Differential Toxicity of Water-Soluble Versus Water-Insoluble Components of Cowshed PM2.5 on Ovarian Granulosa Cells and the Regulatory Role of Txnip in Overall Toxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Cowshed PM2.5 Sample Collection and Preparation
2.2. Physical and Chemical Properties of Cowshed PM2.5
2.3. Endotoxin Content Assay
2.4. Transcriptomics and Data Analysis
2.5. Culture and Grouping of Rat Ovarian Granulosa Cells
2.6. ELISA
2.7. Oxidative Stress Assessment
2.8. ATP Assay
2.9. Intracellular Ca2+, JC-1 and MitoSOX Assays
2.10. qPCR and mtDNA Copy Number Assay
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
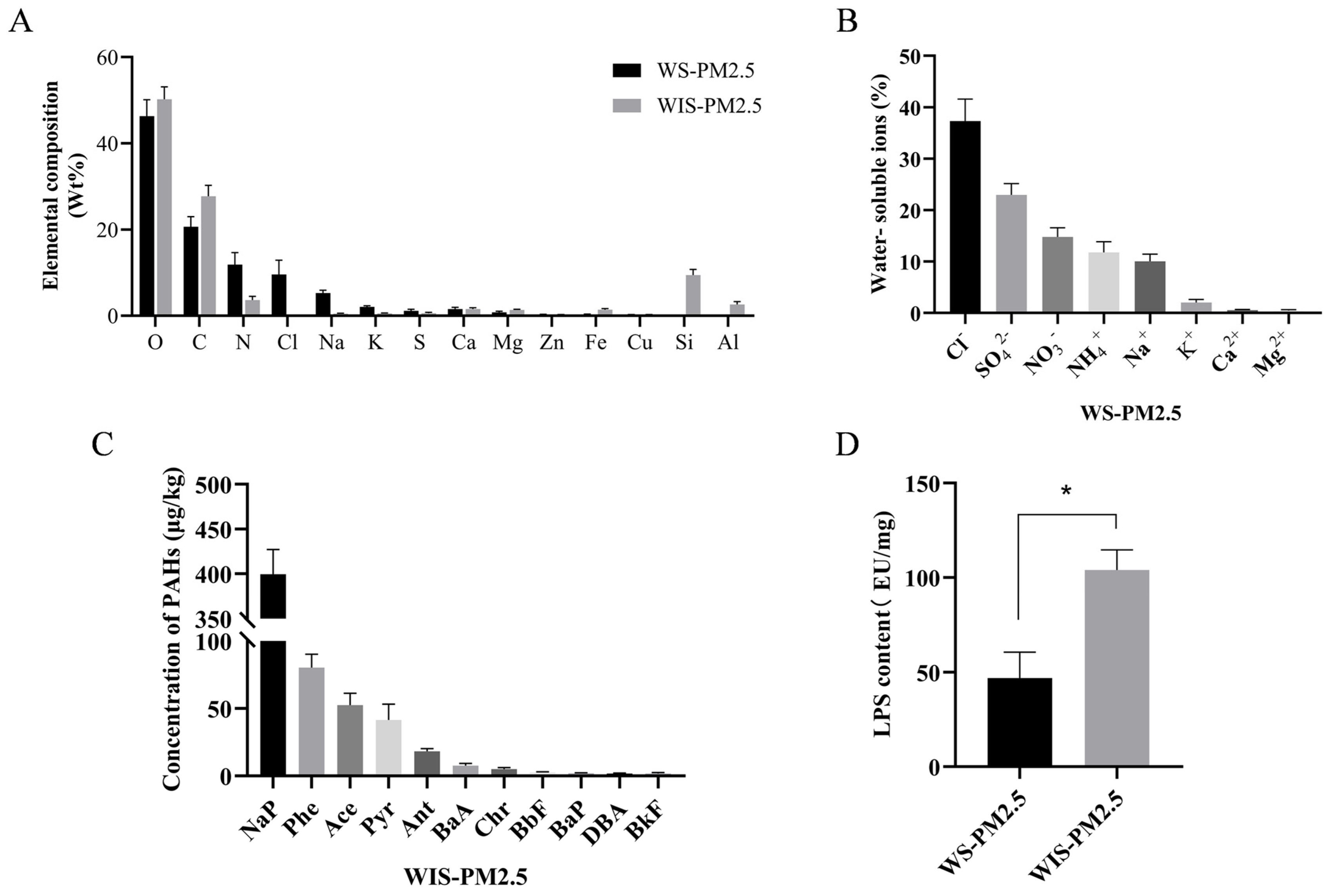
3.1. Chemical Composition and Endotoxin Content Characteristics of Cowshed PM2.5
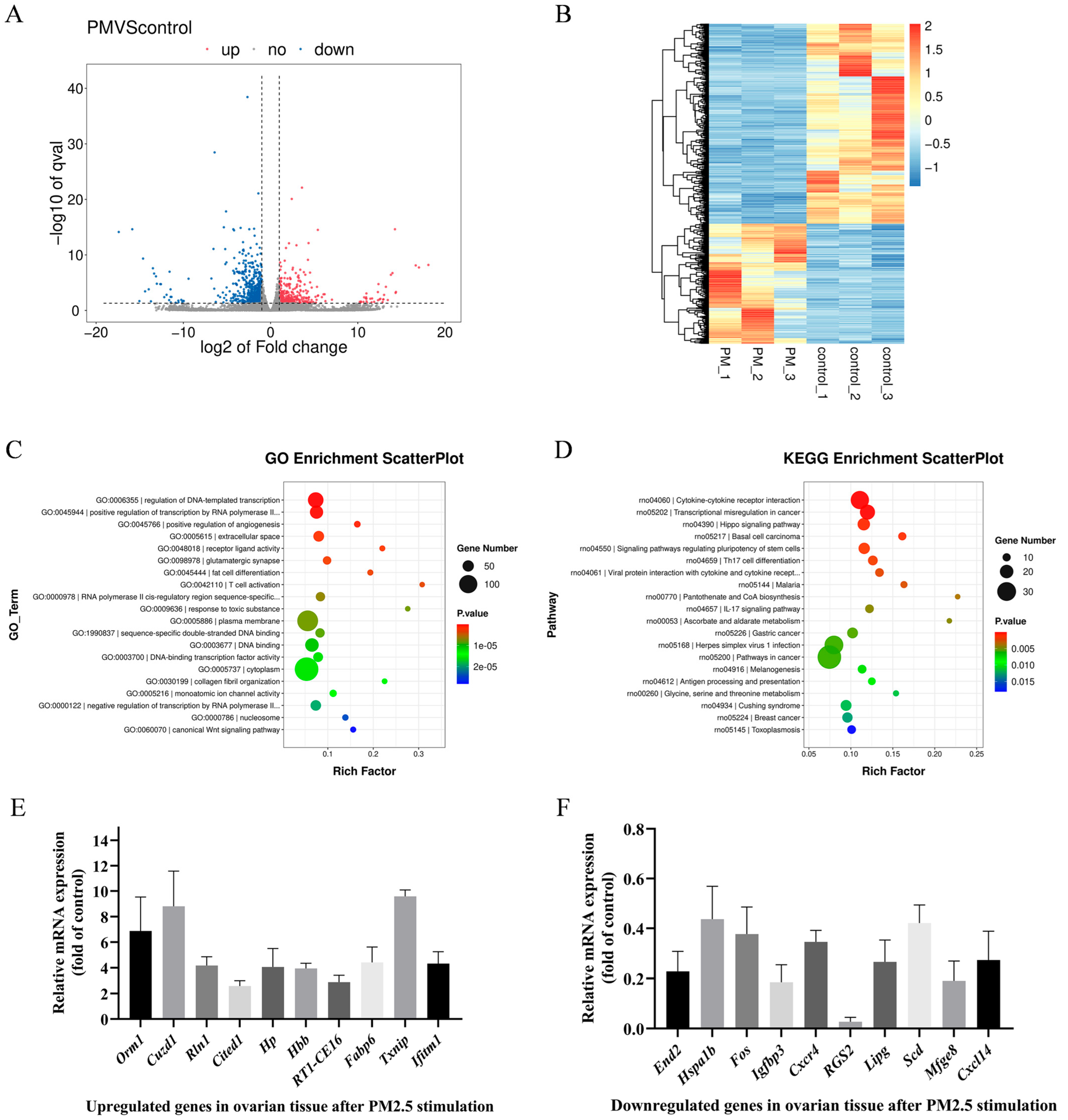
3.2. Cowshed PM2.5-Induced Differential Expression of Ovarian mRNAs in Rats
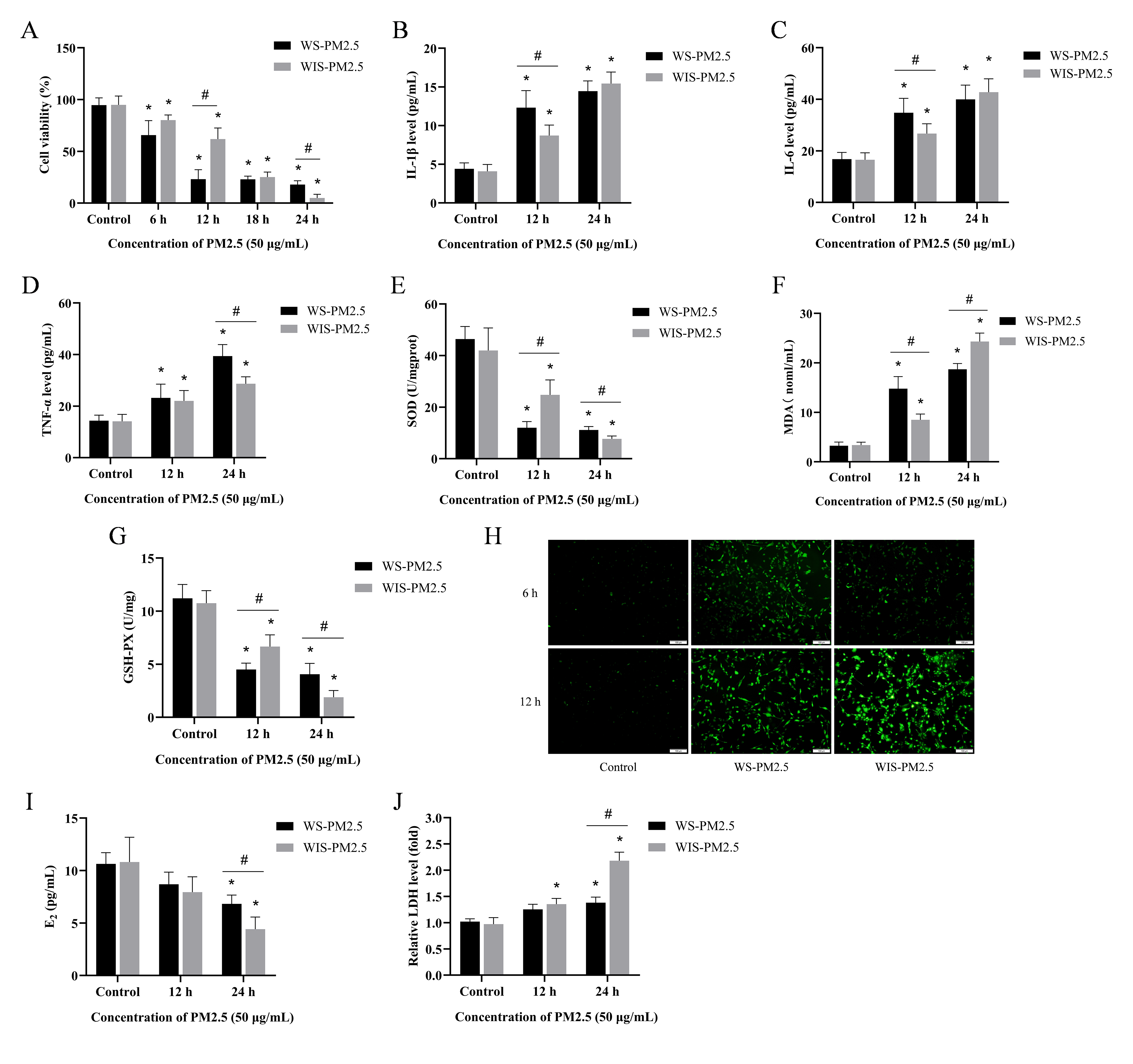
3.3. Water-Soluble Components in Cowshed PM2.5 Had Short-Term Acute Cytotoxicity
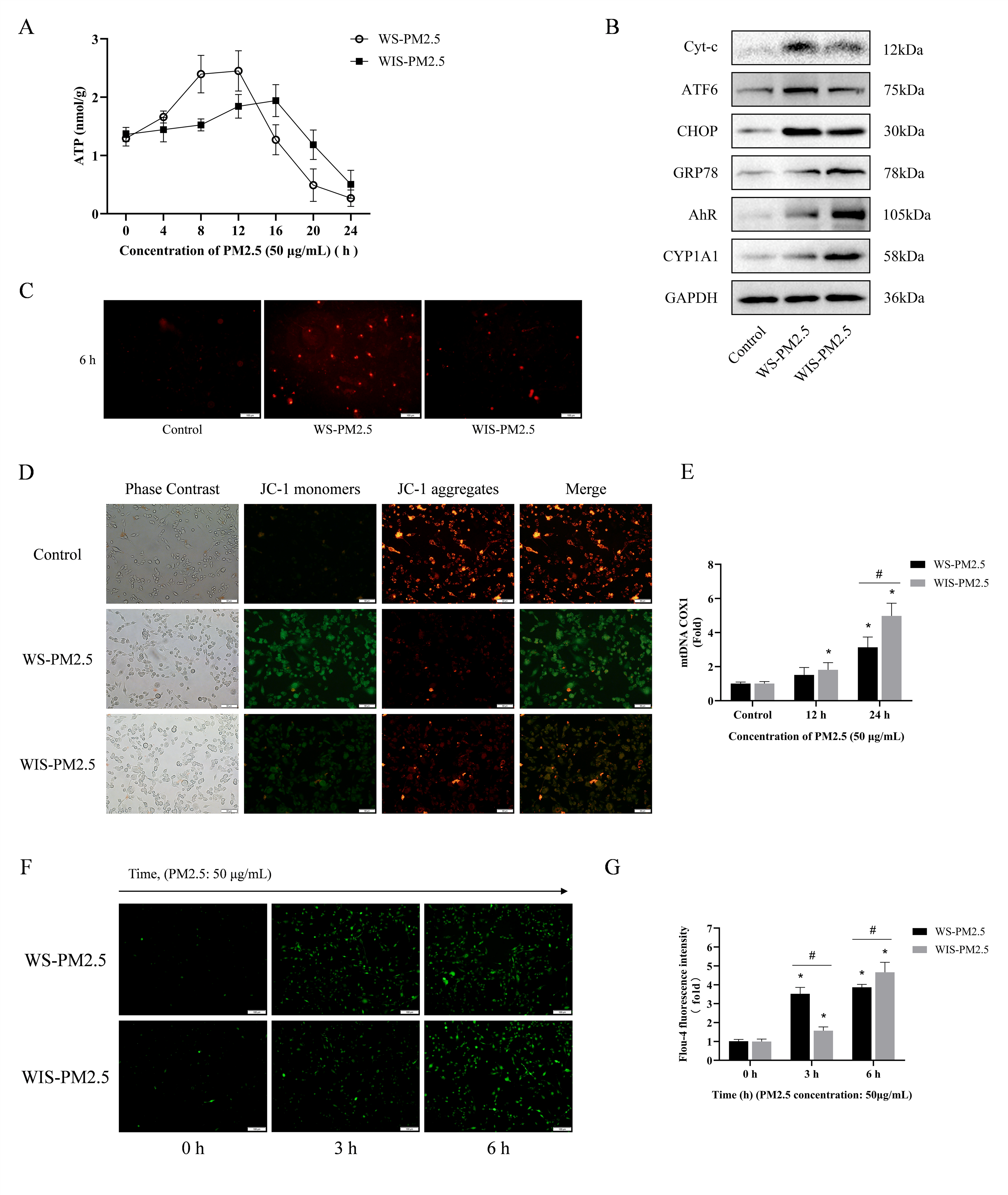
3.4. WS-PM2.5 Induced Rapid Responses in Mitochondrial Dysfunction and Endoplasmic Reticulum Ca2+ Release
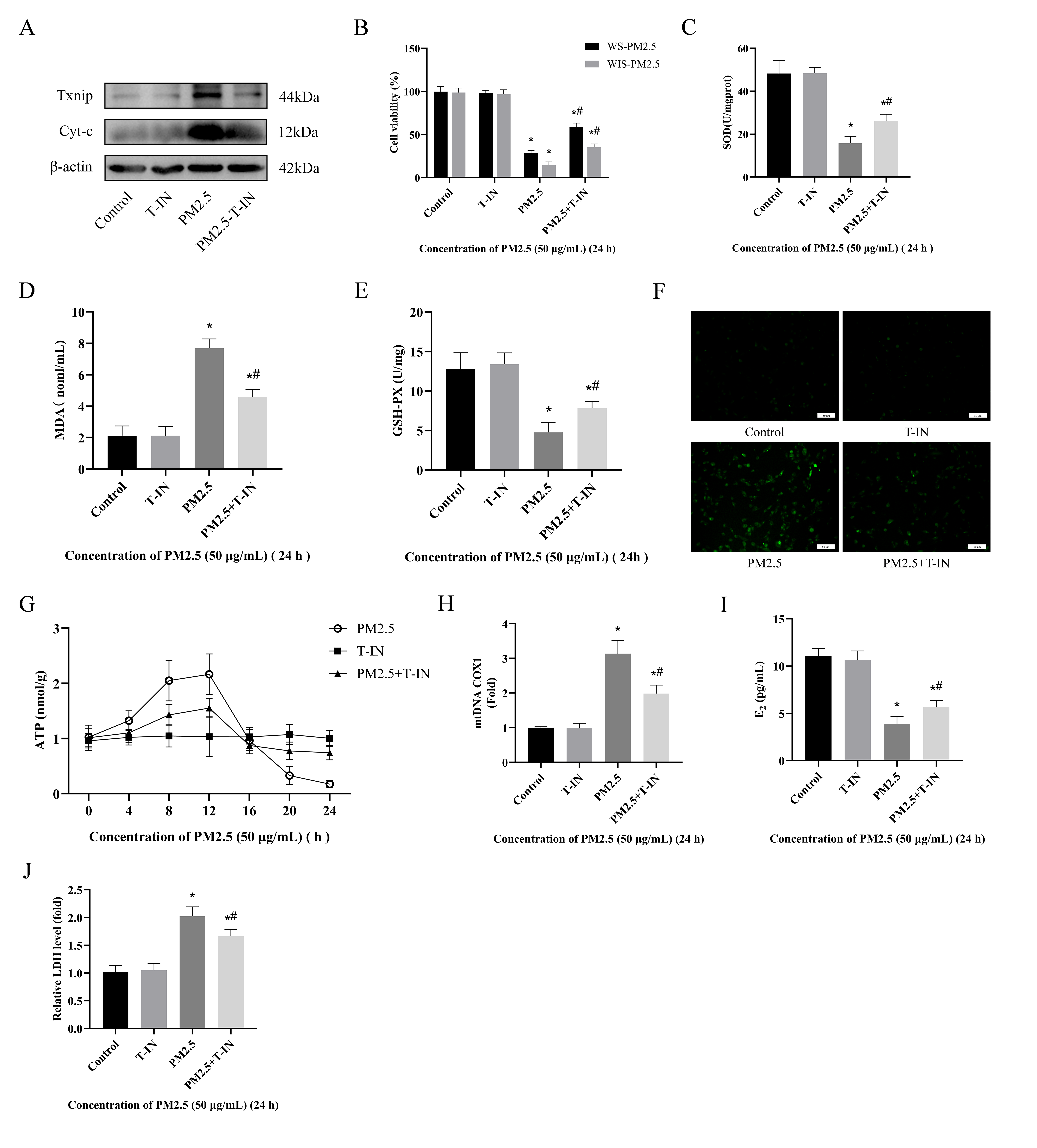
3.5. Inhibition of Txnip Alleviates Cowshed PM2.5-Induced Ovarian Granulosa Cell Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhou, S.; Xi, X. Assessing the short-term exposure risk and mortality burden of dust and fine aerosol PM2.5 in Central Asia. Sci. Total Environ. 2025, 991, 179864. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Zheng, Y.; Tian, J.; Zhang, L. Single-cell RNA sequencing of estrual mice reveals PM2.5-induced uterine cell heterogeneity and reproductive toxicity. Ecotoxicol. Environ. Saf. 2024, 284, 116968. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Zheng, Z.-Y.; Peng, Z.-X.; Ni, H.-G. Global impact of PM2.5 on cardiovascular disease: Causal evidence and health inequities across region from 1990 to 2021. J. Environ. Manag. 2025, 374, 124168. [Google Scholar] [CrossRef]
