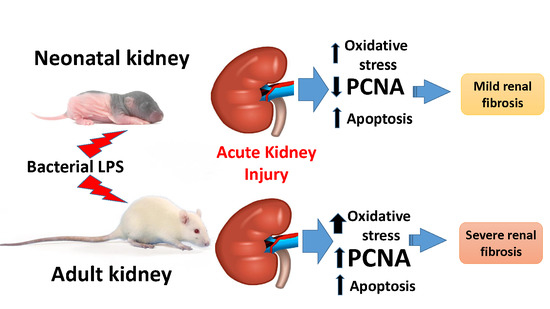
Mechanisms of LPS-Induced Acute Kidney Injury in Neonatal and Adult Rats
Abstract
:1. Introduction
2. Materials and Methods
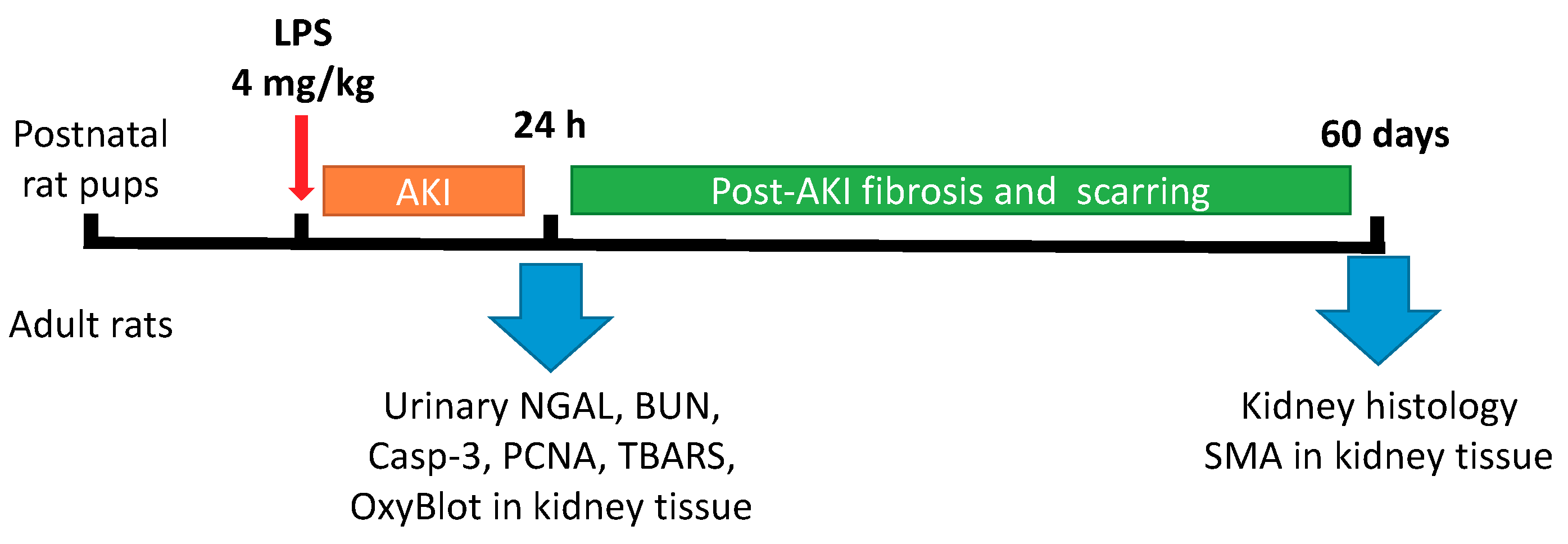
2.1. Animal Experiments
2.2. Western Blotting
2.3. Renal Histology
2.4. Measurement Thiobarbituric Acid-Reactive Substances (TBARS)
2.5. Statistics
3. Results
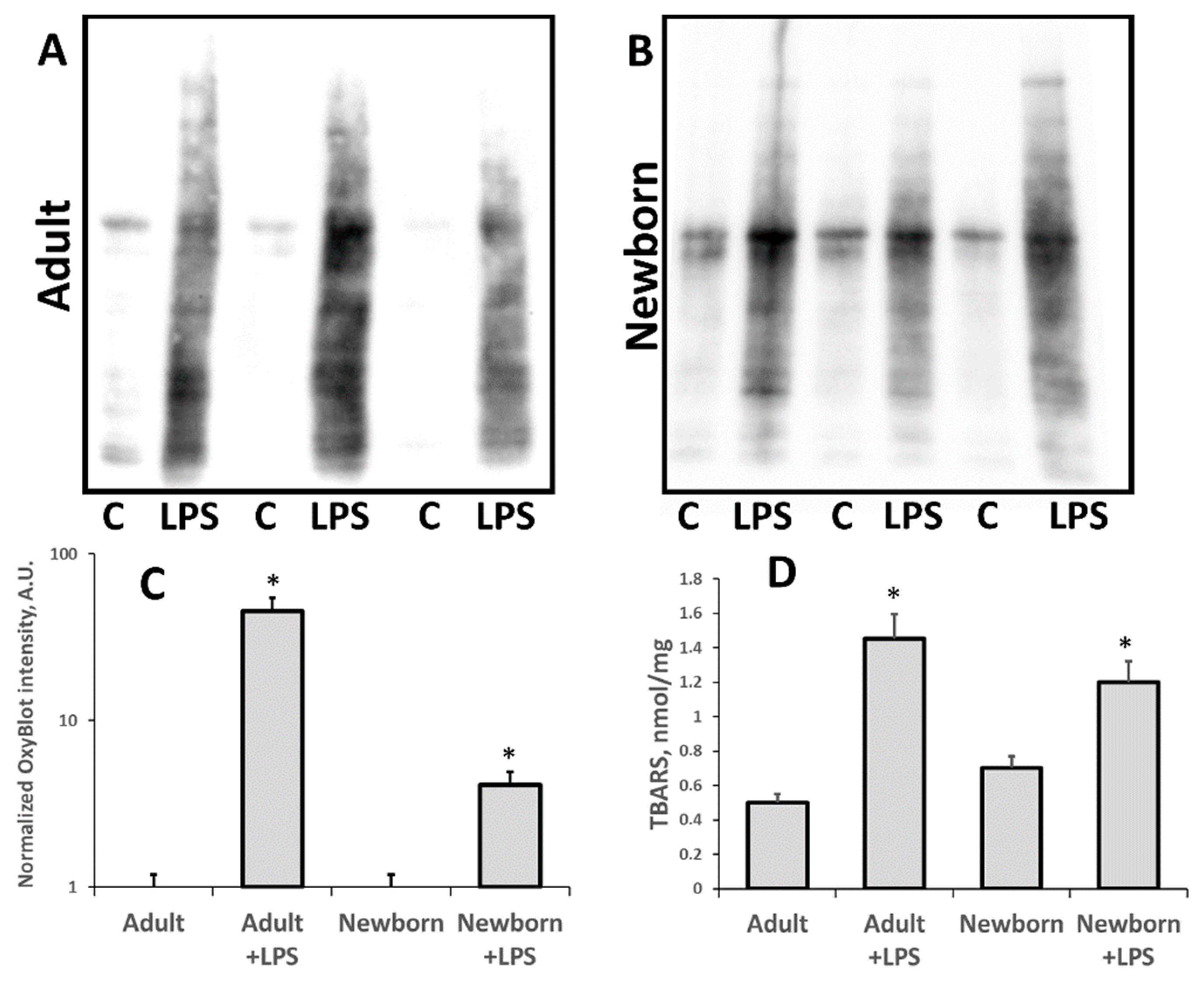
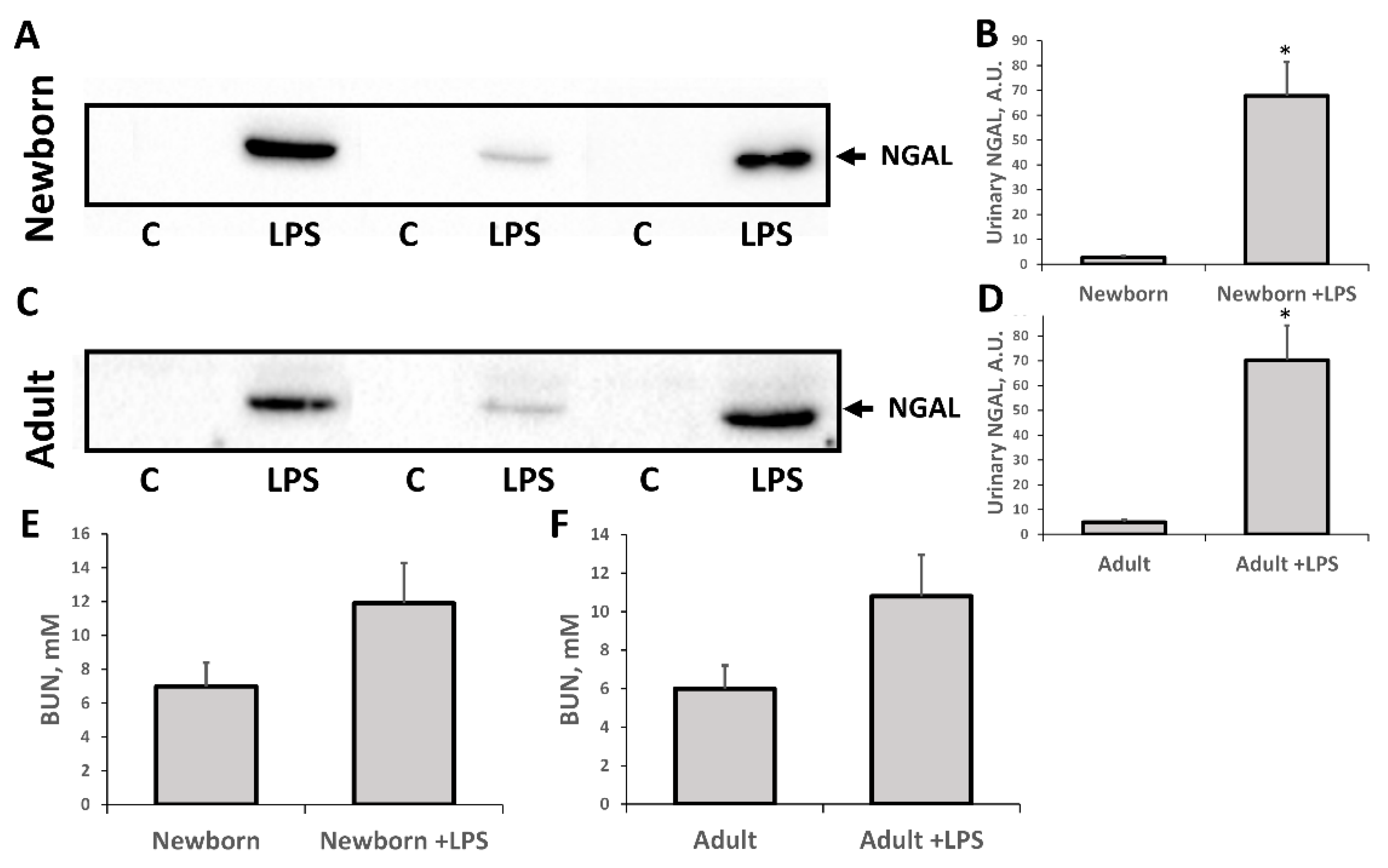
3.1. Oxidative Stress in Kidneys of Neonatal and Adult Rats
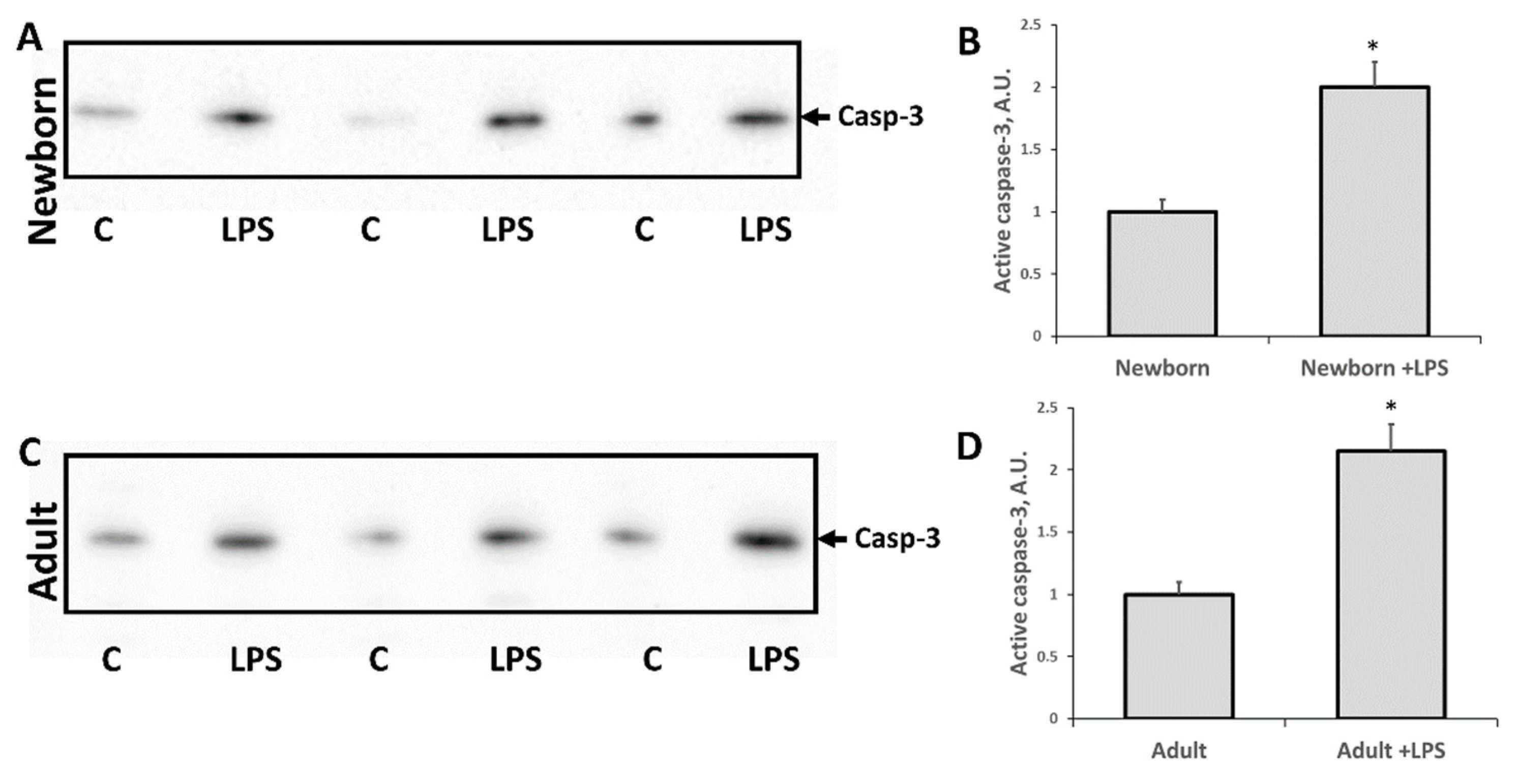
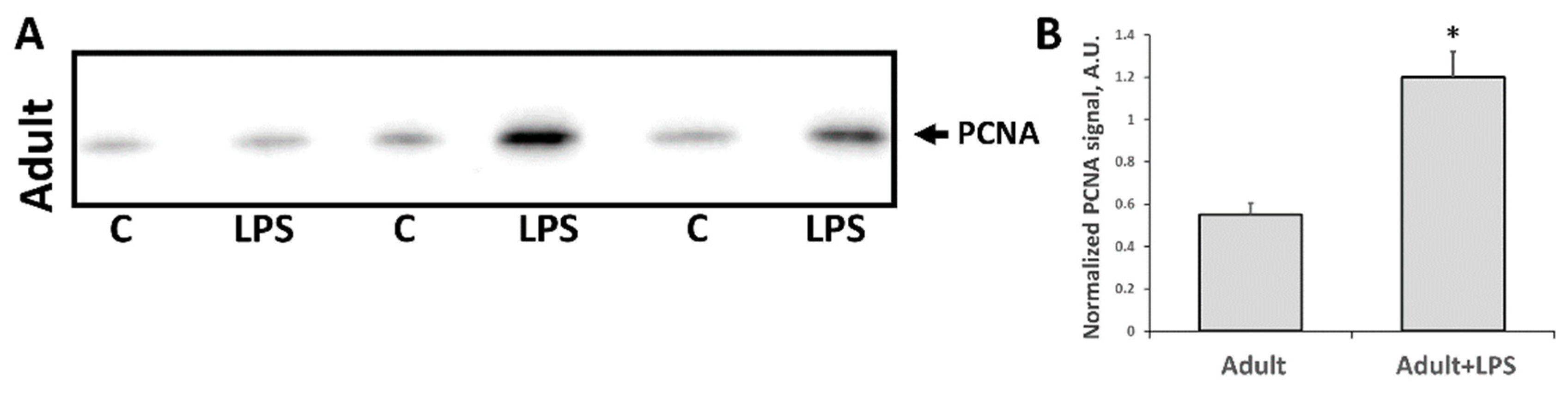
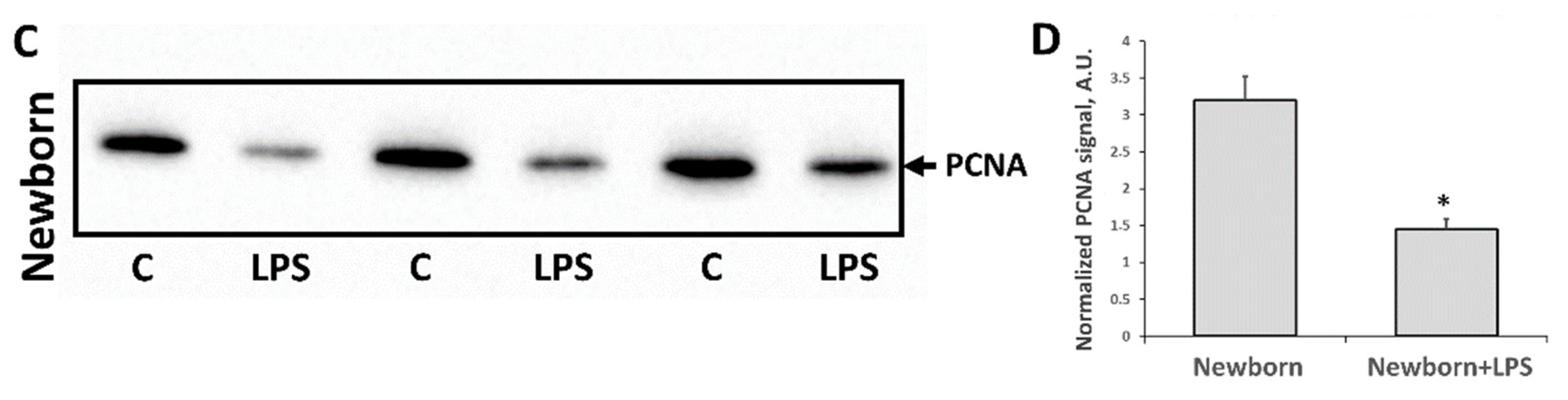
3.2. Changes Associated with LPS-Mediated Oxidative Stress
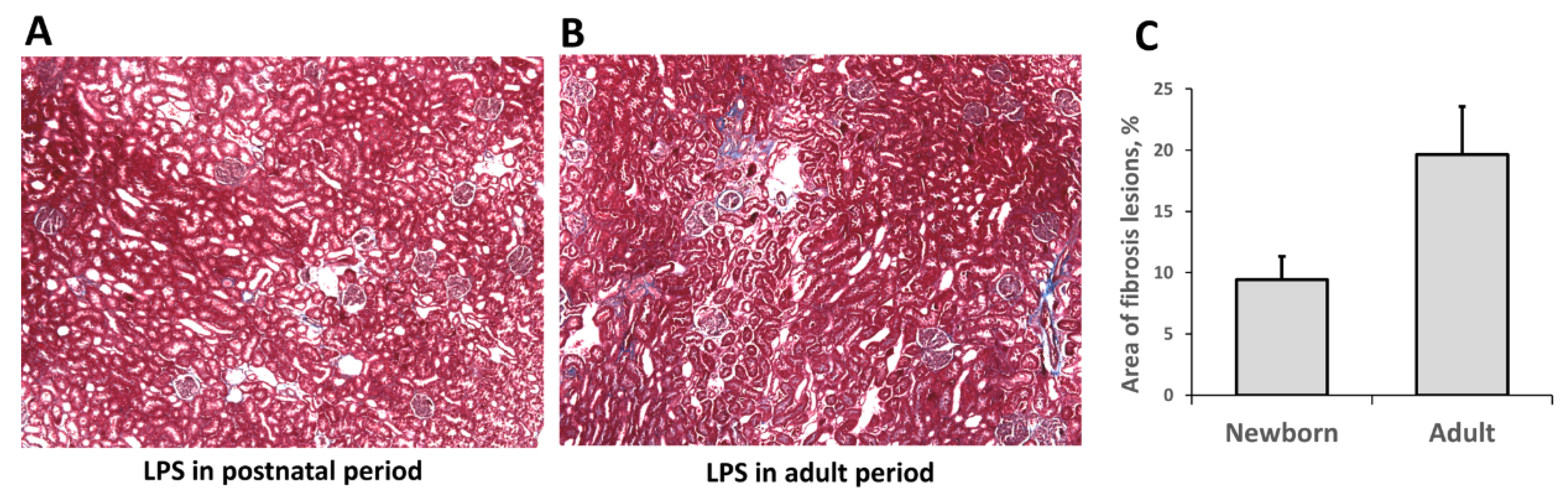
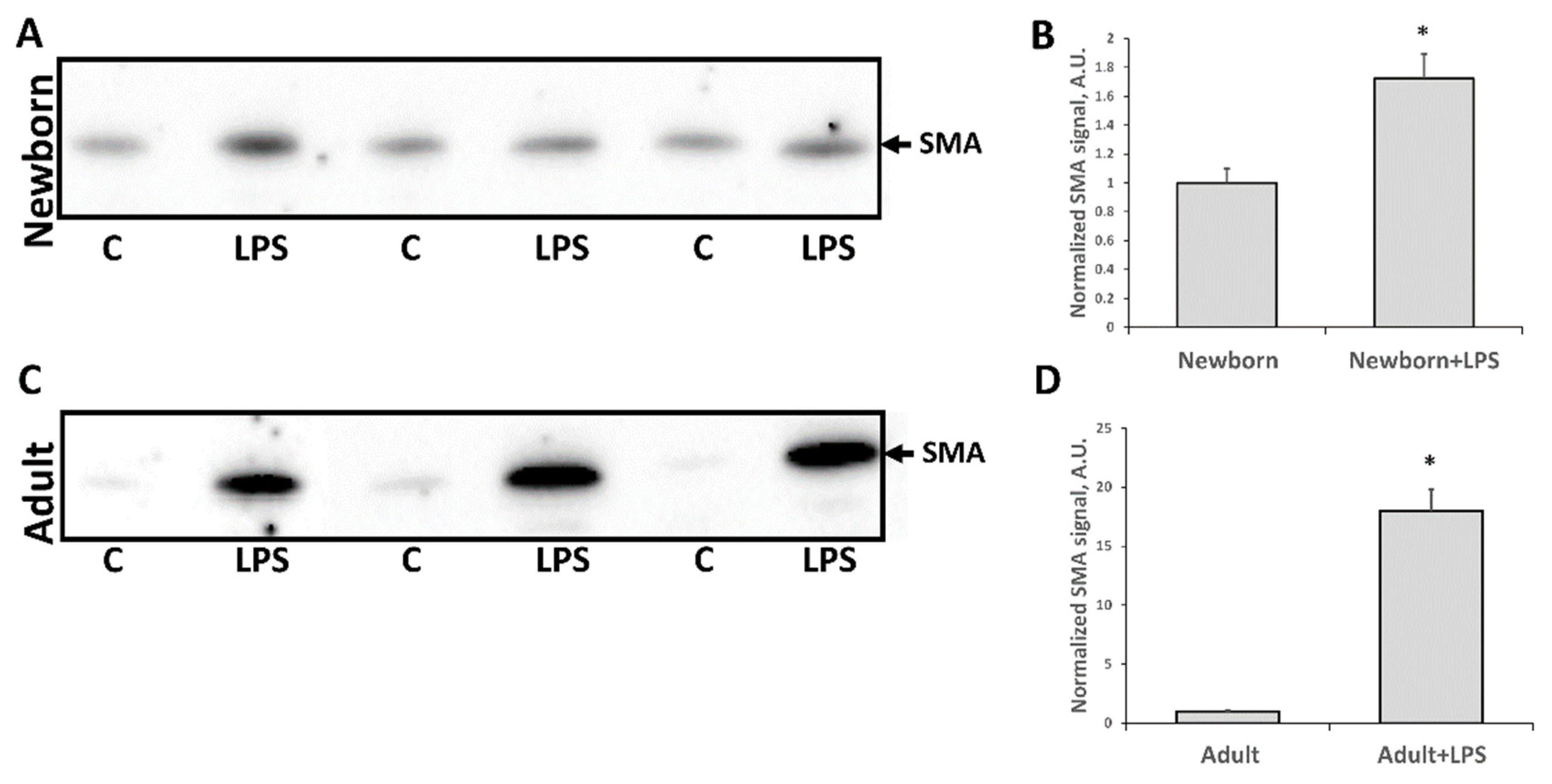
3.3. Fibrosis Formation after Acute Kidney Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Edmond, K.M.; Kortsalioudaki, C.; Scott, S.; Schrag, S.J.; Zaidi, A.K.; Cousens, S.; Heath, P.T. Group B streptococcal disease in infants aged younger than 3 months: Systematic review and meta-analysis. Lancet 2012, 379, 547–556. [Google Scholar] [CrossRef]
