Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Preparation of the Flavonoids Microparticles
2.2.2. Characterization of Flavonoid Microparticles.
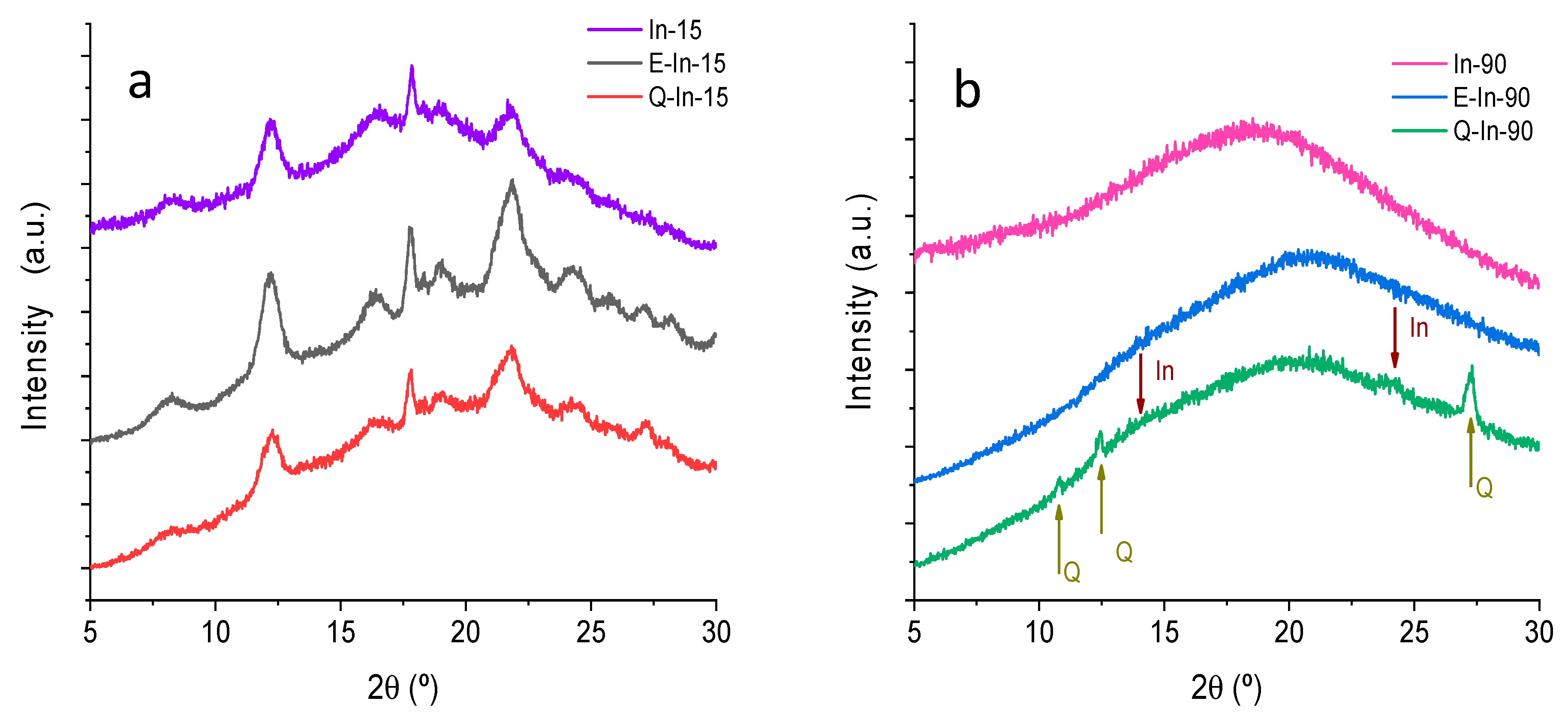
X-ray Diffraction Analysis
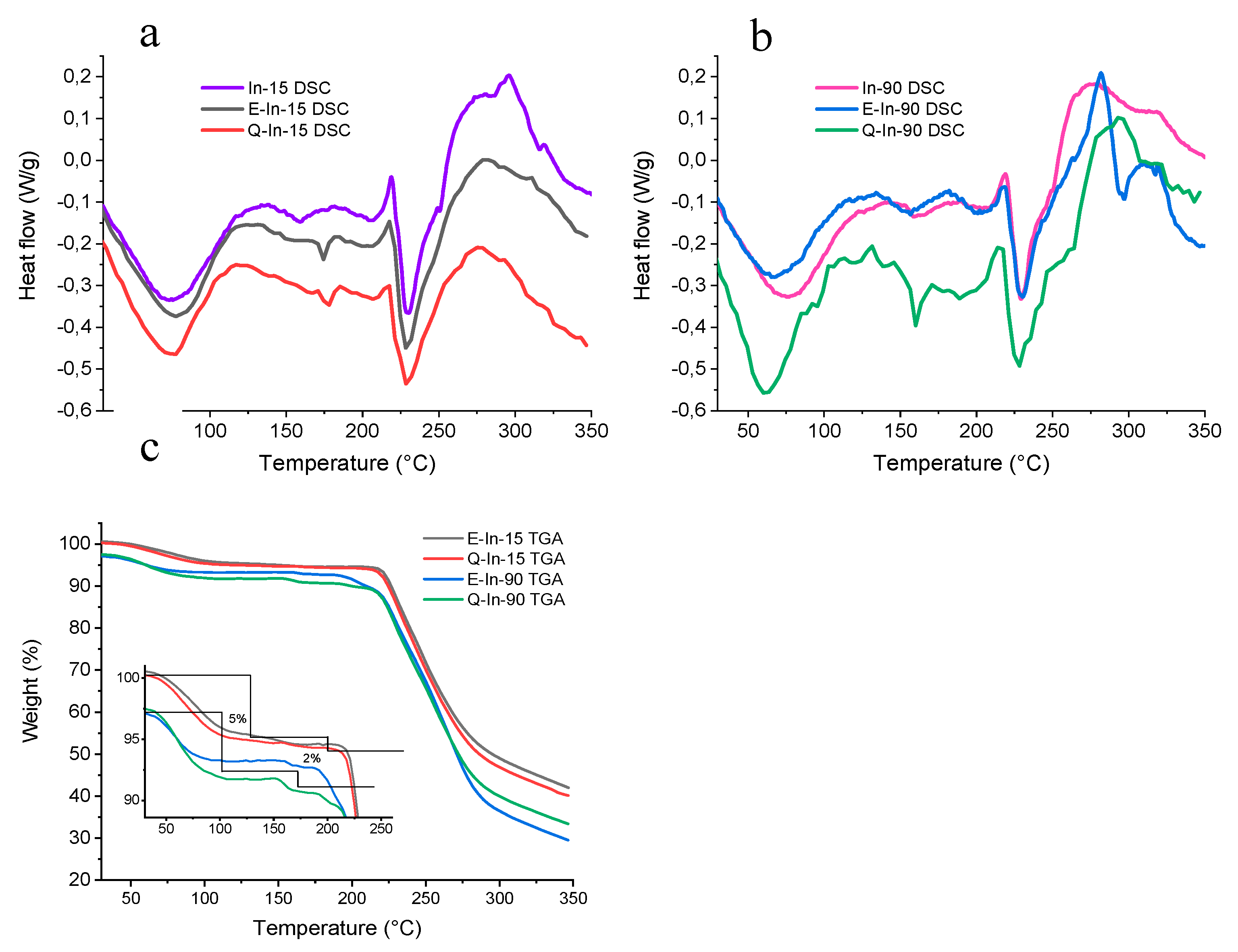
Differential Scanning Calorimetry
Encapsulation Efficiency and Recovery of Flavonoids
Moisture Content, Hygroscopicity and Water Activity (aw)
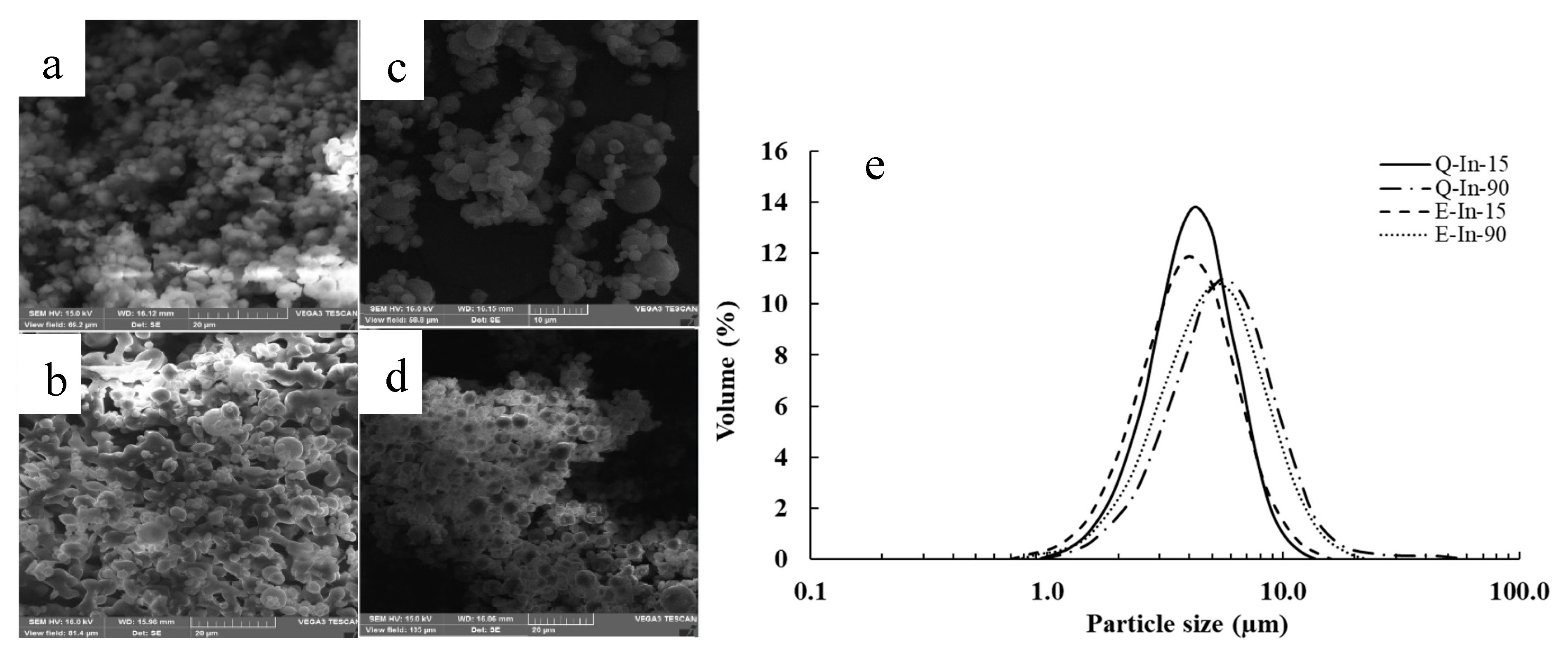
Morphology and Particle Size of the Microparticles
2.2.3. Oxidation Assays
Rancimat
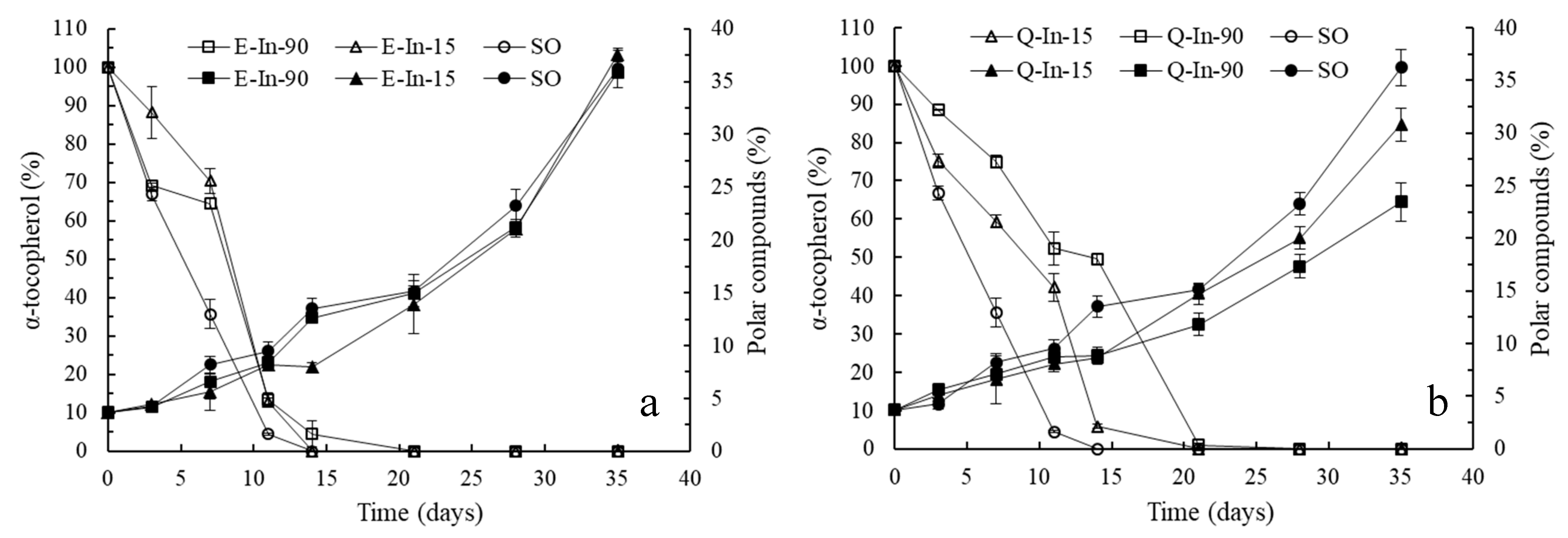
Long-Term Assays
2.2.4. Statistical Analysis
3. Results
3.1. Characterization of the Microparticles
3.1.1. X-ray Diffraction Analysis
3.1.2. Thermal Analysis (TGA/DSC)
3.1.3. Encapsulation Efficiency and Recovery of Flavonoids
3.1.4. Moisture Content, Hygroscopicity, and aw
3.1.5. Morphology and Particle Size of the Microparticles
3.2. Oxidation Assays
3.2.1. Rancimat
3.2.2. Long-Term Oxidation Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Min, D.B.; Boff, J.M. Lipid oxidation of edible oil. In Food Lipids: Chemistry, Nutrition, and Biotechnology, 2nd ed.; Min, D.B., Akoh, C.C., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 335–364. [Google Scholar]
- Ito, N.; Hiroze, M.; Fukushima, G.; Tauda, H.; Shira, T.; Tatmatsu, M. Studies on antioxidant; their carcinogenic and modifying effects on chemical carcinogenesis. Food Chem. Toxicol. 1986, 24, 1071–1082. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Pedrielli, P.; Skibstead, L. Antioxidant synergy and regeneration effect quercetin, (−)-epichatequin, and (+)-catechin on α-tocopherol in homogeneous solutions of peroxidating methyl linoleate. J. Agric. Food Chem. 2002, 50, 7138–7144. [Google Scholar] [CrossRef] [PubMed]
