Abstract
A 52-year-old female presented to Integrated Health Options Clinic in October 2014 with a history of relapsed acute myeloid leukaemia (AML, diagnosed in 2009 and relapsed in 2014). Intravenous(IV) vitamin C therapy was initiated (in 2014) following completion of chemotherapy as an alternative to haematopoietic stem cell transplantation. IV vitamin C was administered twice weekly at a dose of 70 g/infusion. Within 4 weeks of initiation of IV vitamin C therapy, there was a dramatic improvement in the patient’s blood indices with platelet cell counts increasing from 25 × 109/L to 196 × 109/L and white blood cell counts increasing from 0.29 × 109/L to 4.0 × 109/L, with further improvements observed over the next 18 months. Furthermore, there was a clear and sustained improvement in the patient’s health-related quality of life scores assessed using a validated questionnaire. She has remained healthy and in complete remission until the present day. This case study highlights the benefits of IV vitamin C as a supportive therapy for previously relapsed AML.
1. Introduction
Up to 70% of acute myeloid leukaemia (AML) patients treated with chemotherapy will relapse and overall five year survival is about 25% [1,2]. Treatment options for relapsed AML are currently limited to haematopoietic stem cell (HSC) transplantation [3]. Two recent studies have indicated a role for vitamin C in suppressing leukaemia progression through epigenetic mechanisms [4,5]. Vitamin C was found to enhance HSC differentiation and decrease the number of circulating blast cells and leukaemia progression in animal models resulting in improved survival. Animals with vitamin C deficiency were found to have higher levels of HSCs with enhanced self-renewal function, which increases susceptibility to leukaemia. Patients with haematological malignancies have lower vitamin C status than control subjects, with up to 58% exhibiting deficiency [6,7], and further depletion of vitamin C can occur following chemotherapy and HSC transplantation [8,9]. Thus, administration of vitamin C to deficient patients may help to restore normal HSC function and differentiation. Herein we report on a case of relapsed AML who underwent regular IV vitamin C administration instead of HSC transplantation.
2. Case Report
The patient signed a consent form allowing the presentation of her case. On 5 May 2009, a 47-year-old female patient was admitted to Auckland Hospital, New Zealand, and diagnosed with Acute Myeloid Leukaemia (AML, bone marrow 75% blasts, normal karyotype, NPM+, FLT3+ with allelic ratio 0.01). She was prescribed daunorubicin/cytarabine (ARA-C) induction, and a Groshong line was inserted. She underwent four cycles of chemotherapy with DA (daunorubicin, cytarabine) × 2MACE (amsacrine, cytarabine, etoposide) and MidAC (mitoxantrone, cytarabine). This was complicated by a presumed Aspergillus invasive pulmonary infection and was treated with oral Voriconazole for three months. A goitre was noted on admission and she was diagnosed with hypothyroidism (thyroid-stimulating hormone (TSH) 54 mU/L, thyroxine (T4) 7.7 pmol/L). She had positive thyroid antibodies and was prescribed thyroxine 50 mcg/day.
Following every cycle of chemotherapy, she was readmitted with a neutropenic fever, which required antibiotic therapy. On 9 May 2009, she developed a neutropenic fever and was started on empiric cefepime and gentamycin while awaiting blood culture results. On those antibiotics, she developed diarrhoea and a rash on her legs, which was diagnosed as Erythema multiforme and thought to be caused by either the cefepime or allopurinol, which she had also been prescribed. Both drugs were discontinued, and she was trialled on meropenem. This drug is a beta-lactam antibiotic given by IV for more serious infections.
By 14 May 2009, her fever persisted and no obvious cause was found at that time. High-resolution computed tomography (HRCT) confirmed that the chest was normal. IV Amphotericin B was commenced. Finally, Staphylococcus epidermidis was grown from a blood culture sample taken from the Groshong line. Amphotericin B was discontinued and IV vancomycin commenced. Her fever was resolved and she was discharged home with IV vancomycin for a further five days. Her platelet count prior to discharge was <10 × 109/L. She was given a platelet transfusion prior to her discharge.
