Dysregulated Expression of Canonical and Non-Canonical Glycolytic Enzyme Isoforms in Peripheral Blood from Subjects with Alcohol Use Disorder and from Individuals with Acute Alcohol Consumption
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Studies, Literature Review, and Gene Selection
2.2. Subjects
2.2.1. Sample of Patients with Alcohol Use Disorders (AUD)
2.2.2. Samples from Patients with Acute Alcohol Consumption (AAC)
2.3. Sample Processing
2.4. In Vitro Cultures of Mouse Astrocytes Exposed to Alcohol
2.4.1. Mice
2.4.2. Collection and Primary Culture of Astrocytes
2.5. RNA Extraction, Reverse Transcription, and Real-Time PCR (RT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Bioinformatics Studies, Literature Review, and Gene Selection
3.2. Characteristics of the Study Cohort
3.2.1. Patients with AUD
3.2.2. Patients with AAC
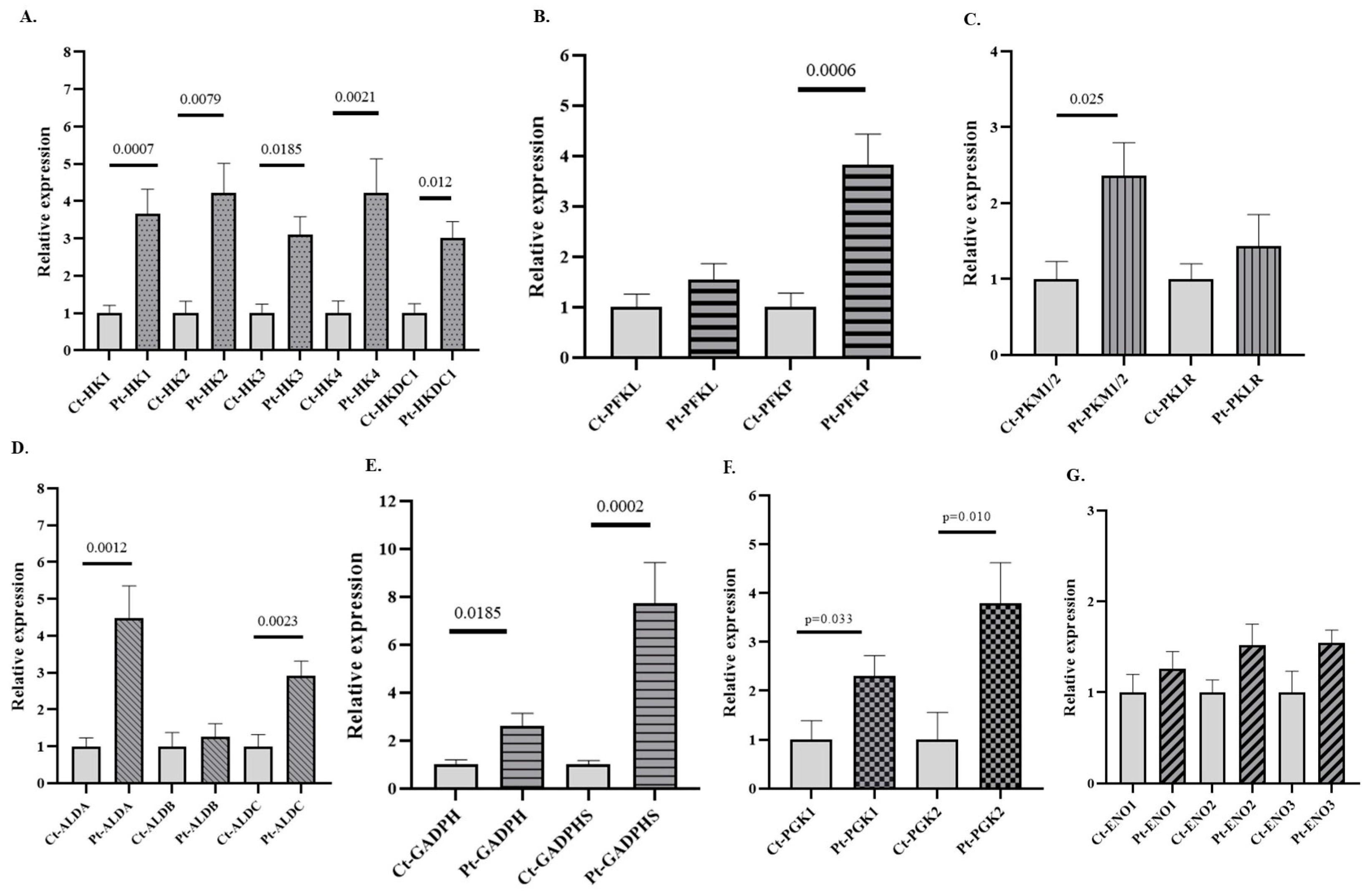
3.3. Expression of GEs in PB of Patients with AUD
3.4. Expression of GEs in PB of Patients with AAC
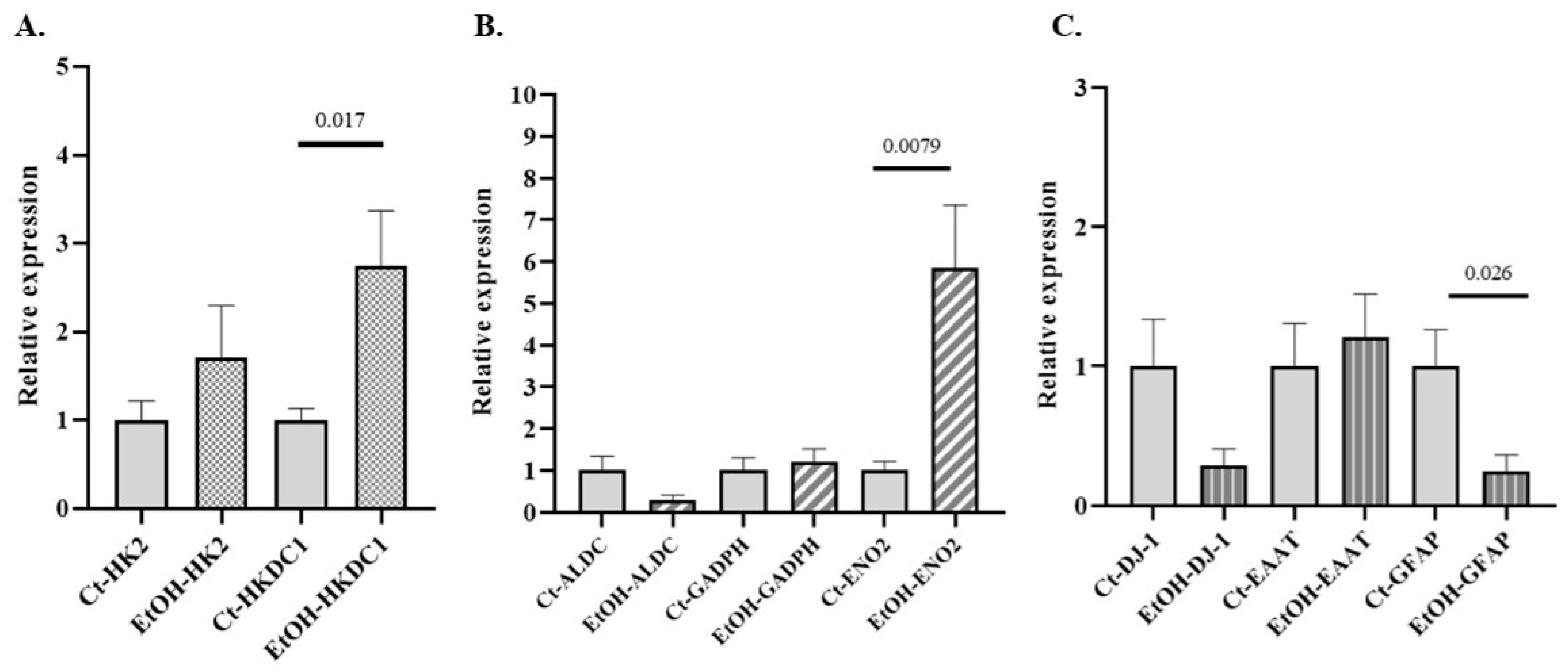
3.5. Expression of GEs in In Vitro Cultures of Mouse Astrocytes Exposed to Alcohol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NIAAA National Institute on Alcohol Abuse and Alcoholism (NIAAA). Available online: https://www.niaaa.nih.gov/ (accessed on 22 March 2025).
- WHO Alcohol. Available online: https://www.who.int/news-room/fact-sheets/detail/alcohol (accessed on 22 March 2025).
- AECC Evita el Consumo de Alcohol. Available online: https://www.contraelcancer.es/es/todo-sobre-cancer/prevencion/evita-consumo-alcohol (accessed on 22 March 2025).
- Tsermpini, E.E.; Plemenitaš Ilješ, A.; Dolžan, V. Alcohol-Induced Oxidative Stress and the Role of Antioxidants in Alcohol Use Disorder: A Systematic Review. Antioxidants 2022, 11, 1374. [Google Scholar] [CrossRef]
- Simon, L.; Molina, P.E. Cellular Bioenergetics: Experimental Evidence for Alcohol-Induced Adaptations. Function 2022, 3, zqac039. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate Metabolism in Human Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Dodson, M.; Darley-Usmar, V.; Zhang, J. Cellular Metabolic and Autophagic Pathways: Traffic Control by Redox Signaling. Free Radic. Biol. Med. 2013, 63, 207–221. [Google Scholar] [CrossRef]
- Massey, V.; Parrish, A.; Argemi, J.; Moreno, M.; Mello, A.; García-Rocha, M.; Altamirano, J.; Odena, G.; Dubuquoy, L.; Louvet, A.; et al. Integrated Multiomics Reveals Glucose Use Reprogramming and Identifies a Novel Hexokinase in Alcoholic Hepatitis. Gastroenterology 2021, 160, 1725–1740.e2. [Google Scholar] [CrossRef]
- Ledig, M.; Doffoel, M.; Doffoel, S.; Kopp, P.; Bockel, R.; Mandel, P. Blood Cell Superoxide Dismutase and Enolase Activities as Markers of Alcoholic and Nonalcoholic Liver Diseases. Alcohol 1988, 5, 387–391. [Google Scholar] [CrossRef]
- Ward, R.; Roderique-Davies, G.; Hughes, H.; Heirene, R.; Newstead, S.; John, B. Alcohol-Related Brain Damage: A Mixed-method Evaluation of an Online Awareness-raising Programme for Frontline Care and Support Practitioners. Drug Alcohol Rev. 2023, 42, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Pirela, M.; Andrade-Alviárez, D.; Rojas, V.; Marcos, M.; Salete-Granado, D.; Chacón-Arnaude, M.; Pérez-Nieto, M.Á.; Kemmerling, U.; Concepción, J.L.; Michels, P.A.M.; et al. Exploring Glycolytic Enzymes in Disease: Potential Biomarkers and Therapeutic Targets in Neurodegeneration, Cancer and Parasitic Infections. Open Biol. 2025, 15, 240239. [Google Scholar] [CrossRef]
- Burns, J.S.; Manda, G. Metabolic Pathways of the Warburg Effect in Health and Disease: Perspectives of Choice, Chain or Chance. Int. J. Mol. Sci. 2017, 18, 2755. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Hu, S.; Wang, X. The Regulatory Roles and Clinical Significance of Glycolysis in Tumor. Cancer Commun. 2024, 44, 761–786. [Google Scholar] [CrossRef] [PubMed]
