Region-Dependent Responses to Oxygen–Glucose Deprivation and Melatonin in Neonatal Brain Organotypic Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Organotypic Slice Cultures
2.2. Oxygen-Glucose Deprivation and Reperfusion (OGDR)
2.3. Preparation of Melatonin Treatment
2.4. Experimental Groups
- (1)
- Control: Slices maintained under normal culture conditions with complete medium and no treatment.
- (2)
- OGDR: Slices subjected to oxygen–glucose deprivation followed by 24 h of reperfusion, to mimic HI and reperfusion-induced injury.
- (3)
- OGDR + MEL: Slices subjected to oxygen–glucose deprivation and treated with 50 μM melatonin followed by 24 h of reperfusion to assess the potential protective effects of melatonin against HI and reperfusion-induced injury.
2.5. Cell Death Quantification
2.6. Glutathione (GSH) and Oxidized Glutathione (GSSG) Quantification
2.7. Proteome Profile
2.8. Statistical Analysis
3. Results
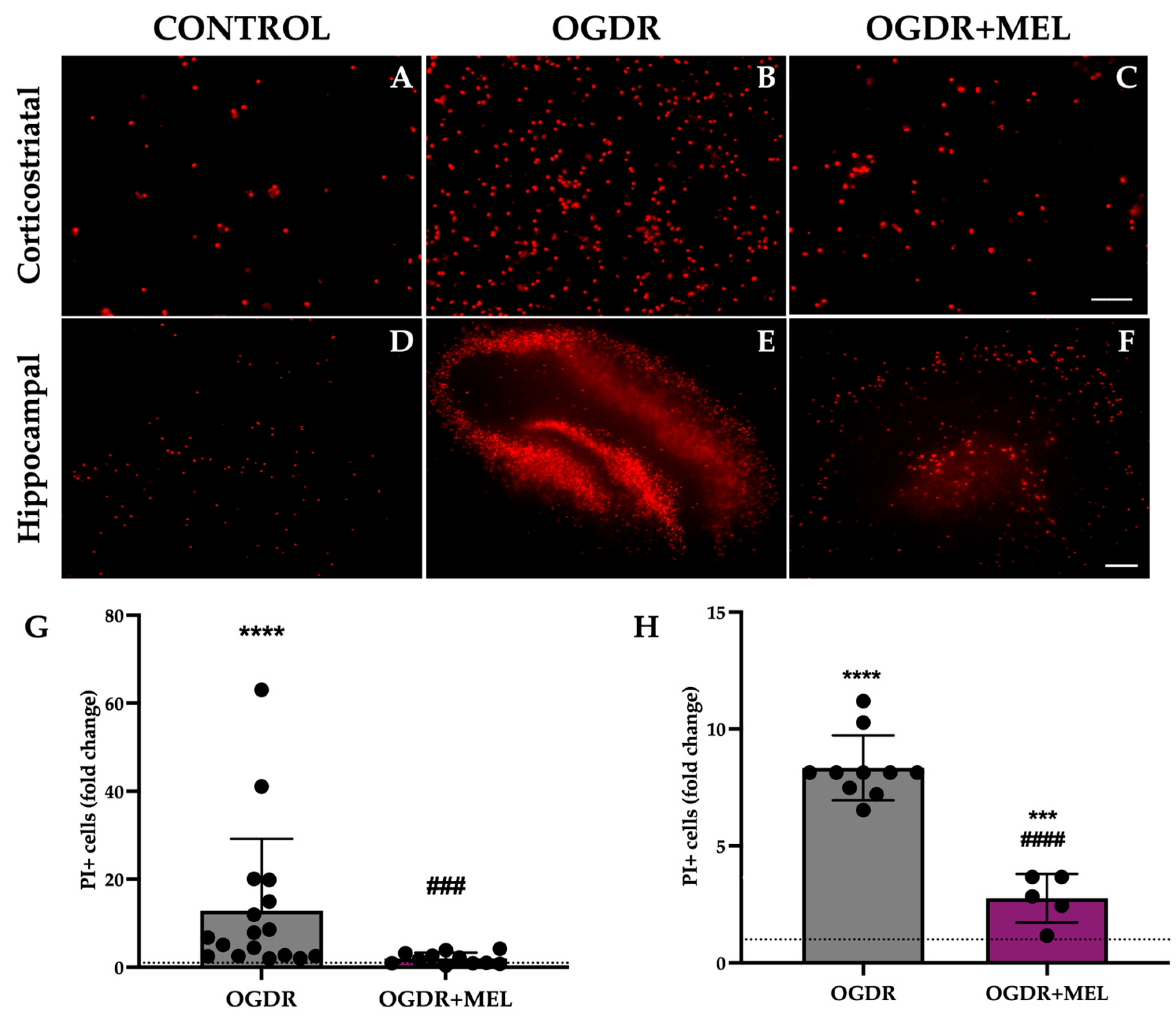
3.1. Cell Death
3.2. Oxidative Stress
3.3. Inflammatory Response


4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gunn, A.J.; Thoresen, M. Neonatal Encephalopathy and Hypoxic–Ischemic Encephalopathy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 162, pp. 217–237. ISBN 978-0-444-64029-1. [Google Scholar]
- Kurinczuk, J.J.; White-Koning, M.; Badawi, N. Epidemiology of Neonatal Encephalopathy and Hypoxic–Ischaemic Encephalopathy. Early Hum. Dev. 2010, 86, 329–338. [Google Scholar] [CrossRef]
- Yıldız, E.P.; Ekici, B.; Tatlı, B. Neonatal Hypoxic Ischemic Encephalopathy: An Update on Disease Pathogenesis and Treatment. Expert Rev. Neurother. 2017, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological Outcomes at 18 Months of Age after Moderate Hypothermia for Perinatal Hypoxic Ischaemic Encephalopathy: Synthesis and Meta-Analysis of Trial Data. BMJ 2010, 340, c363. [Google Scholar] [CrossRef] [PubMed]
- Tagin, M.A.; Woolcott, C.G.; Vincer, M.J.; Whyte, R.K.; Stinson, D.A. Hypothermia for Neonatal Hypoxic Ischemic Encephalopathy: An Updated Systematic Review and Meta-Analysis. Arch. Pediatr. Adolesc. Med. 2012, 166, 558–566. [Google Scholar] [CrossRef]
- Wassink, G.; Davidson, J.O.; Dhillon, S.K.; Zhou, K.; Bennet, L.; Thoresen, M.; Gunn, A.J. Therapeutic Hypothermia in Neonatal Hypoxic-Ischemic Encephalopathy. Curr. Neurol. Neurosci. Rep. 2019, 19, 2. [Google Scholar] [CrossRef]
