Identification and Antioxidant Activity of the Extracts of Eugenia uniflora Leaves. Characterization of the Anti-Inflammatory Properties of Aqueous Extract on Diabetes Expression in an Experimental Model of Spontaneous Type 1 Diabetes (NOD Mice)
Abstract
:1. Introduction
2. Experimental Section
2.1. Extraction of Phenolic Compounds in the Eugenia uniflora Leaves
2.2. Chemical Analysis
2.2.1. Determination of Antioxidant Activity of Extracts
2.2.2. Determination of Total Phenolic Compounds
2.2.3. Identification of Hydrolysable Tannins
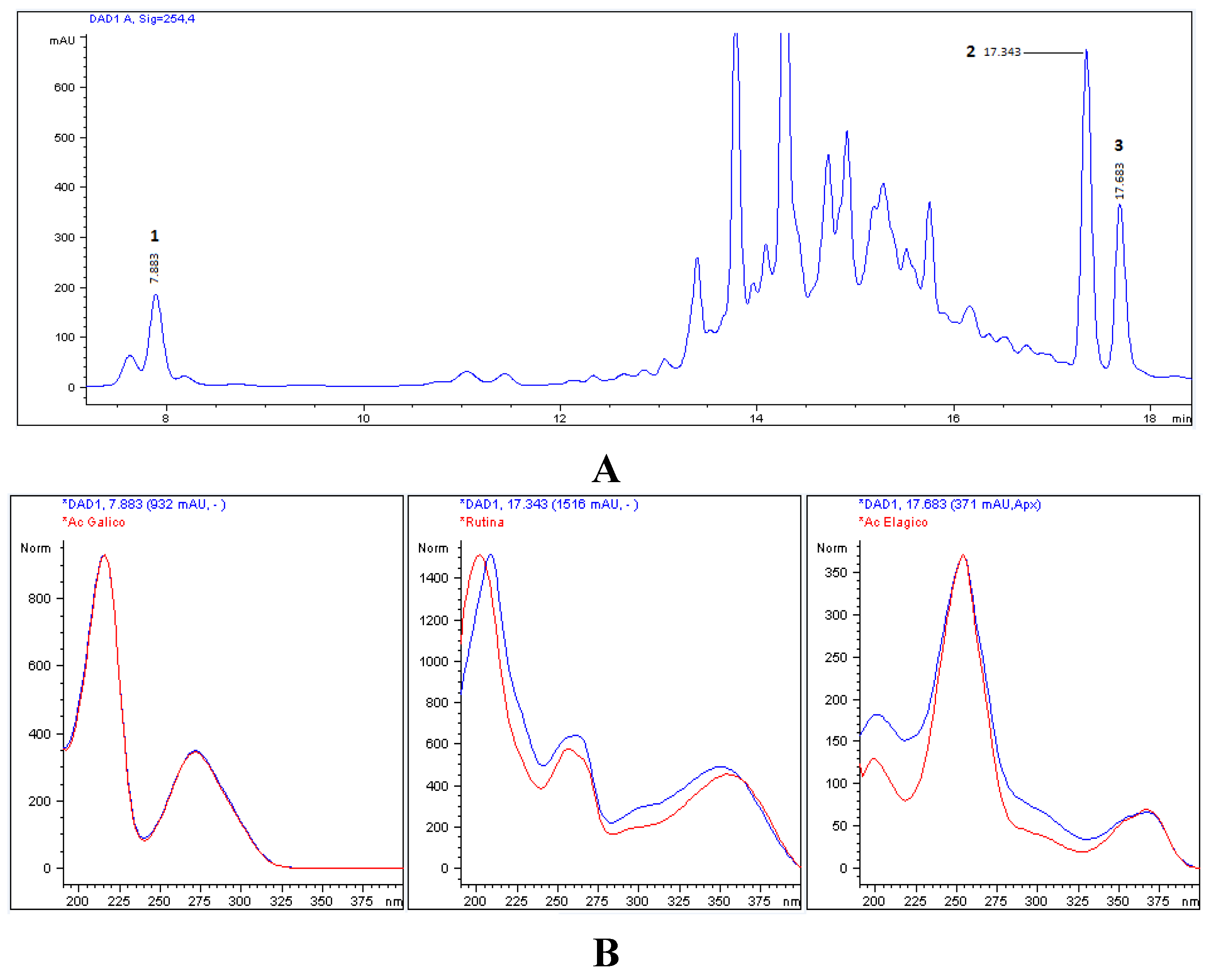
2.3. Chromatographic Analysis (HPLC)
2.4. Biological Assays: NOD Mice Treatment with E. uniflora Leaves
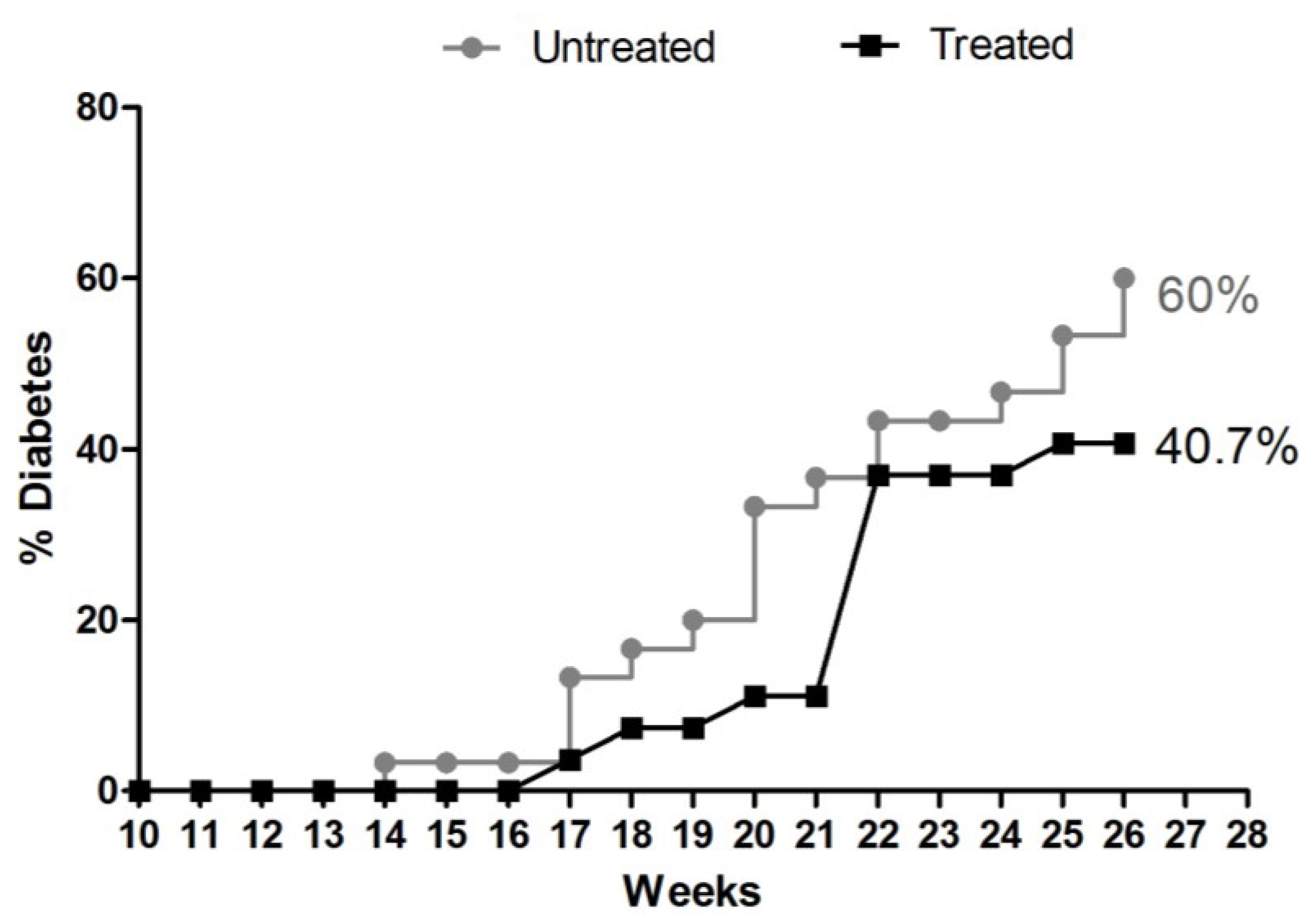
2.4.1. Experimental Design
2.4.2. Diagnosis of Acute Diabetes and Animal Sacrifice
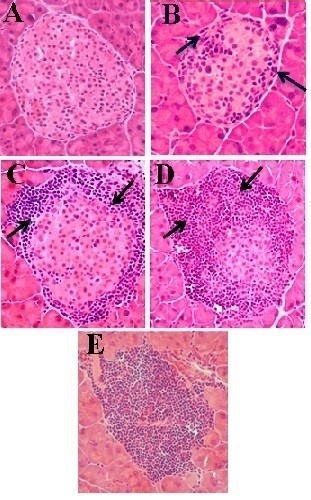
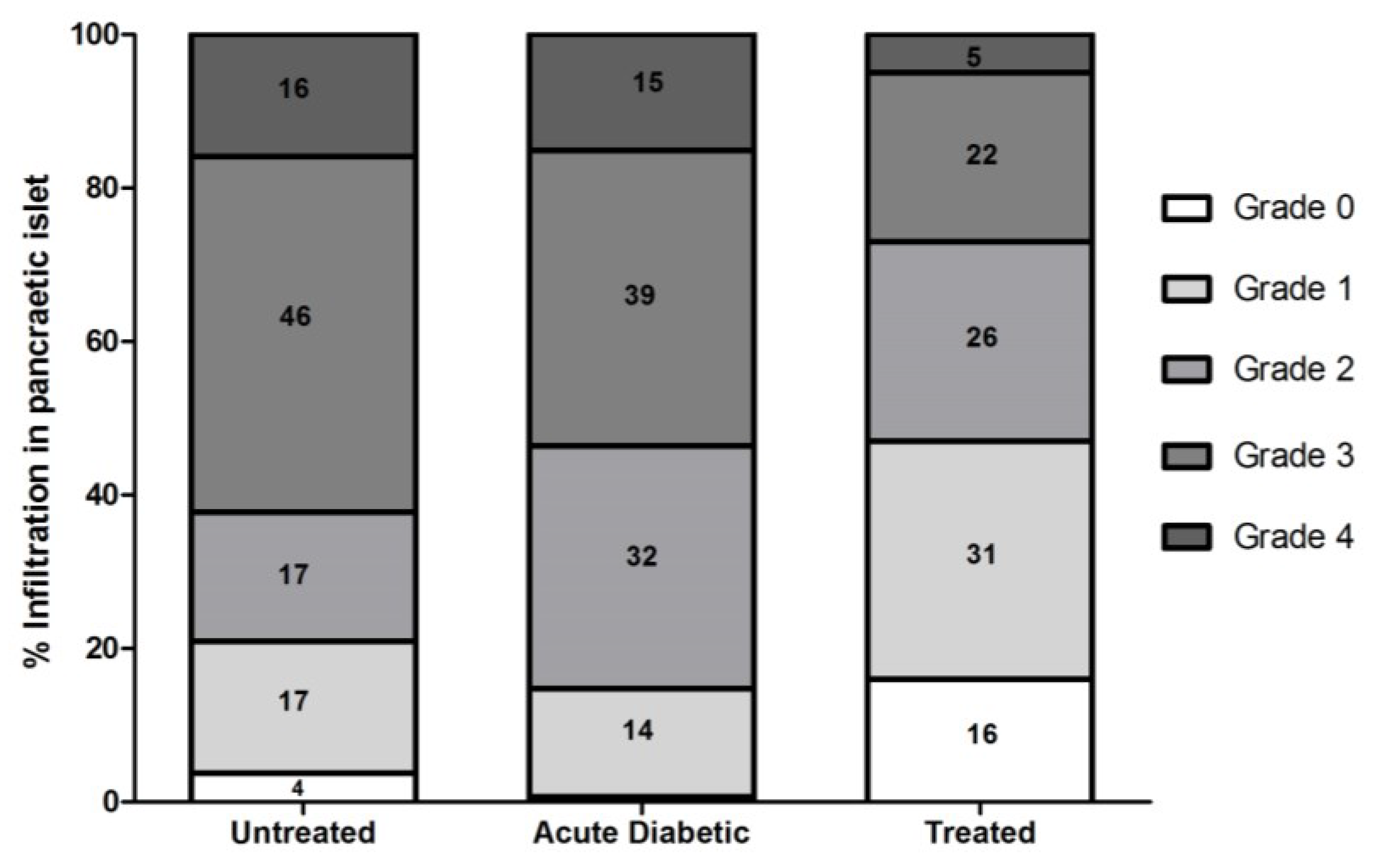
2.4.3. Histological Analysis
2.4.4. Detection of Serum Insulin Levels

2.4.5. Glucose Tolerance Test
2.4.6. Reduced Glutathione Analysis
2.4.7. Determination of Serum Antioxidant Activity and Lipid Peroxidation Assay
2.5. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.2. Chromatographic Characterization (HPLC)
| Phenolic Compounds | Aqueous | Ethanol | Methanol/Acetone |
|---|---|---|---|
| Gallic acid | 6402 ± 222.7 a,b | 7361 ± 115.7 a | 2677 ± 32.3 b |
| Rutin | 10026 ± 97.2 b | 18542 ± 142.6 a,b | 20210 ± 25.2 a |
| Ellagic acid | 2462 ± 55.4 b | 4869±48.8 a | 3389 ± 40.5 a,b |
3.3. Biological Assays: NOD Mice Treated with E. uniflora Leaves
3.3.1. Consumption of Water and Aqueous Extract of E. uniflora and Cumulative Frequency of Incidence of DM-1
3.3.2. Effect of Aqueous Extract of E. uniflora on Inflammation and Infiltration Index in Islet
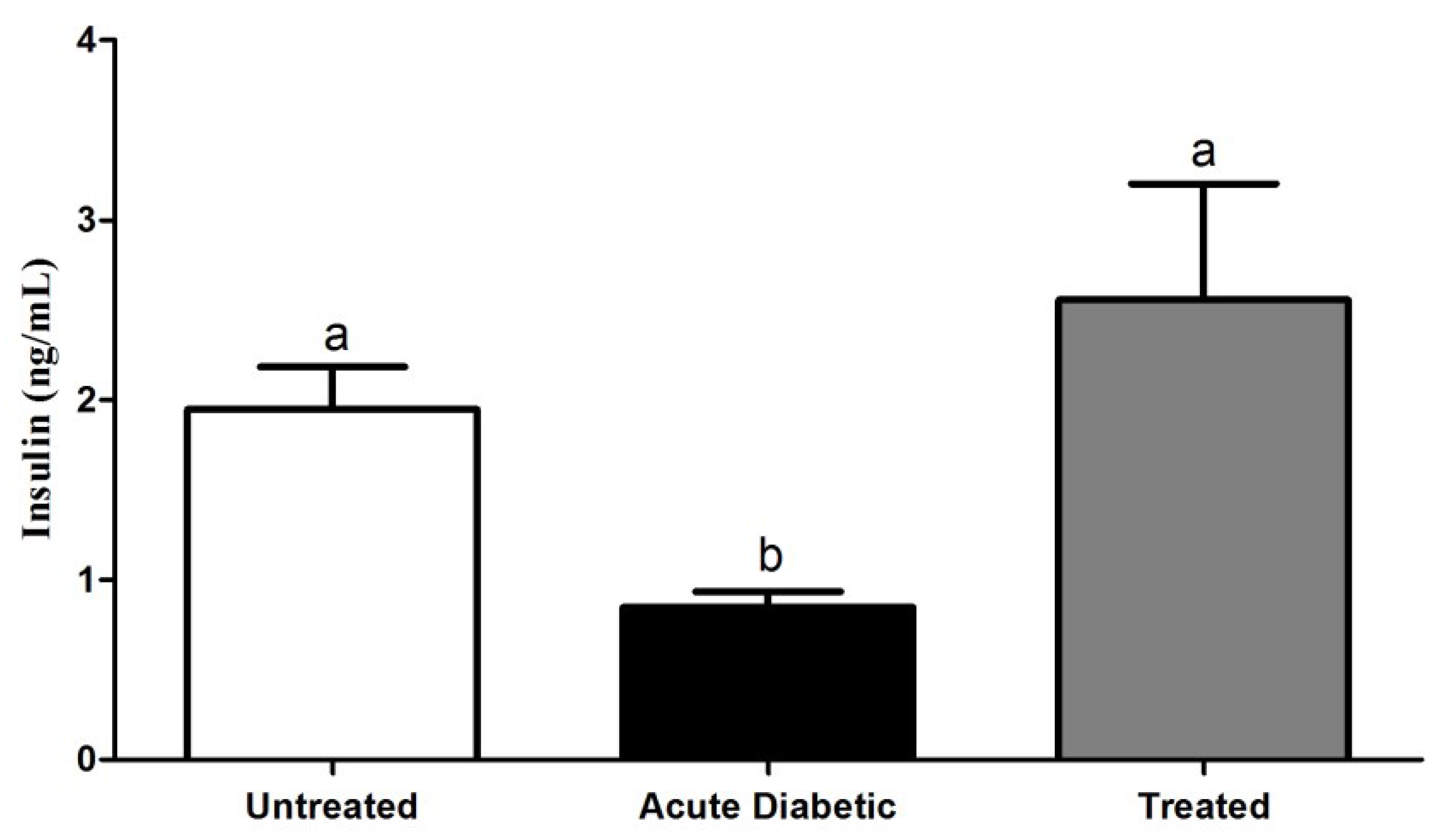