- Didiasova, M.; Schaefer, L.; Wygrecka, M. When Place Matters: Shuttling of Enolase-1 Across Cellular Compartments. Front. Cell Dev. Biol. 2019, 7, 61. [Google Scholar] [CrossRef]
- Rojas-Pirela, M.; Andrade-Alviárez, D.; Rojas, V.; Kemmerling, U.; Cáceres, A.J.; Michels, P.A.; Concepción, J.L.; Quiñones, W. Phosphoglycerate Kinase: Structural Aspects and Functions, with Special Emphasis on the Enzyme from Kinetoplastea. Open Biol. 2020, 10, 200302. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Zhou, J.; Chen, M.; Qiu, L.; Li, Y.; Zhang, W.; Guo, R.; Lei, N.; Chang, L. Bioinformatics Analysis of the Role of Aldolase A in Tumor Prognosis and Immunity. Sci. Rep. 2022, 12, 11632. [Google Scholar] [CrossRef]
- Zoccarato, A.; Nabeebaccus, A.A.; Oexner, R.R.; Santos, C.X.C.; Shah, A.M. The Nexus Between Redox State and Intermediary Metabolism. FEBS J. 2022, 289, 5440–5462. [Google Scholar] [CrossRef]
- Fuentes-Lemus, E.; Usgame, K.; Fierro, A.; López-Alarcón, C. Enzymes of Glycolysis and the Pentose Phosphate Pathway as Targets of Oxidants: Role of Redox Reactions on the Carbohydrate Catabolism. Redox Biochem. Chem. 2025, 11, 100049. [Google Scholar] [CrossRef]
- Selvaraj, N.R.; Nandan, D.; Nair, B.G.; Nair, V.A.; Venugopal, P.; Aradhya, R. Oxidative Stress and Redox Imbalance: Common Mechanisms in Cancer Stem Cells and Neurodegenerative Diseases. Cells 2025, 14, 511. [Google Scholar] [CrossRef]
- Bell, S.M.; Burgess, T.; Lee, J.; Blackburn, D.J.; Allen, S.P.; Mortiboys, H. Peripheral Glycolysis in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8924. [Google Scholar] [CrossRef]
- Lopes de Melo, J.M.; Laursen, J.C.; Søndergaard-Heinrich, N.; Bull Rasmussen, I.K.; Hansen, C.S.; Frimodt-Møller, M.; Rossing, P.; Størling, J. Increased Mitochondrial Proton Leak and Glycolysis in Peripheral Blood Mononuclear Cells in Type-1-Diabetes. Heliyon 2022, 8, e12304. [Google Scholar] [CrossRef]
- Ferguson, L.B.; Mayfield, R.D.; Messing, R.O. RNA Biomarkers for Alcohol Use Disorder. Front. Mol. Neurosci. 2022, 15, 1032362. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, R.D.; Harris, R.A. Gene Expression Profiling in Blood: New Diagnostics in Alcoholism and Addiction? Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2009, 34, 250–251. [Google Scholar] [CrossRef] [PubMed]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s Conserved Domain Database. Nucleic Acids Res. 2015, 43, D222–D226. [Google Scholar] [CrossRef]
- Finn, R.D.; Attwood, T.K.; Babbitt, P.C.; Bateman, A.; Bork, P.; Bridge, A.J.; Chang, H.-Y.; Dosztányi, Z.; El-Gebali, S.; Fraser, M.; et al. InterPro in 2017-beyond Protein Family and Domain Annotations. Nucleic Acids Res. 2017, 45, D190–D199. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 Years of the SMART Protein Domain Annotation Resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Sønderby, C.K.; Sønderby, S.K.; Nielsen, H.; Winther, O. DeepLoc: Prediction of Protein Subcellular Localization Using Deep Learning. Bioinformatics 2017, 33, 3387–3395. [Google Scholar] [CrossRef]
- Chou, K.-C.; Shen, H.-B. Cell-PLoc: A Package of Web Servers for Predicting Subcellular Localization of Proteins in Various Organisms. Nat. Protoc. 2008, 3, 153–162. [Google Scholar] [CrossRef]
- Thul, P.J.; Lindskog, C. The Human Protein Atlas: A Spatial Map of the Human Proteome. Protein Sci. Publ. Protein Soc. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Ibáñez, F.; Ureña-Peralta, J.R.; Costa-Alba, P.; Torres, J.-L.; Laso, F.-J.; Marcos, M.; Guerri, C.; Pascual, M. Circulating MicroRNAs in Extracellular Vesicles as Potential Biomarkers of Alcohol-Induced Neuroinflammation in Adolescence: Gender Differences. Int. J. Mol. Sci. 2020, 21, 6730. [Google Scholar] [CrossRef]
- Anders, Q.S.; Klauss, J.; Rodrigues, L.C.d.M.; Nakamura-Palacios, E.M. FosB mRNA Expression in Peripheral Blood Lymphocytes in Drug Addicted Patients. Front. Pharmacol. 2018, 9, 1205. [Google Scholar] [CrossRef]