- Greco, P.; Nencini, G.; Piva, I.; Scioscia, M.; Volta, C.A.; Spadaro, S.; Neri, M.; Bonaccorsi, G.; Greco, F.; Cocco, I.; et al. Pathophysiology of Hypoxic–Ischemic Encephalopathy: A Review of the Past and a View on the Future. Acta Neurol. Belg. 2020, 120, 277–288. [Google Scholar] [CrossRef]
- Martini, S.; Castellini, L.; Parladori, R.; Paoletti, V.; Aceti, A.; Corvaglia, L. Free Radicals and Neonatal Brain Injury: From Underlying Pathophysiology to Antioxidant Treatment Perspectives. Antioxidants 2021, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Odorcyk, F.K.; Kolling, J.; Sanches, E.F.; Wyse, A.T.S.; Netto, C.A. Experimental Neonatal Hypoxia Ischemia Causes Long Lasting Changes of Oxidative Stress Parameters in the Hippocampus and the Spleen. J. Perinat. Med. 2018, 46, 433–439. [Google Scholar] [CrossRef]
- Jan, M.M.S. Melatonin for the Treatment of Handicapped Children with Severe Sleep Disorders. Pediatr. Neurol. 2000, 23, 229–232. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological Effects in Humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Alvarez, A.; Lacalle, J.; Hilario, E. Histological Study of the Protective Effect of Melatonin on Neural Cells after Neonatal Hypoxia-Ischemia. Histol. Histopathol. 2012, 27, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Carloni, S.; Perrone, S.; Buonocore, G.; Longini, M.; Proietti, F.; Balduini, W. Melatonin Protects from the Long-term Consequences of a Neonatal Hypoxic-ischemic Brain Injury in Rats. J. Pineal Res. 2008, 44, 157–164. [Google Scholar] [CrossRef]
- Robertson, N.J.; Faulkner, S.; Fleiss, B.; Bainbridge, A.; Andorka, C.; Price, D.; Powell, E.; Lecky-Thompson, L.; Thei, L.; Chandrasekaran, M.; et al. Melatonin Augments Hypothermic Neuroprotection in a Perinatal Asphyxia Model. Brain 2013, 136, 90–105. [Google Scholar] [CrossRef]
- Welin, A.-K.; Svedin, P.; Lapatto, R.; Sultan, B.; Hagberg, H.; Gressens, P.; Kjellmer, I.; Mallard, C. Melatonin Reduces Inflammation and Cell Death in White Matter in the Mid-Gestation Fetal Sheep Following Umbilical Cord Occlusion. Pediatr. Res. 2007, 61, 153–158. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic Brain Slice Cultures: A Review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Pellegrini-Giampietro, D.E.; Facchinetti, F. Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy. Biomedicines 2022, 10, 937. [Google Scholar] [CrossRef]
- Carloni, S.; Facchinetti, F.; Pelizzi, N.; Buonocore, G.; Balduini, W. Melatonin Acts in Synergy with Hypothermia to Reduce Oxygen-Glucose Deprivation-Induced Cell Death in Rat Hippocampus Organotypic Slice Cultures. Neonatology 2018, 114, 364–371. [Google Scholar] [CrossRef]
