Potential Application of Prunus armeniaca L. and P. domestica L. Leaf Essential Oils as Antioxidant and of Cholinesterases Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Essential Oils’ Preparation
2.3. Gas Chromatography (GC-FID) and Gas Chromatography-Mass Spectrometry (GC-MS) Analyses
2.4. In Vitro Antioxidant Activity (DPPH, ABTS, β-Carotene Bleaching Tests)
2.5. RACI Determination
2.6. Bioassay for Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibitory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Essential Oils
3.2. Bioactivities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sanabria-Castro, A.; Alvarado-Echeverria, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Kokjohn, T.A.; Clarke, S.G.; Sierks, M.R.; Maarouf, C.L.; Serrano, G.E.; Sabbagh, M.S.; Beach, T.G. APP/Aβ structural diversity and Alzheimer’s disease pathogenesis. Neurochem. Int. 2017, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Fotiou, D.; Kaltsatou, A.; Tsiptsios, D.; Nakou, M. Evaluation of the cholinergic hypothesis in Alzheimer’s disease with neuropsychological methods. Aging Clin. Exp. Res. 2015, 27, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Bonesi, M.; Menichini, F.; Loizzo, M.R. Recent knowledge on medicinal plants as source of cholinesterase inhibitors for the treatment of dementia. Mini Rev. Med. Chem. 2016, 16, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of AD. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Oniszczuk, A.; Oniszczuk, T.; Waksmundzka-Hajnos, M. The influence of common free radicals and antioxidants on development of Alzheimer’s disease. Biomed. Pharmacother. 2016, 78, 39–49. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Dobetsberger, C.; Buchbauer, G. Actions of essential oils on the central nervous system: An updated review. Flavour Fragr. J. 2011, 26, 300–316. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enz. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Menichini, F.; Tundis, R.; Bonesi, M.; Conforti, F.; Nadjafi, F.; Statti, G.A.; Frega, N.G.; Menichini, F. In vitro biological activity of Salvia leriifolia Benth essential oil relevant to the treatment of Alzheimer’s disease. J. Oleo Sci. 2009, 58, 443–446. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Ben Jemia, M.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Mastellone, V.; Colica, C.; Menichini, F. Comparative study on the antioxidant capacity and cholinesterase inhibitory activity of Citrus aurantifolia Swingle, C. aurantium L. and C. bergamia Risso & Poit peel essential oils. J. Food Sci. 2012, 71, H40–H46. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Bonesi, M.; Menichini, F.; De Luca, D.; Colica, C.; Menichini, F. Evaluation of Citrus aurantifolia peel and leaves extracts for their chemical composition, antioxidant and anti-cholinesterase activities. J. Sci. Food Agric. 2012, 92, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Veličković, D.T.; Ristić, M.S.; Karabegović, I.T.; Stojičević, S.S.; Nikolić, N.Č.; Lazić, M.L. Plum (Prunus domestica) and walnut (Juglans regia): Volatiles and fatty oils. Adv. Technol. 2016, 5, 10–16. [Google Scholar]