- Ge, X.; Song, C.; Zhu, C.; Ding, Y.; Liu, M.; Yang, J.; Tian, C.; Zhang, J.; Wu, L.; Zhu, L.; et al. Association of ambient fine particulate matter (PM2.5) and its constituents with risk of pulmonary nodules in a lung cancer screening project. Environ. Res. 2025, 285, 122409. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, Y.; Li, M.; Wu, Y.; Yan, W.; Dai, J.; Wu, M.; Ding, W.; Zhang, J.; Zhang, F.; et al. Maternal exposure to PM2.5 decreases ovarian reserve in neonatal offspring mice through activating PI3K/AKT/FoxO3a pathway and ROS-dependent NF-κB pathway. Toxicology 2022, 481, 153352. [Google Scholar] [CrossRef]
- He, J.-L.; Liu, R.-L.; Hu, Y.-L.; Yao, Q.-Z.; Xu, Z.-L.; Geng, L.-H.; Wang, T.; Luo, X.; Yao, Y.-L.; Zhang, Y.-J.; et al. Association of long-term PM2.5 and its components exposure with ovarian hyperstimulation syndrome risk in assisted reproductive technology patients. Environ. Pollut. 2025, 380, 126569. [Google Scholar] [CrossRef]
- Ma, Z.; Du, X.; Zhao, C.; Sun, Y.; Jia, Y.; Liang, X.; Yu, X.; Gao, Y. Protective role of RGS2 in PM2.5-induced ovarian injury in rats: Modulation of Gq/11 signaling to maintain granulosa cell [Ca2+]i stability. Ecotoxicol. Environ. Saf. 2025, 302, 118537. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, X.; Liu, H.; Xu, K.; Yang, Y.; Yang, M.; Wei, D.; Zhao, S.; Jiao, X.; Zhao, Q.; et al. Associations of long-term exposure to fine particulate matter and its components with ovarian aging: Evidence from a cross-sectional study in China. J. Hazard. Mater. 2025, 494, 138589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, S.; Lu, Y.; Qi, J.; Li, X.; Gao, S.; Qi, X.; Tan, J. Association of ambient PM2.5 and its components with in vitro fertilization outcomes: The modifying role of maternal dietary patterns. Ecotoxicol. Environ. Saf. 2024, 282, 116685. [Google Scholar] [CrossRef]
- Liu, M.; Huang, Y.; Sun, Y.; Sun, Y.; Ren, X.; Xuan, Y.; Yun, Y.; Bai, G.; Jiang, F. Analysis of antibiotic resistance genes and potential host microorganisms in atmospheric PM2.5 in the Beijing urban area. J. Environ. Chem. Eng. 2025, 13, 115456. [Google Scholar] [CrossRef]
- Xin, H.; Gao, M.; Wang, X.; Qiu, T.; Guo, Y.; Zhang, L. Animal farms are hot spots for airborne antimicrobial resistance. Sci. Total Environ. 2022, 851, 158050. [Google Scholar] [CrossRef]