- Lana, D.; Gerace, E.; Magni, G.; Cialdai, F.; Monici, M.; Mannaioni, G.; Giovannini, M.G. Hypoxia/Ischemia-Induced Rod Microglia Phenotype in CA1 Hippocampal Slices. Int. J. Mol. Sci. 2022, 23, 1422. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Mazzantini, C.; Lana, D.; Davolio, P.L.; Giovannini, M.G.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Cannabidiol but Not Δ9-Tetrahydrocannabinol in Rat Hippocampal Slices Exposed to Oxygen-Glucose Deprivation: Studies with Cannabis Extracts and Selected Cannabinoids. Int. J. Mol. Sci. 2021, 22, 9773. [Google Scholar] [CrossRef]
- Landucci, E.; Filippi, L.; Gerace, E.; Catarzi, S.; Guerrini, R.; Pellegrini-Giampietro, D.E. Neuroprotective Effects of Topiramate and Memantine in Combination with Hypothermia in Hypoxic-Ischemic Brain Injury in Vitro and in Vivo. Neurosci. Lett. 2018, 668, 103–107. [Google Scholar] [CrossRef]
- Gerace, E.; Landucci, E.; Scartabelli, T.; Moroni, F.; Pellegrini-Giampietro, D.E. Rat Hippocampal Slice Culture Models for the Evaluation of Neuroprotective Agents. In Neurotrophic Factors; Skaper, S.D., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 846, pp. 343–354. ISBN 978-1-61779-535-0. [Google Scholar]
- McKenna, M.; Filteau, J.R.; Butler, B.; Sluis, K.; Chungyoun, M.; Schimek, N.; Nance, E. Organotypic Whole Hemisphere Brain Slice Models to Study the Effects of Donor Age and Oxygen-Glucose-Deprivation on the Extracellular Properties of Cortical and Striatal Tissue. J. Biol. Eng. 2022, 16, 14. [Google Scholar] [CrossRef]
- Parmentier, C.E.J.; De Vries, L.S.; Groenendaal, F. Magnetic Resonance Imaging in (Near-)Term Infants with Hypoxic-Ischemic Encephalopathy. Diagnostics 2022, 12, 645. [Google Scholar] [CrossRef]
- Wisnowski, J.L.; Wintermark, P.; Bonifacio, S.L.; Smyser, C.D.; Barkovich, A.J.; Edwards, A.D.; De Vries, L.S.; Inder, T.E.; Chau, V. Neuroimaging in the Term Newborn with Neonatal Encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101304. [Google Scholar] [CrossRef]
- Soria, F.N.; Fernandez-Ballester, M. Rat Organotypic Cultures for AAV-Mediated Vital Labeling of the Extracellular Matrix (Version 2). protocols.io, 2024. Available online: https://dx.doi.org/10.17504/protocols.io.e6nvwdnzzlmk/v2 (accessed on 18 December 2025).
- Allen, D. Protein Array Tool (Version 2.0.0.1). MATLAB Central File Exchange, 2020. Available online: https://www.mathworks.com/matlabcentral/fileexchange/35128-protein-array-tool (accessed on 18 December 2025).