- Watson, R.S.; Carcillo, J.A.; Linde-Zwirble, W.T.; Clermont, G.; Lidicker, J.; Angus, D.C. The epidemiology of severe sepsis in children in the United States. Am. J. Respir. Crit. Care Med. 2003, 167, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Celes, M.R.N.; Prado, C.M.; Rossi, M.A. Sepsis: Going to the heart of the matter. Pathobiology 2012, 80, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Tissières, P.; Ochoda, A.; Dunn-Siegrist, I.; Drifte, G.; Morales, M.; Pfister, R.; Berner, M.; Pugin, J. Innate immune deficiency of extremely premature neonates can be reversed by interferon-γ. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Aneja, R.K.; Carcillo, J.A. Differences between adult and pediatric septic shock. Minerva Anestesiol. 2011, 77, 986–992. [Google Scholar] [PubMed]
- Basha, S.; Surendran, N.; Pichichero, M. Immune responses in neonates. Expert Rev. Clin. Immunol. 2014, 10, 1171–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, N.; Müller, W.; Resch, B. Neonates presenting with temperature symptoms: Role in the diagnosis of early onset sepsis. Pediatr. Int. 2012, 54, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, C.; Gloning, A.; Pruenster, M.; Frommhold, D.; Bierschenk, S.; Genzel-Boroviczeny, O.; von Andrian, U.H.; Quackenbush, E.; Sperandio, M. Neutrophil and endothelial adhesive function during human fetal ontogeny. J. Leukoc. Biol. 2013, 93, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Filias, A.; Theodorou, G.L.; Mouzopoulou, S.; Varvarigou, A.A.; Mantagos, S.; Karakantza, M. Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.R.; Qing, G.; Byers, D.M.; Stadnyk, A.W.; Al-Hertani, W.; Bortolussi, R. Role of MyD88 in Diminished Tumor Necrosis Factor Alpha Production by Newborn Mononuclear Cells in Response to Lipopolysaccharide. Infect. Immun. 2004, 72, 1223–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förster-Waldl, E.; Sadeghi, K.; Tamandl, D.; Gerhold, B.; Hallwirth, U.; Rohrmeister, K.; Hayde, M.; Prusa, A.R.; Herkner, K.; Boltz-Nitulescu, G.; et al. Monocyte toll-like receptor 4 expression and LPS-induced cytokine production increase during gestational aging. Pediatr. Res. 2005, 58, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Al-Hertani, W.; Sen, R.Y.; Byers, D.M.; Bortolussi, R. Human newborn polymorphonuclear neutrophils exhibit decreased levels of MyD88 and attenuated p38 phosphorylation in response to lipopolysaccharide. Clin. Investig. Med. 2007, 30, 44–53. [Google Scholar] [CrossRef]
- Sadeghi, K.; Berger, A.; Langgartner, M.; Prusa, A.; Hayde, M.; Herkner, K.; Pollak, A.; Spittler, A.; Förster-Waldl, E. Immaturity of Infection Control in Preterm and Term Newborns Is Associated with Impaired Toll-Like Receptor Signaling. J. Infect. Dis. 2007, 195, 296–302. [Google Scholar] [CrossRef] [PubMed]