- Arcidiacono, C.; Rapisarda, P.; Palella, M.; Longo, M.V.; Moscato, A.; D’Urso, P.R.; Ferrante, M.; Fiore, M. Compliance with the Verification of Environmental Technologies for Agricultural Production Protocol in Ammonia and Particulate Matter Monitoring in Livestock Farming: Development and Validation of the Adherence VERA Index. Environments 2026, 13, 24. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zhang, Y.; Lee, J.; Su, J.; Gates, R.S. Characterization of trace elements and ions in PM10 and PM2.5 emitted from animal confinement buildings. Atmos. Environ. 2011, 45, 7096–7104. [Google Scholar] [CrossRef]
- Basini, G.; Ramoni, R.; Grolli, S.; Bussolati, S.; Assogna, L.; Grasselli, F. Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells. Animals 2026, 16, 81. [Google Scholar] [CrossRef]
- Bai, L.; Fu, P.; Dong, C.; Li, Z.; Yue, J.; Li, X.; Cao, Q.; Han, Y.; Zhang, S.; Li, R. Study of association between embryo growth arrest (EGA) and atmospheric fine particulate matter pollution (PM2.5) and spatial metabolomics of villi derived from pregnant women. J. Hazard. Mater. 2025, 485, 136833. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.; Hong, Y.; Chen, J.; Li, H.-B.; Li, H.; Yao, X.; Mehmood, T.; Feng, X.; Luo, X.-S. Comparative in vitro toxicological effects of water-soluble and insoluble components of atmospheric PM2.5 on human lung cells. Toxicol. Vitr. 2024, 98, 105828. [Google Scholar] [CrossRef]
- Cao, Y.; Hu, L.; Chen, R.; Chen, Y.; Liu, H.; Wei, J. Unfolded protein response-activated NLRP3 inflammasome contributes to pyroptotic and apoptotic podocyte injury in diabetic kidney disease via the CHOP-TXNIP axis. Cell. Signal. 2025, 130, 111702. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M.; et al. Epigenetic regulation of TXNIP-mediated oxidative stress and NLRP3 inflammasome activation contributes to SAHH inhibition-aggravated diabetic nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, R.; Wu, W.; Luan, M.; Li, X.; Liu, C.; Zhang, L.; Liu, Y.; Tan, F.; Han, X.; et al. Fine particulate matter induces cardiac fibrosis via the CHOP/TXNIP/NLRP3 pathway in C57 BL/6 mice. Int. Immunopharmacol. 2025, 161, 115073. [Google Scholar] [CrossRef]
- Zhu, R.; Yang, L.; Li, Y. TXNIP participates in the pathogenesis of PCOS by regulating glycolysis in granulosa cells. Biochem. Biophys. Res. Commun. 2025, 775, 152149. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Cao, M.; Li, F.; Tian, M.; Ren, H.; Chi, Q.; Huang, Q. Atmospheric PM2.5 induce autophagy and autophagic flux blockage in HUVEC cells via ROS/TXNIP signaling: Important role of metal components. J. Hazard. Mater. 2023, 445, 130623. [Google Scholar] [CrossRef]