- Pekkarinen, S.; Heinonen, M.; Hopia, A. Flavonoids quercetin, myricetin, kaemferol and (+)-catechin as antioxidant in methyl linoleate. J. Sci. Food Agric. 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Su, Y.; Xu, J.; Ng, C.; Leung, L.; Huang, Y.; Chen, Z. Antioxidant activity of tea theaflavins and methylated catechins in canola oil. J. Am. Oil Chem. Soc. 2004, 81, 269–274. [Google Scholar] [CrossRef]
- Wanasundara, U.; Shahidi, F. Stabilization of canola oil with flavonoids. Food Chem. 1994, 50, 393–396. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Oreopoulou, V. Defining the role of flavonoid structure on cottonseed oil stabilization: Study of A- and C-ring substitution. J. Am. Oil Chem. Soc. 2007, 84, 129–136. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Sayeed, S. Effect of flavonoids on the oxidative stability of corn oil during deep frying. Int. J. Food Sci. Technol. 2008, 43, 1850–1854. [Google Scholar] [CrossRef]
- Huber, G.; Rupasinghe, H.; Shahidi, F. Inhibition of oxidation of omega-3 polyunsaturated fatty acid and fish oil by quercetin glycosides. Food Chem. 2009, 117, 290–295. [Google Scholar] [CrossRef]
- Nieto, S.; Garrido, A.; Sanhueza, J.; Loyola, L.; Morales, G.; Leighton, F.; Valenzuela, A. Flavonoids as stabilizer in fish oil: An alternative to synthetic antioxidants. J. Am. Oil Chem. Soc. 1993, 70, 773–778. [Google Scholar] [CrossRef]
- Wanasundara, U.; Shahidi, F. Stabilization of marine oils with flavonoids. J. Food Lipids 1998, 5, 183–186. [Google Scholar] [CrossRef]