- Solís-Solís, H.M.; Calderón-Santoyo, M.; Schorr-Galindo, S.; Luna-Solano, G.; Ragazzo-Sánchez, J.A. Characterization of aroma potential of apricot varieties using different extraction techniques. Food Chem. 2007, 105, 829–837. [Google Scholar] [CrossRef]
- Göğüşs, F.; Özel, M.Z.; Lewis, A.C. The effect of various drying techniques on apricot volatiles analysed using direct thermal desorption-GC-TOF/MS. Talanta 2007, 73, 321–325. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.S.; Mc Donald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- De Menezes Patrício Santos, C.C.; Stiebbe Salvadori, M.; Gomes Mota, V.; Muratori Costa, L.; Antonia Cardoso de Almeida, A.; Lopes de Oliveira, G.A.; Pereira Costa, J.; Pergentino de Sousa, D.; Mendes de Freitas, R.; Nóbrega de Almeida, R. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 949452. [Google Scholar]
- Syad, A.N.; Rajamohamed, B.S.; Shunmugaiah, K.P.; Kasi, P.D. Neuroprotective effect of the marine macroalga Gelidiella acerosa: Identification of active compounds through bioactivity-guided fractionation. Pharm. Biol. 2016, 54, 2073–2081. [Google Scholar] [CrossRef]
- Perry, N.S.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, M.; Watanabe, H.; Umemoto, K.; Kameoka, K. Inhibition of acetylcholinesterase activity by essential oils of Mentha Species. J. Agric. Food Chem. 1998, 46, 3431–3434. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Loizzo, M.R.; Bonesi, M.; Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Acetylcholinesterase and butyrylcholinesterase inhibition of ethanolic extract and monoterpenes from Pimpinella anisoides V Brig. (Apiaceae). Fitoterapia 2009, 80, 297–300. [Google Scholar] [CrossRef] [PubMed]
| Compound | RI a | Relative Amount (%) | |||||
|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | ||
| (E)-2-Hexenal | 854 | 3.54 ± 0.21 | 4.44 ± 0.42 | 4.87 ± 0.40 | - | - | - |
| α-Pinene | 938 | 0.64 ± 0.01 | 0.58 ± 0.02 | 0.42 ± 0.06 | 0.23 ± 0.02 | 0.18 ± 0.01 | 0.16 ± 0.01 |
| Benzaldehyde | 963 | - | - | - | 5.66 ± 0.3 | 5.06 ± 0.20 | 4.32 ± 0.12 |
| β-Pinene | 980 | 0.76 ± 0.02 | 0.77 ± 0.02 | 0.65 ± 0.01 | tr | tr | tr |
| Limonene | 1030 | 2.54 ± 0.03 | 2.44 ± 0.02 | 2.87 ± 0.05 | - | - | - |
| Linalool | 1098 | 4.54 ± 0.01 | 4.44 ± 0.05 | 4.81 ± 0.02 | - | - | - |
| p-Mentha-2,4(8)-diene | 1142 | - | - | - | tr | tr | 2.01 ± 0.32 |
| trans-Caryophyllene | 1415 | 0.20 ± 0.01 | 0.18 ± 0.02 | 0.15 ± 0.01 | 0.13 ± 0.01 | 0.15 ± 0.02 | 0.16 ± 0.03 |
| trans-β-Farnesene | 1441 | - | - | - | tr | tr | 2.13 ± 0.22 |
| (E)-β-Ionone | 1484 | 1.22 ± 0.11 | 1.12 ± 0.1 | 1.14 ± 0.50 | - | - | - |
| γ-Cadinene | 1515 | 4.76 ± 0.80 | 0.67 ± 0.03 | 0.70 ± 0.02 | 0.71 ± 0.04 | tr | tr |
| δ-Cadinene | 1526 | 4.73 ± 0.75 | 0.78 ± 0.07 | 0.56 ± 0.03 | 0.42 ± 0.02 | tr | tr |
| Tetradecanoic acid | 1768 | - | - | - | 1.52 ± 0.5 | 1.45 ± 0.14 | 1.40 ± 0.22 |
| (Z)-Phytol | 1950 | 19.92 ± 1.52 | 19.88 ± 2.1 | 19.42 ± 1.84 | 25.83 ± 2.6 | 12.81 ± 1.4 | 9.36 ± 1.10 |
| Hexadecanoic acid | 1969 | - | - | - | 7.67 ± 0.84 | 7.58 ± 1.23 | 6.33 ± 1.20 |