- Yang, Z.; Han, S.; Zhang, T.; Kusumanchi, P.; Huda, N.; Tyler, K.; Chandler, K.; Skill, N.J.; Tu, W.; Shan, M.; et al. Transcriptomic Analysis Reveals the Messenger RNAs Responsible for the Progression of Alcoholic Cirrhosis. Hepatol. Commun. 2022, 6, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Legaki, E.; Dovrolis, N.; Moscholiou, N.; Koutromanos, I.; Vassilopoulos, E.; Dakanalis, A.; Gazouli, M.; Tzavellas, E. Altered Expression of Neuroplasticity-Related Genes in Alcohol Addiction and Treatment. Int. J. Mol. Sci. 2024, 25, 11349. [Google Scholar] [CrossRef]
- Fernández-Regueras, M.; Carbonell, C.; Salete-Granado, D.; García, J.-L.; Gragera, M.; Pérez-Nieto, M.-Á.; Morán-Plata, F.-J.; Mayado, A.; Torres, J.-L.; Corchete, L.-A.; et al. Predominantly Pro-Inflammatory Phenotype with Mixed M1/M2 Polarization of Peripheral Blood Classical Monocytes and Monocyte-Derived Macrophages among Patients with Excessive Ethanol Intake. Antioxidants 2023, 12, 1708. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Busquets-Garcia, A.; Hebert-Chatelain, E.; Serrat, R.; Vicente-Gutierrez, C.; Ioannidou, C.; Gómez-Sotres, P.; Lopez-Fabuel, I.; Resch-Beusher, M.; Resel, E.; et al. Glucose Metabolism Links Astroglial Mitochondria to Cannabinoid Effects. Nature 2020, 583, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic Diseases and Conditions Related to Alcohol Use. Alcohol Res. Curr. Rev. 2013, 35, 155–173. [Google Scholar]
- Danshina, P.V.; Geyer, C.B.; Dai, Q.; Goulding, E.H.; Willis, W.D.; Kitto, G.B.; McCarrey, J.R.; Eddy, E.M.; O’Brien, D.A. Phosphoglycerate Kinase 2 (PGK2) Is Essential for Sperm Function and Male Fertility in Mice. Biol. Reprod. 2010, 82, 136–145. [Google Scholar] [CrossRef]
- Morris, N.L.; Michael, D.N.; Crotty, K.M.; Chang, S.S.; Yeligar, S.M. Alcohol-Induced Glycolytic Shift in Alveolar Macrophages Is Mediated by Hypoxia-Inducible Factor-1 Alpha. Front. Immunol. 2022, 13, 865492. [Google Scholar] [CrossRef]
- Gallegos, E.M.; Simon, L.; Molina, P.E. Chronic Binge Alcohol Mediated Hepatic Metabolic Adaptations in SIV-Infected Female Rhesus Macaques. Alcohol Alcohol. 2024, 59, agae060. [Google Scholar] [CrossRef]
- Mamczur, P.; Gamian, A.; Kolodziej, J.; Dziegiel, P.; Rakus, D. Nuclear Localization of Aldolase A Correlates with Cell Proliferation. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2013, 1833, 2812–2822. [Google Scholar] [CrossRef]
- Pasala, S.; Barr, T.; Messaoudi, I. Impact of Alcohol Abuse on the Adaptive Immune System. Alcohol Res. Curr. Rev. 2015, 37, 185–197. [Google Scholar]
- Soto-Heredero, G.; Gómez de Las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis—A Key Player in the Inflammatory Response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D. The Immunologic Warburg Effect: Evidence and Therapeutic Opportunities in Autoimmunity. WIREs Syst. Biol. Med. 2020, 12, e1486. [Google Scholar] [CrossRef]
- Palsson-McDermott, E.M.; Curtis, A.M.; Goel, G.; Lauterbach, M.A.R.; Sheedy, F.J.; Gleeson, L.E.; van den Bosch, M.W.M.; Quinn, S.R.; Domingo-Fernandez, R.; Johnston, D.G.W.; et al. Pyruvate Kinase M2 Regulates Hif-1α Activity and IL-1β Induction and Is a Critical Determinant of the Warburg Effect in LPS-Activated Macrophages. Cell Metab. 2015, 21, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.I.; Chen, X.; Nagy, L.E. Redox Signaling and the Innate Immune System in Alcoholic Liver Disease. Antioxid. Redox Signal. 2011, 15, 523–534. [Google Scholar] [CrossRef]
- Londhe, P.; Yu, P.Y.; Ijiri, Y.; Ladner, K.J.; Fenger, J.M.; London, C.; Houghton, P.J.; Guttridge, D.C. Classical NF-κB Metabolically Reprograms Sarcoma Cells Through Regulation of Hexokinase 2. Front. Oncol. 2018, 8, 104. [Google Scholar] [CrossRef]
- Gill, J.G.; Leef, S.N.; Ramesh, V.; Martin-Sandoval, M.S.; Rao, A.D.; West, L.; Muh, S.; Gu, W.; Zhao, Z.; Hosler, G.A.; et al. A Short Isoform of Spermatogenic Enzyme GAPDHS Functions as a Metabolic Switch and Limits Metastasis in Melanoma. Cancer Res. 2022, 82, 1251–1266. [Google Scholar] [CrossRef]