- Wu, T.; Yen, F.; Lin, L.; Tsai, T.; Lin, C.; Cham, T. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int. J. Pharmacol. 2008, 346, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S.; Labbett, D.; Emin, M.; Konczak, I.; Lundin, L. Delivering polyphenols for healthy ageing. Nutr. Diet. 2008, 65, S48–S52. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2017, 59, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kosaraju, S. Colon targeted delivery systems: Review of polysaccharides for encapsulation and delivery. Crit. Rev. Food Sci. Nutr. 2005, 45, 251–258. [Google Scholar] [CrossRef]
- Robert, P.; Gorena, T.; Romero, N.; Sepulveda, E.; Chávez, J.; Saenz, C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int. J. Food Sci. Technol. 2010, 45, 1386–1394. [Google Scholar] [CrossRef]
- Deng, Z.Y.; Yu, Y. Process for producing oil-soluble microencapsulated tea polyphenols. Fine Spec. Chem. 2004, 16, 8–15. [Google Scholar]
- Wu, C.; Chen, L.; Li, W. Preparation of microencapsulation quercetin and its antioxidative properties in oils. Food Ind. 2005, 3, 27–29. [Google Scholar]
- Palma, M.; García, P.; Márquez-Ruiz, G.; Vergara, C.; Robert, P. Release kinetics of flavonoids in methyl linoleate from microparticles designed with inulin and channelizing agent. Food Res. Int. 2014, 64, 99–105. [Google Scholar] [CrossRef][Green Version]
- Morelo, G.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Robert, P. Design of flavonoid microparticles with channel forming properties to improve oxidative stability of sunflower oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1700041. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Deroanne, C.; Paquot, M.; Fougnies, C.; Lambrechts, J.; Blecker, C. Characterization of the physical state of spray-dried Inulin. Food Biophys. 2007, 2, 83–92. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Paquot, M.; Deroanne, C.; Fougnies, C.; Blecker, C.S. Effect of water uptake on amorphous inulin properties. Food Hydrocoll. 2009, 23, 922–927. [Google Scholar] [CrossRef]
