mRNA and protein levels of BDNF were highly significant in skeletal muscle, suggesting a role of BDNF in repair mechanisms and possibly satellite cell activation [18]. Indeed, the ablation of BDNF in skeletal muscle was associated with a marked decrease in the satellite cell number, suggesting that BDNF could be an important regulator at the early stage of muscle repair via the activation of satellite cells [19].
The effects of exercise on BDNF production could be systemic however, exercise mediated changes in BDNF levels in the central nervous system have been the most studied to date. BNDF acts on certain neurons in the central and peripheral nervous system, regulating existing neurons and stimulating the growth and differentiation of new ones [20]. As one of the main regulators of brain metabolism, BNDF plays the main role in the development of synaptic plasticity and thus has attracted the attention of researchers interested in neurodegenerative diseases. Pre-clinical trials have shown correlations between depressive behaviors and decreased BNDF levels in the hippocampus [21]. Similar results exist regarding Parkinson’s [22] and Alzheimer’s disease [23]. In experimental models, the brain levels of BDNF and other neurotrophins decreased after exposure to toxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTPT) and 6-hydroxydopamine (6-OHDA) [8,9,24,25,26,27].
It has been reported that BDNF is involved in neural survival and differentiation [28] which could be closely linked to cellular energy levels. Indeed, it has been shown that BDNF can enhance glucose uptake in response to increased energy needs by stimulating the expression of GLUT3 [29]. Thus, BDNF mediated enhanced metabolism can cover the energy cost of increased amino acid uptake and associated protein synthesis necessary for neuronal differentiation, as well as the branching of axons and dendrites [29,30]. Indeed, energy costs of protein synthesis are significantly higher than the turnover cost of oligonucleotides or lipids [31]. However, the greatest extent of energy expenditure in the brain is consumed by action potential related events [32] and BDNF is also an active regulator of synaptic transmission [33]. Based on this, it is not surprising that the expression of BDNF is under neuronal activity-dependent calcium signaling [34]. Indeed, it is well documented that physical exercise-induced beneficial structural and functional effects are associated with the upregulation of BDNF levels.
The results of the effect of resistance training on BDNF levels are diverse. Studies in humans showed that no alterations [35,36,37] or increases [38,39,40,41,42,43] were reported in the level of circulating BDNF. One study observed a difference between the sexes and an increase in BDNF levels in males, but not in females, was noted [44]. It has been suggested that the brain is the main source of the increased BDNF level in circulation after endurance exercise [45,46]. However, the source of increased BDNF in the circulation after resistance training is still unknown. Due to the difficulties of resistance training methods in animal models, BDNF studies with resistance training are limited. When Wistar rats were subjected to four weeks of progressive strength exercise in a vertical ladder apparatus, increased neurogenesis was suggested based on Ki-67-positive cells, but no change in BDNF level was reported in the hippocampus [47]. We have investigated the effects of aerobic and strength training on BDNF levels and neuroplasticity, and found that both endurance and resistance training results in similar stimulating effects on BDNF levels in rats [48].
3. Oxidative Stress in the Brain and Resistance Exercise
The brain is highly sensitive to oxidative stress due to its high levels of phospholipids and polyunsaturated fatty acids [6]. These molecules are prone to oxidation, which leads to the production of abundant reactive oxygen species (ROS) [49]. Moreover, the brain has low levels of antioxidant enzymes and certain regions, like the striatum, have high iron levels that facilitate the formation of ROS [49,50,51,52]. Consequently, the brain’s susceptibility to oxidative stress reduces BDNF levels, since these are influenced by changes in the brain redox state [6,53].
Aerobic physical training has been used in different studies in humans [54,55,56,57] and animals [9,58,59,60,61,62,63,64,65,66] to reduce oxidative stress and maintain the brain redox balance as well as increase the BDNF levels [6]. The influence of aerobic exercise on the brain redox system has been widely reported by different researchers but regarding the cerebral role of resistance exercise, the results are not fully understood. Although several studies have been conducted to verify the effects of exercise on different brain functions and mechanisms, it is only in recent years that the role of resistance exercise has effectively drawn the attention of researchers. For example, in the last 10 years several studies have reported the neuroprotective capacity of resistance exercise, however, few of these considered oxidative stress (see Table 1).

Table 1.
Recent pre-clinical (part A) and clinical (part B) studies related to the effects of resistance (or strength) training on the brain.
Although the cellular mechanisms involved in the regulation of brain oxidative stress by resistance exercise are not fully understood, it is possible to speculate that the adaptive changes induced by resistance training from muscle involve the up-regulation of antioxidant and brain redox regulation from different proteins and pathways, such as the mammalian target of rapamycin (mTOR), a serine/threonine kinase important for cell growth, proliferation, and survival of brain [78] as well as the cAMP-response element-binding protein (CREB), an intracellular protein that regulates the expression of genes that are important in dopaminergic neurons [79]. Both mTOR and CREB are responsible for enhanced translation initiation from AKT (protein kinase B) phosphorylation, which leads to both muscle and brain BDNF expression and activation [80,81,82].
