Heat Tolerance in Magallana hongkongensis: Integrative Analysis of DNA Damage, Antioxidant Defense, and Stress Gene Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Temperature Tolerance Testing
2.3. Thermal Stress and Sampling
2.4. Comet Assay
2.5. RNA Extraction, cDNA Synthesis, and Real-Time Quantitative PCR
2.6. Determination of Enzyme Activities Related to the Antioxidant System
2.7. Statistical Analysis
3. Results
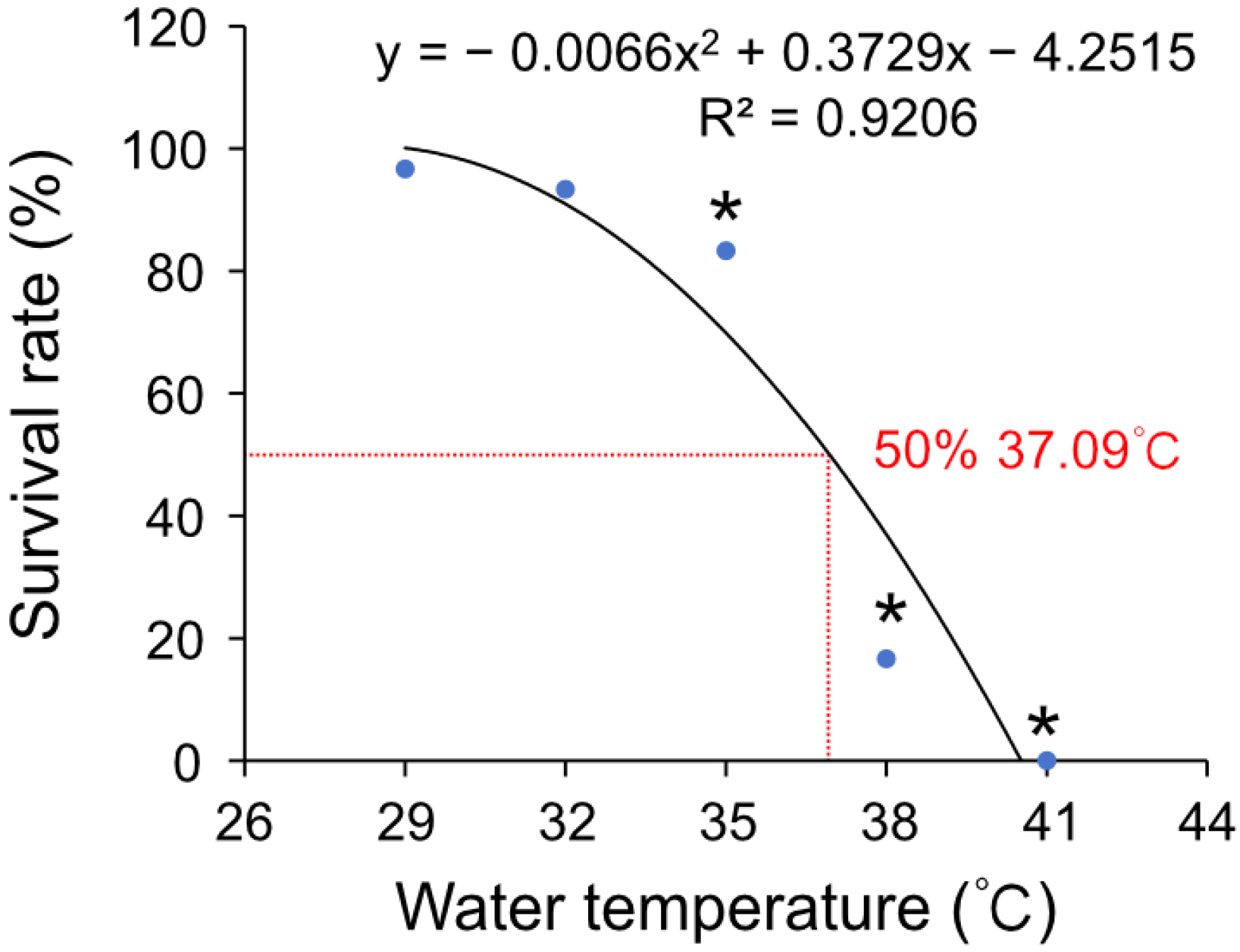
3.1. The Effects of High Temperature Stress on the Survival Rate of Hong Kong Oysters
3.2. DNA Damage After Heat Stress
3.3. Changes in Antioxidant Enzyme Activity in Response to Heat Stress
3.4. Changes in mRNA Expression of Apoptosis-Related Genes
3.5. Changes in mRNA Expression of Inflammation-Related Genes
3.6. Changes in mRNA Expression of HSP Member Family Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, W.; Li, J.; Gao, Y.; Mao, Y.; Jiang, Z.; Du, M.; Zhang, Y.; Fang, J. Effects of Temperature Change on Physiological and Biochemical Responses of Yesso Scallop, Patinopecten yessoensis. Aquaculture 2016, 451, 463–472. [Google Scholar] [CrossRef]
- Copedo, J.S.; Webb, S.C.; Ragg, N.L.C.; Ericson, J.A.; Venter, L.; Schmidt, A.J.; Delorme, N.J.; Alfaro, A.C. Histopathological Changes in the Greenshell Mussel, Perna canaliculus, in Response to Chronic Thermal Stress. J. Therm. Biol. 2023, 117, 103699. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, C.; Tan, K.; Wang, B.; Huang, R.; Wen, J.; Xu, B.; Liu, X.; Lichu, L.; Zheng, H. Variation of Lipids and Fatty Acids in Noble Scallop Chlamys nobilis under Low Temperature Stress. Aquaculture 2022, 554, 738121. [Google Scholar] [CrossRef]
- Manríquez, P.H.; Jara, M.E.; Seguel, M.E.; Torres, R.; Alarcon, E.; Lee, M.R. Ocean Acidification and Increased Temperature Have Both Positive and Negative Effects on Early Ontogenetic Traits of a Rocky Shore Keystone Predator Species. PLoS ONE 2016, 11, e0151920. [Google Scholar] [CrossRef]
- Seuront, L.; Nicastro, K.R.; Zardi, G.I.; Goberville, E. Decreased Thermal Tolerance under Recurrent Heat Stress Conditions Explains Summer Mass Mortality of the Blue Mussel Mytilus edulis. Sci. Rep. 2019, 9, 17498. [Google Scholar] [CrossRef]
- White, J.D.; Hamilton, S.K.; Sarnelle, O. Heat-Induced Mass Mortality of Invasive Zebra Mussels (Dreissena polymorpha) at Sublethal Water Temperatures. Can. J. Fish. Aquat. Sci. 2015, 72, 1221–1229. [Google Scholar] [CrossRef]
- Heo, J.M.; Kim, S.S.; Kim, D.Y.; Lee, S.W.; Lee, J.S.; Kang, M.H.; Kim, S.E. Impact of Exposure Temperature Rise on Mass Mortality of Tidal Flat Pacific Oysters. Front. Mar. Sci. 2023, 10, 1275521. [Google Scholar] [CrossRef]
- White, R.H.; Anderson, S.; Booth, J.F.; Braich, G.; Draeger, C.; Fei, C.; Harley, C.D.G.; Henderson, S.B.; Jakob, M.; Lau, C.A.; et al. The Unprecedented Pacific Northwest Heatwave of June 2021. Nat. Commun. 2023, 14, 727. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ford, S.E.; Yang, H.; Zhang, G.; Zhang, F.; Guo, X. Studies on Mass Summer Mortality of Cultured Zhikong Scallops (Chlamys farreri Jones et Preston) in China. Aquaculture 2005, 250, 602–615. [Google Scholar] [CrossRef]