- Rohozinski, J.; Anderson, M.L.; Broaddus, R.E.; Edwards, C.L.; Bishop, C.E. Spermatogenesis Associated Retrogenes Are Expressed in the Human Ovary and Ovarian Cancers. PLoS ONE 2009, 4, e5064. [Google Scholar] [CrossRef]
- Yara, S.; Lavoie, J.-C.; Levy, E. Oxidative Stress and DNA Methylation Regulation in the Metabolic Syndrome. Epigenomics 2015, 7, 283–300. [Google Scholar] [CrossRef]
- Guo, D.; Meng, Y.; Jiang, X.; Lu, Z. Hexokinases in Cancer and Other Pathologies. Cell Insight 2023, 2, 100077. [Google Scholar] [CrossRef]
- Mokuda, O.; Tanaka, H.; Hayashi, T.; Ooka, H.; Okazaki, R.; Sakamoto, Y. Ethanol Stimulates Glycogenolysis and Inhibits Both Glycogenesis via Gluconeogenesis and from Exogenous Glucose in Perfused Rat Liver. Ann. Nutr. Metab. 2004, 48, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.F.; Matschinsky, F.M. Ethanol Metabolism: The Good, the Bad, and the Ugly. Med. Hypotheses 2020, 140, 109638. [Google Scholar] [CrossRef]
- Zapater, J.L.; Lednovich, K.R.; Khan, M.W.; Pusec, C.M.; Layden, B.T. Hexokinase Domain-Containing Protein-1 in Metabolic Diseases and Beyond. Trends Endocrinol. Metab. TEM 2022, 33, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Farooq, Z.; Jorgensen, J.; Layden, B.T. 6562 Understanding the Role of the Novel Hexokinase HKDC1 in Alzheimer’s Disease. J. Endocr. Soc. 2024, 8, bvae163.990. [Google Scholar] [CrossRef]
- Horvat, S.; Kos, J.; Pišlar, A. Multifunctional Roles of γ-Enolase in the Central Nervous System: More than a Neuronal Marker. Cell Biosci. 2024, 14, 61. [Google Scholar] [CrossRef]
- Vallés, S.; Pitarch, J.; Renau-Piqueras, J.; Guerri, C. Ethanol Exposure Affects Glial Fibrillary Acidic Protein Gene Expression and Transcription During Rat Brain Development. J. Neurochem. 1997, 69, 2484–2493. [Google Scholar] [CrossRef]
- Siragusa, M.; Thöle, J.; Bibli, S.; Luck, B.; Loot, A.E.; de Silva, K.; Wittig, I.; Heidler, J.; Stingl, H.; Randriamboavonjy, V.; et al. Nitric Oxide Maintains Endothelial Redox Homeostasis through PKM2 Inhibition. EMBO J. 2019, 38, e100938. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Tan-Sah, V.P.; Ding, E.Y.; Smith, J.M.; Miyamoto, S. Hexokinase-II Positively Regulates Glucose Starvation-Induced Autophagy through TORC1 Inhibition. Mol. Cell 2014, 53, 521–533. [Google Scholar] [CrossRef]
- Ruiter-Lopez, L.; Khan, M.A.S.; Wang, X.; Song, B.-J. Roles of Oxidative Stress and Autophagy in Alcohol-Mediated Brain Damage. Antioxidants 2025, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Stankevic, E.; Israelsen, M.; Juel, H.B.; Madsen, A.L.; Ängquist, L.; Aldiss, P.S.J.; Torp, N.; Johansen, S.; Hansen, C.D.; Hansen, J.K.; et al. Binge Drinking Episode Causes Acute, Specific Alterations in Systemic and Hepatic Inflammation-Related Markers. Liver Int. 2023, 43, 2680–2691. [Google Scholar] [CrossRef]
- Waszkiewicz, N.; Galińska-Skok, B.; Nestsiarovich, A.; Kułak-Bejda, A.; Wilczyńska, K.; Simonienko, K.; Kwiatkowski, M.; Konarzewska, B. Neurobiological Effects of Binge Drinking Help in Its Detection and Differential Diagnosis from Alcohol Dependence. Dis. Markers 2018, 2018, 5623683. [Google Scholar] [CrossRef]
- NCBI. National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 27 February 2025).
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef] [PubMed]
- HPA. The Human Protein Atlas. 2025. Available online: https://www.proteinatlas.org/ (accessed on 27 February 2025).
- De Jesus, A.; Keyhani-Nejad, F.; Pusec, C.M.; Goodman, L.; Geier, J.A.; Stoolman, J.S.; Stanczyk, P.J.; Nguyen, T.; Xu, K.; Suresh, K.V.; et al. Hexokinase 1 cellular localization regulates the metabolic fate of glucose. Mol. Cell. 2022, 82, 1261–1277.e9. [Google Scholar] [CrossRef] [PubMed]
