Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Plant Materials
2.3. Preparation of Extracts
2.4. Determination of Total Phenolic Contents
2.5. Determination of Total Flavonoid Contents
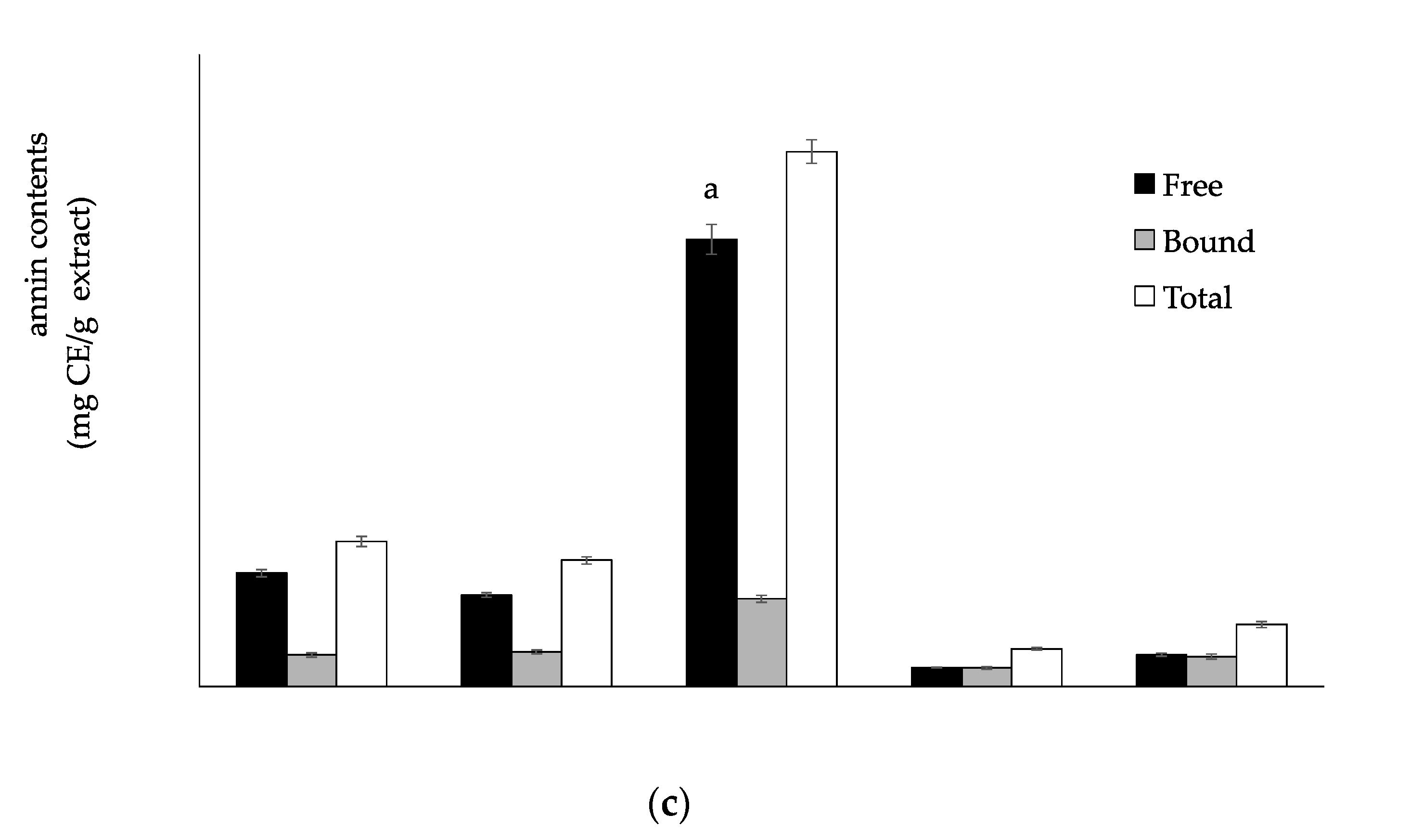
2.6. Determination of Total Tannin Contents
2.7. Fractionation of the Inner Skin Extract by Column Chromatography
2.8. DPPH Radical Scavenging Activity
2.9. ABTS Radical Scavenging Activity
2.10. Reducing Power
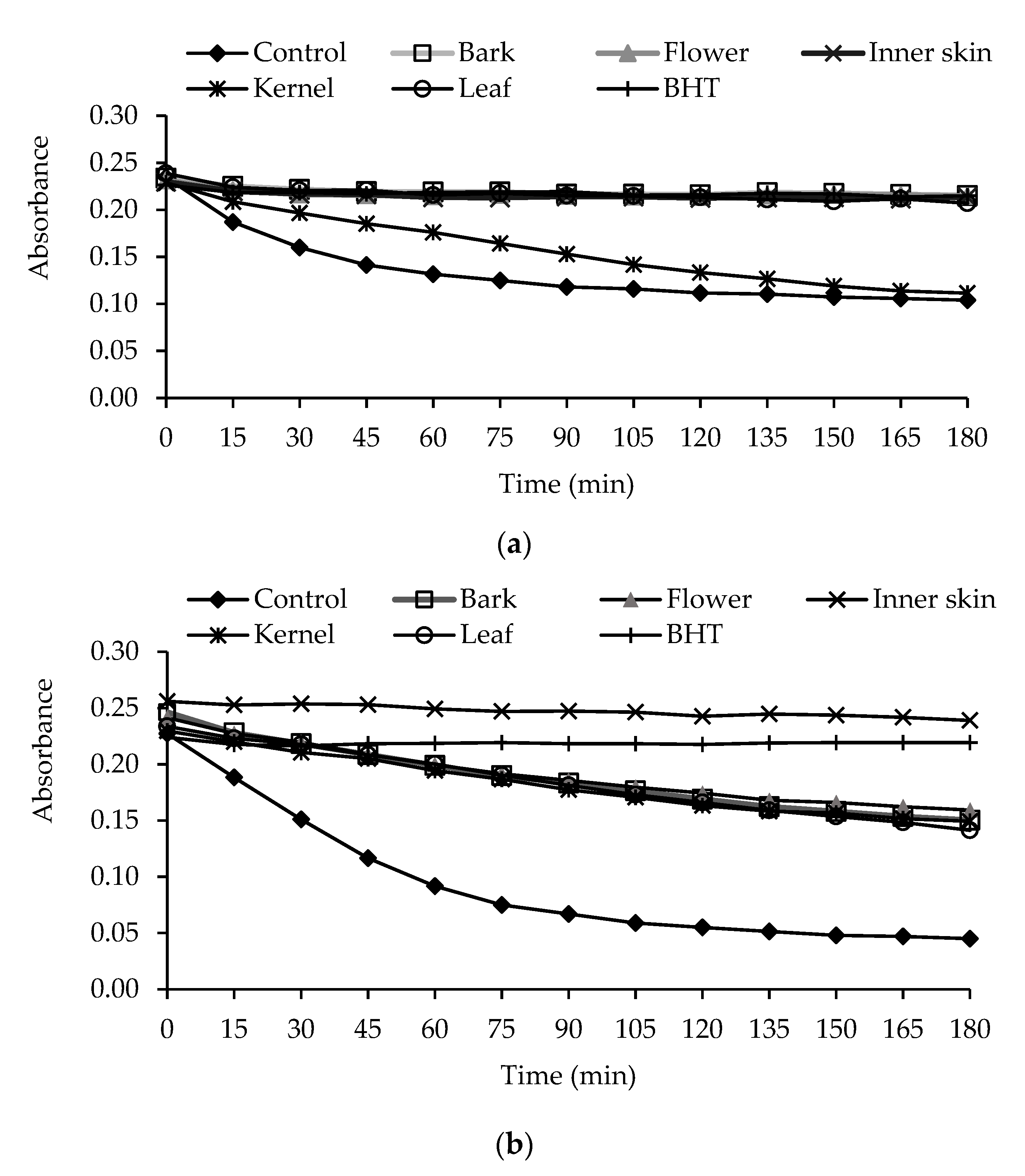
2.11. β-Carotene Bleaching System
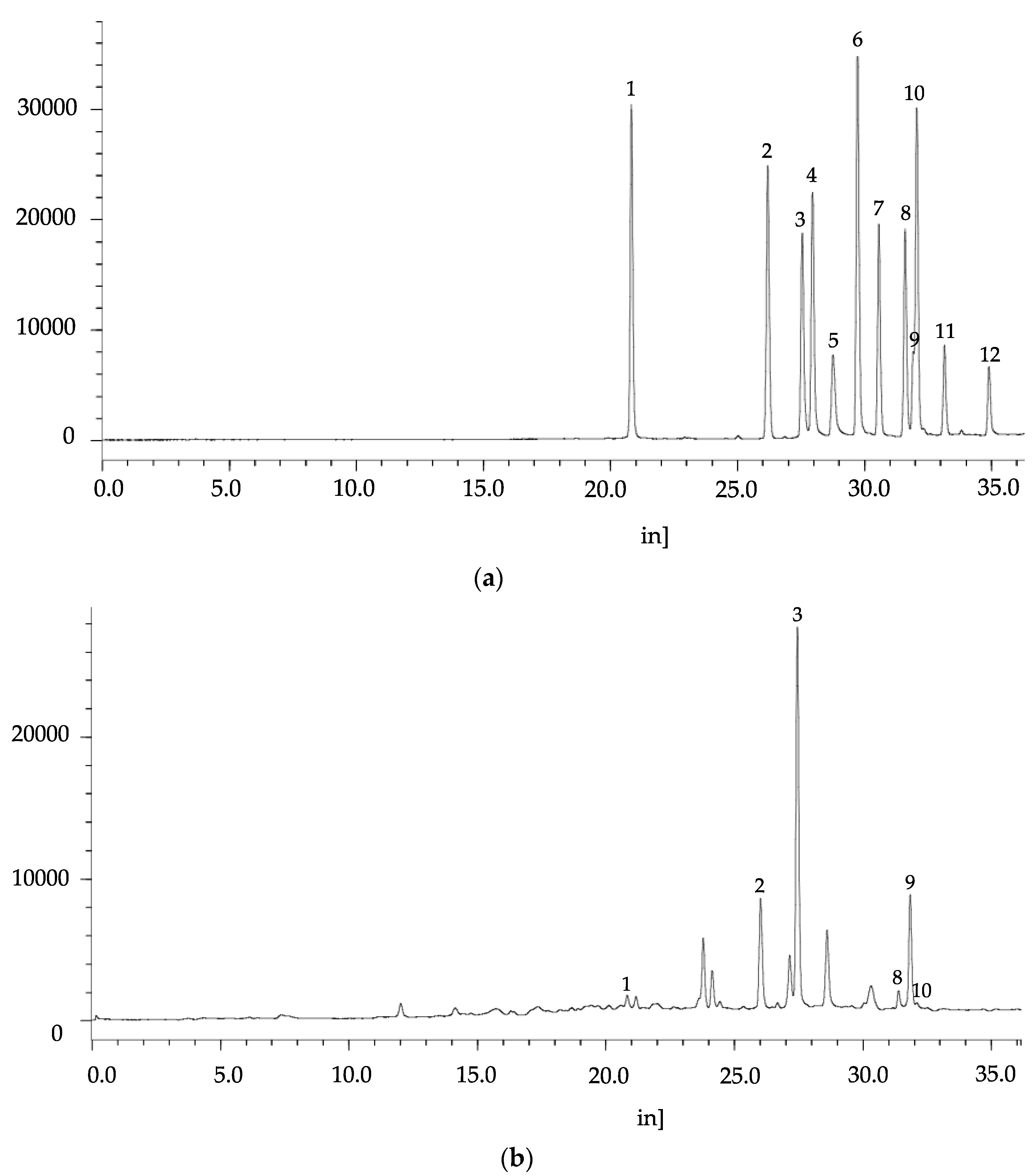
2.12. Identification and Quantification of Phenolic Compounds by HPLC
2.13. Statistical Analysis
3. Results
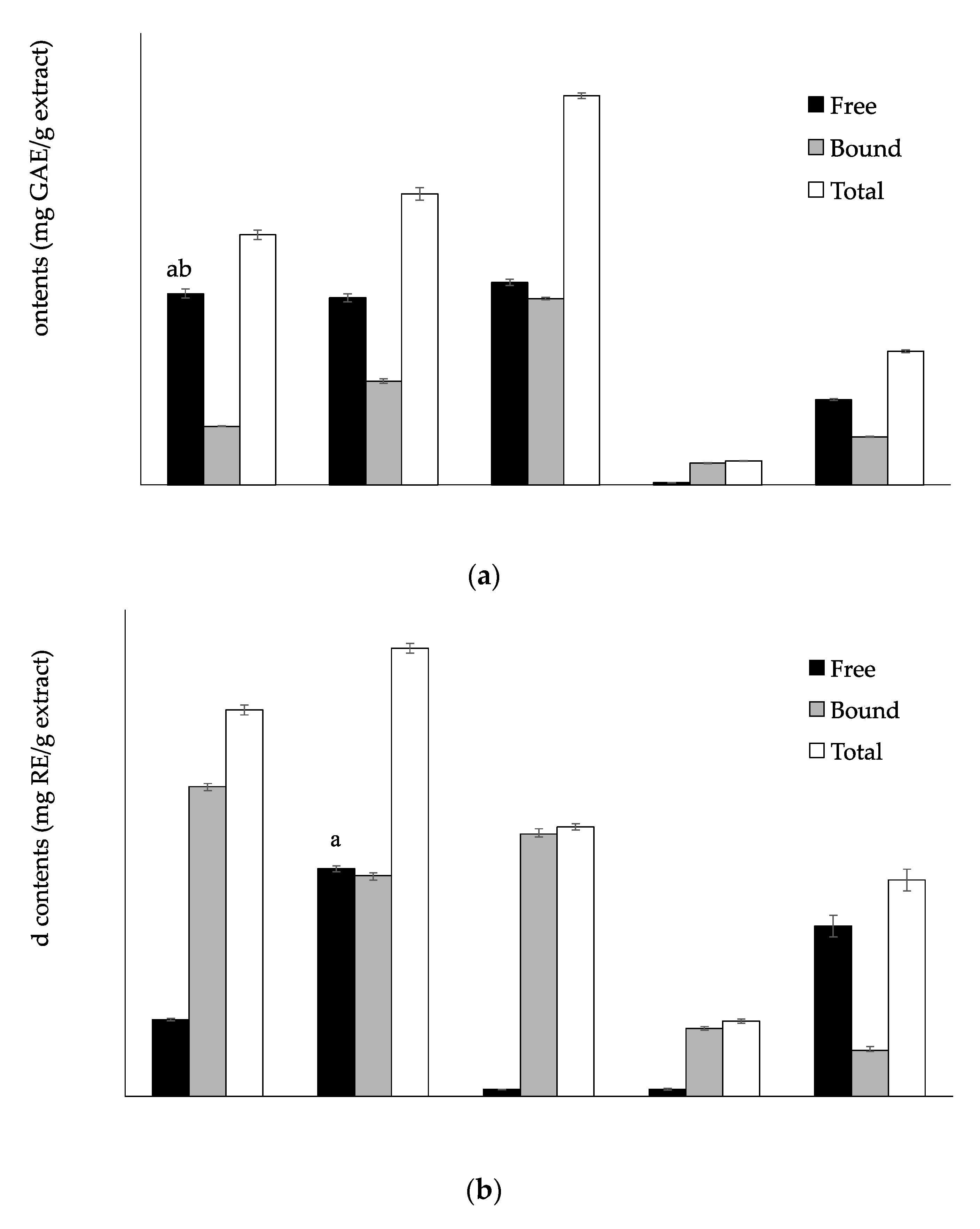
3.1. Total Phenolic, Flavonoid and Tannin Contents
3.2. Antioxidant Activity
3.3. Correlations between Antioxidant Activity and Phenolic Contents
3.4. Identification of Individual Phenolic Acids and Flavonoids of C. crenata
3.5. Fractionation of Free Phenolic Extract of Inner Skins by Column Chromatography
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sapota, K.; Park, S.E.; Kim, J.E.; Kim, S.; Choi, H.S.; Chun, H.S.; Kim, S.J. Antioxidant and antimelanogenic properties of chestnut flower extract. Biosci. Biotechnol. Biochem. 2010, 74, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Choi, E.J.; Cheong, H.; Kim, Y.R.; Ryu, S.Y.; Kim, K.M. Anti-allergic actions of the leaves of Castanea crenata and isolation of an active component responsible for the inhibition of mast cell degranulation. Arch. Pharm. Res. 1999, 22, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Tanaky, K.; Takada, N.; Yutaka, S.; Hirabayashi, T. Comparison of phenolic content of easily removed pellicle of Japanese Chestnut “porotan” with other Japanese and Chinese chestnut cultivars. J. Jpn. Soc. Hortic. Sci. 2010, 79, 258–262. [Google Scholar] [CrossRef]