- McGreal, E.P.; Hearne, K.; Spiller, O.B. Off to a slow start: Under-development of the complement system in term newborns is more substantial following premature birth. Immunobiology 2012, 217, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Wynn, J.L.; Levy, O. Role of innate host defenses in susceptibility to early-onset neonatal sepsis. Clin. Perinatol. 2010, 37, 307–337. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, C.S.; Baumbusch, M.A.; Dulay, A.T.; Oliver, E.A.; Lee, S.; Zhao, G.; Bhandari, V.; Ehrenkranz, R.A.; Weiner, C.P.; Madri, J.A.; et al. Characterization of RAGE, HMGB1, and S100β in inflammation-induced preterm birth and fetal tissue injury. Am. J. Pathol. 2009, 175, 958–975. [Google Scholar] [CrossRef] [PubMed]
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; et al. Acute renal failure in critically ill patients: A multinational, multicenter study. J. Am. Med. Assoc. 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Karajala-Subramanyam, V.; Lee, M.; Yende, S.; Kong, L.; Carter, M.; Angus, D.C.; Kellum, J.A. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010, 77, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagshaw, S.M.; Uchino, S.; Bellomo, R.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; et al. Septic acute kidney injury in critically ill patients: Clinical characteristics and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.M.; Drusano, G.L.; Ambrose, P.G.; Bhavnani, S.M.; Bertino, J.S.; Nafziger, A.N.; Louie, A. Back to the Future: Using Aminoglycosides Again and How to Dose Them Optimally. Clin. Infect. Dis. 2007, 45, 753–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordooei Javan, A.; Shokouhi, S.; Sahraei, Z. A review on colistin nephrotoxicity. Eur. J. Clin. Pharmacol. 2015, 71, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mergenhagen, K.A.; Borton, A.R. Vancomycin nephrotoxicity: A review. J. Pharm. Pract. 2014, 27, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Bajčetić, M.; Spasić, S.; Spasojević, I. Redox therapy in neonatal sepsis: Reasons, targets, strategy, and agents. Shock 2014, 42, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Romeo, C.; Eaton, S.; Spitz, L.; Pierro, A. Nitric oxide inhibits neonatal hepatocyte oxidative metabolism. J. Pediatr. Surg. 2000, 35, 44–48. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pavlenko, T.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Silachev, D.N.; Sukhikh, G.T.; Zorov, D.B. The role of oxidative stress in acute renal injury of newborn rats exposed to hypoxia and endotoxin. FEBS J. 2017, 284, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Morosanova, M.