BDNF release from muscle contraction reaches the brain and binds to Tropomyosin receptor kinase B (TrkB) to induce phosphorylation of different cascades of signaling pathways: PI3K/AKT/mTOR, Pi3K/AKT/CREB, Pi3k/ERK/CREB, and phospholipase Cγ (PLCγ)/CamKII/CREB. The activation of these different signaling pathways results in the additional secretion of the BDNF. In additional, it is possible that the brain mTOR and CREB signaling are also important targets of resistance exercise. These observations are supported partially by previous studies that showed PI3K/mTOR signaling [83] and elevated BDNF levels binds the TRKb receptors and PKC/CREB [48,84] after resistance exercise.
The BDNF leads to the activation of nuclear factor erythroid 2-related factor 2 (Nrf2) [85], which regulates the expression of detoxification enzymes and antioxidants to protect brain cells from oxidants, electrophiles, and inflammatory agents [86], as well as to maintain the mitochondrial function, cellular redox, and protein homeostasis [87]. Nrf2 is a cellular regulator of antioxidant defense systems [88]. Under physiological conditions, the Nrf2 is linked in the cytoplasm to the Kelch-like ECH-associated protein 1 (keap1). Nrf2 translocates to the nucleus in response to oxidative stress or when electrophilic molecules that covalently modify cysteine residues present in the thiol-rich KEAP1 protein by oxidation or alkylation [89,90] where it binds to specific DNA sites termed anti-oxidant response elements (ARE) to initiate the transcription of cytoprotective genes such as heme oxygenase-1 (HO-1), superoxide dismutase (SOD), glutathione S-transferase (GST), NAD(P)H: quinone oxidoreductase 1 (NQO1), and γ-glutamatecysteine ligase (GCL) [91]. This mechanism is summarized in Figure 2.
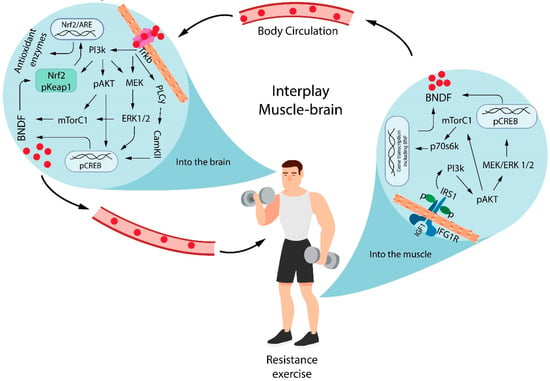
Figure 2.
Interplay between muscle and brain in BDNF-mediated redox regulation. Resistance exercise induces BDNF generation from CREB and mTor phosphorylation by the Pi3K/AKT signaling pathway. The BDNF release from muscle contraction reaches the brain and binds the TrkB receptor to induce the phosphorylation of different cascades of signaling pathways, which results in the additional secretion of BDNF. Brain BDNF leads to the activation of Nrf2, which regulates the expression of antioxidants molecules. BDNF =brain-derived neurotrophic factor; IGF1 = insulin-like growth factor 1; IGF1R = insulin-like growth factor 1 receptor; Pi3K = phosphatidylinositol 3-kinase; IRS1 = Insulin receptor substrate 1; pAKT = protein kinase B phosphorylated; MEK = mitogen-activated protein kinase; ERK = extracellular signal–regulated kinase; CREB = cAMP-response element-binding protein phosphorylated; mTORC1 = mammalian target of rapamycin complex 1; p70s6k = ribosomal protein S6 kinase beta-1; TrkB = Tropomyosin receptor kinase B; PLCγ = phospholipase C gamma; CamKII = calcium/calmodulin-dependent protein kinase II; ARE = antioxidant response element; pKeap1 = Kelch-like ECH-associated protein 1 phosphorylated; Nrf2 = nuclear factor erythroid 2-related factor 2.
Previous studies have showed that strength training promotes the upregulation of Nrf2 in the central nervous system after experimental autoimmune encephalomyelitis (EAE) induction [7]. Similar results were previously reported by Aguiar et al. [66], who demonstrated that moderate-intensity physical exercise protected the 6-OHDA-induced loss of tyrosine hydroxylase immunolabeling and activated the Nrf2-ARE pathway in the nigrostriatal pathway. Regulation of antioxidant enzyme activity by resistance exercise has not been the focus of many studies. In one of the few studies, Park et al. [71] showed that resistance exercise training increased SOD1 activity in the hypothalamus of rats with type II diabetes (T2DM) and that it could contribute to hypothalamus redox regulation under T2DM conditions. Souza et al., [7] showed that animals with EAE, undertaking resistance training, showed no changes in SOD activity, but a modulation in the content of glutathione and glutathione peroxidase activity was observed. The authors suggest that these exercise-modulating effects on the glutathione system may be associated with the regulation of mechanisms controlled by Nrf2 phosphorylation. These results suggest the possibility of a regulatory mechanism induced by resistance exercise which modulates the actions of BDNF and provides evidence of resistance training-induced brain redox modulation.