- Ma, Z.; Du, X.; Sun, Y.; Sun, K.; Zhang, X.; Wang, L.; Zhu, Y.; Basang, W.; Gao, Y. RGS2 attenuates alveolar macrophage damage by inhibiting the Gq/11-Ca2+ pathway during cowshed PM2.5 exposure, and aberrant RGS2 expression is associated with TLR2/4 activation. Toxicol. Appl. Pharmacol. 2024, 487, 116976. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef]
- Qi, Z.; Song, Y.; Ding, Q.; Liao, X.; Li, R.; Liu, G.; Tsang, S.; Cai, Z. Water soluble and insoluble components of PM2.5 and their functional cardiotoxicities on neonatal rat cardiomyocytes in vitro. Ecotoxicol. Environ. Saf. 2019, 168, 378–387. [Google Scholar] [CrossRef]
- Zhang, X.; Man, X.; Zhang, Q.; Zhu, L.; Chen, L.; Zhu, C.; Ci, X.; Yu, X. Melatonin protects against particulate matter-induced ovarian dysfunction by activating the Nrf2 signaling pathway to alleviate ferroptosis. Life Sci. 2024, 359, 123200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Li, H.; Wang, H.; Du, D.; Huang, H. ATF3 mediates PM2.5-induced apoptosis and inflammation in ovarian granulosa cells. J. Ovarian Res. 2024, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, Z.; Hao, P.; Ji, S.; Gao, Y. Characteristics and health impacts of bioaerosols in animal barns: A comprehensive study. Ecotoxicol. Environ. Saf. 2024, 278, 116381. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Du, X.; Sun, Y.; Jia, Y.; Liang, X.; Gao, Y. Attenuation of PM2.5-Induced Lung Injury by 4-Phenylbutyric Acid: Maintenance of [Ca2+]i Stability between Endoplasmic Reticulum and Mitochondria. Biomolecules 2024, 14, 1135. [Google Scholar] [CrossRef]
- Liu, X.; Yan, L.; Wang, J.; Effah, C.Y.; Lan, H.; Ding, L.; Wu, Y. Mass spectrometry analysis of PM2.5 in poultry farms and the cytotoxicity and metabolism perturbation of BEAS-2B cells. Anal. Bioanal. Chem. 2025, 417, 3371–3382. [Google Scholar] [CrossRef]
- Yi, W.; Liu, G.; Wang, M.; Wang, J.; Chen, D.; Shen, J. Increased nitrogen deposition and airborne particulate matter pollution in the vicinity of intensive animal farms caused by ammonia emissions. Agric. Ecosyst. Environ. 2025, 387, 109634. [Google Scholar] [CrossRef]