3.3.3. Effects of Aqueous Extract on Serum Insulin Concentration
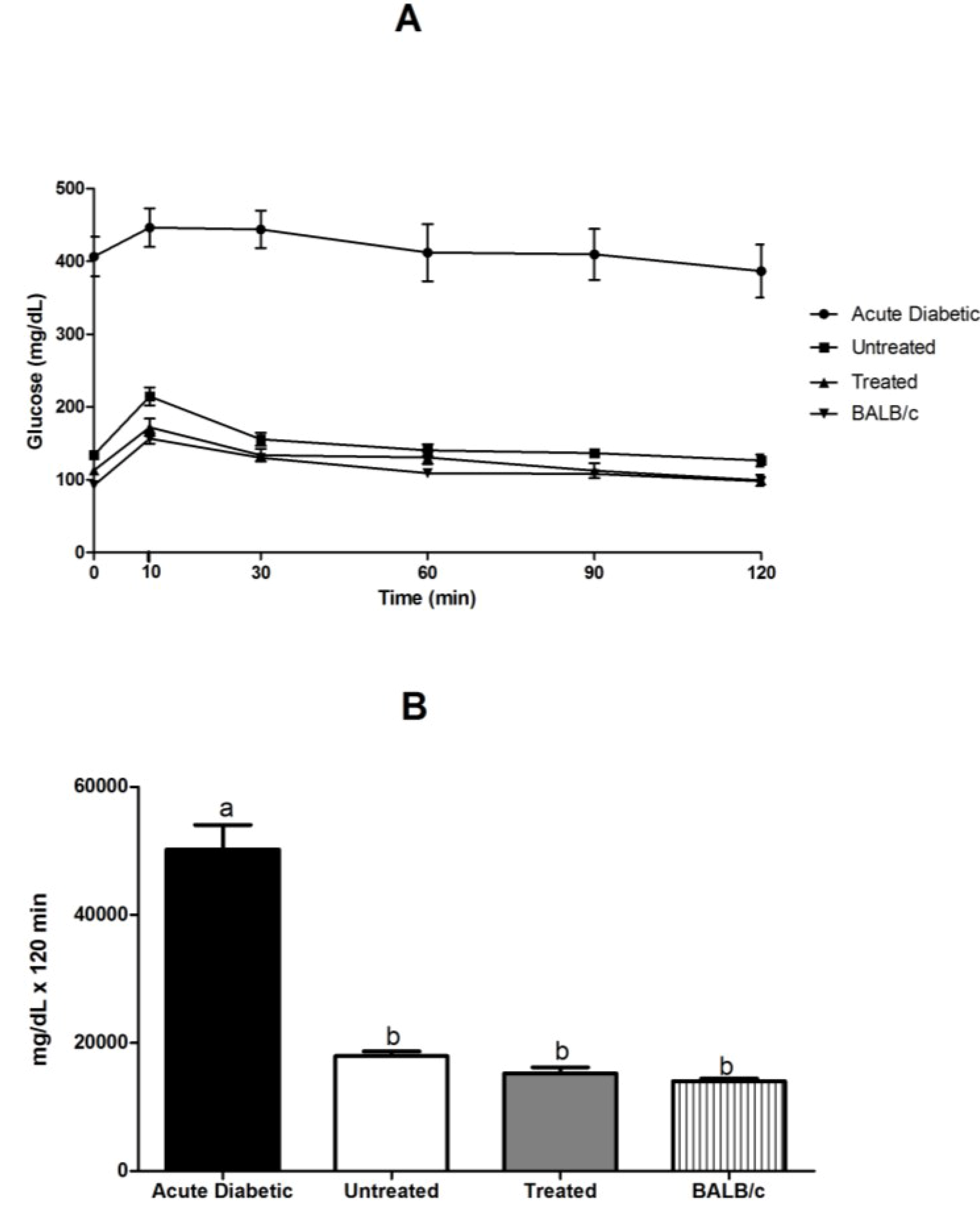
3.3.4. Glucose Tolerance Test in NOD and BALB/c Mice
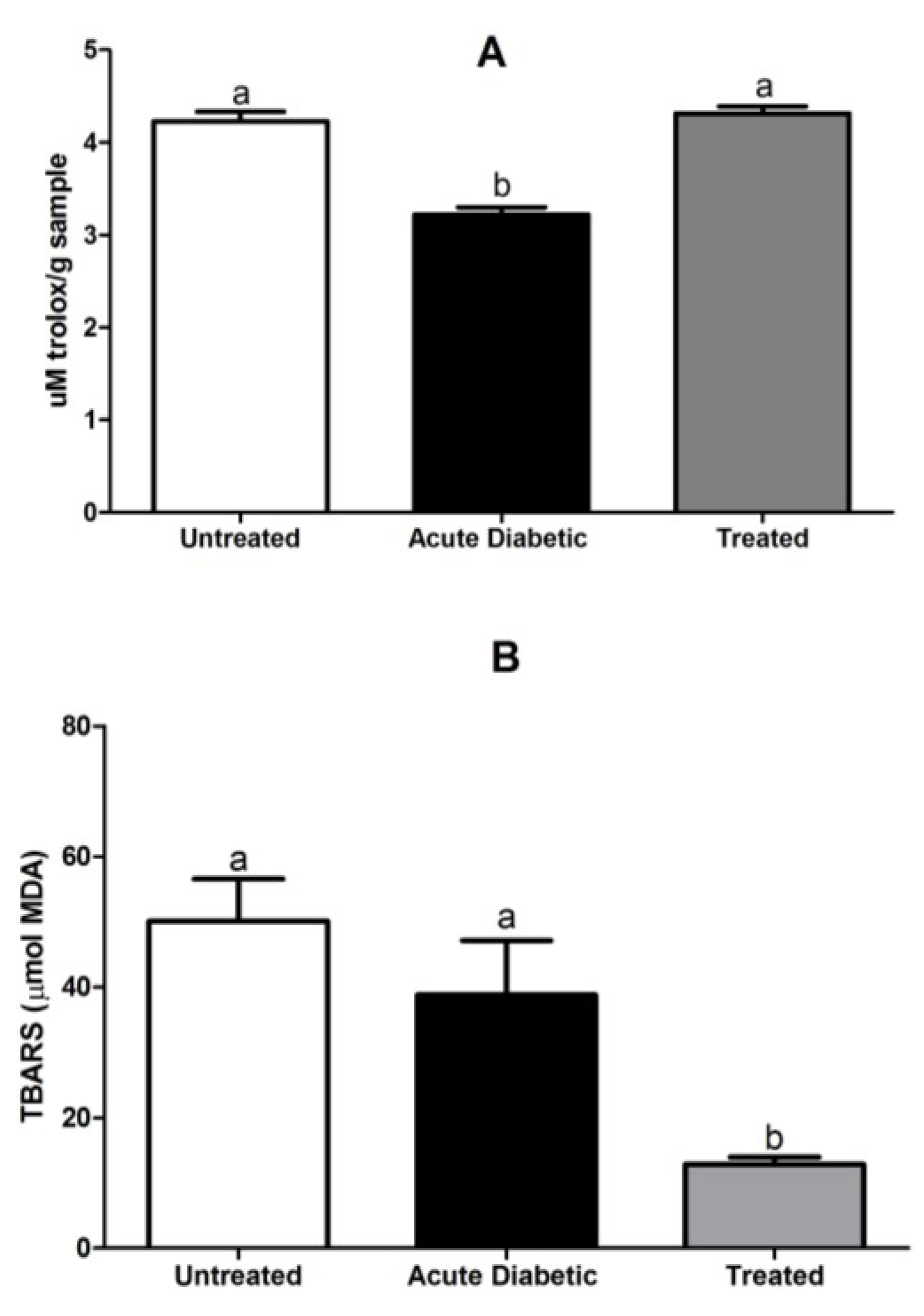
3.3.5. Reduced Glutathione Analysis

3.3.6. Determination of Serum Antioxidant Activity and Lipid Peroxidation Assay
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lorenzi, H.; Matos, F.J.A. Plantas Medicinais no Brasil Nativas e Exóticas, 2nd ed.; Instituto Plantarum de Estudos da Flora Ltda: Nova Odessa-SP, Brazil, 2002; p. 512. (In Portuguese) [Google Scholar]
- Auricchio, M.T.; Bacchi, E.M. Folhas de Eugenia uniflora L. (pitanga): Propriedades farmacobotânicas, químicas e farmacológicas. Rev. Inst. Adolfo. Lutz. 2003, 62, 55–61. (In Portuguese) [Google Scholar]
- Temple, N.J. Antioxidants and Disease: More Question than Answers. Nutr. Res. 200, 20, 449–459. [Google Scholar] [CrossRef]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [PubMed]
- Aherne, S.A.; Obrien, N.M. Dietery flavonols: Chemistry, food content, and, metabolism. Nutr. Res. 2002, 18, 75–81. [Google Scholar]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.C.; Lean, M.E.; Crozler, A. Extraction of Phenolics and Changes in Antioxidant Activity of Red Wines during Vinification. J Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Vieira, K.P.; Zollner, A.R.A.S.L.; Malaguti, C.; Vilella, C.A.; Zollner, R.L. Ganglioside GM1 effects on the expression of nerve growth factor (NGF), Trk-A receptor, proinflammatory cytokines and on autoimmune diabetes onset in non-obese diabetic (NOD) mice. Cytokine 2008, 42, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Kunimoto, K.; Muraoka, Y.; Mizushima, Y.; Katagiri, K.; Tochino, Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980, 29, 1–13. [Google Scholar] [PubMed]
