Integrated Metabolomics and Transcriptomics Reveals Metabolic Pathway Changes in Common Carp Muscle Under Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish, Experimental Design and Sampling
2.2. Measurement of Amino Acids and Fatty Acids in Muscle
2.3. Non-Targeted Metabolome Analysis
2.4. Transcriptome Analysis
2.5. Statistical Analysis
3. Results
3.1. Amino Acid and Fatty Acid Composition in Muscle
3.2. Metabolomics Analysis in Muscle
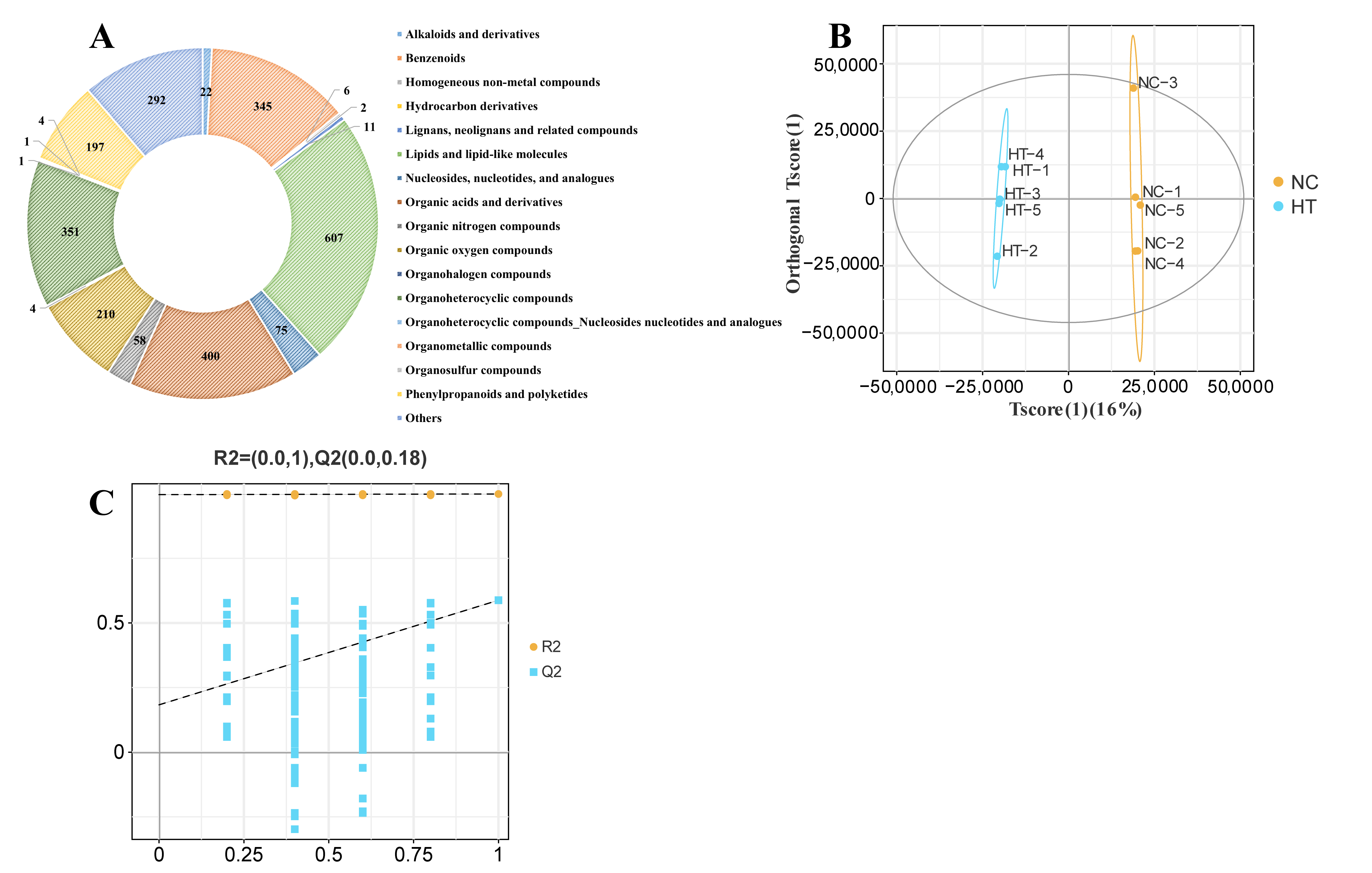
3.2.1. Metabolite Identification
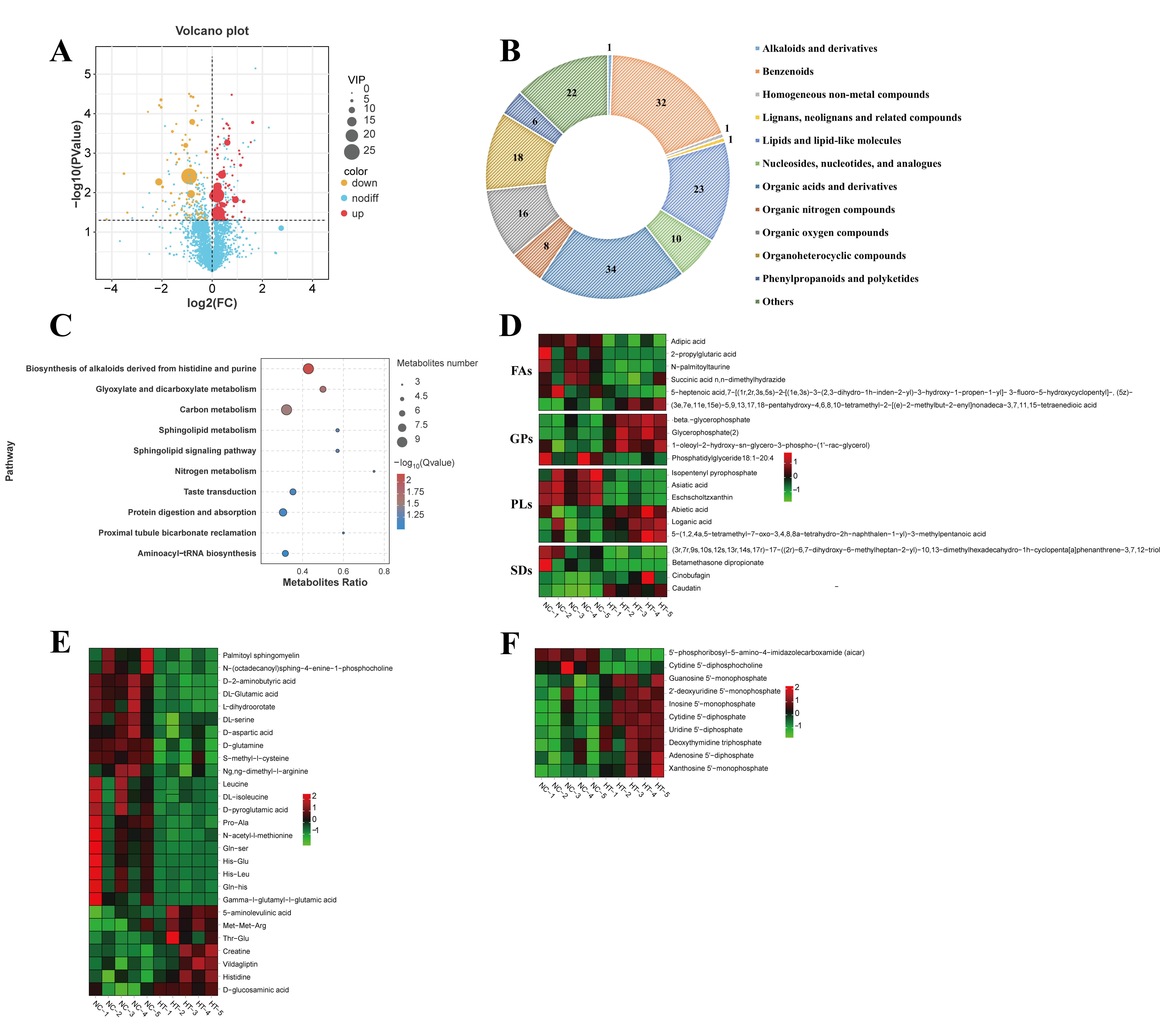
3.2.2. Metabolite Differential Analysis
3.3. Transcriptomic Analysis in Muscle
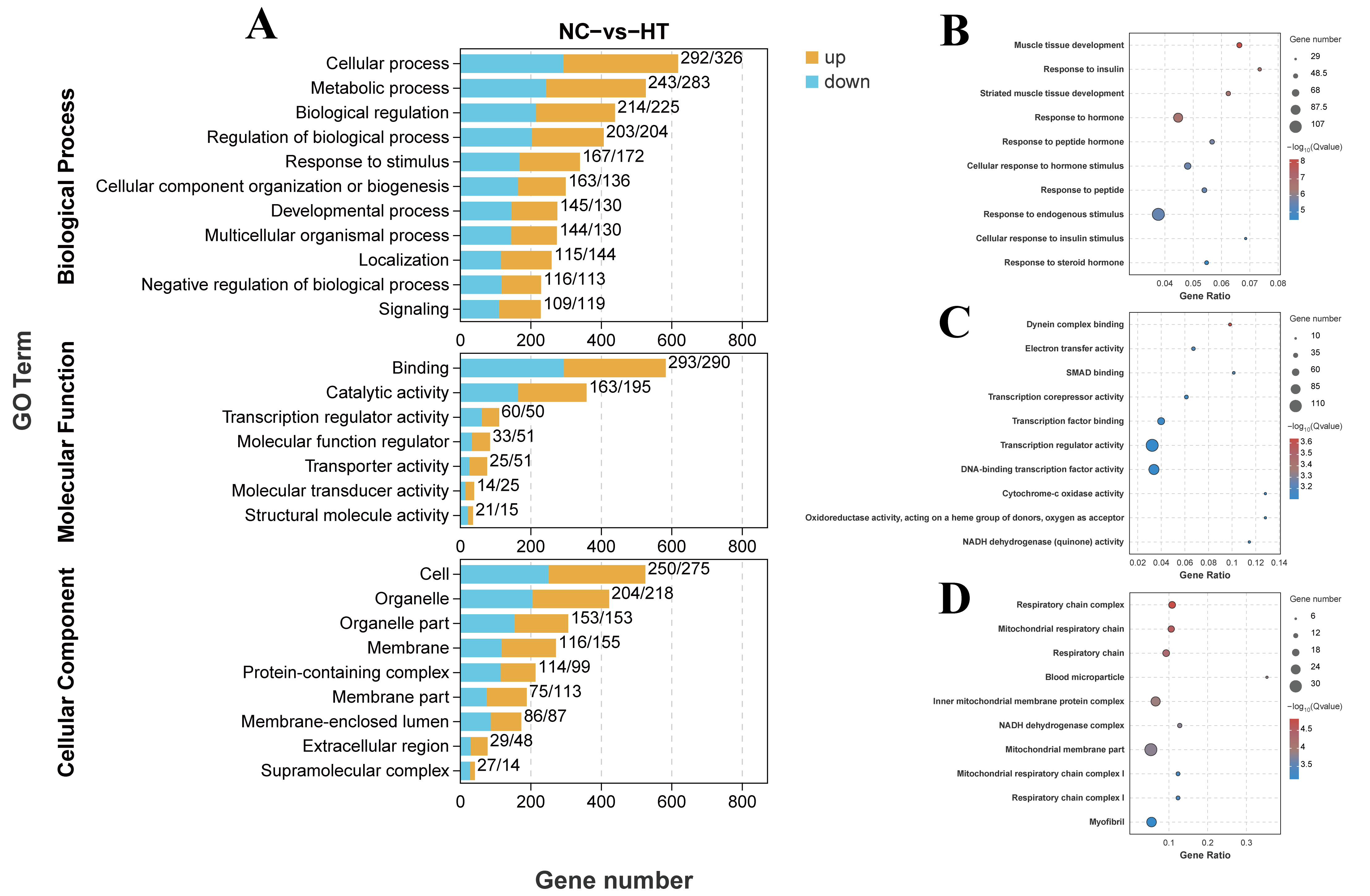
3.3.1. Differential Analysis
3.3.2. Enrichment Analysis of GO and KEGG
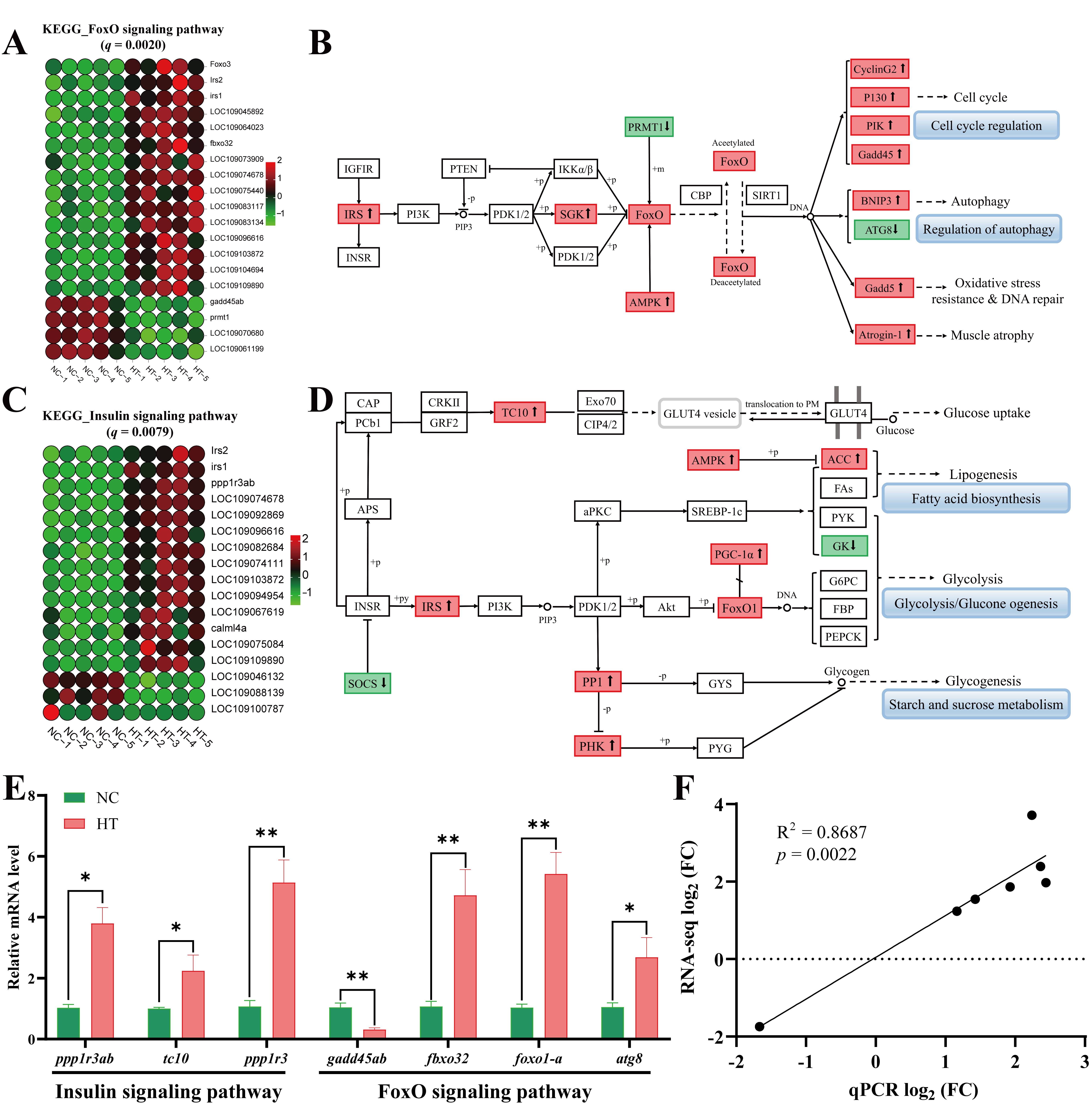
3.3.3. Alterations of Pathways of FoxO and Insulin
3.3.4. Alterations of Metabolism-Related Pathways
4. Discussion
4.1. The Effect of Oxidative Stress on Muscle Nutrient Quality
4.2. The Effects of Oxidative Stress on Muscle Metabolite Composition
4.3. The Effects of Oxidative Stress on Muscle Metabolism-Related Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ueki, R.; Imaizumi, Y.; Iwamoto, Y.; Sakugawa, H.; Takeda, K. Factors controlling the degradation of hydrogen peroxide in river water, and the role of riverbed sand. Sci. Total. Environ. 2020, 716, 136971. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.M.; Zang, Y.; Hua, X.Y.; Jiang, X.; Liang, D.P.; Guo, Z.Y. Effect of dissolved organic matter on the generation of H2O2 in natural biofilm systems under illumination. Chem. J. Chin. Univ. 2019, 40, 800–808. [Google Scholar]
- Clark, C.D.; de Bruyn, W.; Jones, J.G. Photoproduction of hydrogen peroxide in aqueous solution from model compounds for chromophoric dissolved organic matter (CDOM). Mar. Pollut. Bull. 2014, 79, 54–60. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Fischer, A.M. Sunlight-induced photochemistry of humic substances in natural waters: Major reactive species. ACS Symp. Ser. 1989, 219, 333–362. [Google Scholar]
- Santos, A.A.; Guedes, D.O.; Barros, M.U.G.; Oliveira, S.; Pacheco, A.B.F.; Azevedo, S.; Magalhaes, V.F.; Pestana, C.J.; Edwards, C.; Lawton, L.A.; et al. Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res. 2021, 197, 117069. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Shi, X.; Zhang, M.; Liu, C.; Chen, K. Comparison of algal harvest and hydrogen peroxide treatment in mitigating cyanobacterial blooms via an in situ mesocosm experiment. Sci. Total. Environ. 2019, 694, 133721. [Google Scholar] [CrossRef]
- Sousa, K.S.; Souto-Neto, J.A.; Medeiros, A.P.M.; Oliveira, T.P.R.; Rebouças, J.S.; Rosa, I.M.D. Hydrogen peroxide in seahorse aquaculture: Determining safe exposure levels using non-invasive biomarkers of stress. Aquaculture 2023, 564, 8. [Google Scholar] [CrossRef]
- Pedersen, L.F.; Pedersen, P.B. Hydrogen peroxide application to a commercial recirculating aquaculture system. Aquac. Eng. 2012, 46, 40–46. [Google Scholar] [CrossRef]
- Bögner, D.; Bögner, M.; Schmachtl, F.; Bill, N.; Halfer, J.; Slater, M.J. Hydrogen peroxide oxygenation and disinfection capacity in recirculating aquaculture systems. Aquac. Eng. 2021, 92, 11. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.L.; Cao, L.P.; Jeney, G.; Yin, G.J. A model of oxidative stress-induced liver injury in tilapia (Oreochromis niloticus). In Proceedings of the 2018 Annual Academic Conference of the China Society of Fisheries, Xi’an, China, 10 August 2018; p. 1. [Google Scholar]
- He, Q.; Jia, R.; Cao, L.P.; Du, J.L.; Gu, Z.Y.; Galina, J.; Xu, P.; Yin, G.J. Antioxidative status and immune response in common carp (Cyprinus carpio) under oxidative stress. J. Fish. China 2021, 45, 33–43. [Google Scholar]
- Hao, X.; Liu, M.C.; Zhang, X.; Yu, H.; Fang, Z.Y.; Gao, X.X.; Chen, M.; Shao, Q.; Gao, W.W.; Lei, L.; et al. Thioredoxin-2 suppresses hydrogen peroxide-activated nuclear factor kappa B signaling via alleviating oxidative stress in bovine adipocytes. J. Dairy Sci. 2024, 107, 4045–4055. [Google Scholar] [CrossRef]