On 25 May 2009, she was readmitted with neutropenic fever. She was again treated with meropenem and vancomycin. She had haemoglobin (Hb) 88 g/L and platelets 20 × 109/L on admission. She was transfused with red blood cells and platelets but developed a reaction to the platelet transfusion, which was managed with hydrocortisone and cetirizine. After two days she was discharged on augmentin for five days and a further two days of vancomycin. The four cycles of chemotherapy were completed by October 2009.
She underwent regular review by the Hematology Clinic until 5 May 2014 when she was discharged from the clinic in complete remission. She remained healthy until August 2014 when she again developed a neutropenia and thrombocytopenia. A bone marrow aspirate on 20 August 2014 revealed a relapsed AML with 61% blasts of similar morphology and cell marker expression as the initial diagnosis. At this time she also had borderline glucose intolerance with HbA1c of 40 mmol/mol. She saw a haematologist, who suggested more intensive chemotherapy, and allogeneic bone marrow transplant. The Haematologist stated, “without a transplant, cure is extremely unlikely and even with a transplant, she is faced with significant transplant related morbidity and an appreciable relapse risk, but with a potential cure opportunity”.
The patient decided to undergo further chemotherapy but rejected a bone marrow transplant. Induction chemotherapy was commenced on 29 August 2014. Bone marrow biopsy on 1 October 2014, showed complete remission of her AML (with 4% blasts). She had no further chemotherapy.
Intravenous Vitamin C Therapy
On the 10 October 2014, the patient had her first consultation at Integrated Health Options clinic, Auckland, New Zealand. Her Hb at that visit was 93 g/L and platelets 27 × 109/L. There was a discussion with the patient, in the presence of her daughter, about the clinic’s protocol that IV vitamin C infusions are not routinely offered to people with a platelet count of less than 50 × 109/L. After informed consent, she chose to have the IV vitamin C infusions. Her G6PD was normal (tested due to potential haemolytic anaemia with G6PD deficiency). She commenced IV vitamin C therapy on the 7 November 2014 and was prescribed twice weekly IV vitamin C infusions at 70 g/infusion. Her initial pre-infusion plasma vitamin C concentration, measured prior to her first test dose of IV vitamin C on 7 November 2014, was 0.66 mg/dL (37.5 µmol/L). This plasma concentration is considered inadequate, with ≥50 µmol/L (0.88 mg/dL) being adequate and ≥70 µmol/L (1.23 mg/dL) being saturating [10,11]. Post-infusion plasma vitamin C levels were regularly assessed to confirm that the proposed ascorbic acid therapeutic level was achieved (Table 1) [12]. The patient underwent weekly complete blood counts (CBC) and renal function tests, and was also prescribed additional oral supplements (Table 2).

Table 1.
Intravenous vitamin C dosage infused into the patient and post-infusion ascorbic acid therapeutic level in blood plasma.

Table 2.
Supplementary medication taken concurrently with IV vitamin C infusion by patient.
Prior to initiation of IV vitamin C treatments, the patient’s Hb was 107 g/L, platelets 25 × 109/L and white blood cells (WBC) 0.29 × 109/L. By the 19 December 2014, 42 days after beginning IV vitamin C infusions, her Hb had increased to 111 g/L, platelets to 196 × 109/L (Figure 1) and WBC to 4 × 109/L (Figure 2). On the 7 September 2017 her Hb was 124 g/L, platelet counts were 227 × 109/L, and WBC count was 6.5 × 109/L with a normal differential.