- Ronkart, S.N.; Deroanne, C.; Paquot, M.; Fougnies, C.; Blecker, C.S. Impact of the crystallisation pathway of inulin on its mono-hydrate to hemi-hydrate thermal transition. Food Chem. 2010, 119, 317–322. [Google Scholar] [CrossRef]
- Ahmed, I.; Khan Niazi, M.B.; Jahan, Z.; Naqvi, S.R. Effect of drying parameters on the physical, morphological and thermal properties of spray dried inulin. J. Polym. Eng. 2018, 38, 775–783. [Google Scholar] [CrossRef]
- Xu, H.; Ince, B.S.; Cebe, P. Development of the crystallinity and rigid amorphous fraction in cold-crystallized isotactic polystyrene. J. Polym. Sci. B 2003, 41, 3026–3036. [Google Scholar] [CrossRef]
- Blundell, D.J.; Osborn, B.N. The morphology of poly(aryl-ether-ether-ketone). Polymer 1983, 24, 953–958. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 16th ed.; AOAC: Gaythersburg, MD, USA, 1996. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1245–1252. [Google Scholar] [CrossRef]
- AOCS. Oil stability index. In Official Methods and Recommended Practices of American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1994; Method Cd 12b-92. [Google Scholar]
- IUPAC. Standard Method 2.507: Determination of Polar Compounds in Frying Fats. In Standard Methods for the Analysis of Oils, Fats and Derivatives, 7th ed.; International Union of Pure and Applied Chemistry, Pergamon Press: Oxford, UK, 1992. [Google Scholar]
- Encina, C.; Márquez-Ruiz, G.; Holgado, F.; Giménez, B.; Vergara, C.; Robert, P. Effect of spray-drying with organic solvents on the encapsulation, release and stability of fish oil. Food Chem. 2018, 263, 283–291. [Google Scholar] [CrossRef]