- He, Q.H.; Feng, W.R.; Chen, X.; Xu, Y.F.; Zhou, J.; Li, J.L.; Xu, P.; Tang, Y.K. H2O2-induced oxidative stress responses in Eriocheir sinensis: Antioxidant defense and immune gene expression dynamics. Antioxidants 2024, 13, 524. [Google Scholar] [CrossRef]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Chen, Z.D.; Xing, T.; Li, J.L.; Zhang, L.; Jiang, Y.; Gao, F. Oxidative stress induced by hydrogen peroxide promotes glycolysis by activating CaMKK/LKB1/AMPK pathway in broiler breast muscle. Poult. Sci. 2022, 101, 10. [Google Scholar] [CrossRef]
- Golnarnik, G.; Thiede, B.; Soland, T.M.; Galtung, H.K.; Haug, T.M. Hydrogen peroxide-induced oxidative stress alters protein expression in two rat salivary acinar cell lines. Arch. Oral Biol. 2025, 175, 16. [Google Scholar] [CrossRef]
- Hwang, B.O.; Kim, Y.K.; Nam, Y.K. Effect of hydrogen peroxide exposures on mucous cells and lysozymes of gill tissues of olive flounder Paralichthys olivaeceus. Aquac. Res. 2016, 47, 433–444. [Google Scholar] [CrossRef]
- Mou, Y.T.; Li, B.; Hou, Y.R.; Jia, R.; Zhu, J. Effect of Chronic Hydrogen Peroxide Exposure on Ion Transport in Gills of Common Carp (Cyprinus carpio). Fishes 2023, 8, 134. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.L.; Cao, L.P.; Li, Y.; Johnson, O.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Antioxidative, inflammatory and immune responses in hydrogen peroxide-induced liver injury of tilapia (GIFT, Oreochromis niloticus). Fish Shellfish Immunol. 2019, 84, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhong, L.; Fan, Y.D.; Zhang, J.Z.; Dai, J.H.; Zhong, H.; Fu, G.H.; Hu, Y. Taurine inhibits hydrogen peroxide-induced oxidative stress, inflammatory response and apoptosis in liver of Monopterus albus. Fish Shellfish Immunol. 2022, 128, 536–546. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Chen, K.J.; Fan, Y.D.; Xie, K.; Zhang, J.Z.; Dai, J.H.; Hu, Y. Sanguinarine attenuates hydrogen peroxide-induced toxicity in liver of Monopterus albus: Role of oxidative stress, inflammation and apoptosis. Fish Shellfish Immunol. 2022, 125, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Zhang, X.X.; Zhao, L.L.; Liu, S.H. Multi-omics profiling reveals the molecular mechanisms of H2O2-induced detrimental effects on Thamnaconus septentrionalis. BMC Genom. 2024, 25, 16. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, L.Z.; Xiong, S.B.; You, J.; Liu, R.; Xu, D.F.; Huang, Q.L.; Ma, H.W.; Yin, T. A comprehensive review of the mechanisms on fish stress affecting muscle qualities: Nutrition, physical properties, and flavor. Compr. Rev. Food. Sci. Food Saf. 2024, 23, 23. [Google Scholar] [CrossRef]
- Tanaka, K.; Farooqui, A.A.; Siddiqi, N.J.; Alhomida, A.S.; Ong, W.Y. Effects of Docosahexaenoic Acid on Neurotransmission. Biomol. Ther. 2012, 20, 152–157. [Google Scholar] [CrossRef]
- Kew, S.; Mesa, M.D.; Tricon, S.; Buckley, R.; Minihane, A.M.; Yaqoob, P. Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am. J. Clin. Nutr. 2004, 79, 674–681. [Google Scholar] [CrossRef]
- Hosomi, R. Health Benefits of Dietary Docosahexaenoic Acid-and Eicosapentaenoic Acid-enriched Glycerophospholipids from Marine Sources. J. Oleo Sci. 2025, 74, 12. [Google Scholar] [CrossRef] [PubMed]
- Sohn, J.H.; Ohshima, T. Control of lipid oxidation and meat color deterioration in skipjack tuna muscle during ice storage. Fish. Sci. 2010, 76, 703–710. [Google Scholar] [CrossRef]
- Qi, X.J.; Yin, M.Y.; Qiao, Z.H.; Li, Z.Z.; Yu, Z.; Chen, M.; Xiao, T.; Wang, X.C. Freezing and frozen storage of aquatic products: Mechanism and regulation of protein oxidation. Food Sci. Technol. 2022, 42, 9. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Z.D.; Fu, S.J. Variations in temperature acclimation effects on glycogen storage, hypoxia tolerance and swimming performance with seasonal acclimatization in juvenile Chinese crucian carp. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2015, 185, 16–23. [Google Scholar] [CrossRef]
- Dutta, P.; Dutta, M. Analysis of Fatty Acid Composition in the Flesh of Boal (Wallagu attu). Biosci. Biotechnol. Res. Asia 2019, 16, 477–481. [Google Scholar] [CrossRef]
- GB5009.124-2016; National Food Safety Standard—Determination of Amino Acids in National Food Safety Standards. National Health and Family Planning Commission of the China: Beijing, China, 2016.