- Towfighi, J.; Mauger, D.; Vannucci, R.C.; Vannucci, S.J. Influence of Age on the Cerebral Lesions in an Immature Rat Model of Cerebral Hypoxia–Ischemia: A Light Microscopic Study. Dev. Brain Res. 1997, 100, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sapolsky, R.M.; Giffard, R.G. Differential Sensitivity of Murine Astrocytes and Neurons from Different Brain Regions to Injury. Exp. Neurol. 2001, 169, 416–424. [Google Scholar] [CrossRef]
- Ianevski, A.; Cámara-Quílez, M.; Wang, W.; Suganthan, R.; Hildrestrand, G.; Grini, J.V.; Døskeland, D.S.; Ye, J.; Bjørås, M. Early Transcriptional Responses Reveal Cell Type-Specific Vulnerability and Neuroprotective Mechanisms in the Neonatal Ischemic Hippocampus. Acta Neuropathol. Commun. 2025, 13, 147. [Google Scholar] [CrossRef]
- Nakajima, W.; Ishida, A.; Lange, M.S.; Gabrielson, K.L.; Wilson, M.A.; Martin, L.J.; Blue, M.E.; Johnston, M.V. Apoptosis Has a Prolonged Role in the Neurodegeneration after Hypoxic Ischemia in the Newborn Rat. J. Neurosci. 2000, 20, 7994–8004. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; Sheldon, R.A.; Flock, D.L.; Ferriero, D.M.; Martin, L.J.; O’Riordan, D.P.; Northington, F.J. Neonatal Mice Lacking Functional Fas Death Receptors Are Resistant to Hypoxic–Ischemic Brain Injury. Neurobiol. Dis. 2004, 17, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Yigitkanli, K.; Zheng, Y.; Pekcec, A.; Lo, E.H.; Van Leyen, K. Increased 12/15-Lipoxygenase Leads to Widespread Brain Injury Following Global Cerebral Ischemia. Transl. Stroke Res. 2017, 8, 194–202. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Hilario, E.; Álvarez, F.J.; Álvarez, A. Apoptotic Cell Death Correlates With ROS Overproduction and Early Cytokine Expression After Hypoxia–Ischemia in Fetal Lambs. Reprod. Sci. 2012, 19, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Meibach, R.C.; Ross, D.A.; Cox, R.D.; Glick, S.D. The Ontogeny of Hippocampal Energy Metabolism. Brain Res. 1981, 204, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Khakpai, F.; Zarrindast, M.R.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A. The Role of Glutamatergic Pathway between Septum and Hippocampus in the Memory Formation. EXCLI J. 2013, 12, 41–51. [Google Scholar]
- Schmidt-Kastner, R.; Freund, T.F. Selective Vulnerability of the Hippocampus in Brain Ischemia. Neuroscience 1991, 40, 599–636. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Yin, B.; Barrionuevo, G.; Weber, S.G. Optimized Real-Time Monitoring of Glutathione Redox Status in Single Pyramidal Neurons in Organotypic Hippocampal Slices during Oxygen–Glucose Deprivation and Reperfusion. ACS Chem. Neurosci. 2015, 6, 1838–1848. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Zalewska, T. Insights Into the Neuroinflammatory Responses After Neonatal Hypoxia-Ischemia. J. Neuropathol. Exp. Neurol. 2017, 76, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Z.; Wang, D.; Zhang, T.; Sun, B.; Mu, L.; Wang, J.; Liu, Y.; Kong, Q.; Liu, X.; et al. Accumulation of Natural Killer Cells in Ischemic Brain Tissues and the Chemotactic Effect of IP-10. J. Neuroinflamm. 2014, 11, 79. [Google Scholar] [CrossRef]
- Alam, A.; Thelin, E.P.; Tajsic, T.; Khan, D.Z.; Khellaf, A.; Patani, R.; Helmy, A. Cellular Infiltration in Traumatic Brain Injury. J. Neuroinflamm. 2020, 17, 328. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Tu, Y.-F.; Lin, Y.-C.; Huang, C.-C. CXCL5 Signaling Is a Shared Pathway of Neuroinflammation and Blood–Brain Barrier Injury Contributing to White Matter Injury in the Immature Brain. J. Neuroinflamm. 2016, 13, 6. [Google Scholar] [CrossRef]