4. Resistance Exercise and Cognition
There is not much evidence regarding the effects of resistance training on higher brain functions such as cognition, executive function, and attention. The primary outcome of major clinical trials was muscle strength [92,93,94], fall prevention [95], and the neuronal effects were secondary outcomes. These clinical studies focused on healthy or unhealthy aged people using various neurological tests such as the Working Memory Index for working memory [92], the Rey Auditory Verbal Learning Test for declarative memory [96,97], Trail Making Test Part B, Verbal Digits Backward Test, and Stroop Color-Word Test for executive functions [95,96,97], as well as the Alzheimer’s Disease Assessment Scale (ADAS-Cog) and Mini-mental State Examination for cognitive impairments [93,94,97]. The most consistent result of resistance training was the improvement of the executive function in the elderly with no differences between the sexes. Three studies using male rats and a vertical ladder where the cognitive enhancer effects of resistance training were associated with neurogenesis [47], an improved IGF-1 pathway in young rats [98], and BDNF/TrκB signaling in aged rats [48].
Cognition is the ability to process information through perception, knowledge acquired through experience and context, and personal characteristics that integrate all information to interact with the environment. Aging and many neurological diseases impair cognitive functions, from mild cognitive impairment to severe dementia with Alzheimer’s disease being the most prevalent dementia [99]. Some muscle strengthening programs have shown cognitive enhancer effects in these populations [92,93,94,96]. The characteristics of the resistance training program design are described in Table 2. All studies were successful in strengthening muscle [92,94,96]. The Study of Mental and Resistance Training (SMART) program improved cognitive impairments associated with Alzheimer’s disease, as assessed by the ADAS-Cog instrument in community-dwelling adults [93,94]. The resistance training improved declarative memory of elderly women, even after two years of follow-up [96]. Declarative (or explicit) memory dependent on the hippocampus and related cortex are crucial for the memory of facts, events, faces, and environments [100]. However, muscle strengthening did not modify hippocampal atrophy of aged women, but reduced cortical white matter atrophy [96].

Table 2.
Resistance training for cognitive enhancer effects in the elderly.
Preclinical experimental studies focused on the effects of resistance training in the hippocampus of adult male rats. Cassilhas and colleagues [98] demonstrated that resistance training improves spatial memory and serum IGF-1 levels in adult male rats. They also demonstrated an exercise-induced increase in IGF-1R and AKT phosphorylation, as well as in density of IGF-1, synapsin, and synaptophysin in the hippocampus of rats. Neurotrophin TRκB receptor levels were not modified. Gomes and colleagues [47] showed that resistance training increased neurogenesis and decreased apoptosis signaling in the dentate gyrus of adult male rats, however BDNF levels were not modified in the dentate gyrus and CA3 area of the hippocampus after exercise. Vilela et al., [48] demonstrated that resistance training improved spatial memory and strengthened neurotrophin signaling in the hippocampus, unlike young animals [47,98]. All of these well-designed animal and human studies reinforce the feasibility and cognitive benefits of resistance training on elderly cognition.
Clinical studies have also demonstrated the benefits of strength exercise in the executive function [93,94,95], a set of cognitive processes necessary for behavior control that facilitate the attainment of chosen goals. Cuttler and colleagues [101] demonstrated that a single strength exercise session in healthy youths improved prospective memory (the ability to remember intended actions in the future). The home-based Otago Exercise Program demonstrated that resistance training improved response inhibition by 12.8% in the elderly in fall-dedicated clinics, while control sedentary presented a deterioration of response inhibition [95,102], which is important for self-control and resistance to temptations and impulsiveness [103].
Cognitive inhibition is strongly associated with working memory. The core executive function of working memory involves storing information and mentally working with it [96,103]. Aging declines speed of processing, working memory, inhibitory function, and long-term memory, as well as decreases brain structure size and white matter integrity [104]. The home-based Strong for Life program improved verbal working memory of older adults with at least one disability [92]. However, these cognitive benefits were observed in the elderly who reached moderate-high resistance training intensities and not in the elderly who performed low-intensity exercise. Working memory supports inhibitory control and temporarily stored information is crucial for goal behavior with inhibition of errors. The opposite is also true. Inhibitory control supports working memory, goal objective needs focus and resists noise. Impaired working memory during aging is also associated with decreased processing speed, which is the efficiency with which an individual is able to perceive and act upon a stimulus [105]. Yoon et al. [97] demonstrated that resistance training in the elderly without major health problems improved processing speed. The only mechanism reported for the executive cognitive enhancer effects of resistance training in the elderly is the reduction of cortical white matter atrophy, as previously described [96]. Figure 3 depicts that resistance training improves serum IGF-1 levels and hippocampal IGF-1 signaling. Muscle strengthening also boosts cognitive and executive functions.
