Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro
Abstract
1. Introduction
2. Materials/Methods
2.1. Cell Lines
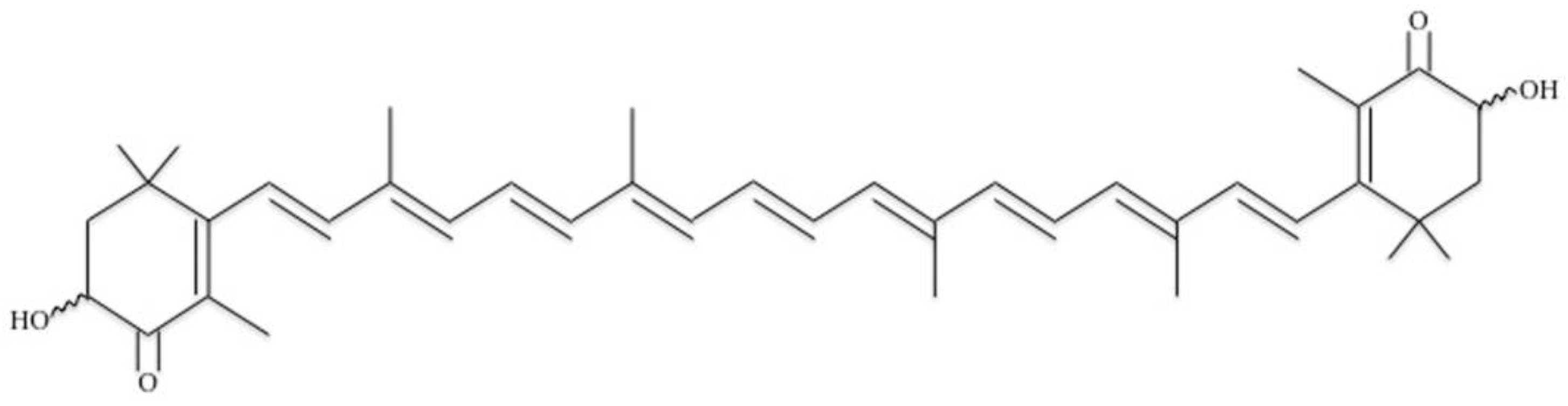
2.2. Astaxanthin
2.3. Migration Assays
2.4. Proliferation Assays
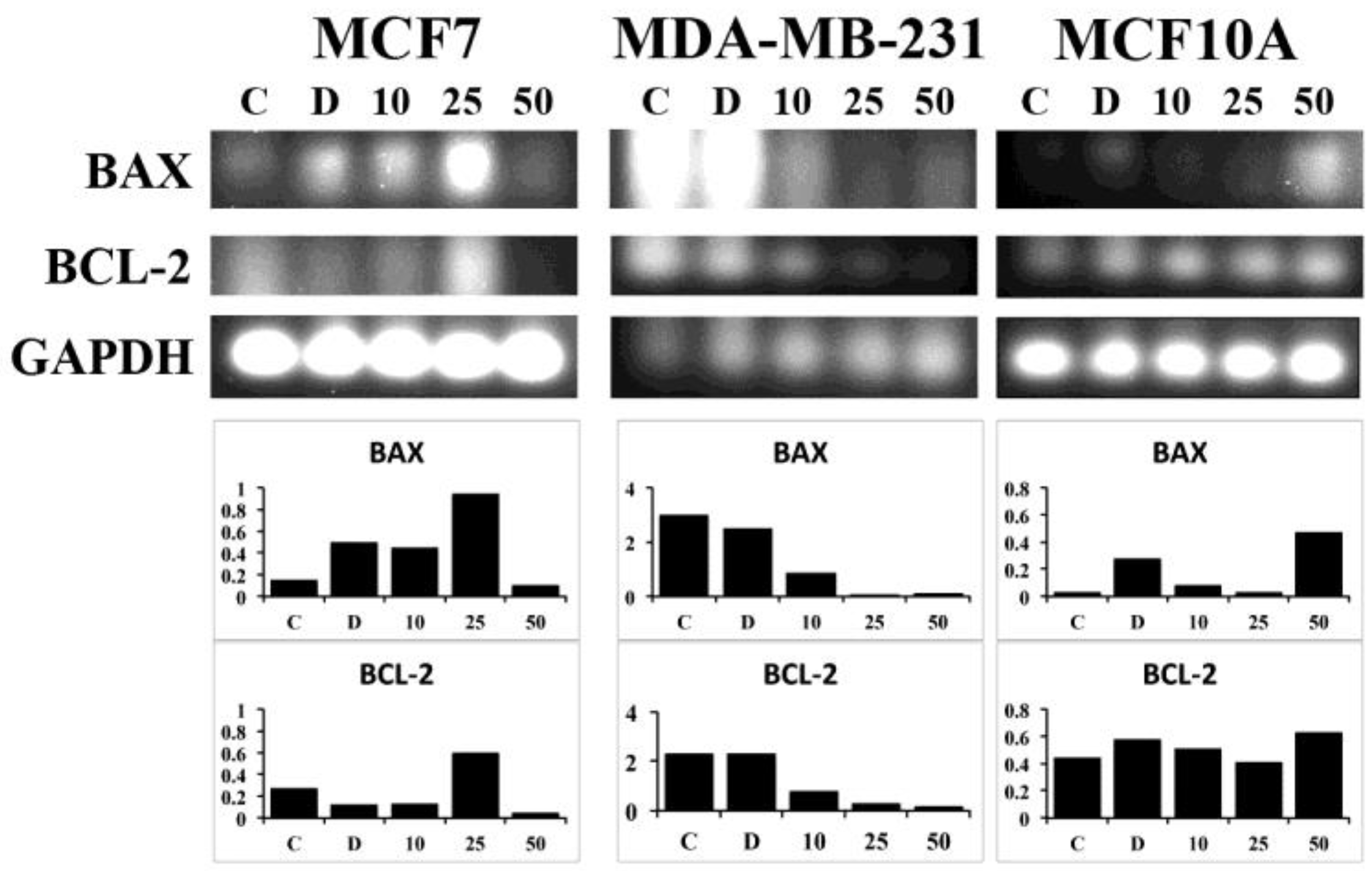
2.5. RT-PCR
2.6. Statistical Analyses
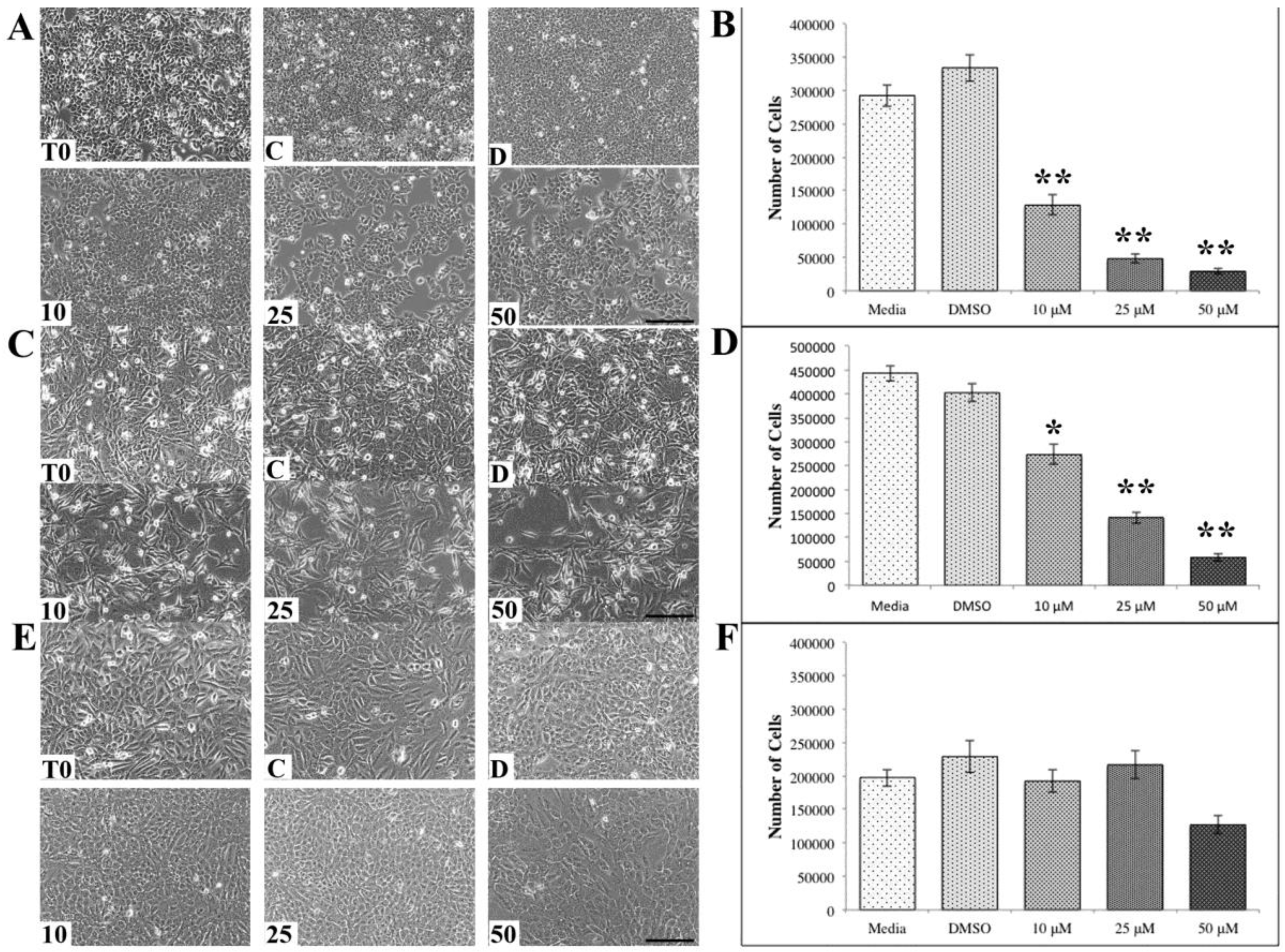
3. Results
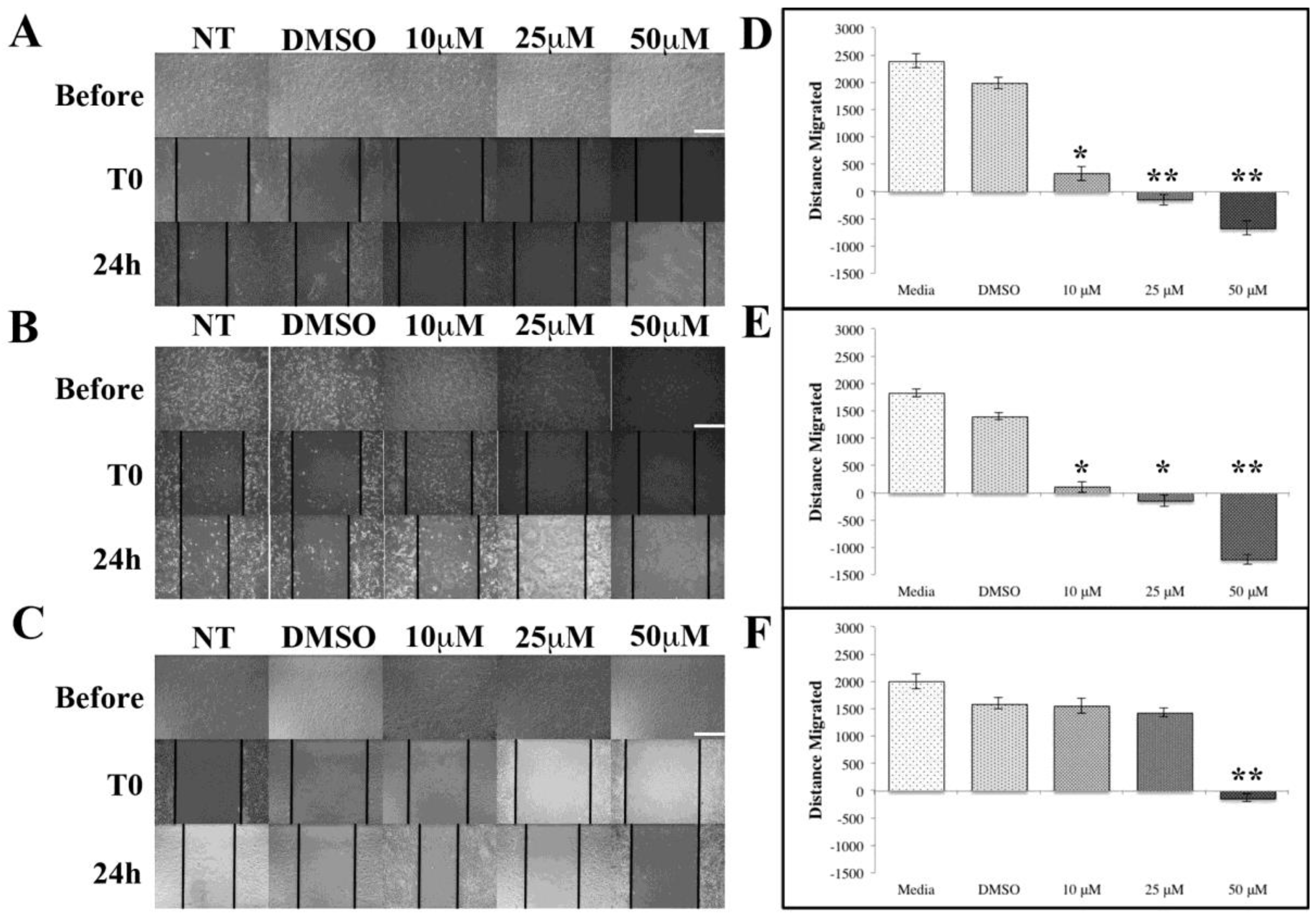
ASX Inhibits Breast Cancer Cell Migration
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol. 2009, 5, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chu, A.; Luo, Q.; Wu, M.; Shi, X.; Chen, Y. The Protective Effect of Astaxanthin on Cognitive Function via Inhibition of Oxidative Stress and Inflammation in the Brains of Chronic T2DM Rats. Front. Pharmacol. 2018, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, X.; Xiang, Q.; Meng, X.; Peng, Y.; Du, N.; Liu, Z.; Sun, Q.; Wang, C.; Liu, X. Astaxanthin alleviates brain aging in rats by attenuating oxidative stress and increasing BDNF levels. Food Funct. 2014, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, L.; Aggarwal, A. Astaxanthin inhibits cytokines production and inflammatory gene expression by suppressing IkappaB kinase-dependent nuclear factor kappaB activation in pre and postpartum Murrah buffaloes during different seasons. Vet. World 2018, 11, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Anarjan, N.; Nehdi, I.A.; Tan, C.P. Influence of astaxanthin, emulsifier and organic phase concentration on physicochemical properties of astaxanthin nanodispersions. Chem. Cent. J. 2013, 7, 127. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Barrow, C.J. Critical review of encapsulation methods for stabilization and delivery of astaxanthin. J. Food Bioact. 2018, 1, 104–115. [Google Scholar]