- Colomeu, T.C.; Figueiredo, D.; Cazarin, C.B.; Schumacher, N.S.G.; Marostica, M.R., Jr.; Meletti, L.M.; Zoller, R.L. Antioxidant and anti-diabetic potential of Passiflora alata Curtis aqueous leaves extract in type 1 diabetes mellitus (NOD-mice). Int. Immunopharmacol. 2013, 18, 106–115. [Google Scholar] [PubMed]
- Spagolla, L.C.; Santos, M.M.; Passos, L.M.L.; Aguiar, C.L. Extração alcóolica de fenólicos e flavonóides totais de mirtilo Rabbiteye (Vaccinium ashei) e sua atividade antioxidante. Rev. Ciênc. Farm. Básica Apl. 2009, 30, 59–64. (In Portuguese) [Google Scholar]
- Larrauri, J.A.; Ruperez, P.; Saura Calixto, F. Mango peel fibres with antioxidant activity. Z. Lebensm. Unters. Forsch. 1997, 205, 39–42. [Google Scholar] [CrossRef]
- Brand-Willians, W.; Cuverlier, M.E.; Berset, C. Use of a free radical method to evaluated antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Benzie, S.E.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary as determined by a modified ferric reducing/abtioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [PubMed]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of scavenging activity of phenolic compounds using the ABTS assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef] [PubMed]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczpa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2007, 15, 137–149. [Google Scholar] [CrossRef]
- Viana, F.C.; Santana, A.C.M.; Moura, R.M.X. Identificação fitoquímica de flavonóides e taninos em folhas de pitanga (Eugenia uniflora L.) utilizadas tradicionalmente na região sul da Bahia. Interfaces Pharm. Ciênc. Saúde 2012, 1, 28–37. (In Portuguese) [Google Scholar]