- Kang, L.; Yu, H.; Yang, X.; Zhu, Y.; Bai, X.; Wang, R.; Cao, Y.; Xu, H.; Luo, H.; Lu, L.; et al. Neutrophil Extracellular Traps Released by Neutrophils Impair Revascularization and Vascular Remodeling after Stroke. Nat. Commun. 2020, 11, 2488. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil Extracellular Traps in Ischemic Stroke Thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9–Positive Neutrophil Infiltration Is Associated to Blood–Brain Barrier Breakdown and Basal Lamina Type IV Collagen Degradation During Hemorrhagic Transformation After Human Ischemic Stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lee, H.; Lee, H.-K.; Kim, I.-D.; Lee, J.-K. Neutrophil Extracellular Trap Induced by HMGB1 Exacerbates Damages in the Ischemic Brain. Acta Neuropathol. Commun. 2019, 7, 94. [Google Scholar] [CrossRef]
- Terao, Y.; Ohta, H.; Oda, A.; Nakagaito, Y.; Kiyota, Y.; Shintani, Y. Macrophage Inflammatory Protein-3alpha Plays a Key Role in the Inflammatory Cascade in Rat Focal Cerebral Ischemia. Neurosci. Res. 2009, 64, 75–82. [Google Scholar] [CrossRef]
- Yilmaz, G.; Granger, D.N. Leukocyte Recruitment and Ischemic Brain Injury. Neuromol. Med. 2010, 12, 193–204. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular Endothelial Growth Factor Signaling in Hypoxia and Inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Wang, X.; Siren, A.-L.; Liu, Y.; Yue, T.-L.; Barone, F.C.; Feuerstein, G.Z. Upregulation of Intercellular Adhesion Molecule 1 (ICAM-1) on Brain Microvascular Endothelial Cells in Rat Ischemic Cortex. Mol. Brain Res. 1994, 26, 61–68. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Zhang, L.; Tsang, W.; Soltanian-Zadeh, H.; Morris, D.; Zhang, R.; Goussev, A.; Powers, C.; Yeich, T.; Chopp, M. Correlation of VEGF and Angiopoietin Expression with Disruption of Blood–Brain Barrier and Angiogenesis after Focal Cerebral Ischemia. J. Cereb. Blood Flow Metab. 2002, 22, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Jickling, G.C.; Liu, D.; Ander, B.P.; Stamova, B.; Zhan, X.; Sharp, F.R. Targeting Neutrophils in Ischemic Stroke: Translational Insights from Experimental Studies. J. Cereb. Blood Flow Metab. 2015, 35, 888–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory Mechanisms of Blood-Brain Barrier Damage in Ischemic Stroke. Am. J. Physiol.-Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Gidday, J.M.; Gasche, Y.G.; Copin, J.-C.; Shah, A.R.; Perez, R.S.; Shapiro, S.D.; Chan, P.H.; Park, T.S. Leukocyte-Derived Matrix Metalloproteinase-9 Mediates Blood-Brain Barrier Breakdown and Is Proinflammatory after Transient Focal Cerebral Ischemia. Am. J. Physiol.-Heart Circ. Physiol. 2005, 289, H558–H568. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, N.; Svedin, P.; Garnotel, R.; Favrais, G.; Loron, G.; Schwendiman, L.; Hagberg, H.; Morville, P.; Mallard, C.; Gressens, P. Increased MMP-9 and TIMP-1 in Mouse Neonatal Brain and Plasma and in Human Neonatal Plasma after Hypoxia–Ischemia: A Potential Marker of Neonatal Encephalopathy. Pediatr. Res. 2012, 71, 63–70. [Google Scholar] [CrossRef]
- Abellanas, M.A.; Zamarbide, M.; Basurco, L.; Luquin, E.; Garcia-Granero, M.; Clavero, P.; San Martin-Uriz, P.; Vilas, A.; Mengual, E.; Hervas-Stubbs, S.; et al. Midbrain Microglia Mediate a Specific Immunosuppressive Response under Inflammatory Conditions. J. Neuroinflamm. 2019, 16, 233. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial Brain Region−dependent Diversity and Selective Regional Sensitivities to Aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Szaflarski, J.; Burtrum, D.; Silverstein, F.S. Cerebral Hypoxia-Ischemia Stimulates Cytokine Gene Expression in Perinatal Rats. Stroke 1995, 26, 1093–1100. [Google Scholar] [CrossRef]