- Blaha, C.S.; Ramakrishnan, G.; Jeon, S.-M.; Nogueira, V.; Rho, H.; Kang, S.; Bhaskar, P.; Terry, A.R.; Aissa, A.F.; Frolov, M.V.; et al. A non-catalytic scaffolding activity of hexokinase 2 contributes to EMT and metastasis. Nat. Commun. 2022, 13, 899. [Google Scholar] [CrossRef]
- Ciscato, F.; Ferrone, L.; Masgras, I.; Laquatra, C.; Rasola, A. Hexokinase 2 in Cancer: A Prima Donna Playing Multiple Characters. Int. J. Mol. Sci. 2021, 22, 4716. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Qin, Q.; Wang, D.; Zhao, J.; Gao, H.; Yuan, X.; Zhang, J.; Zou, Y.; Mao, Z.; et al. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson’s disease. Neurobiol. Dis. 2022, 163, 105605. [Google Scholar] [CrossRef]
- Lowes, W.; Walker, M.; Alberti, K.G.; Agius, L. Hexokinase isoenzymes in normal and cirrhotic human liver: Suppression of glucokinase in cirrhosis. Biochim. Biophys Acta 1998, 1379, 134–142. [Google Scholar] [CrossRef]
- Preller, A.; Wilson, J.E. Localization of the type III isozyme of hexokinase at the nuclear periphery. Arch. Biochem. Biophys. 1992, 294, 482–492. [Google Scholar] [CrossRef]
- Zhu, M.; Tang, X.; Xu, J.; Gong, Y. Identification of HK3 as a promising immunomodulatory and prognostic target in sepsis-induced acute lung injury. Biochem. Biophys. Res. Commun. 2024, 706, 149759. [Google Scholar] [CrossRef]
- van der Kooij, M.A.; Rojas-Charry, L.; Givehchi, M.; Wolf, C.; Bueno, D.; Arndt, S.; Tenzer, S.; Mattioni, L.; Treccani, G.; Hasch, A.; et al. Chronic social stress disrupts the intracellular redistribution of brain hexokinase 3 induced by shifts in peripheral glucose levels. J. Mol. Med. Berl. Ger. 2022, 100, 1441–1453. [Google Scholar] [CrossRef]
- Iynedjian, P.B. Molecular physiology of mammalian glucokinase. Cell. Mol. Life Sci. CMLS 2009, 66, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Shiota, C.; Coffey, J.; Grimsby, J.; Grippo, J.F.; Magnuson, M.A. Nuclear import of hepatic glucokinase depends upon glucokinase regulatory protein, whereas export is due to a nuclear export signal sequence in glucokinase. J. Biol. Chem. 1999, 274, 37125–37130. [Google Scholar] [CrossRef] [PubMed]
- Arden, C.; Baltrusch, S.; Agius, L. Glucokinase regulatory protein is associated with mitochondria in hepatocytes. FEBS Lett. 2006, 580, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, L.P.; Gastaldelli, A.; Vetter, D.; Patman, G.L.; Pascoe, L.; Hannivoort, R.A.; Lee, U.E.; Fiel, I.; Muñoz, U.; Ciociaro, D.; et al. Glucokinase links Krüppel-like factor 6 to the regulation of hepatic insulin sensitivity in nonalcoholic fatty liver disease. Hepatology 2012, 55, 1083–1093. [Google Scholar] [CrossRef]
- Khan, M.W.; Terry, A.R.; Priyadarshini, M.; Ilievski, V.; Farooq, Z.; Guzman, G.; Cordoba-Chacon, J.; Ben-Sahra, I.; Wicksteed, B.; Layden, B.T. The hexokinase «HKDC1» interaction with the mitochondria is essential for liver cancer progression. Cell Death Dis. 2022, 13, 660. [Google Scholar] [CrossRef]
- Cui, M.; Yamano, K.; Yamamoto, K.; Yamamoto-Imoto, H.; Minami, S.; Yamamoto, T.; Matsui, S.; Kaminishi, T.; Shima, T.; Ogura, M.; et al. HKDC1, a target of TFEB, is essential to maintain both mitochondrial and lysosomal homeostasis, preventing cellular senescence. Proc. Natl. Acad. Sci. USA 2024, 121, e2306454120. [Google Scholar] [CrossRef]
- Broxmeyer, H.E. All in for nuclear PFKP–induced CXCR4 metastasis: A T cell acute lymphoblastic leukemia prognostic marker. J. Clin. Investig. 2021, 131, e151295. [Google Scholar] [CrossRef]
- Wu, X.Y.; Peng, S.; Li, X.T.; Chen, S.W.; Wei, Y.; Ye, Y.T.; Zhou, C.Z.; Zhong, Z.K.; Gao, L.Z.; Jin, C.Y.; et al. PFKP inhibition protects against pathological cardiac hypertrophy by regulating protein synthesis. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167542. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, L.; Fang, C.; Liang, H.; Ma, J. A comprehensive prognostic and immunological implications of PFKP in pan-cancer. Cancer Cell Int. 2024, 24, 310. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Dou, X.; Wan, J.; Zhou, J.; Li, T.; Yu, J.; Ye, F. Integrative metabolomics and proteomics reveal the effect and mechanism of Zi Qi decoction on alleviating liver fibrosis. Sci. Rep. 2024, 14, 28943. [Google Scholar] [CrossRef]