- Kim, J.H.; Chang, M.J.; Choi, H.D.; Youn, Y.K.; Kim, J.T.; Oh, J.M.; Shin, W.G. Protective effects of Haematococcus astaxanthin on oxidative stress in healthy smokers. J. Med. Food 2011, 14, 1469–1475. [Google Scholar] [CrossRef] [PubMed]
- Camera, E.; Mastrofrancesco, A.; Fabbri, C.; Daubrawa, F.; Picardo, M.; Sies, H.; Stahl, W. Astaxanthin, canthaxanthin and beta-carotene differently affect UVA-induced oxidative damage and expression of oxidative stress-responsive enzymes. Exp. Dermatol. 2009, 18, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gao, H.; Zhang, L.; Chen, C.; Yang, W.; Deng, Q.; Huang, Q.; Huang, F. A combination of flaxseed oil and astaxanthin alleviates atherosclerosis risk factors in high fat diet fed rats. Lipids Health Dis. 2014, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.F.; Andrei, S.; Cernea, C.; Taulescu, M.; Catoi, C. Effects of astaxanthin supplementation on chemically induced tumorigenesis in Wistar rats. Acta Vet. Scand. 2012, 54, 50. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Kim, H.W.; Mathison, B.D.; Hayek, M.G.; Massimino, S.; Reinhart, G.A.; Chew, B.P. Astaxanthin uptake in domestic dogs and cats. Nutr. Metab. 2010, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Li, H.Y.; Wu, J.S.; Niu, Q.; Duan, W.H.; Han, Q.Z.; Ji, W.M.