- AOCS. Method Ce 8–89: Determination of tocopherols and Tocotrienols in Vegetable Oils and Fats by HPLC. In Official Methods and Recommended Practices of American Oil Chemists’ Society, 4th ed.; AOCS Press: Champaign, IL, USA, 1993. [Google Scholar]
- André, I.; Putaux, J.L.; Chanzy, H.; Taravel, F.R.; Timmermans, J.W.; de Wit, D. Single crystals of inulin. Int. J. Biol. Macromol. 1996, 18, 195–204. [Google Scholar] [CrossRef]
- Hazra, M.; Mandal, D.D.; Mandal, T.; Bhuniya, S.; Ghosh, M. Designing polymeric microparticulate drug delivery system for hydrophobic drug quercetin. Saudi Pharm. J. 2015, 23, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Tanwar, Y.S.; Singh, D.; Rawat, M.S.M. Phosphatidylcholine complex in improving oral drug delivery of epicatechin: Preparation and characterization. BDDD 2014, 1, 46–55. [Google Scholar]
- Moreira da Costa, E.; Barbosa Filho, J.M.; Gomes do Nascimento, T.; Oliveira Macêdo, R. Thermal characterization of the quercetin and rutin flavonoids. Thermochim. Acta 2002, 392–393, 79–84. [Google Scholar] [CrossRef]
- Hébette, C.L.M.; Delcour, J.A.; Koch, M.H.J.; Booten, K.; Kleppinger, R.; Mischenkod, N.; Reynaers, H. Complex melting of semi-crystalline chicory (Cichorium intybus L.) root inulin. Carbohydr. Res. 1998, 310, 65–75. [Google Scholar] [CrossRef]
- Bohm, A.; Kaiser, I.; Trebstein, A.; Henle, T. Heat-induced degradation of inulin. Eur. Food Res. Technol. 2005, 220, 466–471. [Google Scholar] [CrossRef]
- Sansone, F.; Picerno, P.; Mencherini, T.; Villeco, F.; D’Ursi, A.M.; Aquino, R.P.; Lauro, M.R. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J. Food Eng. 2011, 103, 188–196. [Google Scholar] [CrossRef]
- Cuvelier, M.; Bondet, V.; Berset, C. Behavior of phenolic antioxidants in a partitioned medium: Structure–activity relationship. J. Am. Oil Chem. Soc. 2000, 77, 819–824. [Google Scholar] [CrossRef]