- GB5009.168-2016; National Food Safety Standard—Determination of Fatty Acids in National Food Safety Standards. National Health and Family Planning Commission of the China: Beijing, China, 2016.
- Cao, X.L.; Cui, H.; Ji, X.Y.; Lu, Y.Y.J.; Kang, Q.X.; Lu, R.H.; Zhang, Y.R.; Xu, X.X.; Chen, J.J. Branched-chain amino acids target miR-203a/fosb axis to promote skeletal muscle growth in Common Carp (Cyprinus carpio). Aquac. Nutr. 2025, 2025, 19. [Google Scholar] [CrossRef]
- Bifari, F.; Ruocco, C.; Decimo, I.; Fumagalli, G.; Valerio, A.; Nisoli, E. Amino acid supplements and metabolic health: A potential interplay between intestinal microbiota and systems control. Genes Nutr. 2017, 12, 12. [Google Scholar] [CrossRef]
- D’Antona, G.; Tedesco, L.; Ruocco, C.; Corsetti, G.; Ragni, M.; Fossati, A.; Saba, E.; Fenaroli, F.; Montinaro, M.; Carruba, M.O.; et al. A peculiar formula of essential amino acids prevents rosuvastatin myopathy in mice. Antioxid. Redox Signal. 2016, 25, 595–608. [Google Scholar] [CrossRef]
- Aquilani, R.; Zuccarelli, G.C.; Dioguardi, F.S.; Baiardi, P.; Frustaglia, A.; Rutili, C.; Comi, E.; Catani, M.; Iadarola, P.; Viglio, S.; et al. Effects of oral amino acid supplementation on long-term-care-acquired infections in elderly patients. Arch. Gerontol. Geriatr. 2011, 52, E123–E128. [Google Scholar] [CrossRef]
- Zhao, M.J.; Li, A.; Zhang, K.X.; Wang, W.; Zhang, G.F.; Li, L. The role of the balance between energy production and ammonia detoxification mediated by key amino acids in divergent hypersaline adaptation among crassostrea oysters. Environ. Res. 2024, 248, 10. [Google Scholar] [CrossRef]
- Rong, Y.R.; Li, B.; Hou, Y.R.; Zhang, L.Q.; Jia, R.; Zhu, J. Influences of stocking density on antioxidant status, nutrients composition, and lipid metabolism in the muscles of Cyprinus carpio under rice-fish co-culture. Antioxidants 2024, 13, 849. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.Y.; Jia, R.; Li, B.; Zhou, L.J.; Zhu, J.; Hou, Y.R. Effects of Different Stocking Densities on Snail Bellamya purificata Foot Muscle Nutritional Quality and Metabolic Function. Animals 2024, 14, 3618. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Q.; Cao, Y.-M.; Hou, M.-X.; Zhao, R.; Chen, Y.-J.; Yu, S.-T.; Wang, K.-K.; Zhang, Q.; Li, S.-J. Genome-wide association analysis identifies genetic variants associated with muscle fatty acids and amino acids in grass carp (Ctenopharyngodon idella). Agric. Commun. 2024, 2, 100043. [Google Scholar] [CrossRef]
- Han, X.Z.; Wang, J.Y.; Li, B.S.; Song, Z.D.; Li, P.Y.; Huang, B.S.; Wang, C.Q.; Sun, Y.Z.; Wang, X.Y.; Hao, T.T. Analyses of regulatory network and discovery of potential biomarkers for Korean rockfish (Sebastes schlegelii) in responses to starvation stress through transcriptome and metabolome. Comp. Biochem. Physiol. D-Genom. Proteom. 2023, 46, 13. [Google Scholar] [CrossRef]
- Ren, X.Y.; Yu, Z.X.; Xu, Y.; Zhang, Y.B.; Mu, C.M.; Liu, P.; Li, J. Integrated transcriptomic and metabolomic responses in the hepatopancreas of kuruma shrimp (Marsupenaeus japonicus) under cold stress. Ecotoxicol. Environ. Saf. 2020, 206, 9. [Google Scholar] [CrossRef]
- Fan, X.P.; Zhang, J.S.; Guo, Q.Y.; Du, H.; Qin, X.M.; Liu, S.C. Effect of CO2 anesthesia on water-free live-transport of the grouper (Epinephelus fuscoguttatus♀ × Epinephelus laceolatus♂). J. Guangdong Ocean Univ. 2021, 41, 73–81. [Google Scholar]
- Zhang, T.H.; Zhang, L.Z.; Yin, T.; You, J.; Liu, R.; Huang, Q.L.; Shi, L.; Wang, L.; Liao, T.; Wang, W.S.; et al. Recent understanding of stress response on muscle quality of fish: From the perspective of industrial chain. Trends Food Sci. Technol. 2023, 140, 14. [Google Scholar] [CrossRef]
- Jin, S.B.; Xu, M.J.; Gao, X.B.; Jiang, S.F.; Xiong, Y.W.; Zhang, W.Y.; Qiao, H.; Wu, Y.; Fu, H.T. Effects of alkalinity exposure on antioxidant status, metabolic function, and immune response in the hepatopancreas of Macrobrachium nipponense. Antioxidants 2024, 13, 129. [Google Scholar] [CrossRef]
- Wu, Y.W.; Zhao, M.M.; Xiong, G.Q.; Sun, W.Q.; Wu, W.J.; Ding, A.Z.; Chen, S.; Wang, L.; Shi, L. Effects of hypoxia on meat qualities and muscle metabolism in rainbow trout (Oncorhynchus mykiss) during short-time transportation and its relief by reoxygenation. Aquaculture 2023, 570, 11. [Google Scholar] [CrossRef]