- Ishaq, A.R.; Younis, T.; Lin, S.; Usman, M.; Wang, T.; Chen, Z.S. Phosphofructokinase-1 in Cancer: A Promising Target for Diagnosis and Therapy. Recent Pat. Anticancer Drug Discov. in press. 2024. [Google Scholar]
- García, M.; Pujol, A.; Ruzo, A.; Riu, E.; Ruberte, J.; Arbós, A.; Serafín, A.; Albella, B.; Felíu, J.E.; Bosch, F.; et al. Phosphofructo-1-kinase deficiency leads to a severe cardiac and hematological disorder in addition to skeletal muscle glycogenosis. PLoS Genet. 2009, 5, e1000615. [Google Scholar] [CrossRef]
- Nakamura, N.; Mori, C.; Eddy, E.M. Molecular complex of three testis-specific isozymes associated with the mouse sperm fibrous sheath: Hexokinase 1, phosphofructokinase M, and glutathione S-transferase mu class 5. Biol. Reprod. 2010, 82, 504–515. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Chen, T.; Lin, Y.; Gong, J.; Xu, Q.; Wang, J.; Li, J.; Meng, Y.; Li, Y.; et al. Caveolin-1 depletion attenuates hepatic fibrosis via promoting SQSTM1-mediated PFKL degradation in HSCs. Free Radic. Biol. Med. 2023, 204, 95–107. [Google Scholar] [CrossRef]
- Kohnhorst, C.L.; Kyoung, M.; Jeon, M.; Schmitt, D.L.; Kennedy, E.L.; Ramirez, J.; Bracey, S.M.; Luu, B.T.; Russell, S.J.; An, S. Identification of a multienzyme complex for glucose metabolism in living cells. J. Biol. Chem. 2017, 292, 9191–9203. [Google Scholar] [CrossRef]
- Yu, S.; Xue, Y.; Chen, Y.; Cao, Y.; Yang, Y.; Ge, X.; Cai, X. The multifaceted roles of aldolase A in cancer: Glycolysis, cytoskeleton, translation and beyond. Hum. Cell 2025, 38, 45. [Google Scholar] [CrossRef] [PubMed]
- Sobanski, T.; Suraweera, A.; Burgess, J.T.; Richard, I.; Cheong, C.M.; Dave, K.; Rose, M.; Adams, M.N.; O’bYrne, K.J.; Richard, D.J.; et al. The fructose-bisphosphate, Aldolase A (ALDOA), facilitates DNA-PKcs and ATM kinase activity to regulate DNA double-strand break repair. Sci. Rep. 2023, 13, 15171. [Google Scholar] [CrossRef]
- Zhao, N.; Xu, H. Pan-cancer analysis of aldolase B gene as a novel prognostic biomarker for human cancers. Medicine 2023, 102, e33577. [Google Scholar] [CrossRef]
- Yin, C.; Zhang, C.; Wang, Y.; Liu, G.; Wang, N.; Liang, N.; Zhang, L.; Tu, Q.; Lv, J.; Jiang, H.; et al. ALDOB/KAT2A interactions epigenetically modulate TGF-β expression and T cell functions in hepatocellular carcinogenesis. Hepatol. Baltim. Md. 2025, 81, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Cui, Q. Diverse roles of aldolase enzymes in cancer development, drug resistance and therapeutic approaches as moonlighting enzymes. Med. Oncol. 2024, 41, 224. [Google Scholar] [CrossRef]
- Wang, F.; Xu, C.S.; Chen, W.H.; Duan, S.W.; Xu, S.J.; Dai, J.J.; Wang, Q.W.; Zhu, L.Q. Identification of Blood-Based Glycolysis Gene Associated with Alzheimer’s Disease by Integrated Bioinformatics Analysis. J. Alzheimers Dis. JAD 2021, 83, 163–178. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Cao, X.; Tan, L.; Jia, B.; Chen, R.; Li, J. GAPDH: A common housekeeping gene with an oncogenic role in pan-cancer. Comput. Struct. Biotechnol. J. 2023, 21, 4056–4069. [Google Scholar] [CrossRef]
- Cornett, K.; Puderbaugh, A.; Back, O.; Craven, R. GAPDH in neuroblastoma: Functions in metabolism and survival. Front. Oncol. 2022, 12, 979683. [Google Scholar] [CrossRef]
- Miki, K.; Qu, W.; Goulding, E.H.; Willis, W.D.; Bunch, D.O.; Strader, L.F.; Perreault, S.D.; Eddy, E.M.; O’BRien, D.A. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 16501–16506. [Google Scholar] [CrossRef]