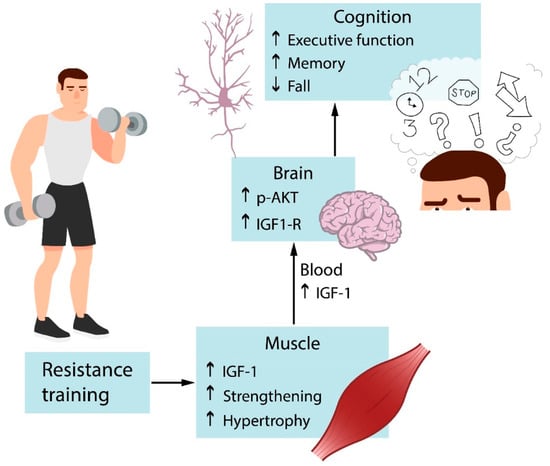
Figure 3.
Effects of resistance exercise on cognition. Resistance training activates IGF-1 signaling in muscles, increasing muscle mass and strength. IGF-1 produced by muscles reaches the brain via circulation and binds to specific receptors that lead to the activation of signaling pathways. It acts on specific targets and results in improved cognition. IGF-1 = Insulin growth factor.
5. Conclusions
The effects of resistance exercise on the brain are not yet fully understood due to the few studies available so far. Although promising, these studies do not allow for a definitive conclusion regarding the effects resistance exercise on the brain’s redox and cognitive mechanisms. In some cases, associations were speculative and further investigations are required. In this regard, future studies in animals and humans may fill these gaps and contribute to further understanding the effects of resistance exercise on the brain.
Author Contributions
R.A.P., A.S.A.J., and Z.R. contributed by searching and all contributed to the manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Acknowledgments
This study was supported by OTKA (112810) and National Excellence Program (126823), and Scientific Excellence Program TUDFO/51757/2019-ITM, at the University of Physical Education, Innovation and Technology Ministry, Hungary, grants awarded to Z.R., National Council of Technological and Scientific Development-CNPq/Brazil, and Coordination for the Improvement of Higher Education Personnel-CAPES/Brazil. We also thank Rafael Barreto Pinho for the final artwork of figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Mantoani, L.C.; Dell’Era, S.; MacNee, W.; Rabinovich, R.A. Physical activity in patients with COPD: The impact of comorbidities. Expert Rev. Respir. Med. 2017, 11, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, G.I.; Febbraio, M.A. The immunomodulating role of exercise in metabolic disease. Trends Immunol. 2014, 35, 262–269. [Google Scholar] [CrossRef]
- Hamer, M.; Chida, Y. Physical activity and risk of neurodegenerative disease: A systematic review of prospective evidence. Psychol. Med. 2009, 39, 3–11. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical exercise, reactive oxygen species and neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Tuon, T.; Souza, P.S.; Santos, M.F.; Pereira, F.T.; Pedroso, G.S.; Luciano, T.F.; De Souza, C.T.; Dutra, R.C.; Silveira, P.C.L.; Pinho, R.A. Physical Training Regulates Mitochondrial Parameters and Neuroinflammatory Mechanisms in an Experimental Model of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2015, 2015, 261809. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Dal Pont, G.C.; Paganini, C.S.; Pozzi, B.G.; Luciano, T.F.; Souza, P.S.; Quevedo, J.; Souza, C.T.; Pinho, R.A. Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res. Bull. 2014, 108, 106–112. [Google Scholar] [CrossRef]
- Jensen, C.S.; Bahl, J.M.; Østergaard, L.B.; Høgh, P.; Wermuth, L.; Heslegrave, A.; Zetterberg, H.; Heegaard, N.H.H.; Hasselbalch, S.G.; Simonsen, A.H. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 2019, 121, 91–98. [Google Scholar] [CrossRef]
- Radak, Z.; Ihasz, F.; Koltai, E.; Goto, S.; Taylor, A.W.; Boldogh, I. The redox-associated adaptive response of brain to physical exercise. Free Radic. Res. 2014, 48, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [PubMed]
- Egan, B.; Zierath, J.R. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013, 17, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Secher, N.H.; Seifert, T.; Van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef]
- Varendi, K.; Mätlik, K.; Andressoo, J.-O. From microRNA target validation to therapy: Lessons learned from studies on BDNF. Cell. Mol. Life Sci. 2015, 72, 1779–1794. [Google Scholar] [CrossRef]
- Mandel, A.L.; Ozdener, H.; Utermohlen, V. Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Arch. Oral Biol. 2009, 54, 689–695. [Google Scholar] [CrossRef]
- Halievski, K.; Nath, S.; Katsuno, M.; Adachi, H.; Sobue, G.; Breedlove, S.; Lieberman, A.; Jordan, C. Disease Affects Bdnf Expression in Synaptic and Extrasynaptic Regions of Skeletal Muscle of Three SBMA Mouse Models. Int. J. Mol. Sci. 2019, 20, 1314. [Google Scholar] [CrossRef]
- Omura, T.; Sano, M.; Omura, K.; Hasegawa, T.; Doi, M.; Sawada, T.; Nagano, A. Different expressions of BDNF, NT3, and NT4 in muscle and nerve after various types of peripheral nerve injuries. J. Peripher. Nerv. Syst. 2005, 10, 293–300. [Google Scholar] [CrossRef]