- Bermejo-Nogales, A.; Nederlof, M.; Benedito-Palos, L.; Ballester-Lozano, G.F.; Folkedal, O.; Olsen, R.E.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Metabolic and transcriptional responses of gilthead sea bream (Sparus aurata L.) to environmental stress: New insights in fish mitochondrial phenotyping. Gen. Comp. Endocrinol. 2014, 205, 305–315. [Google Scholar] [CrossRef]
- Xu, J.; Shi, M.L.; Chen, L.T.; Chi, S.Y.; Zhang, S.; Cao, J.M.; Tan, B.P.; Xie, S.W. Muscular lipidomics and transcriptomics reveal the effects of bile acids on lipid metabolism in high-fat diet-fed grouper. Fish Physiol. Biochem. 2024, 50, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.M.; Yi, K.; Qian, X.L.; Niu, X.J.; Sun, Y.Z.; Ye, J.D. Growth and metabolic responses of juvenile grouper (Epinephelus coioides) to dietary methionine/cystine ratio at constant sulfur amino acid levels. Aquaculture 2020, 518, 9. [Google Scholar] [CrossRef]
- Kim, M.G.; Yang, I.; Lee, H.S.; Lee, J.Y.; Kim, K. Lipid-modifying effects of krill oil vs fish oil: A network meta-analysis. Nutr. Rev. 2020, 78, 699–708. [Google Scholar] [CrossRef]
- Garcia, C.; Andersen, C.J.; Blesso, C.N. The role of lipids in the regulation of immune responses. Nutrients 2023, 15, 3899. [Google Scholar] [CrossRef]
- Chen, X.-X.; Ouyang, S.-H.; Kurihara, H.; Li, Y.-F.; Wu, Y.-P.; He, R.-R. The role of lipid peroxidation in modulating immune function. Yaoxue Xuebao 2023, 58, 3230–3241. [Google Scholar] [CrossRef]
- Esmaeili, M.; Hosseini, H.; Zare, M.; Akhavan, S.R.; Rombenso, A. Early mild stress along with lipid improves the stress responsiveness of Oscar (Astronotus ocellatus). Aquac. Nutr. 2022, 2022, 17. [Google Scholar] [CrossRef] [PubMed]
- Babu, T.; Yun, E.J.; Kim, S.; Kim, D.H.; Liu, K.H.; Kim, S.R.; Kim, K.H. Engineering Escherichia coli for the production of adipic acid through the reversed β-oxidation pathway. Process Biochem. 2015, 50, 2066–2071. [Google Scholar] [CrossRef]
- Loizides-Mangold, U. On the future of mass-spectrometry-based lipidomics. FEBS J. 2013, 280, 2817–2829. [Google Scholar] [CrossRef]
- Jia, R.; Hou, Y.R.; Feng, W.R.; Nomingerel, M.; Li, B.; Zhu, J. Multi-omics analysis to understand the effects of dietary proanthocyanidins on antioxidant capacity, muscle nutrients, lipid metabolism, and intestinal microbiota in Cyprinus carpio. Antioxidants 2023, 12, 2095. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Ma, A.J.; Yang, S.S.; Huang, Z.H. Integrated metabolome and transcriptome analyses revealing the effects of thermal stress on lipid metabolism in juvenile turbot Scophthalmus maximus. J. Therm. Biol. 2021, 99, 8. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Nie, M.M.; Song, H.B.; Xu, D.D.; You, F. Physiological responses to cold and starvation stresses in the liver of yellow drum (Nibea albiflora) revealed by LC-MS metabolomics. Sci. Total Environ. 2020, 715, 15. [Google Scholar] [CrossRef]
- Li, L.L.; Liu, Z.; Quan, J.Q.; Lu, J.H.; Zhao, G.Y.; Sun, J. Metabonomics analysis reveals the protective effect of nano-selenium against heat stress of rainbow trout (Oncorhynchus mykiss). J. Proteom. 2022, 259, 13. [Google Scholar] [CrossRef]
- Ding, Y.; Sha, W.B.; Sun, Y.F.; Cheng, Y.X. Effects of acute low-temperature stress on respiratory metabolism, antioxidants, and metabolomics of red swamp crayfish, Procambarus clarkii. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2025, 278, 10. [Google Scholar] [CrossRef]
- Salamanca, N.; Giráldez, I.; Morales, E.; de La Rosa, I.; Herrera, M. Phenylalanine and tyrosine as feed additives for reducing stress and enhancing welfare in Gilthead Seabream and Meagre. Animals 2021, 11, 45. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Wang, L.R.; Xu, J.X.; Zheng, J.J.; Xu, Y.Y.; Jin, Z.; Feng, D.; Zhang, M.; Yu, M.; et al. Multi-omics integrative analysis reveals the molecular mechanisms of muscle adaptive changes in largemouth bass (Micropterus salmoides) under water flow stress in recirculating aquaculture. Aquaculture 2025, 599, 12. [Google Scholar] [CrossRef]