- Dai, P.; Shen, D.; Tang, Q.; Huang, K.; Li, C. PM2.5 from a broiler breeding production system: The characteristics and microbial community analysis. Environ. Pollut. 2020, 256, 113368. [Google Scholar] [CrossRef]
- Shen, D.; Wu, S.; Dai, P.Y.; Li, Y.S.; Li, C.M. Distribution of particulate matter and ammonia and physicochemical properties of fine particulate matter in a layer house. Poult. Sci. 2018, 97, 4137–4149. [Google Scholar] [CrossRef]
- Ventura, L.M.B.; Mateus, V.L.; de Almeida, A.C.S.L.; Wanderley, K.B.; Taira, F.T.; Saint’Pierre, T.D.; Gioda, A. Chemical composition of fine particles (PM2.5): Water-soluble organic fraction and trace metals. Air Qual. Atmos. Health 2017, 10, 845–852. [Google Scholar] [CrossRef]
- Zhao, T.; Cao, Y.; Cai, Q.; Bai, Y.; Huo, P.; Zhang, L.; Zhang, Y. Multiple spectroscopic insights into the coupling effect of transition metals and water-soluble organic compounds on the oxidative potential of PM2.5 during haze and clean days. J. Hazard. Mater. 2025, 496, 139406. [Google Scholar] [CrossRef]
- Luo, X.S.; Huang, W.; Shen, G.; Pang, Y.; Tang, M.; Li, W.; Zhao, Z.; Li, H.; Wei, Y.; Xie, L.; et al. Source differences in the components and cytotoxicity of PM2.5 from automobile exhaust, coal combustion, and biomass burning contributing to urban aerosol toxicity. Atmos. Chem. Phys. 2024, 24, 1345–1360. [Google Scholar] [CrossRef]
- Roy, R.; Jan, R.; Gunjal, G.; Bhor, R.; Pai, K.; Satsangi, P.G. Particulate matter bound polycyclic aromatic hydrocarbons: Toxicity and health risk assessment of exposed inhabitants. Atmos. Environ. 2019, 210, 47–57. [Google Scholar] [CrossRef]
- Dai, P.; Ding, M.; Yu, J.; Gao, Y.; Wang, M.; Ling, J.; Dong, S.; Zhang, X.; Zeng, X.; Sun, X. The Male Reproductive Toxicity Caused by 2-Naphthylamine Was Related to Testicular Immunity Disorders. Toxics 2024, 12, 342. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, H.; Zhu, Y.; Xie, S.; Shao, X.; Wang, C.; Chung, S.K.; Zhang, Z.; Hao, K. Exposure to Airborne PM2.5 Water-Soluble Inorganic Ions Induces a Wide Array of Reproductive Toxicity. Environ. Sci. Technol. 2024, 58, 4092–4103. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, J.; Song, J.; Liang, P.; Zheng, K.; Xiao, G.; Liu, L.; Zouboulis, C.C.; Lei, T. Particulate matter 2.5 regulates lipid synthesis and inflammatory cytokine production in human SZ95 sebocytes. Int. J. Mol. Med. 2017, 40, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tang, W.; Wang, L.; Xue, W.; Yao, W.; Zhong, Y.; Qiu, X.; Li, Y.; Chen, Y.; Wang, H.; et al. Transcriptomics changes and the candidate pathway in human macrophages induced by different PM2.5 extracts. Environ. Pollut. 2021, 289, 117890. [Google Scholar] [CrossRef] [PubMed]
- Leung, F.; Soosaipillai, A.; Kulasingam, V.; Diamandis, E.P. CUB and zona pellucida-like domain-containing protein 1 (CUZD1): A novel serological biomarker for ovarian cancer. Clin. Biochem. 2012, 45, 1543–1546. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferri-Borgogno, S.; Zhu, Y.; Sheng, J.; Burks, J.K.; Gomez, J.A.; Wong, K.K.; Wong, S.T.C.; Mok, S.C. Spatial Transcriptomics Depict Ligand–Receptor Cross-talk Heterogeneity at the Tumor-Stroma Interface in Long-Term Ovarian Cancer Survivors. Cancer Res. 2023, 83, 14. [Google Scholar] [CrossRef]
- Bergamini, A.; Tassi, E.; Wignall, J.; Bocciolone, L.; Candiani, M.; Potenza, A.; Manfredi, F.; Taccagni, G.; Scalisi, F.; Doglioni, C.; et al. 1900P—Activated effector T cells co-expressing multiple inhibitory receptors (IRs) are enriched in the tumor immune microenvironment in high grade serous ovarian cancer (HGSOC). Ann. Oncol. 2019, 30, v770. [Google Scholar] [CrossRef]
- Shen, J.; Xu, F.; Liu, T.; Ye, Y.; Xu, S. NAD+ Metabolism-Mediated SURF4-STING Axis Enhances T-Cell Anti-Tumor Effects in the Ovarian Cancer Microenvironment. Cell Death Dis. 2025, 16, 640. [Google Scholar] [CrossRef]
- Csibi, A.; Blenis, J. Hippo–YAP and mTOR pathways collaborate to regulate organ size. Nat. Cell Biol. 2012, 14, 1244–1245. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, T.; Liu, C.; Wu, H.; Weng, L.; Cai, J.; Liang, N.; Ge, H. Correlation between ovarian follicular development and Hippo pathway in polycystic ovary syndrome. J. Ovarian Res. 2024, 17, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tumaneng, K.; Guan, K.-L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ezzell, R.M.; Tompkins, R.G.; Warren, H.S. Cellular Distribution of Endotoxin after Injection of Chemically Purified Lipopolysaccharide Differs from That after Injection of Live Bacteria. J. Infect. Dis. 1994, 169, 95–104. [Google Scholar] [CrossRef]