- Atala, E.; Fuentes, J.; Wehrhahn, M.J.; Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 2017, 234, 479–485. [Google Scholar] [CrossRef]
- Martín-Polvillo, M.; Márquez-Ruiz, G.; Dobarganes, M.C. Oxidative stability of sunflower oils differing in unsaturation degree during long-term storage at room temperature. J. Am. Oil Chem. Soc. 2004, 81, 577–583. [Google Scholar] [CrossRef]
- Marinova, E.; Toneva, A.; Yanishlieva, N. Synergistic antioxidant effect of α-tocopherol and myricetin on the autoxidation of triacylglycerols of sunflower oil. Food Chem. 2008, 106, 628–633. [Google Scholar] [CrossRef]
- Zhou, B.; Jia, Z.; Chen, Z.; Yang, L.; Wu, L.; Liu, Z. Synergistic antioxidant effect of green tea polyphenols with α-tocopherol on free radical initiated peroxidation of linoleic acid in micelles. J. Chem. Soc. Perkin Trans. 2 2000, 4, 785–791. [Google Scholar] [CrossRef]
- Pazos, M.; Andersen, M.; Medina, I.; Skibsted, L. Efficiency of natural phenolic compounds regenerating α-tocopherol from α-tocopheroxyl radical. J. Agric. Food Chem. 2007, 55, 3661–3666. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ruiz, G.; Dobarganes, M.C. Analysis of non-volatile lipid oxidation compounds by high-performance size-exclusion chromatography. In Analysis of Lipid Oxidation; Kamal-Eldin, A., Pokorny, J., Eds.; AOCS Press: Champaign, IL, USA, 2005; pp. 40–69. [Google Scholar]
- Palma, M.; Márquez-Ruiz, G.; García, P.; Holgado, F.; Vergara, C.; Giménez, B.; Robert, P. Effect of spray-dried flavonoid microparticles on oxidative stability of methyl linoleate as lipid model system. J. Am. Oil Chem. Soc. 2017, 94, 99–105. [Google Scholar] [CrossRef]
| Parameters | In-15 | In-90 | E-In-15 | E-In-90 | Q-In-15 | Q-In-90 |
|---|---|---|---|---|---|---|