- Zhu, J.Q.; Shi, W.J.; Zhao, R.; Gu, C.; Shen, H.; Li, H.; Wang, L.B.; Cheng, J.; Wan, X.H. Integrated physiological, transcriptome, and metabolome analyses of the hepatopancreas of Litopenaeus vannamei under cold stress. Comp. Biochem. Physiol. D-Genom. Proteom. 2024, 49, 19. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Zhang, L.B.; Yang, H.S.; Sun, L.A. Adaptation to hypoxic stress involves amino acid metabolism: A case in sea cucumber. Environ. Pollut. 2023, 330, 9. [Google Scholar] [CrossRef]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–935. [Google Scholar] [CrossRef]
- Wu, G.Y.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Abe, H. Role of histidine-related compounds as intracellular proton buffering constituents in vertebrate muscle. Biochem.-Mosc. 2000, 65, 757–765. [Google Scholar]
- Tian, Y.Q.; Guo, C.; Zhang, X.S.; Xie, S.C.; Zhou, Q.C.; Luo, J.X.; Zhu, T.T.; Yang, Y.H.; Li, X.K.; Jin, M. Effects of dietary histidine level on growth, antioxidant capacity and TOR signaling pathway in juvenile swimming crabs, Portunus trituberculatus. Aquacult. Rep. 2023, 33, 10. [Google Scholar] [CrossRef]
- Yang, P.F.; Deng, F.F.; Yuan, M.D.; Chen, M.; Zeng, L.; Ouyang, Y.A.; Chen, X.B.; Zhao, B.; Yang, Z.; Tian, Z.M. Metabolomics reveals the defense mechanism of histidine supplementation on high-salt exposure-induced hepatic oxidative stress. Life Sci. 2023, 314, 10. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef]
- Klivenyi, P.; Calingasan, N.Y.; Starkov, A.; Stavrovskaya, I.G.; Kristal, B.S.; Yang, L.C.; Wieringa, B.; Beal, M.F. Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiol. Dis. 2004, 15, 610–617. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, Y.J.; Li, Y.Z.; Tian, J.B.; Zhu, Y.J.; Qian, G.Y.; Chen, Z.F.; Li, C.Y.; Wang, W. Effects of creatine treatment at low temperature on the development of ovarian follicles before sexual maturity in Pelodiscus sinensis. Aquaculture 2025, 600, 10. [Google Scholar] [CrossRef]
- Yan, B.A.; Luo, L.J.; Zhang, Y.D.; Men, J.; Guo, Y.Y.; Wu, S.M.; Han, J.; Zhou, B.S. Detrimental effects of glyphosate on muscle metabolism in grass carp (Ctenopharyngodon idellus). Aquat. Toxicol. 2024, 276, 8. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.Y.; Du, J.H.; Zhu, W.L.; Li, Q.Y.; Chen, X.L.; Chen, X.H.; Liu, H.; Zhou, X.Y.; Zhao, Y.Z.; et al. Integrated analysis of physiological, transcriptomic and metabolomic responses and tolerance mechanism of nitrite exposure in Litopenaeus vannamei. Sci. Total Environ. 2020, 711, 10. [Google Scholar] [CrossRef]
- Janero, D.R.; Hreniuk, D.; Sharif, H.M.; Prout, K.C. Hydroperoxide-induced oxidative stress alters pyridine nucleotide metabolism in neonatal heart muscle cells. Am. J. Physiol. 1993, 264, C1401–C1410. [Google Scholar] [CrossRef]
- Leonard, J.A.; Cope, W.G.; Barnhart, M.C.; Bringolf, R.B. Metabolomic, behavioral, and reproductive effects of the synthetic estrogen 17 α-ethinylestradiol on the unionid mussel Lampsilis fasciola. Aquat. Toxicol. 2014, 150, 103–116. [Google Scholar] [CrossRef]
- Xing, Y.F.; Zhu, X.Y.; Duan, Y.F.; Huang, J.H.; Nan, Y.X.; Zhang, J.S. Toxic effects of nitrite and microplastics stress on histology, oxidative stress, and metabolic function in the gills of Pacific white shrimp, Litopenaeus vannamei. Mar. Pollut. Bull. 2023, 187, 10. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Z.M.; Liu, C.; Zhou, J.; Huang, Z.P.; Duan, Y.L.; Zhang, L.; Ke, H.Y.; Du, J.; Mou, C.Y.; et al. An integrated analysis of transcriptome and metabolome to reveal the effects of temperature stress on energy metabolism and physiological responses in Schizothorax wangchiachii muscles. Aquaculture 2024, 591, 12. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; He, K.; Liu, Q.; Luo, J.; Zhang, D.M.; Liang, J.; Liao, L.; Yang, S. MiRNA-mRNA integration analysis reveals the regulatory roles of miRNAs in the metabolism of largemouth bass (Micropterus salmoides) livers during acute hypoxic stress. Aquaculture 2020, 526, 10. [Google Scholar] [CrossRef]
- Cheng, C.H.; Su, Y.L.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Chen, X.L.; Guo, Z.X. Nitrite-induced oxidative stress, histopathology, and transcriptome changes in the Mud Crab (Scylla paramamosain). Isr. J. Aquac.-Bamidgeh 2019, 71, 12. [Google Scholar]