- Chen, W.; Ge, P.; Lu, Z.; Liu, X.; Cao, M.; Yan, Z.; Chen, M. Acute exposure to seasonal PM2.5 induces toxicological responses in A549 cells cultured at the air-liquid interface mediated by oxidative stress and endoplasmic reticulum stress. Environ. Res. 2024, 248, 118283. [Google Scholar] [CrossRef]
- Yi, W.; Cheng, J.; Song, J.; Pan, R.; Liang, Y.; Sun, X.; Li, Y.; Wu, Y.; Yan, S.; Jin, X.; et al. Associations of polycyclic aromatic hydrocarbons, water-soluble ions and metals in PM2.5 with liver function: Evidence from schizophrenia cohort. Sci. Total Environ. 2023, 868, 161624. [Google Scholar] [CrossRef]
- Kurowska, P.; Berthet, L.; Ramé, C.; Węgiel, M.; Maślanka, A.; Guérif, F.; Froment, P.; Rak, A.; Dupont, J. Polycyclic aromatic hydrocarbons in human granulosa cells: First in vivo presence and positive correlation with body mass index and in vitro ovarian cell steroidogenesis regulation. Environ. Toxicol. Pharmacol. 2025, 113, 104611. [Google Scholar] [CrossRef]
- Dong, W.; Wang, L.; Thornton, C.; Scheffler, B.E.; Willett, K.L. Benzo(a)pyrene decreases brain and ovarian aromatase mRNA expression in Fundulus heteroclitus. Aquat. Toxicol. 2008, 88, 289–300. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Guo, L.; Chen, Q.; Gao, X.; Li, P.-h.; Tang, N.; Guo, X.; Deng, F.; Wu, S. Effects of short-term personal exposure to air pollution on platelet mitochondrial DNA methylation levels and the potential mitigation by L-arginine supplementation. J. Hazard. Mater. 2021, 417, 125963. [Google Scholar] [CrossRef]
- Wang, K.; Wen, Y.; Fu, X.; Wei, S.; Liu, S.; Chen, M. mtDNA regulates cGAS-STING signaling pathway in adenomyosis. Free Radic. Biol. Med. 2024, 216, 80–88. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, H.; Wang, X.; Wang, Q.-C.; Zhang, C.; Wang, J.-Q.; Wang, Y.-H.; An, C.-Q.; Yang, K.-Y.; Wang, Y.; et al. TMCO1 is essential for ovarian follicle development by regulating ER Ca2+ store of granulosa cells. Cell Death Differ. 2018, 25, 1686–1701. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, B.; Chen, Q.; Li, X.; Zhu, X.; Duan, M.; Zhang, M.; Liu, Z.; Wen, X.; Guo, J.; et al. HIIT and MICT mitigate endothelial dysfunction in early atherosclerotic mice via PCSK9 inhibition. Sci. Rep. 2025, 15, 30411. [Google Scholar] [CrossRef]