| F/In ratio | - | - | 1:41 | 1:41 | 1:43 | 1:43 |
| Infeed temperature (°C) | 15 ± 1 | 90 ± 2 | 15 ± 1 | 90 ± 2 | 15 ± 1 | 90 ± 2 |
| Inlet temperature (°C) | 160 | 160 | 160 | 160 | 160 | 160 |
| Crystallinity index (%) | 55.7 | 18.6 | 61.2 | 1.73 | 60.0 | 2.30 |
| EE Flavonoids (%) | - | - | 68.8 ± 1.0 c | 41.6 ± 0.7 a | 67.8 ± 1.9 c | 51.1 ± 2.8 b |
| R Flavonoids (%) | - | - | 82.0 ± 2.0 c | 79.1 ± 0.5 b,c | 75.7 ± 1.9 a | 78.1 ± 1.0 b |
| Total flavonoids (mg/g) | - | - | 19.5 ± 0.48 b | 18.8 ± 0.1 b | 17.2 ± 0.1 a | 17.7 ± 0.2 a |
| Moisture (%) | 5.7 ± 0.3 a | 8.5 ± 0.1 c | 6.2 ± 0.1 a | 7.2 ± 0.1 b | 6.3 ± 0.1 a | 7.0 ± 0.3 b |
| Water activity (aw) | 0.15 ± 0.03 a | 0.13 ± 0.0 a | 0.22 ± 0.1 b | 0.27 ± 0.01 c | 0.27 ± 0.1 c | 0.42 ± 0.1 d |
| Hygroscopicity (g/100g) | 25.0 ± 0.1 a | 46.7 ± 1.6 b | 25.4 ± 0.2 a | 47.0 ± 0.2 b | 24.9 ± 0.5 a | 47.4 ± 0.5 b |
| D(4,3) (μm) | - | - | 4.4 ± 0.01 a | 5.6 ± 0.05 b | 4.4 ± 0.03 a | 6.1 ± 0.4 c |
| Systems | Induction Period (IP) (h) | Protection Factor (PF) |
|---|---|---|
| ML | 10.7 ± 0.2 a | - |
| ML+E-In-15 | 12.0 ± 1.6 a,b | 1.12 |
| ML+E-In-90 | 12.7 ± 0.1 b | 1.19 |
| ML+Q-In-15 | 97.5 ± 2.2 c | 9.11 |
| ML+Q-In-90 | 144.1 ± 1.5 d | 13.4 |
| SO | 175.3 ± 6.6 a | - |
| SO+E-In-15 | 225.4 ± 4.3 b | 1.28 |
| SO+E-In-90 | 235.6 ± 4.4 b,c | 1.34 |
| SO+Q-In-15 | 249.2 ± 7.3 c | 1.42 |
| SO+Q-In-90 | 294.0 ± 1.6 d | 1.68 |
| Systems | Days | OxTM (%) | TD (%) | TP (%) | DG (%) | FFA (%) |
|---|---|---|---|---|---|---|
| SO | 0 | 3.0 ± 1.0 a,b | 0.2 ± 0.06 a | 0.0 ± 0.01 a | 0.9 ± 0.42 a | 0.2 ± 0.12 a |
| SO+E-In-15 | 0 | 3.0 ± 0.03 a,b | 0.2 ± 0.01 a,b | 0.04 ± 0.01 a,b | 0.9 ± 0.05 a | 0.2± 0.01 a |
| SO+E-In-90 | 0 | 2.5 ± 0.01 a | 0.2 ± 0.05 a,b | 0.1 ± 0.01 a | 1.1 ± 0.07 a | 0.2 ± 0.02 a |