- Mizutani, T.; Shizuka, F.; Matsuzawa, T.; Amano, Y.; Arikawa, Y. Anti-glycation activity of Japanese chestnut (Castanea crenata) inner skin extract is beneficial for type 2 diabetes in a rat model. J. Anti-Aging Med. 2014, 10, 112–119. [Google Scholar]
- Noh, J.R.; Gang, G.T.; Kim, Y.H.; Yang, K.J.; Lee, H.S.; Oh, W.K.; Song, K.S.; Lee, C.H. Antioxidant effects of the chestnut (Castanea crenata) inner shell extract in t-BHP-treated HepG2 cells, and CCl4-and high-fat diet-treated mice. Food Chem. Toxicol. 2009, 44, 3177–3183. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Gutiérrez-uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods: A review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; de Camargo, A.C.; Fereidoon, S. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Živkovíc, J.; Zekovíc, Z.; Mujíc, I.; Tumbas, V.; Cvetkovíc, D.; Spasojevíc, I. Antioxidant properties of phenolics in Castanea sativa Mill. Extracts. Biotecnology 2009, 47, 421–427. [Google Scholar]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Ham, J.S.; Kim, H.Y.; Lim, S.T. Antioxidant and deodorizing activities of phenolic components in chestnut inner shell extracts. Ind. Crops Prod. 2015, 73, 99–105. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Bento, A.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Castanea sativa Mill. Flowers amongst the most powerful antioxidant matrices: A phytochemical approach in decoctions and infusions. BioMed Res. Int. 2014, 2014, 232956. [Google Scholar] [CrossRef] [PubMed]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark sample (Castanea sativa Mill.) by HPLC-DAD-MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Yang, H.E.; Lee, S.C.; Chung, J.H.; Jo, B.K.; Kim, H.P.; Heo, M.Y. Antioxidant activity of the extract from the inner shell of chestnut. Toxicol. Appl. Pharmacol. 2005, 13, 150–155. [Google Scholar]