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Pulkova, N.V.; Galkina, S.I.; Skulachev, V.P.; Zorov, D.B. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta 2011, 1812, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, J.A. Histological and Histochemical Methods: Theory and Practice, 3rd ed.; Butterworth-Heinemann: Boston, MA, USA; Oxford, UK, 1999. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [PubMed]
- Balbi, A.P.C.; Costa, R.S.; Coimbra, T.M. Postnatal renal development of rats from mothers that received increased sodium intake. Pediatr. Nephrol. 2004, 19, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, T.M.; Raivio, K.O.; Saksela, M.; Kinnula, V.L. Expression and developmental profile of antioxidant enzymes in human lung and liver. Am. J. Respir. Cell Mol. Biol. 1998, 19, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Hayashibe, H.; Asayama, K.; Dobashi, K.; Kato, K. Prenatal development of antioxidant enzymes in rat lung, kidney, and heart: Marked increase in immunoreactive superoxide dismutases, glutathione peroxidase, and catalase in the kidney. Pediatr. Res. 1990, 27, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, V.; Miller, N.J.; Milner, A.D.; Rice-Evans, C.A. Bilirubin and ascorbate antioxidant activity in neonatal plasma. FEBS Lett. 1994, 349, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Nassi, N.; Ponziani, V.; Becatti, M.; Galvan, P.; Donzelli, G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr. Int. 2009, 51, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.D. Respiratory Gas Exchange in the Placenta. In Comprehensive Physiology; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 351–401. ISBN 9780470650714. [Google Scholar]
- Saugstad, O.D. Mechanisms of tissue injury by oxygen radicals: Implications for neonatal disease. Acta Paediatr. Int. J. Paediatr. 1996, 85, 1–4. [Google Scholar] [CrossRef]
- Buonocore, G.; Groenendaal, F. Anti-oxidant strategies. Semin. Fetal Neonatal Med. 2007, 12, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Tsukimori, K.; Hata, K.; Satoh, S.; Nakano, H. The characterization of superoxide production of human neonatal neutrophil. Early Hum. Dev. 2001, 65, 11–19. [Google Scholar] [CrossRef]
- Doi, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Star, R.A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Investig. 