- Brune, M.; Rossander-Hultén, L.; Hallberg, L.; Gleerup, A.; Sandberg, A.S. Iron Absorption from Bread in Humans: Inhibitng Efects of Cereal Fiber, Phytate and Inositol Phosphates with Diferent Numbers of Phosphate Groups1. Hum. Clin. Nutr. 1992, 122, 442–449. [Google Scholar]
- Schons, P.F.; Edi, F.R.; Battestin, V.; Macedo, G.A. Effect of enzymatic treatment on tannins and phytate in sorghum (Sorghum bicolor) and its nutritional study in rats. Food Sci. Technol. 2011, 46, 1253–1258. [Google Scholar] [CrossRef]
- Pavin, E.J.; Zollner, R.L. Implantação da linhagem NOD mice (camundongos diabéticos não-obesos) no Brasil: Contribuição deste modelo animal ao estudo do diabetes mellitus insulino-dependente e outras doenças autoimunes. Arq. Bras. Endocrinol. Metab. 1994, 38, 105–108. (In Portuguese) [Google Scholar]
- Suarez-Pinzon, W.L.; Szabo, C.; Rabinovitch, A. Development of autoimmune diabetes in NOD mice is associated with the formation of peroxynitrite in pancreatic islet beta-cells. Diabetes 1997, 46, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Signore, A.; Pozzilli, P.; Gale, E.A.; Andreani, D.; Beverley, P.C. The natural history of lymphocyte subsets infiltrating the pancreas of NOD mice. Diabetologia 1989, 32, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Oliveira, D.; Vilella, C.A.; Zanin, M.E.; Castro, G.M.; Moreira-Filho, D.C.; Zollner, R.L. Kinetics of TNF-alpha and IFN-gamma mRNA expression in islets and spleen of NOD mice. Braz. J. Med. Biol. Res. 2002, 35, 1347–1355. (In Portuguese) [Google Scholar] [CrossRef]
- Leiter, E.H. The NOD mouse: A model for insulin-dependent diabetes mellitus. Curr. Protoc. Immunol. 2001. [Google Scholar] [CrossRef]
- Andrikopoulos, S.; Blair, A.R.; Deluca, N.; Fam, B.C.; Proietto, J. Evaluating the glucose tolerance test in mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1323–E1332. [Google Scholar] [PubMed]