| SO+Q-In-15 | 0 | 3.2 ± 0.1 a,b | 0.3 ± 0.01 b | 0.1 ± 0.01 b | 1.1 ± 0.1 a | 0.3 ± 0.01 a |
| SO+Q-In-90 | 0 | 3.8 ± 0.6 a,b | 0.3 ± 0.06 b | 0.1 ± 0.02 a,b | 1.2 ± 0.28 a | 0.3 ± 0.1 a |
| SO | 35 | 16.3 ± 0.6 a | 10.1 ± 0.4 b | 8.2 ± 0.3 b | 1.22 ± 0.4 a | 0.33 ± 0.0 a |
| SO+E-In-15 | 35 | 14.4 ± 0.5 a | 9.9 ± 0.07 b | 10.8 ± 0.1 d | 1.0 ± 0.02 a | 0.2 ± 0.01 a |
| SO+E-In-90 | 35 | 16.7 ± 0.8 a | 9.8 ± 0.2 b | 8.0 ± 1.2 c | 1.1 ± 0.06 a | 0.3 ± 0.05 a |
| SO+Q-In-15 | 35 | 16.5 ± 1.5 a | 7.7 ± 1.6 b | 5.3 ± 1.6 b | 1.0 ± 0.05 a | 0.3 ± 0.12 a |
| SO+Q-In-90 | 35 | 15.8 ± 2.0 a | 4.4 ± 2.1 a | 1.9 ± 1.0 a | 1.0 ± 0.1 a | 0.3 ± 0.10 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelo, G.; Giménez, B.; Márquez-Ruiz, G.; Holgado, F.; Romero-Hasler, P.; Soto-Bustamante, E.; Robert, P. Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices. Antioxidants 2019, 8, 520. https://doi.org/10.3390/antiox8110520
Morelo G, Giménez B, Márquez-Ruiz G, Holgado F, Romero-Hasler P, Soto-Bustamante E, Robert P. Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices. Antioxidants. 2019; 8(11):520. https://doi.org/10.3390/antiox8110520
Chicago/Turabian StyleMorelo, Guibeth, Begoña Giménez, Gloria Márquez-Ruiz, Francisca Holgado, Patricio Romero-Hasler, Eduardo Soto-Bustamante, and Paz Robert. 2019. "Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices" Antioxidants 8, no. 11: 520. https://doi.org/10.3390/antiox8110520
APA StyleMorelo, G., Giménez, B., Márquez-Ruiz, G., Holgado, F., Romero-Hasler, P., Soto-Bustamante, E., & Robert, P. (2019). Influence of the Physical State of Spray-Dried Flavonoid-Inulin Microparticles on Oxidative Stability of Lipid Matrices. Antioxidants, 8(11), 520. https://doi.org/10.3390/antiox8110520