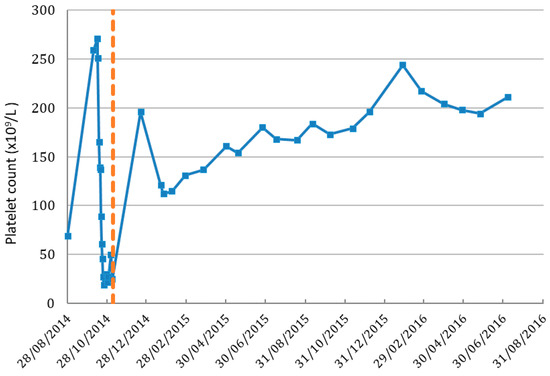
Figure 1.
Platelet cell count in patient before and after IV vitamin C treatment. Dashed line indicates the IV vitamin C commenced date. Normal range of platelet count is 150–400 (×109/L).
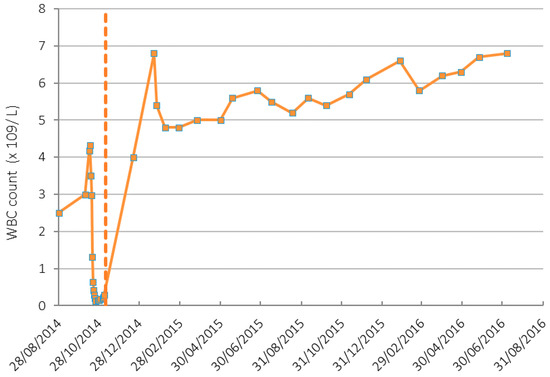
Figure 2.
White blood cell (WBC) count in patient before and after IV vitamin C treatment. Dashed line indicates the IV vitamin C commenced date. Normal range of WBC count is 4.5–11 (×109/L).
The quality of life (QoL) of the patient was measured before and throughout the IV vitamin C therapy using the validated European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), which has been used previously to assess QoL following IV vitamin C therapy [16,17]. Six weeks after starting IV vitamin C infusions, her global health status score had increased from 58 to 83, her overall functional score had increased from 89 to 98, and her overall symptom score had dropped from 39 to 8 (Table 3). This was a marked improvement of her QoL, which has been maintained until the present time.

Table 3.
Quality of life score measured using the EORTC QLQ-C30.
The patient continued twice weekly IV vitamin C infusions of 70 g until August 2015, when the patient felt so well that she decided to reduce the frequency of her IV vitamin C infusions to once weekly. As of 21 September 2017, she has had 141 IV vitamin C infusions. Her blood vitamin C level (AATL) post-infusion averaged at 360 mg/dL (targeted therapeutic range is 350–400 mg/dL) [14,15]. She has now cut back to a monthly infusion but maintains her oral intake of vitamin C as well as the other supplements. The patient remains clinically very well and has remained in complete remission.
3. Discussion
The use of complementary and alternative medicine is substantial in the general population [18]. High-dose IV vitamin C, in particular, is sought after by many cancer patients [19]. The rationale for IV administration is the ability to bypass the regulated intestinal uptake of oral vitamin C, thus resulting in 20-fold higher plasma concentrations with IV infusion [20]. These high concentrations of vitamin C are able to indirectly generate hydrogen peroxide through a transition metal ion-dependent mechanism, and this is thought to specifically target and kill cancer cells [21]. IV administration may also be required to facilitate diffusion of vitamin C into tumours with subsequent modulation of important cell signalling pathways [22,23]. Although evidence for the efficacy of vitamin C against solid tumours is currently limited, recent research is indicating a possible role for vitamin C in modulating certain haematological malignancies that are sensitive to the epigenetic regulating effects of vitamin C [4,5]. Vitamin C is able to upregulate the activity of specific tumour suppressor epigenetic enzymes (TETs), which contain mutations in a proportion of AML patients [24,25].