- Clow, C.; Jasmin, B.J. Brain-derived neurotrophic factor regulates satellite cell differentiation and skeltal muscle regeneration. Mol. Biol. Cell 2010, 21, 2182–2190. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Martinowich, K.; Manji, H.; Lu, B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007, 10, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.B.; Soderstrom, K.; Bakay, R.A.E.; Kordower, J.H. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010, 184, 237–264. [Google Scholar] [PubMed]
- Wang, Z.-H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.-Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef] [PubMed]
- Marais, L.; Stein, D.J.; Daniels, W.M.U. Exercise increases BDNF levels in the striatum and decreases depressive-like behavior in chronically stressed rats. Metab. Brain Dis. 2009, 24, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Tripanichkul, W.; Gerdprasert, O.; Jaroensuppaperch, E. Estrogen reduces BDNF level, but maintains dopaminergic cell density in the striatum of MPTP mouse model. Int. J. Neurosci. 2010, 120, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Lesemann, A.; Reinel, C.; Hühnchen, P.; Pilhatsch, M.; Hellweg, R.; Klaissle, P.; Winter, C.; Steiner, B. MPTP-induced hippocampal effects on serotonin, dopamine, neurotrophins, adult neurogenesis and depression-like behavior are partially influenced by fluoxetine in adult mice. Brain Res. 2012, 1457, 51–69. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Luciano, T.; Trom, C.B.; Silva, L.A.; Quevedo, J.; Souza, C.T.; Lira, F.S.; Pinho, R.A. Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 2012, 227, 305–312. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Vega-Rivera, N.M.; Babu, H.; Ramírez-Rodríguez, G.B. Brain-Derived Neurotrophic Factor Induces Cell Survival and the Migration of Murine Adult Hippocampal Precursor Cells During Differentiation In Vitro. Neurotox. Res. 2017, 31, 122–135. [Google Scholar] [CrossRef]
- Burkhalter, J.; Fiumelli, H.; Allaman, I.; Chatton, J.-Y.; Martin, J.-L. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J. Neurosci. 2003, 23, 8212–8220. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Ames, A. CNS energy metabolism as related to function. Brain Res. Brain Res. Rev. 2000, 34, 42–68. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Balkowiec, A.; Katz, D.M. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J. Neurosci. 2002, 22, 10399–10407. [Google Scholar] [CrossRef] [PubMed]
- Goekint, M.; De Pauw, K.; Roelands, B.; Njemini, R.; Bautmans, I.; Mets, T.; Meeusen, R. Strength training does not influence serum brain-derived neurotrophic factor. Eur. J. Appl. Physiol. 2010, 110, 285–293. [Google Scholar] [CrossRef]
- Forti, L.N.; Njemini, R.; Beyer, I.; Eelbode, E.; Meeusen, R.; Mets, T.; Bautmans, I. Strength training reduces circulating interleukin-6 but not brain-derived neurotrophic factor in community-dwelling elderly individuals. Age 2014, 36, 9704. [Google Scholar] [CrossRef]
- Hvid, L.G.; Nielsen, M.K.F.; Simonsen, C.; Andersen, M.; Caserotti, P. Brain-derived neurotrophic factor (BDNF) serum basal levels is not affected by power training in mobility-limited older adults—A randomized controlled trial. Exp. Gerontol. 2017, 93, 29–35. [Google Scholar] [CrossRef]
- Yarrow, J.F.; White, L.J.; McCoy, S.C.; Borst, S.E. Training augments resistance exercise induced elevation of circulating brain derived neurotrophic factor (BDNF). Neurosci. Lett. 2010, 479, 161–165. [Google Scholar] [CrossRef]
- Coelho, F.M.; Pereira, D.S.; Lustosa, L.P.; Silva, J.P.; Dias, J.M.D.; Dias, R.C.D.; Queiroz, B.Z.; Teixeira, A.L.; Teixeira, M.M.; Pereira, L.S.M. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef]
- Kim, H.; Song, B.; So, B.; Lee, O.; Song, W.; Kim, Y. Increase of circulating BDNF levels and its relation to improvement of physical fitness following 12 weeks of combined exercise in chronic patients with schizophrenia: A pilot study. Psychiatry Res. 2014, 220, 792–796. [Google Scholar] [CrossRef]