- Hartree, E.F. Determination of the lowry method that gives a linear photometric response. Anal. Biochem. 1972, 48, 422–427. [Google Scholar] [CrossRef]
- Faure, P.; Lafond, J.L. Measurement of plasma sulfhydryl and carbonyl groups as a possible indicator of protein oxidation. In Analysis of Free Radicals in Biological Systems, 1st ed.; Birkhäuser: Basel, Switzerland, 1995; pp. 237–248. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Cazarin, B.B.C.; de Silva, J.K.; Colomeu, T.C.; Batista, A.G.; Meletti, L.M.M.; Paschoal, J.A.R.; Junoir, S.B.; Braga, P.A.C.; Reyes, F.G.R.; et al. Intake of passiflora edulis leaf extract improves antioxidant and anti-inflammatory status in rats with 2,4,6-trinitrobenzenesulphonic acid induced colitis. J. Funct. Foods 2015, 17, 576–586. [Google Scholar]
- Sucupira, N.R.; Silva, A.B.; Pereira, G.; Costa, J.N. Methods for Measuring Antioxidant Activity of Fruits. UNOPAR Cient. Ciênc. Biol. Saúde 2012, 14, 263–269. [Google Scholar]
- Degáspari, C.M.; Waszczynskyj, N. Antioxidants properties of phenolic compounds. Visão Acad. Curitiba. 2004, 5, 35–40. [Google Scholar]
- Siddhuraju, P.; Becker, K. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jimenez, J.; Calixto, F.S. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Bagetti, M.; Facco, E.M.P.; Piccolo, J.; Hirsch, G.E.; Rodriguez-Amaya, D.; Kobori, C.N.; Vizzotto, M.; Emanuelli, T. Physicochemical characterization and antioxidant capacity of pitanga. Ciênc. Tecnol. Aliment. 2011, 31, 147–154. (In Portuguese) [Google Scholar] [CrossRef]