The purpose of this case study was to document the benefits of IV vitamin C as a supportive therapy for previously relapsed AML. In this case, after IV vitamin C treatment, the patient regained normal values for absolute circulating neutrophil count (>1 × 109/L) and platelets count (>100 × 109/L). Cell culture studies have indicated that vitamin C can restore normal tumour suppressor expression profiles and the maturation of HSCs [4,26]. Although the epigenetic (TET mutation) status of the patient was not assessed, it is possible that she had the mutations which respond to vitamin C treatment. The patient remains clinically very well. In addition to vitamin supplements, the patient also incorporated a healthy lifestyle and dietary changes, such as vegetarian diet, yoga, meditation, homeopathic remedies, and herbal remedies (e.g., mistletoe, and black cumin seed oil). These supplements and lifestyle changes may have contributed to the dramatic results achieved by this patient. Therefore, the relative contribution of IV vitamin C therapy could not be singled out. It is also possible that vitamin C may work synergistically with other supplements. Thus, in this case, it was observed that chemotherapy followed by IV vitamin C therapy, combined with a healthy diet and other supplements, supported ongoing patient quality of life and remission of AML.
Administration of chemotherapeutic drugs to patients can result in depletion of vitamin C levels and negatively impact on the patient’s health-related quality of life [17]. Thus, administration of vitamin C to these patients may contribute to improved quality of life through replenishing the essential nutrient and counteracting the toxic side effects of chemotherapy. Indeed, the patient had an inadequate vitamin C status initially, and we observed a marked improvement in the patient’s health-related quality of life following initiation of IV vitamin C therapy, which has been maintained until the present time. The incidence of AML increases dramatically with age and prognosis for the elderly is poor with only 10% surviving five years after diagnosis [2]. As a result of the toxic effects of therapy, including myelosuppression and an increased risk of infection, chemotherapy may not be offered to the very elderly, in which case IV vitamin C may be a viable palliative option for these patients due to its relative safety. Furthermore, a recent trial of low gram dose IV vitamin C administered with DCAG (decitabine, granulocyte colony stimulating factor, cytarabine, aclarubicin) in elderly acute myeloid leukaemia patients showed an increased incidence of complete remission after first induction and prolonged overall survival compared with DCAG alone [27].
4. Conclusions
Administration of high-dose IV vitamin C to previously relapsed AML patients may help to restore normal blood cell counts and function. Improved quality of life is also observed. Future clinical trials of IV vitamin C for haematological cancers should target patients with the relevant (TET) mutations, or endeavour to include sub-group analysis of patients with these mutations.
Author Contributions
M.N.F. treated the patient and collected samples, A.A., S.P. and M.G.F. analysed samples and data, M.N.F. and A.C.C. wrote the paper, which was edited by A.A., S.P. and M.G.F.
Funding
This research received no external funding.
Acknowledgments
We thank the patient for giving us permission to present her results. A.C.C. is supported by a Health Research Council of New Zealand Sir Charles Hercus Health Research Fellowship (#16/037).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yin, J.A.; Grimwade, D. Minimal residual disease evaluation in acute myeloid leukaemia. Lancet 2002, 360, 160–162. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Bishop, K.; Kosary, C.L.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; et al. SEER Cancer Statistics Review, 1975–2014; National Cancer Institute: Bethesda, MD, USA, 2016.