- Sun, Y.; Xu, D.Y.; Chen, X.; Zhou, J.M.; Jiang, C.W.; Huang, Z.Q.; Qi, D.M. Deciphering the transcriptional regulatory networks of FOX genes in nitrite-induced spleen injury in largemouth bass. Aquat. Ecol. 2025, 59, 1–20. [Google Scholar] [CrossRef]
- Benayoun, B.A.; Caburet, S.; Veitia, R.A. Forkhead transcription factors: Key players in health and disease. Trends Genet. 2011, 27, 224–232. [Google Scholar] [CrossRef]
- Eijkelenboom, A.; Burgering, B.M. FOXOs: Signalling integrators for homeostasis maintenance. Nat. Rev. Mol. Cell Biol. 2013, 14, 83–97. [Google Scholar] [CrossRef]
- Jin, J.L.; Wang, Y.; Wu, Z.X.; Hergazy, A.; Lan, J.F.; Zhao, L.J.; Liu, X.L.; Chen, N.; Lin, L. Transcriptomic analysis of liver from grass carp (Ctenopharyngodon idellus) exposed to high environmental ammonia reveals the activation of antioxidant and apoptosis pathways. Fish Shellfish Immunol. 2017, 63, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wu, S.J.; Huang, J.Q.; Zhao, L. Integration of physiological, miRNA-mRNA interaction and functional analysis reveals the molecular mechanism underlying hypoxia stress tolerance in crucian carp (Carassius auratus). Faseb J. 2024, 38, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Ma, A.J.; Yang, S.S.; Liu, X.F.; Zhao, T.T.; Zhang, J.S.; Wang, X.A.; Sun, Z.B.; Liu, Z.F.; Xu, R.J. Transcriptome analysis and weighted gene co-expression network reveals potential genes responses to heat stress in turbot Scophthalmus maximus. Comp. Biochem. Physiol. D-Genom. Proteom. 2020, 33, 9. [Google Scholar] [CrossRef]
- Zhi, H.Y.; Bi, D.N.; Zheng, D.; Lu, Q.Y.; Wang, H.L.; Wang, Y.; Lv, Y.; Lou, D.D.; Hu, Y. The role of BNIP3 and blocked autophagy flux in arsenic-induced oxidative stress-induced liver injury in rats. Biol. Trace Elem. Res. 2024, 202, 4054–4064. [Google Scholar] [CrossRef]
- Ma, Y.M.; Hossen, M.M.; Huang, J.J.; Yin, Z.H.; Du, J.; Ye, Z.Z.; Zeng, M.Y.; Huang, Z. Growth arrest and DNA damage-inducible 45: A new player on inflammatory diseases. Front. Immunol. 2025, 16, 17. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.B.; Li, C.; Yan, S.P.; Li, Q.; Luo, X.M.; Zhang, S.H.; Zhang, Y.C.; Yao, L.R. Liver transcriptome of largemouth bass (Micropterus salmoides) under acute ammonia nitrogen stress. Acta Hydrobiol. Sin. 2024, 48, 713–724. [Google Scholar]
- Lennicke, C.; Cochemé, H.M. Redox regulation of the insulin signalling pathway. Redox Biol. 2021, 42, 14. [Google Scholar] [CrossRef]
- Smith, G.R.; Shanley, D.P. Computational modelling of the regulation of insulin signalling by oxidative stress. BMC Syst. Biol. 2013, 7, 19. [Google Scholar] [CrossRef]
- Feng, W.R.; Xu, Y.F.; Su, S.Y.; Yu, F.; Li, J.L.; Jia, R.; Song, C.Y.; Li, H.X.; Xu, P.; Tang, Y.K. Transcriptomic analysis of hydrogen peroxide-induced liver dysfunction in Cyprinus carpio: Insights into protein synthesis and metabolism. Sci. Total Environ. 2024, 917, 12. [Google Scholar] [CrossRef]
- Jia, R.; Wang, L.; Hou, Y.R.; Feng, W.R.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, physiological parameters, redox status and lipid metabolism of Micropterus salmoides in integrated rice-fish farming systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Y.; Wen, H.S.; Zhang, C.; Zhang, Y.H.; Wang, L.Y.; Sun, D.L.; Zhang, K.Q.; Qi, X.; Xia, Y.; et al. Dynamics of proximate composition, fatty acid profile and transcriptome response to low-temperature stress in muscle tissues of spotted sea bass (Lateolabrax maculatus). Aquaculture 2025, 599, 12. [Google Scholar] [CrossRef]
- Lu, J.H.; Quan, J.Q.; Zhou, J.; Liu, Z.; Ding, J.P.; Shang, T.T.; Zhao, G.Y.; Li, L.L.; Zhao, Y.C.; Li, X.R.; et al. Combined transcriptomics and metabolomics to reveal the effects of copper exposure on the liver of rainbow trout (Oncorhynchus mykiss). Ecotoxicol. Environ. Saf. 2024, 284, 14. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.Y.; Wang, J.Y.; Cao, Q.S.; Yang, H.; Zhang, Y.Y. Transcriptomic analysis of lipid metabolism in zebrafish offspring of parental long-term exposure to bisphenol A. Environ. Sci. Pollut. Res. 2023, 30, 51654–51664. [Google Scholar] [CrossRef]
- Fanale, D.; Amodeo, V.; Caruso, S. The interplay between metabolism, PPAR signaling pathway, and cancer. PPAR Res. 2017, 2017, 2. [Google Scholar] [CrossRef]