| Manoyl oxide | 1989 | 6.53 ± 1.10 | 5.45 ± 1.13 | 5.21 ± 1.23 | - | - | - |
| Tricosane | 2300 | 2.30 ± 0.34 | 2.30 ± 0.55 | 2.39 ± 0.42 | 6.14 ± 0.02 | 6.33 ± 0.20 | 8.55 ± 0.92 |
| Tetracosane | 2400 | 1.97 ± 0.21 | 2.02 ± 0.11 | 2.06 ± 0.20 | 9.13 ± 1.6 | 8.88 ± 0.02 | 4.82 ± 0.94 |
| Pentacosane | 2500 | 7.39 ± 0.64 | 2.28 ± 0.20 | 3.93 ± 0.31 | 16.83 ± 1.3 | 16.30 ± 0.02 | 15.78 ± 1.7 |
| Heptacosane | 2700 | 10.14 ± 1.21 | 10.45 ± 1.7 | 11.61 ± 1.45 | 8.94 ± 0.20 | 4.58 ± 0.40 | 3.99 ± 0.23 |
| Nonacosane | 2900 | 21.76 ± 1.53 | 21.25 ± 1.1 | 21.11 ± 1.42 | 11.08 ± 1.60 | 3.86 ± 0.63 | 3.68 ± 0.42 |
| Triacontane | 3000 | - | - | - | 2.21 ± 0.26 | 2.11 ± 0.52 | 5.06 ± 0.71 |
| Entriacontane | 3100 | - | - | - | - | - | 4.06 ± 0.31 |
| Essential oil | DPPH Test | ABTS Test | β-Carotene-Bleaching Test | RACI | |
|---|---|---|---|---|---|
| P. armeniaca | |||||
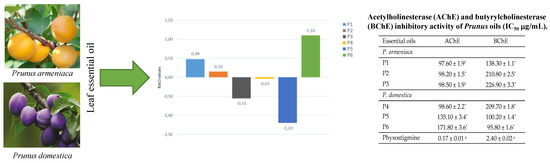
| P1 | 83.86 ± 1.25 * | 0.47 ± 0.07 * | 28.33 ± 0.48 * | 20.73 ± 0.54 * | 0.48 |
| P2 | 105.76 ± 2.81 * | 0.54 ± 0.06 * | 23.05 ± 0.65 * | 18.55 ± 0.57 * | 0.16 |
| P3 | 80.05 ± 2.44 * | 0.57 ± 0.04 * | 22.64 ± 0.46 * | 17.30 ± 0.47 * | −0.55 |
| P. domestica | |||||
| P4 | 88.33 ± 1.90 * | 0.45 ± 0.07 * | 14.75 ± 0.56 * | 16.19 ± 0.54 * | −0.03 |
| P5 | 73.78 ± 1.64 * | 0.48 ± 0.05 * | 14.68 ± 0.51 * | 13.07 ± 0.70 * | −1.20 |
| P6 | 103.32 ± 3.26 * | 0.50 ± 0.04 * | 11.15 ± 0.43 * | 11.39 ± 0.53 * | 1.10 |
| Positive control | |||||
| Ascorbic acid | 5.01 ± 0.83 | 1.70 ± 0.11 | |||
| Propyl gallate | 1.0 ± 0.04 | 1.0 ± 0.03 | |||
| Essential Oils | AChE | BChE |
|---|---|---|
| P. armeniaca | ||
| P1 | 97.60 ± 1.94 * | 138.30 ± 1.13 * |
| P2 | 98.20 ± 1.51 * | 210.80 ± 2.50 * |
| P3 | 98.50 ± 1.96 * | 226.90 ± 3.31 * |
| P. domestica | ||
| P4 | 98.60 ± 2.20 * | 209.70 ± 1.82 * |
| P5 | 135.10 ± 3.45 * | 100.20 ± 1.42 * |
| P6 | 171.80 ± 3.63 * | 95.80 ± 1.60 * |
| Positive control | ||
| Physostigmine | 0.17 ± 0.01 | 2.40 ± 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonesi, M.; Tenuta, M.C.; Loizzo, M.R.; Sicari, V.; Tundis, R. Potential Application of Prunus armeniaca L. and P. domestica L. Leaf Essential Oils as Antioxidant and of Cholinesterases Inhibitors. Antioxidants 2019, 8, 2. https://doi.org/10.3390/antiox8010002
Bonesi M, Tenuta MC, Loizzo MR, Sicari V, Tundis R. Potential Application of Prunus armeniaca L. and P. domestica L. Leaf Essential Oils as Antioxidant and of Cholinesterases Inhibitors. Antioxidants. 2019; 8(1):2. https://doi.org/10.3390/antiox8010002
Chicago/Turabian StyleBonesi, Marco, Maria Concetta Tenuta, Monica R. Loizzo, Vincenzo Sicari, and Rosa Tundis. 2019. "Potential Application of Prunus armeniaca L. and P. domestica L. Leaf Essential Oils as Antioxidant and of Cholinesterases Inhibitors" Antioxidants 8, no. 1: 2. https://doi.org/10.3390/antiox8010002
APA StyleBonesi, M., Tenuta, M. C., Loizzo, M. R., Sicari, V., & Tundis, R. (2019). Potential Application of Prunus armeniaca L. and P. domestica L. Leaf Essential Oils as Antioxidant and of Cholinesterases Inhibitors. Antioxidants, 8(1), 2. https://doi.org/10.3390/antiox8010002