- Appelbaum, F.R. Hematopoietic cell transplantation beyond first remission. Leukemia 2002, 16, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 function blocks aberrant self-renewal and leukemia progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef] [PubMed]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Wodzig, W.K.; Walczak, M.; Germeraad, W.T.; Bos, G.M. Ascorbic acid serum levels are reduced in patients with hematological malignancies. Results Immunol. 2016, 6, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ohtani, H.; Zhou, W.; Orskov, A.D.; Charlet, J.; Zhang, Y.W.; Shen, H.; Baylin, S.B.; Liang, G.; Gronbaek, K.; et al. Vitamin C increases viral mimicry induced by 5-aza-2′-deoxycytidine. Proc. Natl. Acad. Sci. USA 2016, 113, 10238–10244. [Google Scholar] [CrossRef] [PubMed]
- Nannya, Y.; Shinohara, A.; Ichikawa, M.; Kurokawa, M. Serial profile of vitamins and trace elements during the acute phase of allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.; Roberts, C.H.; Gupta, G.; Fisher, B.J.; Leslie, K.; Simmons, G.L.; Wiedle, C.M.; McCarty, J.M.; Clark, W.B.; Chung, H.M.; et al. Low plasma vitamin C levels in patients undergoing stem cell transplantation (Abstract). Biol. Blood Marrow Transplant. 2017, 23, S286–S287. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Poulsen, H.E. Is vitamin C supplementation beneficial? Lessons learned from randomised controlled trials. Br. J. Nutr. 2010, 103, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on dietary reference values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar]
- Welsh, J.L.; Wagner, B.A.; van’t Erve, T.J.; Zehr, P.S.; Berg, D.J.; Halfdanarson, T.R.; Yee, N.S.; Bodeker, K.L.; Du, J.; Roberts, L.J., 2nd; et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): Results from a phase I clinical trial. Cancer Chemother. Pharmacol. 2013, 71, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. Measurement of ascorbic acid and dehydroascorbic acid in biological samples. Curr. Protoc. Toxicol. 2002. [Google Scholar] [CrossRef]
- Mikirova, N.A.; Casciari, J.J.; Hunninghake, R.E.; Riordan, N.H. Intravenous ascorbic acid protocol for cancer patients: Scientific rationale, pharmacology, and clinical experience. Funct. Foods Health Dis. 2013, 3, 344–366. [Google Scholar]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Vissers, M.C.M.; Cook, J.S. The effect of intravenous vitamin C on cancer- and chemotherapy-related fatigue and quality of life. Front. Oncol. 2014, 4, 283. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.; Rees, R. The prevalence of complementary and alternative medicine use among the general population: A systematic review of the literature. Complement. Ther. Med. 2000, 8, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, A.Y.; Chen, Q.; Espey, M.G.; Drisko, J.; Levine, M. Vitamin C: Intravenous use by complementary and alternative medicine practitioners and adverse effects. PLoS ONE 2010, 5, e11414. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: Implications for oral and intravenous use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Parrow, N.L.; Leshin, J.A.; Levine, M. Parenteral ascorbate as a cancer therapeutic: A reassessment based on pharmacokinetics. Antioxid. Redox Signal. 2013, 19, 2141–2156. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C.; Hicks, K.O. Pharmacokinetic modeling of ascorbate diffusion through normal and tumor tissue. Free Radic. Biol. Med. 2014, 77, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, C.; Vissers, M.C. Ascorbate as a co-factor for Fe- and 2-oxoglutarate dependent dioxygenases: Physiological activity in tumor growth and progression. Front. Oncol. 2014, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, D.; Pelosi, E.; Castelli, G.; Lo-Coco, F.; Testa, U. Mechanisms of anti-cancer effects of ascorbate: Cytotoxic activity and epigenetic modulation. Blood Cells Mol. Dis. 2018, 69, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Delhommeau, F.; Dupont, S.; Della Valle, V.; James, C.; Trannoy, S.; Masse, A.; Kosmider, O.; Le Couedic, J.P.; Robert, F.; Alberdi, A.; et al. Mutation in TET2 in myeloid cancers. N. Engl. J. Med. 2009, 360, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Huijskens, M.J.; Walczak, M.; Koller, N.; Briede, J.J.; Senden-Gijsbers, B.L.; Schnijderberg, M.C.; Bos, G.M.; Germeraad, W.T. Technical advance: Ascorbic acid induces development of double-positive T cells from human hematopoietic stem cells in the absence of stromal cells. J. Leukoc. Biol. 2014, 96, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhu, H.; Huang, J.; Zhu, Y.; Hong, M.; Zhu, H.; Zhang, J.; Li, S.; Yang, L.; Lian, Y.; et al. The synergy of vitamin C with decitabine activates TET2 in leukemic cells and significantly improves overall survival in elderly patients with acute myeloid leukemia. Leuk. Res. 2018, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).