- Ozawa, T.; Kobayashi, S.; Seki, R.; Imagawa, H. A new gallotannin from bark of chestnut tree. Agric. Biol. Chem. 1984, 48, 1411–1416. [Google Scholar] [CrossRef]
- Vekiari, S.A.; Gordon, M.H.; García-Macías, P.; Labrinea, H. Extraction and determination of ellagic acid content in chestnut bark and fruit. Food Chem. 2008, 110, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Bueno-Costa, F.M.; Zambiazi, R.C.; Bohmer, B.W.; Chaves, F.C.; da Silva, W.P.; Zanusso, J.T.; Dutra, I. Antibacterial and antioxidant activity of honeys from the state of Rio Grande do Sul, Brazil. LWT Food Sci. Technol. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Baghdikian, B.; Leddet, V.M.; Mabrouki, F.; Olivier, E.; Cherif, J.K.; Ayadi, M.T. Total phenolic, total flavonoid, tannin content, and antioxidant capacity of Halimium halimifolium (Cistaceae). J. Appl. Pharm. Sci. 2014, 5, 52–57. [Google Scholar]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Essential oils, kava pyrones and phenolic compounds from leaves and rhizomes of Alpinia zerumbet (Pers.) B.L. Burtt. & R.M. Sm. and their antioxidant activity. Food Chem. 2007, 103, 486–494. [Google Scholar]
- Mikulic-Petkovsek, M.; Samoticha, J.; Eler, K.; Stampar, F.; Veberic, R. Traditional elderflower beverages: A rich source of phenolic compounds with high antioxidant activity. J. Agric. Food Chem. 2015, 63, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kumari, N. Comparative determination of phytochemicals and antioxidant activity from leaf and fruit of Sapindus mukorrossi Gaertn.—A valuable medicinal tree. Ind. Crops Prod. 2015, 73, 1–8. [Google Scholar] [CrossRef]