2009, 119, 2868–2878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.K.; Kang, M.J.; Kim, W.; Koh, G.Y. Renal tubule regeneration after ischemic injury is coupled to the up-regulation and activation of cyclins and cyclin dependent kinases. Kidney Int. 1997, 52, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotnikov, E.Y.; Brezgunova, A.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Popkov, V.A.; Silachev, D.N.; Zorov, D.B. Mechanisms of LPS-Induced Acute Kidney Injury in Neonatal and Adult Rats. Antioxidants 2018, 7, 105. https://doi.org/10.3390/antiox7080105
Plotnikov EY, Brezgunova AA, Pevzner IB, Zorova LD, Manskikh VN, Popkov VA, Silachev DN, Zorov DB. Mechanisms of LPS-Induced Acute Kidney Injury in Neonatal and Adult Rats. Antioxidants. 2018; 7(8):105. https://doi.org/10.3390/antiox7080105
Chicago/Turabian StylePlotnikov, Egor Y., Anna A. Brezgunova, Irina B. Pevzner, Ljubava D. Zorova, Vasily N. Manskikh, Vasily A. Popkov, Denis N. Silachev, and Dmitry B. Zorov. 2018. "Mechanisms of LPS-Induced Acute Kidney Injury in Neonatal and Adult Rats" Antioxidants 7, no. 8: 105. https://doi.org/10.3390/antiox7080105
APA StylePlotnikov, E. Y., Brezgunova, A. A., Pevzner, I. B., Zorova, L. D., Manskikh, V. N., Popkov, V. A., Silachev, D. N., & Zorov, D. B. (2018). Mechanisms of LPS-Induced Acute Kidney Injury in Neonatal and Adult Rats. Antioxidants, 7(8), 105. https://doi.org/10.3390/antiox7080105