- Naletova, I.; Schmalhausen, E.; Tomasello, B.; Pozdyshev, D.; Attanasio, F.; Muronetz, V. The role of sperm-specific glyceraldehyde-3-phosphate dehydrogenase in the development of pathologies-from asthenozoospermia to carcinogenesis. Front. Mol. Biosci. 2023, 10, 1256963. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Shen, P.; Ni, Y.; Han, X. The basic functions of phosphoglycerate kinase 1 and its roles in cancer and other diseases. Eur. J. Pharmacol. 2022, 920, 174835. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Meisenhelder, J.; Yang, W.; Hawke, D.H.; Zheng, Y.; Xia, Y.; Aldape, K.; He, J.; Hunter, T.; et al. Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Mol. Cell 2016, 61, 705–719. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Gao, J.; Cheng, Q.; Liu, L.; Cai, R. Activation of Pgk1 Results in Reduced Protein Aggregation in Diverse Neurodegenerative Conditions. Mol. Neurobiol. 2023, 60, 5090–5101. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Zhang, H.; Shen, X.F.; Liu, F.J.; Liu, J.; Wang, W.J. Characteristics of testis-specific phosphoglycerate kinase 2 and its association with human sperm quality. Hum. Reprod. 2016, 31, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Wang, C.; Gu, J.; Zhu, Z. Isoscopoletin inhibits hepatocellular carcinoma cell proliferation via regulating glycolysis-related proteins. PLoS ONE 2024, 19, e0310530. [Google Scholar] [CrossRef]
- Feo, S.; Arcuri, D.; Piddini, E.; Passantino, R.; Giallongo, A. ENO1 gene product binds to the c-myc promoter and acts as a transcriptional repressor: Relationship with Myc promoter-binding protein 1 (MBP-1). FEBS Lett. 2000, 473, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.; Wu, A.; Chen, X.; Tian, Y.; Lin, X. Enolase 1, a Moonlighting Protein, as a Potential Target for Cancer Treatment. Int. J. Biol. Sci. 2021, 17, 3981–3992. [Google Scholar] [CrossRef]
- Nawaz, S.; Hussain, S.; Bilal, M.; Syed, N.; Liaqat, K.; Ullah, I.; Akil, A.A.; Fakhro, K.A.; Ahmad, W. A variant in sperm-specific glycolytic enzyme enolase 4 (ENO4) causes human male infertility. J. Gene Med. 2024, 26, e3583. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hernández, R.; Serrano-Somavilla, A.; Ramos-Leví, A.; Sampedro-Nuñez, M.; Lens-Pardo, A.; De Nova, J.L.M.; Triviño, J.C.; González, M.U.; Torné, L.; Casares-Arias, J.; et al. Integrated miRNA and mRNA expression profiling identifies novel targets and pathological mechanisms in autoimmune thyroid diseases. EBioMedicine 2019, 50, 329–342. [Google Scholar] [CrossRef]
- Hamzic, S.; Kummer, D.; Froehlich, T.K.; Joerger, M.; Aebi, S.; Palles, C.; Thomlinson, I.; Meulendijks, D.; Schellens, J.H.; García-González, X.; et al. Evaluating the role of ENOSF1 and TYMS variants as predictors in fluoropyrimidine-related toxicities: An IPD meta-analysis. Pharmacol. Res. 2020, 152, 104594. [Google Scholar] [CrossRef]
- Wichelecki, D.J.; Froese, D.S.; Kopec, J.; Muniz, J.R.C.; Yue, W.W.; Gerlt, J.A. Enzymatic and structural characterization of rTSγ provides insights into the function of rTSβ. Biochemistry 2014, 53, 2732–2738. [Google Scholar] [CrossRef]
- Holmes, R.S. Bioinformatic studies of vertebrate enolases: Multifunctional genes and proteins. Open Access Bioinforma. 2011, 3, 43–59. [Google Scholar] [CrossRef]
- Liang, P.; Nair, J.R.; Song, L.; McGuire, J.J.; Dolnick, B.J. Comparative genomic analysis reveals a novel mitochondrial isoform of human rTS protein and unusual phylogenetic distribution of the rTS gene. BMC Genom. 2005, 6, 125. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Lee, S.; Kim, W.; Klevstig, M.; Harzandi, A.M.; Sikanic, N.; Arif, M.; Ståhlman, M.; Nielsen, J.; et al. Pyruvate kinase L/R is a regulator of lipid metabolism and mitochondrial function. Metab. Eng. 2019, 52, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Chen, B.; Wang, C.; Xiao, X.; Hu, A.; Liu, W.; Kuang, Y.; Sample, K.M.; Zacksenhaus, E.; Gajendran, B.; et al. FLI1 accelerates leukemogenesis through transcriptional regulation of pyruvate kinase-L/R and other glycolytic genes. Med. Oncol. 2022, 40, 69. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.; Dey, T.; Ashish Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef]