- Deba, F.; Xuan, T.D.; Yasuda, M.; Tawata, S. Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata. Food Control 2008, 19, 346–352. [Google Scholar] [CrossRef]
- Jung, B.S.; Lee, N.K.; Na, D.S.; Yu, H.H.; Paik, H.D. Comparative analysis of the antioxidant and anticancer activities of chestnut inner shell extracts prepared with various solvents. J. Sci. Food Agric. 2015, 96, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Su, B.; Som, D.; Hee, H.; Kim, J.S.; Paik, H.D. The impact of antimicrobial effect of chestnut inner shell extracts against Campylobacter jejuni in chicken meat. LWT Food Sci. Technol. 2016, 65, 746–750. [Google Scholar] [CrossRef]
- Barreira, J.C.M; Ferreira, I.C.F.R; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Moctezuma, C.; Hammerbacher, A.; Heil, M.; Gershenzon, J.; Méndez-Alonzo, R.; Oyama, K. Specific polyphenols and tannins are associated with defense against insect herbivores in the tropical Oak Quercus oleoides. J. Chem. Ecol. 2014, 40, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Köksal, E. Evaluation of reducing power and radical scavenging activities of water and ethanol extracts from sumac (Rhus coriaria L.). Food Res. Int. 2011, 44, 2217–2221. [Google Scholar] [CrossRef]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Antioxidant and antibacterial activities of Rumex japonicas Houtt. Biol. Pharm. Bull. 2005, 28, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Sakthidevi, G.; Mohan, V.R. Total phenolic, flavonoid contents and in vitro antioxidant activity of Dioscorea alata L. tuber. Int. J. Pharm. Sci. Res. 2013, 5, 115–119. [Google Scholar]