- Zhao, T.T.; Ma, A.J.; Huang, Z.H.; Liu, Z.F.; Sun, Z.B.; Zhu, C.Y.; Yang, J.K.; Li, Y.D.; Wang, Q.M.; Qiao, X.W.; et al. Transcriptome analysis reveals that high temperatures alter modes of lipid metabolism in juvenile turbot (Scophthalmus maximus) liver. Comp. Biochem. Physiol. D-Genom. Proteom. 2021, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Khan, B.; Hussain, S.; Wang, Y.H.; Mai, W.J.; Hou, Y.Z. Permethrin exposure impacts zebrafish lipid metabolism via the KRAS-PPAR-GLUT signaling pathway, which is mediated by oxidative stress. Aquat. Toxicol. 2024, 273, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, X.W.; Zhu, H.; Zhang, Q.J.; He, Y.; Shen, Y.B.; Xu, X.Y.; Li, J.L. The effects of exposure to microplastics on grass carp (Ctenopharyngodon idella) at the physiological, biochemical, and transcriptomic levels. Chemosphere 2022, 286, 11. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhang, Z.; Guan, Y.Q. Physiological and transcriptional analysis of Chinese soft-shelled turtle (Pelodiscus sinensis) in response to acute nitrite stress. Aquat. Toxicol. 2021, 237, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fu, J.P.; Tang, Q.Y.; Wang, H.; Lin, C.G.; Wei, L.L. Combined transcriptomics and metabolomics analysis reveals lipid metabolic disruption in swamp eel (Monopterus albus) under chronic waterborne copper exposure. Aquat. Toxicol. 2023, 259, 15. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; Abdulkader, F. Textbook oxidative phosphorylation needs to be rewritten. Trends Biochem.Sci. 2025, 50, 87–88. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Gao, T.; Wang, Q.; Sun, H.R.; Liu, Y.; Li, J.T.; He, Y.Y. Physiological adaptation of fenneropenaeus chinensis in response to saline-alkaline stress revealed by a combined proteomics and metabolomics method. Biology 2024, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Y.; Jia, S.T.; Gao, B.Q.; Zhou, Q.S.; Xu, Y.; Liu, P.; Li, J. Application of proteomics and metabolomics to assess ammonia stress response and tolerance mechanisms of juvenile ornate rock lobster Panulirus ornatus. Sci. Total. Environ. 2022, 837, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Yang, C.; Zhang, S.; Rong, L.Y.; Yang, X.F.; Wu, Z.X.; Sun, W.T. Metabolic changes and stress damage induced by ammonia exposure in juvenile Eriocheir sinensis. Ecotoxicol. Environ. Saf. 2021, 223, 11. [Google Scholar] [CrossRef]
- Wang, L.; Guan, T.Y.; Wang, G.L.; Gu, J.Y.; Wu, N.; Zhu, C.K.; Wang, H.; Li, J.L. Effects of copper on gill function of juvenile oriental river prawn (Macrobrachium nipponense): Stress and toxic mechanism. Aquat. Toxicol. 2023, 261, 13. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Q.Q.; Hu, M.; Zhou, X.Y.; Guan, T.Y.; Wu, N.; Zhu, C.K.; Wang, H.; Wang, G.L.; Li, J.L. Toxic mechanisms of nanoplastics exposure at environmental concentrations on juvenile red swamp crayfish (Procambarus clarkii): From multiple perspectives. Environ. Pollut. 2024, 352, 13. [Google Scholar] [CrossRef]
- Jia, R.; Hou, Y.R.; Zhou, L.J.; Zhang, L.Q.; Li, B.; Zhu, J. Comparative transcriptome analysis reveals the impact of a high-fat diet on hepatic metabolic function in Tilapia (Oreochromis niloticus). Animals 2024, 14, 3204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.G.; Zhao, T.; Hogstrand, C.; Ye, H.M.; Xu, X.J.; Luo, Z. Oxidized fish oils increased lipid deposition via oxidative stress-mediated mitochondrial dysfunction and the CREB1-Bcl2-Beclin1 pathway in the liver tissues and hepatocytes of yellow catfish. Food Chem. 2021, 360, 11. [Google Scholar] [CrossRef]
- Zheng, S.K.; Zhang, Q.; Shi, X.L.; Luo, C.Y.; Chen, J.S.; Zhang, W.C.; Wu, K.S.; Tang, S.J. Developmental hazards of 2,2′,4,4′-tetrabromodiphenyl ether induced endoplasmic reticulum stress on early life stages of zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2023, 267, 12. [Google Scholar] [CrossRef] [PubMed]




| Amino Acids (g/100 g, ww) | Groups | Percent Difference | p-Value | |
|---|---|---|---|---|
| NC | HT | |||
| Thr | 0.62 ± 0.02 | 0.60 ± 0.02 | −3.23% | 0.364 |
| Val | 0.72 ± 0.03 | 0.68 ± 0.02 | −5.56% | 0.233 |