- Walsh, J.J.; Scribbans, T.D.; Bentley, R.F.; Kellawan, J.M.; Gurd, B.; Tschakovsky, M.E. Neurotrophic growth factor responses to lower body resistance training in older adults. Appl. Physiol. Nutr. Metab. 2016, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high-intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Marston, K.J.; Newton, M.J.; Brown, B.M.; Rainey-Smith, S.R.; Bird, S.; Martins, R.N.; Peiffer, J.J. Intense resistance exercise increases peripheral brain-derived neurotrophic factor. J. Sci. Med. Sport 2017, 20, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015, 70, 144–149. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Seifert, T.; Brassard, P.; Wissenberg, M.; Rasmussen, P.; Nordby, P.; Stallknecht, B.; Adser, H.; Jakobsen, A.H.; Pilegaard, H.; Nielsen, H.B.; et al. Endurance training enhances BDNF release from the human brain. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R372–R377. [Google Scholar] [CrossRef]
- Novaes Gomes, F.G.; Fernandes, J.; Vannucci Campos, D.; Cassilhas, R.C.; Viana, G.M.; D’Almeida, V.; de Moraes Rêgo, M.K.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats Through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef]
- Hwang, O. Role of Oxidative Stress in Parkinson’s Disease. Exp. Neurobiol. 2013, 22, 11. [Google Scholar] [CrossRef]
- Crichton, R.R.; Dexter, D.T.; Ward, R.J. Brain iron metabolism and its perturbation in neurological diseases. J. Neural Transm. 2011, 118, 301–314. [Google Scholar] [CrossRef]
- Lan, A.P.; Chen, J.; Chai, Z.F.; Hu, Y. The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. BioMetals 2016, 29, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Sian-Hülsmann, J.; Mandel, S.; Youdim, M.B.H.; Riederer, P. The relevance of iron in the pathogenesis of Parkinson’s disease. J. Neurochem. 2011, 118, 939–957. [Google Scholar] [CrossRef] [PubMed]
- Siamilis, S.; Jakus, J.; Nyakas, C.; Costa, A.; Mihalik, B.; Falus, A.; Radak, Z. The effect of exercise and oxidant–antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord 2009, 47, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Rasmussen, P.; Evans, K.A.; Bohm, A.M.; Zaar, M.; Nielsen, H.B.; Brassard, P.; Nordsborg, N.B.; Homann, P.H.; Raven, P.B.; et al. Hypoxia compounds exercise-induced free radical formation in humans; partitioning contributions from the cerebral and femoral circulation. Free Radic. Biol. Med. 2018, 124, 104–113. [Google Scholar] [CrossRef]
- Iofrida, C.; Daniele, S.; Pietrobono, D.; Fusi, J.; Galetta, F.; Trincavelli, M.L.; Bonuccelli, U.; Franzoni, F.; Martini, C. Influence of physical exercise on β-amyloid, α-synuclein and tau accumulation: an in vitro model of oxidative stress in human red blood cells. Arch. Ital. Biol. 2017, 155, 33–42. [Google Scholar]
- Roh, H.-T.; Cho, S.-Y.; Yoon, H.-G.; So, W.-Y. Effect of Exercise Intensity on Neurotrophic Factors and Blood-Brain Barrier Permeability Induced by Oxidative-Nitrosative Stress in Male College Students. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 239–246. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Q.; Jiang, H.; Du, J.; Zhou, C.; Yu, S.; Hashimoto, K.; Zhao, M. Impact of aerobic exercise on cognitive impairment and oxidative stress markers in methamphetamine-dependent patients. Psychiatry Res. 2018, 266, 328–333. [Google Scholar] [CrossRef]
- Um, H.S.; Kang, E.B.; Leem, Y.H.; Cho, I.H.; Yang, C.H.; Chae, K.R.; Hwang, D.Y.; Cho, J.Y. Exercise training acts as a therapeutic strategy for reduction of the pathogenic phenotypes for Alzheimer’s disease in an NSE/APPsw-transgenic model. Int. J. Mol. Med. 2008, 22, 529–539. [Google Scholar]
- Speck, A.E.; Tromm, C.B.; Pozzi, B.G.; Paganini, C.S.; Tuon, T.; Silveira, P.C.L.; Aguiar, A.S.; Pinho, R.A. The dose-dependent antioxidant effects of physical exercise in the hippocampus of mice. Neurochem. Res. 2014, 39, 1496–1501. [Google Scholar] [CrossRef]
- Tuon, T.; Valvassori, S.S.; Lopes-Borges, J.; Fries, G.R.; Silva, L.A.; Kapczinski, F.; Quevedo, J.; Pinho, R.A. Effects of moderate exercise on cigarette smoke exposure-induced hippocampal oxidative stress values and neurological behaviors in mice. Neurosci. Lett. 2010, 475, 16–19. [Google Scholar] [CrossRef]
- Aguiar, A.S.; Tuon, T.; Pinho, C.A.; Silva, L.A.; Andreazza, A.C.; Kapczinski, F.; Quevedo, J.; Streck, E.L.; Pinho, R.A. Mitochondrial IV complex and brain neurothrophic derived factor responses of mice brain cortex after downhill training. Neurosci. Lett. 2007, 426, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, R.O.; Gadelha-Filho, C.V.J.; da Costa, A.E.M.; Feitosa, M.L.; de Araújo, D.P.; de Lucena, J.D.; de Aquino, P.E.A.; Lima, F.A.V.; Neves, K.R.T.; de Barros Viana, G.S. The Treadmill Exercise Protects against Dopaminergic Neuron Loss and Brain Oxidative Stress in Parkinsonian Rats. Oxid. Med. Cell. Longev. 2017, 2017, 2138169. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Ying, Z.; Radak, Z.; Gomez-Pinilla, F. Voluntary exercise may engage proteasome function to benefit the brain after trauma. Brain Res. 2010, 1341, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Bori, Z.; Hart, N.; Sárga, L.; Koltai, E.; Radák, Z.; Nyakas, C. Long-term exercise treatment reduces oxidative stress in the hippocampus of aging rats. Neuroscience 2012, 226, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Radák, Z.; Silye, G.; Bartha, C.; Jakus, J.; Stefanovits-Bányai, E.; Atalay, M.; Marton, O.; Koltai, E. The effects of cocoa supplementation, caloric restriction, and regular exercise, on oxidative stress markers of brain and memory in the rat model. Food Chem. Toxicol. 2013, 61, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.S.; Duzzioni, M.; Remor, A.P.; Tristão, F.S.M.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-Intensity Physical Exercise Protects Against Experimental 6-Hydroxydopamine-Induced Hemiparkinsonism Through Nrf2-Antioxidant Response Element Pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.C.O.; Quines, C.B.; Jardim, N.S.; Leite, M.R.; Nogueira, C.W. Resistance exercise reduces memory impairment induced by monosodium glutamate in male and female rats. Exp. Physiol. 2017, 102, 845–853. [Google Scholar] [CrossRef]
- Özbeyli, D.; Sarı, G.; Özkan, N.; Karademir, B.; Yüksel, M.; Çilingir Kaya, Ö.T.; Kasımay Çakır, Ö. Protective effects of different exercise modalities in an Alzheimer’s disease-like model. Behav. Brain Res. 2017, 328, 159–177. [Google Scholar] [CrossRef]
- De Almeida, A.A.; Gomes da Silva, S.; Lopim, G.M.; Vannucci Campos, D.; Fernandes, J.; Cabral, F.R.; Arida, R.M. Resistance Exercise Reduces Seizure Occurrence, Attenuates Memory Deficits and Restores BDNF Signaling in Rats with Chronic Epilepsy. Neurochem. Res. 2017, 42, 1230–1239. [Google Scholar] [CrossRef]
- Henrique, J.S.; França, E.F.; Cardoso, F.D.S.; Serra, F.T.; de Almeida, A.A.; Fernandes, J.; Arida, R.M.; Gomes da Silva, S. Cortical and hippocampal expression of inflammatory and intracellular signaling proteins in aged rats submitted to aerobic and resistance physical training. Exp. Gerontol. 2018, 110, 284–290. [Google Scholar] [CrossRef]
- Park, S.H.; Yoon, J.H.; Seo, D.Y.; Kim, T.N.; Ko, J.R.; Han, J. Resistance Exercise Training Attenuates the Loss of Endogenous GLP-1 Receptor in the Hypothalamus of Type 2 Diabetic Rats. Int. J. Environ. Res. Public Health 2019, 16, 830. [Google Scholar] [CrossRef] [PubMed]
- Farzi, M.A.; Sadigh-Eteghad, S.; Ebrahimi, K.; Talebi, M. Exercise Improves Recognition Memory and Acetylcholinesterase Activity in the Beta Amyloid-Induced Rat Model of Alzheimer’s Disease. Ann. Neurosci. 2019, 25, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Shin, S.K.; Hong, S.B.; Kim, H.J. The effects of strength exercise on hippocampus volume and functional fitness of older women. Exp. Gerontol. 2017, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Conrado de Freitas, M.; Gerosa-Neto, J.; Cholewa, J.M.; Rossi, F.E. Comparison Between Full-Body vs. Split-Body Resistance Exercise on the Brain-Derived Neurotrophic Factor and Immunometabolic Response. J. Strength Cond. Res. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Sanchéz, M.A.; Bustos-Cruz, R.H.; Velasco-Orjuela, G.P.; Quintero, A.P.; Tordecilla-Sanders, A.; Correa-Bautista, J.E.; Triana-Reina, H.R.; García-Hermoso, A.; González-Ruíz, K.; Peña-Guzmán, C.A.; et al. Acute Effects of High Intensity, Resistance, or Combined Protocol on the Increase of Level of Neurotrophic Factors in Physically Inactive Overweight Adults: The BrainFit Study. Front. Physiol. 2018, 9, 741. [Google Scholar] [CrossRef]
- Goldfield, G.S.; Kenny, G.P.; Prud’homme, D.; Holcik, M.; Alberga, A.S.; Fahnestock, M.; Cameron, J.D.; Doucette, S.; Hadjiyannakis, S.; Tulloch, H.; et al. Effects of aerobic training, resistance training, or both on brain-derived neurotrophic factor in adolescents with obesity: The hearty randomized controlled trial. Physiol. Behav. 2018, 191, 138–145. [Google Scholar] [CrossRef]
- Lan, Y.; Huang, Z.; Jiang, Y.; Zhou, X.; Zhang, J.; Zhang, D.; Wang, B.; Hou, G. Strength exercise weakens aerobic exercise-induced cognitive improvements in rats. PLoS ONE 2018, 13, e0205562. [Google Scholar] [CrossRef]
- LiCausi, F.; Hartman, N. Role of mTOR Complexes in Neurogenesis. Int. J. Mol. Sci. 2018, 19, 1544. [Google Scholar] [CrossRef]
- Sakamoto, K.; Karelina, K.; Obrietan, K. CREB: A multifaceted regulator of neuronal plasticity and protection. J. Neurochem. 2011, 116, 1–9. [Google Scholar] [CrossRef]