- Sumczynski, D.; Kotásková, E.; Družbíková, H.; Mlček, J. Determination of contents and antioxidant activity of free and bound phenolics compounds and in vitro digestibility of commercial black and red rice (Oryza sativa L.) varieties. Food Chem. 2016, 211, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Selek, I. Phenolic compounds and antioxidant activities of chestnut (Castanea sativa Mill.) fruits. Qual. Assur. Saf. Crop. 2012, 4, 199–205. [Google Scholar] [CrossRef]
- Narayan, L.C.; Rai, V.R. Anti-HIV-1 Activity of ellagic acid isolated from Terminalia paniculata. Free Radic. Antioxid. 2016, 6, 101–107. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An overview of plant phenolic compounds and their importance in human Nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Ou, H.C.; Hsu, W.C.; Chou, M.M.; Tseng, J.J.; Hsu, S.L.; Tsai, K.L.; Sheu, W.H.H.S. Ellagic acid inhibits oxidized LDL-mediated LOX-1 expression, ROS generation, and inflammation in human endothelial cells. J. Vasc. Surg. 2010, 52, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic acid: Biological properties and biotechnological development for production processes. Afr. J. Biotechnol. 2011, 4518–4523. [Google Scholar]

| Antioxidant Activities | Bark | Flower | Inner Skin | Kernel | Leaf | BHT |
|---|---|---|---|---|---|---|
| Free phenolic extract | ||||||
| DPPH (IC50 μg/mL) | 25.59 ± 0.22 c,d | 29.45 ± 0.22 b | 23.81 ± 0.07 d | NA | 48.98 ± 0.61 a | 27.27 ± 0.53 c |
| ABTS (IC50 μg/mL) | 300.26 ± 3.22 c | 289.45 ± 1.30 c,d | 270.73 ± 5.36 d | >10,000 a | 602.20 ± 9.35 b | 184.18 ± 3.01 e |
| Reducing power (IC50 μg/mL) | 186.07 ± 0.91 d | 197.50 ± 1.04 c | 187.78 ± 1.88 c,d | >10,000 a | 435.72 ± 4.85 b | 185.23 ± 1.46 d |
| Bleaching inhibition (LPI %) | 92.16 ± 0.74 a | 92.79 ± 1.02 a | 93.03 ± 0.06 a | 48.63 ± 9.38 b | 86.77 ± 3.23 a | 95.02 ± 2.28 a |
| Bound phenolic extract | ||||||