| Met | 0.38 ± 0.02 | 0.35± 0.01 | −7.89% | 0.294 |
| Ile | 0.65 ± 0.02 | 0.61 ± 0.02 | −6.15% | 0.232 |
| Leu | 1.17 ± 0.04 | 1.11 ± 0.03 | −5.13% | 0.276 |
| Phe | 0.64 ± 0.03 | 0.60 ± 0.02 | −6.25% | 0.294 |
| Lys | 1.43 ± 0.05 | 1.37 ± 0.04 | −4.20% | 0.375 |
| His | 0.57 ± 0.03 | 0.53 ± 0.02 | −7.02% | 0.348 |
| Arg | 0.84 ± 0.03 | 0.81 ± 0.02 | −3.57% | 0.386 |
| Asp | 1.40 ± 0.06 | 1.31 ± 0.05 | −6.43% | 0.233 |
| Ser | 0.47 ± 0.02 | 0.44 ± 0.01 | −6.38% | 0.199 |
| Glu | 1.85 ± 0.07 | 1.67 ± 0.05 | −9.73% | 0.082 |
| Gly | 0.62 ± 0.02 | 0.60 ± 0.01 | −3.23% | 0.583 |
| Ala | 0.88 ± 0.03 | 0.85 ± 0.03 | −3.41% | 0.484 |
| Cys | 0.16 ± 0.02 | 0.13 ± 0.01 | −18.75% | 0.388 |
| Tyr | 0.46 ± 0.02 | 0.44 ± 0.01 | −4.35% | 0.398 |
| Pro | 0.47 ± 0.02 | 0.45 ± 0.01 | −4.26% | 0.434 |
| EAAs | 5.61 ± 0.21 | 5.31 ± 0.16 | −5.35% | 0.295 |
| CEAAs | 1.40 ± 0.06 | 1.34 ± 0.03 | −4.29% | 0.352 |
| NEAAs | 6.31 ± 0.25 | 5.90 ± 0.17 | −6.50% | 0.218 |
| Fatty Acids (mg/100 g, ww) | Groups | Percent Difference | p-Value | |
|---|---|---|---|---|
| NC | HT | |||
| C16:0 | 103.56 ± 4.23 | 99.84 ± 3.31 | −3.59% | 0.508 |
| C16:1 | 4.98 ± 0.87 | 4.12 ± 1.09 | −17.27% | 0.555 |
| C18:0 | 39.50 ± 1.12 | 38.86 ± 0.68 | −1.62% | 0.639 |
| C18:1n9c | 133.08 ± 12.85 | 121.56 ± 9.25 | −8.66% | 0.488 |
| C18:2n6c | 138.88 ± 10.16 | 125.52 ± 7.99 | −9.62% | 0.441 |
| C18:3n3 | 7.62 ± 0.79 | 6.82 ± 0.59 | −10.50% | 0.832 |
| C20:1 | 6.44 ± 0.65 | 6.24 ± 0.29 | −3.11% | 0.785 |
| C20:2 | 5.92 ± 0.37 | 6.02 ± 0.27 | 1.69% | 0.884 |
| C20:3n6 | 14.20 ± 0.69 | 14.08 ± 0.40 | −0.85% | 0.459 |
| C22:1n9 | 3.12 ± 0.82 | 3.34 ± 1.94 | 7.05% | 0.331 |
| C20:4n6 | 35.80 ± 3.31 | 32.84 ± 1.87 | −8.27% | 0.872 |
| C22:6n3 | 17.70 ± 1.48 | 18.08 ± 1.75 | 2.15% | 0.484 |
| Total SFA | 148.06 ± 4.89 | 138.70 ± 3.37 | −6.32% | 0.481 |
| Total MUFA | 147.62 ± 13.46 | 135.26 ± 9.94 | −8.37% | 0.188 |
| Total PUFA | 220.12 ± 8.36 | 203.36 ± 8.09 | −7.61% | 0.858 |
| Total UFA | 367.74 ± 45.62 | 338.62 ± 37.77 | −7.91% | 0.304 |
| Samples | Raw Data (bp) | Clean Data (bp) | Total Reads | Q20 (%) | Q30 (%) | GC (%) | Total Mapped |
|---|---|---|---|---|---|---|---|
| NC1 | 6,386,695,500 | 6,285,991,447 | 41,054,402 | 98.12 | 95.81 | 50.02 | 93.73% |
| NC2 | 6,411,411,600 | 6,260,831,140 | 41,489,560 | 97.72 | 95.09 | 49.30 | 92.93% |
| NC3 | 5,782,143,300 | 5,679,862,256 | 45,412,678 | 97.89 | 95.41 | 49.94 | 93.79% |
| NC4 | 7,055,086,200 | 6,901,618,956 | 48,139,874 | 97.80 | 95.26 | 49.77 | 93.40% |
| NC5 | 7,412,127,000 | 7,249,214,727 | 37,557,260 | 97.90 | 95.43 | 50.04 | 93.40% |
| HT1 | 7,291,706,100 | 7,100,651,939 | 46,258,780 | 97.64 | 94.88 | 49.87 | 93.08% |
| HT2 | 7,417,197,738 | 7,198,571,166 | 47,574,450 | 97.29 | 94.22 | 49.43 | 92.21% |
| HT3 | 5,718,504,300 | 5,580,781,347 | 36,795,230 | 97.66 | 94.90 | 49.45 | 92.79% |
| HT4 | 6,525,954,900 | 6,357,279,854 | 42,060,676 | 97.60 | 94.90 | 49.05 | 92.27% |
| HT5 | 7,097,538,300 | 6,948,760,394 | 45,946,326 | 97.81 | 95.23 | 49.49 | 92.79% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, B.; Hou, Y.; Zhou, L.; Yang, Q.; Zhang, C.; Li, H.; Zhu, J.; Jia, R. Integrated Metabolomics and Transcriptomics Reveals Metabolic Pathway Changes in Common Carp Muscle Under Oxidative Stress. Antioxidants 2025, 14, 1115. https://doi.org/10.3390/antiox14091115
Liu Y, Li B, Hou Y, Zhou L, Yang Q, Zhang C, Li H, Zhu J, Jia R. Integrated Metabolomics and Transcriptomics Reveals Metabolic Pathway Changes in Common Carp Muscle Under Oxidative Stress. Antioxidants. 2025; 14(9):1115. https://doi.org/10.3390/antiox14091115
Chicago/Turabian StyleLiu, Yongxiang, Bing Li, Yiran Hou, Linjun Zhou, Qiqin Yang, Chengfeng Zhang, Hongwei Li, Jian Zhu, and Rui Jia. 2025. "Integrated Metabolomics and Transcriptomics Reveals Metabolic Pathway Changes in Common Carp Muscle Under Oxidative Stress" Antioxidants 14, no. 9: 1115. https://doi.org/10.3390/antiox14091115
APA StyleLiu, Y., Li, B., Hou, Y., Zhou, L., Yang, Q., Zhang, C., Li, H., Zhu, J., & Jia, R. (2025). Integrated Metabolomics and Transcriptomics Reveals Metabolic Pathway Changes in Common Carp Muscle Under Oxidative Stress. Antioxidants, 14(9), 1115. https://doi.org/10.3390/antiox14091115