- Sousa, C.M.M.; Silva, H.R.; Vierira, G.M., Jr.; Ayres, M.C.C.; da Costa, L.S.; Araújo, D.S.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.M.; Brandão, M.S.; Chaves, M.H. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Quim. Nova. 2007, 30, 351–355. (In Portuguese) [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ. 2010, 33, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Santos, R.M.; Fortes, G.A.C.; Ferri, P.H.; Santos, S.C. Influence of foliar nutrients on phenol levels in leaves of Eugenia uniflora. Rev. Bras. Farmacogn. 2011, 21, 581–586. [Google Scholar] [CrossRef]
- Lee, M.H.; Nishimoto, S.; Yang, L.L.; Yen, K.Y.; Hatano, T.; Yoshida, T.; Okuda, T. Two macrocyclic hydrolysable tannin dimers from Eugenia uniflora. Phytochemistry 1997, 44, 1343–1349. [Google Scholar]
- Oliveira, A.L.; Destandau, E.; Fougere, L.; Lafosse, M. Isolation by pressurised fluid extraction (PFE) and identification using CPC and HPLC/ESI/MS of phenolic compounds from Brazilian cherry seeds (Eugenia uniflora L.). Food Chem. 2014, 145, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Al-Salih, R.M. Clinical Experimental Evidence: Synergistic Effect of Gallic Acid and Tannic Acid as Antidiabetic and Antioxidant Agents. Med. J. 2010, 4, 109–119. [Google Scholar]
- Kamalakkannan, N.; Stanely, M.P.P. Rutin improves glucose homeostasis in streptozotocin diabetic tissues by altering glycolytic and gluconeogenic enzymes. J. Biochem. Molecul. Toxicol. 2006, 20, 96–102. [Google Scholar]
- Gottlieb, M.G.V.; Morassutti, A.L.; Cruz, I.B.M. Transição epidemiológica, estresse oxidativo e doenças crônicas não transmissíveis sob uma perspectiva evolutiva. Sci. Med. 2011, 21, 69–80. (In Portuguese) [Google Scholar]
- Anderson, M.S.; Bluestone, J.A. The NOD mouse: A model of immune dysregulation. Annu. Rev. Immunol. 2005, 23, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Aeschbach, R.; Loliger, J.; Aruoma, O.I. The characterization of Antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef]