- Xue, S.; Luo, Z.; Mao, Y.; Liu, S. A comprehensive analysis of the pyruvate kinase M1/2 (PKM) in human cancer. Gene 2025, 937, 149155. [Google Scholar] [CrossRef] [PubMed]


| Variable | AUD Patients (n = 11) | Controls (n = 7) | p-Value |
|---|---|---|---|
| Age (years) | 54.51 (10.43) | 52.85 (4.38) | 0.64 |
| Male (n*)/Female (n*) | 9 (81.80)/3(27.27) | 4 (57.14)/3 (42.85) | 0.52 |
| Total bilirubin (mg/dL) | 0.80 (0.51) | 0.82 (0.29) | 0.84 |
| AST (U/L) | 117 (98.65) | 17.88 (4.94) | 0.049 |
| ALT (U/L) | 64.27 (43.43) | 27.86 (18) | 0.034 |
| GGT (U/L) | 348.29 (518) | 21.14 (11.23) | 0.17 |
| ALP (U/L) | 91.45 (29.22) | 55.86 (9.55) | 0.003 |
| Proteins (g/dL) | 7.3 (0.70) | 7.50 (0.49) | 0.89 |
| Albumin (g/dL) | 4.5 (0.32) | 4.77 (0.16) | 0.11 |
| Ferritin (ng/mL) | 205 (112.62) | 98.96 (91.62) | 0.15 |
| Hemoglobin (g/dL) | 16.10 (1.46) | 15.11 (1.34) | 0.19 |
| Leukocytes (×103 cells/μL) | 8.77 (3.41) | 6.20 (8.29) | 0.045 |
| Neutrophils (×103 cells/μL) | 5.73 (2.77) | 3.67 (6.16) | 0.043 |
| Platelets (×103 cells/μL) | 252.27 (80.83) | 233.42 (66.40) | 0.62 |
| Total cholesterol (mg/dL) | 175 (42.43) | 197 (34.38) | 0.29 |
| Triglycerides (mg/dL) | 106.2 (55.13) | 90.06 (46.07) | 0.55 |
| Variable | AAC Patients (n = 7) | Controls (n = 7) | p-Value |
|---|---|---|---|
| Age (years) | 18.71 (3.37) | 22.57 (2.37) | 0.043 |
| Male (n*)/Female (n*) | 6 (85.71)/1 (14.18) | 4 (57.14)/3 (42.85) | 0.272 |
| AST (U/L) | 33.29 (13.64) | 18.83 (4.60) | 0.040 |
| ALT (U/L) | 18.29 (4.46) | 23.33 (18.25) | 0.572 |
| ALP (U/L) | 105.01 (54.96) | 60.17 (13.43) | 0.094 |
| GGT (U/L) | 18.71 (5.09) | 15.67 (8.81) | 0.513 |
| LDH | 228.71 (96.88) | 159.67 (11.79) | 0.134 |
| Leukocytes (×103 cells/μL) | 8.19 (1.85) | 5.18 (1.35) | 0.010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Pirela, M.; Salete-Granado, D.; Andrade-Alviárez, D.; Prieto-Rojas, A.; Rodríguez, C.; Aguilar-Sánchez, M.-L.; Puertas-Miranda, D.; Pérez-Nieto, M.-Á.; Rueda-Cala, V.; Pérez, C.; et al. Dysregulated Expression of Canonical and Non-Canonical Glycolytic Enzyme Isoforms in Peripheral Blood from Subjects with Alcohol Use Disorder and from Individuals with Acute Alcohol Consumption. Antioxidants 2025, 14, 1143. https://doi.org/10.3390/antiox14091143
Rojas-Pirela M, Salete-Granado D, Andrade-Alviárez D, Prieto-Rojas A, Rodríguez C, Aguilar-Sánchez M-L, Puertas-Miranda D, Pérez-Nieto M-Á, Rueda-Cala V, Pérez C, et al. Dysregulated Expression of Canonical and Non-Canonical Glycolytic Enzyme Isoforms in Peripheral Blood from Subjects with Alcohol Use Disorder and from Individuals with Acute Alcohol Consumption. Antioxidants. 2025; 14(9):1143. https://doi.org/10.3390/antiox14091143
Chicago/Turabian StyleRojas-Pirela, Maura, Daniel Salete-Granado, Diego Andrade-Alviárez, Alejandro Prieto-Rojas, Cristina Rodríguez, María-Lourdes Aguilar-Sánchez, David Puertas-Miranda, María-Ángeles Pérez-Nieto, Vanessa Rueda-Cala, Candy Pérez, and et al. 2025. "Dysregulated Expression of Canonical and Non-Canonical Glycolytic Enzyme Isoforms in Peripheral Blood from Subjects with Alcohol Use Disorder and from Individuals with Acute Alcohol Consumption" Antioxidants 14, no. 9: 1143. https://doi.org/10.3390/antiox14091143
APA StyleRojas-Pirela, M., Salete-Granado, D., Andrade-Alviárez, D., Prieto-Rojas, A., Rodríguez, C., Aguilar-Sánchez, M.-L., Puertas-Miranda, D., Pérez-Nieto, M.-Á., Rueda-Cala, V., Pérez, C., Quiñones, W., Michels, P. A. M., Almeida, Á., & Marcos, M. (2025). Dysregulated Expression of Canonical and Non-Canonical Glycolytic Enzyme Isoforms in Peripheral Blood from Subjects with Alcohol Use Disorder and from Individuals with Acute Alcohol Consumption. Antioxidants, 14(9), 1143. https://doi.org/10.3390/antiox14091143