| DPPH (IC50 μg/mL) | 103.50 ± 1.70 c | 49.43 ± 0.17 d | 28.41 ± 0.25 e | 1680 ± 8.67 a | 467.93 ± 1.39 b | 27.27 ± 0.53 e |
| ABTS (IC50 μg/mL) | 809.64 ± 0.00 c | 386.46 ± 1.88 e | 538.70 ± 13.30 d | >4000 a | 3193 ± 18.00 b | 184.18 ± 3.01 f |
| Reducing power (IC50 μg/mL) | 726.00 ± 10.10 c | 344.85 ± 3.28 d | 209.56 ± 0.62 e | 3944 ± 29.10 a | 2542 ± 57.50 b | 185.23 ± 1.46 e |
| Bleaching inhibition (LPI %) | 61.02 ± 0.74 b | 66.02 ± 1.14 b | 93.35 ± 2.76 a | 64.96 ± 5.11 b | 60.45 ± 6.75 b | 97.92 ± 1.04 a |
| Correlated Components | TPC | TFC | TTC | DPPH (1/IC50) | ABTS (1/IC50) | Reducing Power (1/IC50) |
|---|---|---|---|---|---|---|
| TFC | 0.20 | |||||
| TTC | 0.64 ** | −0.34 | ||||
| DPPH (1/IC50) | 0.64 ** | 0.04 | 0.56 ** | |||
| ABTS (1/IC50) | 0.91 ** | 0.18 | 0.64 ** | 0.88 ** | ||
| Reducing Power (1/IC50) | 0.99 ** | 0.21 | 0.61 ** | 0.67 ** | 0.94 ** | |
| LPI | 0.71 ** | 0.01 | 0.47 ** | 0.45 * | 0.66 ** | 0.71 ** |
| Phenolic Acids (mg/g Dry Weight) | Retention Time (min) | Bark | Flower | Inner Skin | Kernel | Leaf | Total |
|---|---|---|---|---|---|---|---|
| Free phenolic acids | |||||||
| Gallic acid | 12.80 | 0.14 ± 0.01 b | 1.71 ± 0.05 a | - | - | - | 1.85 |
| Protocatechuic acid | 16.83 | 0.13 ± 0.00 b | 0.41 ± 0.05 a | - | - | - | 0.54 |
| Catechol | 17.78 | - | 1.11 ± 0.04 | - | - | - | 1.11 |
| Vanillin | 22.70 | 0.68 ± 10.09 | - | - | - | - | 0.68 |
| Sinapic acid | 24.81 | - | 0.89 ± 0.00 | - | - | - | 0.89 |
| p-Coumaric | 25.20 | 0.46 ± 0.00 b | - | 2.61 ± 0.15 a | 3.07 | ||
| Benzoic acid | 26.29 | - | 0.65 ± 0.12 | - | - | - | 0.65 |
| Ellagic acid | 28.35 | 1.49 ± 0.06 a | 0.71 ± 0.02 b | 0.51 ± 0.01 c | - | 0.40 ± 0.01 d | 3.11 |
| Total | 2.90 | 5.48 | 3.12 | 0.40 | |||
| Bound phenolic acids | |||||||