- Kalkan, I.H.; Suher, M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak. J. Med. Sci. 2013, 29, 938–942. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Seghrouchni, I.; Drai, J.; Bannier, E.; Riviere, J.; Calmard, P.; Garcia, I.; Orgiazzi, A. Oxidative stress parameters in type I, type II and insulin-treated type 2 diabetes mellitus insulin treatment efficiency. Clin. Chim. Acta 2002, 321, 89–96. [Google Scholar] [CrossRef]
- Dave, G.S.; Kalia, K. Hyperglycemia induced oxidative stress in type-1 and type-2 diabetic patients with and without nephropathy. Cell. Mol. Biol. 2007, 53, 68–78. [Google Scholar] [PubMed]
- Turk, H.M.; Sevinc, A.; Camci, C.; Cigli, A.; Buyukberber, S.; Savli, H.; Bayrakatar, N. Plasma lipid peroxidation products and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Acta Diabetol. 2002, 39, 117–122. [Google Scholar] [CrossRef]
- Nakhaee, A.; Bokaeian, M.; Saravani, M.; Farhangi, A.; Akbarzadeh, A. Attenuation of oxidative stress in streptozotocin-induced diabetic rats by Eucalyptus globulus. Indian J. Clin. Biochem. 2009, 24, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Jelenik, T.; Sequaris, G.; Kaul, K.; Ouwens, D.M.; Phielix, E.; Kotzka, J.; Knebel, B.; Weiß, J.; Reinbeck, A.L.; Janke, L.; et al. Tissue-specific differences in the development of insulin resistance in a mouse model for type 1 diabetes. Diabetes 2014, 63, 3856–3867. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M.; Gorska, M.; Wilk, J.; Kretowski, A. Reduced intake of dietary antioxidants can impair antioxidant status in type 2 diabetes patients. Pol. Arch. Med. Wewn. 2014, 124, 599–607. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schumacher, N.S.G.; Colomeu, T.C.; De Figueiredo, D.; Carvalho, V.D.C.; Cazarin, C.B.B.; Prado, M.A.; Meletti, L.M.M.; Zollner, R.D.L. Identification and Antioxidant Activity of the Extracts of Eugenia uniflora Leaves. Characterization of the Anti-Inflammatory Properties of Aqueous Extract on Diabetes Expression in an Experimental Model of Spontaneous Type 1 Diabetes (NOD Mice). Antioxidants 2015, 4, 662-680. https://doi.org/10.3390/antiox4040662
Schumacher NSG, Colomeu TC, De Figueiredo D, Carvalho VDC, Cazarin CBB, Prado MA, Meletti LMM, Zollner RDL. Identification and Antioxidant Activity of the Extracts of Eugenia uniflora Leaves. Characterization of the Anti-Inflammatory Properties of Aqueous Extract on Diabetes Expression in an Experimental Model of Spontaneous Type 1 Diabetes (NOD Mice). Antioxidants. 2015; 4(4):662-680. https://doi.org/10.3390/antiox4040662
Chicago/Turabian StyleSchumacher, Nayara Simon Gonzalez, Talita Cristina Colomeu, Daniella De Figueiredo, Virginia De Campos Carvalho, Cinthia Baú Betim Cazarin, Marcelo Alexandre Prado, Laura Maria Molina Meletti, and Ricardo De Lima Zollner. 2015. "Identification and Antioxidant Activity of the Extracts of Eugenia uniflora Leaves. Characterization of the Anti-Inflammatory Properties of Aqueous Extract on Diabetes Expression in an Experimental Model of Spontaneous Type 1 Diabetes (NOD Mice)" Antioxidants 4, no. 4: 662-680. https://doi.org/10.3390/antiox4040662
APA StyleSchumacher, N. S. G., Colomeu, T. C., De Figueiredo, D., Carvalho, V. D. C., Cazarin, C. B. B., Prado, M. A., Meletti, L. M. M., & Zollner, R. D. L. (2015). Identification and Antioxidant Activity of the Extracts of Eugenia uniflora Leaves. Characterization of the Anti-Inflammatory Properties of Aqueous Extract on Diabetes Expression in an Experimental Model of Spontaneous Type 1 Diabetes (NOD Mice). Antioxidants, 4(4), 662-680. https://doi.org/10.3390/antiox4040662