- Liu, T.; McDonnell, P.C.; Young, P.R.; White, R.F.; Siren, A.L.; Hallenbeck, J.M.; Barone, F.C.; Feurestein, G.Z. Interleukin-1 Beta mRNA Expression in Ischemic Rat Cortex. Stroke 1993, 24, 1746–1750. [Google Scholar] [CrossRef]
- Wang, X.; Figueroa, B.E.; Stavrovskaya, I.G.; Zhang, Y.; Sirianni, A.C.; Zhu, S.; Day, A.L.; Kristal, B.S.; Friedlander, R.M. Methazolamide and Melatonin Inhibit Mitochondrial Cytochrome C Release and Are Neuroprotective in Experimental Models of Ischemic Injury. Stroke 2009, 40, 1877–1885. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, T.; Hung, C.; Tai, S.; Huang, S.; Chang, C.; Hung, H.; Lee, E. Melatonin Protects Brain against Ischemia/Reperfusion Injury by Attenuating Endoplasmic Reticulum Stress. Int. J. Mol. Med. 2018, 42, 182–192. [Google Scholar] [CrossRef]
- Luchetti, F.; Nasoni, M.G.; Burattini, S.; Mohammadi, A.; Pagliarini, M.; Canonico, B.; Ambrogini, P.; Balduini, W.; Reiter, R.J.; Carloni, S. Melatonin Attenuates Ischemic-like Cell Injury by Promoting Autophagosome Maturation via the Sirt1/FoxO1/Rab7 Axis in Hippocampal HT22 Cells and in Organotypic Cultures. Cells 2022, 11, 3701. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin Stimulates Antioxidant Enzymes and Reduces Oxidative Stress in Experimental Traumatic Brain Injury: The Nrf2–ARE Signaling Pathway as a Potential Mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Han, X.; Zhang, M.; Kou, H.; Liu, H.; Cheng, T. Melatonin Exerts Neuroprotective Effects in Mice with Spinal Cord Injury by Activating the Nrf2/Keap1 Signaling Pathway via the MT2 Receptor. Exp. Ther. Med. 2023, 27, 37. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, H.Y.; Ali, T.; Kim, M.O. Melatonin as a Potential Regulator of Oxidative Stress, and Neuroinflammation: Mechanisms and Implications for the Management of Brain Injury-Induced Neurodegeneration. J. Inflamm. Res. 2021, 14, 6251–6264. [Google Scholar] [CrossRef]
- Alonso-Alconada, D.; Álvarez, A.; Arteaga, O.; Martínez-Ibargüen, A.; Hilario, E. Neuroprotective Effect of Melatonin: A Novel Therapy against Perinatal Hypoxia-Ischemia. Int. J. Mol. Sci. 2013, 14, 9379–9395. [Google Scholar] [CrossRef]
- Feng, Y.; Rhodes, P.G.; Bhatt, A.J. Neuroprotective Effects of Vascular Endothelial Growth Factor Following Hypoxic Ischemic Brain Injury in Neonatal Rats. Pediatr. Res. 2008, 64, 370–374. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Beldarrain, G.; Montejo, U.; Chillida, M.; Alart, J.A.; Álvarez, A.; Alonso-Alconada, D. Region-Dependent Responses to Oxygen–Glucose Deprivation and Melatonin in Neonatal Brain Organotypic Slices. Antioxidants 2026, 15, 13. https://doi.org/10.3390/antiox15010013
Beldarrain G, Montejo U, Chillida M, Alart JA, Álvarez A, Alonso-Alconada D. Region-Dependent Responses to Oxygen–Glucose Deprivation and Melatonin in Neonatal Brain Organotypic Slices. Antioxidants. 2026; 15(1):13. https://doi.org/10.3390/antiox15010013
Chicago/Turabian StyleBeldarrain, Gorane, Unai Montejo, Marc Chillida, Jon Ander Alart, Antonia Álvarez, and Daniel Alonso-Alconada. 2026. "Region-Dependent Responses to Oxygen–Glucose Deprivation and Melatonin in Neonatal Brain Organotypic Slices" Antioxidants 15, no. 1: 13. https://doi.org/10.3390/antiox15010013
APA StyleBeldarrain, G., Montejo, U., Chillida, M., Alart, J. A., Álvarez, A., & Alonso-Alconada, D. (2026). Region-Dependent Responses to Oxygen–Glucose Deprivation and Melatonin in Neonatal Brain Organotypic Slices. Antioxidants, 15(1), 13. https://doi.org/10.3390/antiox15010013