| Gallic acid | 12.80 | 0.29 ± 0.01 c | 2.22 ± 0.01 b | 5.20 ± 0.12 a | - | - | 7.71 |
| Protocatechuic acid | 16.83 | 0.35 ± 0.01 a | 0.15 ± 0.00 b | 0.39 ± 0.00 a | 0.01 ± 0.00 c | 0.90 | |
| Chlorogenic acid | 20.05 | 0.94 ± 0.04 a | - | - | - | 0.82 ± 0.00 a | 1.76 |
| p-Hydroxybenzoic | 20.60 | 0.08 ± 0.01 b | 0.06 ± 0.01 b | 0.14 ± 0.02 a | 0.01 ± 0.00 c | 0.05 ± 0.00 b | 0.34 |
| Vanillic acid | 21.58 | - | 0.05 ± 0.01 a | - | - | 0.04 ± 0.00 a | 0.09 |
| Syringic acid | 22.02 | 1.71 ± 0.06 | - | - | - | - | 1.71 |
| Ferulic acid | 24.50 | - | 0.52 ± 0.00 a | - | 0.01 ± 0.00 c | 0.21 ± 0.01 b | 0.74 |
| Sinapic acid | 24.81 | 1.74 ± 0.03 a | 0.69 ± 0.01 c | - | 0.05 ± 0.00 d | 1.06 ± 0.00 b | 3.54 |
| p-Coumaric | 25.20 | 0.98 ± 0.07 a | - | - | 0.02 ± 0.00 c | 0.36 ± 0.05 b | 1.36 |
| Benzoic acid | 26.29 | - | - | - | - | 0.76 ± 0.00 | 0.76 |
| Ellagic acid | 28.35 | 0.32 ± 0.01 b | 0.94 ± 0.01 a | 0.33 ± 0.01 b | - | 0.09 ± 0.01 c | 1.68 |
| Total | 6.41 | 4.63 | 6.06 | 0.10 | 3.39 | ||
| Flavonoids (mg/g Dry Weight) | Retention Time (min) | Bark | Flower | Inner Skin | Kernel | Leaf | Total |
|---|---|---|---|---|---|---|---|
| Free flavonoids | |||||||
| Isoquercitrin | 26.18 | - | 1.43 ± 0.01 b | - | - | 3.96 ± 0.04 a | 5.39 |
| Myricetin | 27.53 | 1.16 ± 0.10 a | 0.49 ± 0.09 b | - | - | 0.54 ± 0.10 b | 2.19 |
| Fisetin | 27.93 | 0.60 ± 0.00 b | - | - | - | 1.37 ± 0.01 a | 1.97 |
| Kaempferol | 31.58 | - | - | - | - | 0.18 ± 0.02 | 0.18 |
| Rhamnetin | 33.13 | - | 0.49 ± 0.00 | - | - | - | 0.49 |
| Total | 1.76 | 2.41 | - | - | 6.05 | ||
| Bound flavonoids | |||||||
| Esculetin | 20.85 | - | 0.16 ± 0.01 b | 0.61 ± 0.01 a | - | - | 0.77 |
| Isoquercitrin | 26.18 | - | 0.69 ± 0.01 | - | - | - | 0.69 |