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Kim, H. Astaxanthin and β-carotene in Helicobacter pylori-induced Gastric Inflammation: A Mini-review on Action Mechanisms. J. Cancer Prev. 2017, 22, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Yu, H.; Wang, S.; Zhang, C.; Shen, S. Astaxanthin Inhibits PC-3 Xenograft Prostate Tumor Growth in Nude Mice. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, Y.; Minenaka, Y.; Ichimura, M.; Tatsumi, K.; Nadamoto, T.; Urabe, K. Effects of astaxanthin and vitamin C on the prevention of gastric ulcerations in stressed rats. J. Nutr. Sci. Vitaminol. (Tokyo) 2005, 51, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Willen, R.; Wadstrom, T. Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob. Agents Chemother. 2000, 44, 2452–2457. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Hajizadeh Moghaddam, A.; Mir Mohammadrezaei, F. Effect of Astaxanthin on cell viability in T-47D and MDA-MB-231 Breast Cancer Cell Lines. Multidiscip. Cancer Investig. 2017, 1. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, D.; Cheng, K.W.; Chen, F. Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation. Bioresour. Technol. 2018, 247, 610–615. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, C.; Ma, J.; Bryan, L.; Jemal, A. Breast cancer statistics, 2013. CA Cancer J. Clin. 2014, 64, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Nath, A.K.; Tang, Y.; Choi, Y.J.; Debnath, T.; Choi, E.J.; Kim, E.K. Investigation of the Anti-Prostate Cancer Properties of Marine-Derived Compounds. Mar. Drugs 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Anthocyanins in cereals: Composition and health effects. Food Res. Int. 2018, 109, 232–249. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.W.; Inskeep, B.D.; Shah, H.; Park, J.P.; Hay, E.J.; Burg, K.J. Tannic acid preferentially targets estrogen receptor-positive breast cancer. Int. J. Breast Cancer 2013, 2013, 369609. [Google Scholar] [CrossRef] [PubMed]
- Jordan, L.G.; Booth, B.W. HER2(+) breast cancer cells undergo apoptosis upon exposure to tannic acid released from remodeled cross-linked collagen type I. J. Biomed. Mater. Res. A 2018, 106, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ngobili, T.A.; Shah, H.; Park, J.P.; Kwist, K.W.; Inskeep, B.; Burg, K.J.; Booth, B.W. Remodeling of tannic acid crosslinked collagen type I induces apoptosis in ER+ breast cancer cells. Anticancer Res. 2015, 35, 1285–1290. [Google Scholar] [PubMed]
- Metri, K.; Bhargav, H.; Chowdhury, P.; Koka, P.S. Ayurveda for chemo-radiotherapy induced side effects in cancer patients. J. Stem Cells 2013, 8, 115–129. [Google Scholar] [PubMed]
- Numico, G.; Longo, V.; Courthod, G.; Silvestris, N. Cancer survivorship: Long-term side-effects of anticancer treatments of gastrointestinal cancer. Curr. Opin. Oncol. 2015, 27, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Visvanathan, K.; Wolff, A.C. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast 2015, 24 Suppl. 2, S149–S153. [Google Scholar] [CrossRef]
- Yachida, S.; Jones, S.; Bozic, I.; Antal, T.; Leary, R.; Fu, B.; Kamiyama, M.; Hruban, R.H.; Eshleman, J.R.; Nowak, M.A.; et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010, 467, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Joshi, A.A.; Hegde, M.V.; Kadam, S.S. Enterolactone