- Zhao, B.; Ban, F.; Li, Y.; Shi, Q.; Guo, S.; Yi, X.; Wang, H.; Gao, T.; Li, C.; Zhu, G. Exploiting mitochondrial dysfunction to overcome BRAF inhibitor resistance in advanced melanoma: The role of disulfiram as a copper ionophore. Cell Death Dis. 2025, 16, 482. [Google Scholar] [CrossRef]
- Choi, E.-H.; Park, S.-J. TXNIP: A key protein in the cellular stress response pathway and a potential therapeutic target. Exp. Mol. Med. 2023, 55, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Pan, T.; Liu, Z.; McCarthy, C.; Vicencio, J.M.; Cao, L.; Alfano, G.; Suwaidan, A.A.; Yin, M.; Beatson, R.; et al. The role of TXNIP in cancer: A fine balance between redox, metabolic, and immunological tumor control. Br. J. Cancer 2023, 129, 1877–1892. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M. TXNIP Links Redox Circuitry to Glucose Control. Cell Metab. 2007, 5, 412–414. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Ma, Z.; Zhang, X.; Du, X.; Zhao, C.; Jia, Y.; Wang, Y.; Li, X.; Yu, X.; Gao, Y. Differential Toxicity of Water-Soluble Versus Water-Insoluble Components of Cowshed PM2.5 on Ovarian Granulosa Cells and the Regulatory Role of Txnip in Overall Toxicity. Antioxidants 2026, 15, 138. https://doi.org/10.3390/antiox15010138
Ma Z, Zhang X, Du X, Zhao C, Jia Y, Wang Y, Li X, Yu X, Gao Y. Differential Toxicity of Water-Soluble Versus Water-Insoluble Components of Cowshed PM2.5 on Ovarian Granulosa Cells and the Regulatory Role of Txnip in Overall Toxicity. Antioxidants. 2026; 15(1):138. https://doi.org/10.3390/antiox15010138
Chicago/Turabian StyleMa, Zhenhua, Xiqing Zhang, Xiaohui Du, Cuizhu Zhao, Yunna Jia, Ye Wang, Xintian Li, Xiuzhen Yu, and Yunhang Gao. 2026. "Differential Toxicity of Water-Soluble Versus Water-Insoluble Components of Cowshed PM2.5 on Ovarian Granulosa Cells and the Regulatory Role of Txnip in Overall Toxicity" Antioxidants 15, no. 1: 138. https://doi.org/10.3390/antiox15010138
APA StyleMa, Z., Zhang, X., Du, X., Zhao, C., Jia, Y., Wang, Y., Li, X., Yu, X., & Gao, Y. (2026). Differential Toxicity of Water-Soluble Versus Water-Insoluble Components of Cowshed PM2.5 on Ovarian Granulosa Cells and the Regulatory Role of Txnip in Overall Toxicity. Antioxidants, 15(1), 138. https://doi.org/10.3390/antiox15010138