| Myricetin | 27.53 | 0.10 ± 0.03 c | 3.59 ± 0.07 a | - | - | 2.13 ± 0.07 b | 5.82 |
| Morin | 28.79 | 0.37 ± 0.03 b | 0.17 ± 0.00 b | 1.22 ± 0.08 a | - | - | 1.76 |
| Kaempferol | 31.58 | - | 0.09 ± 0.00 | - | - | - | 0.09 |
| Apigenin | 32.08 | - | - | 1.04 ± 0.22 | - | - | 1.04 |
| Total | 0.47 | 4.70 | 2.87 | - | 2.13 | ||
| Fractions | TPC | TFC | TTC | DPPH (IC50) | Gallic Acid | Protocatechuic Acid | p-Coumaric Acid | Ellagic Acid |
|---|---|---|---|---|---|---|---|---|
| F1 | 482.71 ± 19.41 a | 7.44 ± 0.19 c | 58.11 ± 1.91 a | 6.70 ± 0.09 c | 4.27 ± 0.53 a | 0.30 ± 0.01 | - | - |
| F2 | 404.03 ± 2.04 b | 6.93 ± 0.13 c | 59.83 ± 0.82 a | 11.56 ± 0.21 b | 3.74 ± 0.00 a,b | - | - | - |
| F3 | 514.22 ± 8.51 a | 16.07 ± 0.55 b | 60.50 ± 1.27 a | 5.71 ± 0.26 c | 5.17 ± 0.45 a | - | 19.85 ± 2.96 | 4.12 ± 0.08 |
| F4 | 210.67 ± 0.77 c | 22.85 ± 0.55 a | 20.17 ± 0.75 b | 29.09 ± 0.62 a | 1.78 ± 0.09 b | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, L.H.; Minh, T.N. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. https://doi.org/10.3390/antiox6020031
Tuyen PT, Xuan TD, Khang DT, Ahmad A, Quan NV, Tu Anh TT, Anh LH, Minh TN. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants. 2017; 6(2):31. https://doi.org/10.3390/antiox6020031
Chicago/Turabian StyleTuyen, Phung Thi, Tran Dang Xuan, Do Tan Khang, Ateeque Ahmad, Nguyen Van Quan, Truong Thi Tu Anh, La Hoang Anh, and Truong Ngoc Minh. 2017. "Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc" Antioxidants 6, no. 2: 31. https://doi.org/10.3390/antiox6020031
APA StyleTuyen, P. T., Xuan, T. D., Khang, D. T., Ahmad, A., Quan, N. V., Tu Anh, T. T., Anh, L. H., & Minh, T. N. (2017). Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea crenata Sieb. et Zucc. Antioxidants, 6(2), 31. https://doi.org/10.3390/antiox6020031