modulates the ERK/NF-kappaB/Snail signaling pathway in triple-negative breast cancer cell line MDA-MB-231 to revert the TGF-beta-induced epithelial-mesenchymal transition. Cancer Biol. Med. 2018, 15, 137–156. [Google Scholar] [PubMed]
| BAX Reverse primer (3′ antisense) | 5′-CAT CTT CTT CCA GAT GGT GA-3′ |
| BAX Forward primer (5′ sense) | 5′-GTT TCA TCC AGG ATC GAG CAG-3′ |
| BCL-2 Reverse primer (3′ antisense) | 5′-GAG ACA GCC AGG AGA AAT CA-3′ |
| BCL-2 Forward primer (5′ sense) | 5′-CCT GTG GAT GAC TGA GTA CC-3′ |
| GAPDH Reverse primer (3′ antisense) | 5′-ACATCGCTCAGACCCATG-3′ |
| GAPDH Forward primer (5′ sense) | 5′-TGTAGTTGAGGTCAATGAAGGG-3′ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCall, B.; McPartland, C.K.; Moore, R.; Frank-Kamenetskii, A.; Booth, B.W. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants 2018, 7, 135. https://doi.org/10.3390/antiox7100135
McCall B, McPartland CK, Moore R, Frank-Kamenetskii A, Booth BW. Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants. 2018; 7(10):135. https://doi.org/10.3390/antiox7100135
Chicago/Turabian StyleMcCall, Buckley, Connor K. McPartland, Reece Moore, Anastasia Frank-Kamenetskii, and Brian W. Booth. 2018. "Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro" Antioxidants 7, no. 10: 135. https://doi.org/10.3390/antiox7100135
APA StyleMcCall, B., McPartland, C. K., Moore, R., Frank-Kamenetskii, A., & Booth, B. W. (2018). Effects of Astaxanthin on the Proliferation and Migration of Breast Cancer Cells In Vitro. Antioxidants, 7(10), 135. https://doi.org/10.3390/antiox7100135