- Ying, Z.; Roy, R.R.; Zhong, H.; Zdunowski, S.; Edgerton, V.R.; Gomez-Pinilla, F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience 2008, 155, 1070–1078. [Google Scholar] [CrossRef]
- Seki, K.; Yoshida, S.; Jaiswal, M.K. Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen. Res. 2018, 13, 1159–1169. [Google Scholar] [PubMed]
- Browne, C.A.; Lucki, I. Antidepressant effects of ketamine: Mechanisms underlying fast-acting novel antidepressants. Front. Pharmacol. 2013, 4, 161. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, B.A.; Hake, H.S.; Ishiwata, T.; Farmer, C.E.; Loetz, E.C.; Fleshner, M.; Bland, S.T.; Greenwood, B.N. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav. Brain Res. 2017, 323, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Taheri, P.; Keshavarzi, S.; Ebadi, M.; Motaghinejad, M.; Motevalian, M. Neuroprotective Effects of Forced Exercise and Bupropion on Chronic Methamphetamine-induced Cognitive Impairment via Modulation of cAMP Response Element-binding Protein/Brain-derived Neurotrophic Factor Signaling Pathway, Oxidative Stress, and Inflammatory Biomarkers in Rats. Adv. Biomed. Res. 2018, 7, 151. [Google Scholar] [PubMed]
- Ishii, T.; Mann, G. When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons? Neural Regen. Res. 2018, 13, 803. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Hur, W.; Gray, N.S. Small molecule modulators of antioxidant response pathway. Curr. Opin. Chem. Biol. 2011, 15, 162–173. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Lachman, M.E.; Neupert, S.D.; Bertrand, R.; Jette, A.M. The effects of strength training on memory in older adults. J. Aging Phys. Act. 2006, 14, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Gates, N.J.; Valenzuela, M.; Sachdev, P.S.; Singh, N.A.; Baune, B.T.; Brodaty, H.; Suo, C.; Jain, N.; Wilson, G.C.; Wang, Y.; et al. Study of Mental Activity and Regular Training (SMART) in at risk individuals: A randomised double blind, sham controlled, longitudinal trial. BMC Geriatr. 2011, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Mavros, Y.; Gates, N.; Wilson, G.C.; Jain, N.; Meiklejohn, J.; Brodaty, H.; Wen, W.; Singh, N.; Baune, B.T.; Suo, C.; et al. Mediation of Cognitive Function Improvements by Strength Gains After Resistance Training in Older Adults with Mild Cognitive Impairment: Outcomes of the Study of Mental and Resistance Training. J. Am. Geriatr. Soc. 2017, 65, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Donaldson, M.G.; Ahamed, Y.; Graf, P.; Cook, W.L.; Close, J.; Lord, S.R.; Khan, K.M. Otago home-based strength and balance retraining improves executive functioning in older fallers: A randomized controlled trial. J. Am. Geriatr. Soc. 2008, 56, 1821–1830. [Google Scholar] [CrossRef]
- Best, J.R.; Chiu, B.K.; Liang Hsu, C.; Nagamatsu, L.S.; Liu-Ambrose, T. Long-Term Effects of Resistance Exercise Training on Cognition and Brain Volume in Older Women: Results from a Randomized Controlled Trial. J. Int. Neuropsychol. Soc. 2015, 21, 745–756. [Google Scholar] [CrossRef]
- Yoon, D.H.; Lee, J.-Y.; Song, W. Effects of Resistance Exercise Training on Cognitive Function and Physical Performance in Cognitive Frailty: A Randomized Controlled Trial. J. Nutr. Health Aging 2018, 22, 944–951. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Lee, K.S.; Fernandes, J.; Oliveira, M.G.M.; Tufik, S.; Meeusen, R.; de Mello, M.T. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Kelley, B.J.; Petersen, R.C. Alzheimer’s disease and mild cognitive impairment. Neurol. Clin. 2007, 25, 577–609. [Google Scholar] [CrossRef]
- Yavas, E.; Gonzalez, S.; Fanselow, M.S. Interactions between the hippocampus, prefrontal cortex, and amygdala support complex learning and memory. F1000Research 2019, 8, 1292. [Google Scholar] [CrossRef]
- Cuttler, C.; Connolly, C.P.; LaFrance, E.M.; Lowry, T.M. Resist forgetting: Effects of aerobic and resistance exercise on prospective and retrospective memory. Sport. Exerc. Perform. Psychol. 2018, 7, 205–217. [Google Scholar] [CrossRef]
- Gardner, M.M.; Buchner, D.M.; Robertson, M.C.; Campbell, A.J. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001, 30, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Park, D.C.; Reuter-Lorenz, P. The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 2009, 60, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A. Aging and measures of processing speed. Biol. Psychol. 2000, 54, 35–54. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).