- Guo, K.; Ruan, G.; Fan, W.; Wang, Q.; Fang, L.; Luo, J.; Liu, Y. Immune Response to Acute Heat Stress in the Intestine of the Red Swamp Crayfish, Procambarus clarkii. Fish Shellfish Immunol. 2020, 100, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lu, W.; Shang, Y.; Kong, H.; Li, L.; Sui, Y.; Hu, M.; Wang, Y. Combined Effects of Seawater Acidification and High Temperature on Hemocyte Parameters in the Thick Shell Mussel Mytilus coruscus. Fish Shellfish Immunol. 2016, 56, 554–562. [Google Scholar] [CrossRef]
- Wang, J.; Ren, R.M.; Yao, C.L. Oxidative Stress Responses of Mytilus galloprovincialis to Acute Cold and Heat during Air Exposure. J. Molluscan Stud. J. 2018, 84, 285–292. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Luo, S.W.; Wang, A.L. Effects of High Temperature on Biochemical Parameters, Oxidative Stress, DNA Damage and Apoptosis of Pufferfish (Takifugu obscurus). Ecotox. Environ. Safe. 2018, 150, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Guo, S.N.; Zhu, Q.L.; Yuan, S.S.; Zheng, J.L. Heat-Induced Oxidative Stress and Inflammation Involve in Cadmium Pollution History in the Spleen of Zebrafish. Fish Shellfish Immunol. 2018, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, Z.; Wang, L.; Luo, J.; Li, H. Oxidative Stress, Apoptosis Activation and Symbiosis Disruption in Giant Clam Tridacna crocea under High Temperature. Fish Shellfish Immunol. 2019, 84, 451–457. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free Radicals, Natural Antioxidants, and Their Reaction Mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Wang, W.; Gao, L.; Liu, W.; Tian, Z.; Wang, X.; Hu, H. Regulation of Antioxidant Defense in 18Response to Heat Stress in Siberian Sturgeon (Acipenser baerii). Aquaculture 2023, 572, 739551. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, E.; Li, C.; Pan, C.; Zhao, X.; Wang, Y.; Ling, Q. Effects of Heat Stress on Histopathology, Antioxidant Enzymes, and Transcriptomic Profiles in Gills of Pikeperch Sander lucioperca. Aquaculture 2021, 534, 736277. [Google Scholar] [CrossRef]
- Gething, M.J.; Sambrook, J. Protein Folding in the Cell. Nature 1992, 355, 33–45. [Google Scholar] [CrossRef]
- Stetler, R.A.; Gan, Y.; Zhang, W.; Liou, A.K.; Gao, Y.; Cao, G.; Chen, J. Heat Shock Proteins: Cellular and Molecular Mechanisms in the Central Nervous System. Prog. Neurobiol. 2010, 92, 184–211. [Google Scholar] [CrossRef]
- Han, P.; Yan, W.; Liu, X.; Wang, X. Differential Environmental-Induced Heat Stresses Cause the Structural and Molecular Changes in the Spleen of Japanese Flounder (Paralichthys olivaceus). Aquaculture 2024, 581, 740490. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, X.; Wang, Z.; Meng, Z.; Huang, B.; Guan, C. Physiological Response of Juvenile Turbot (Scophthalmus maximus L.) during Hyperthermal Stress. Aquaculture 2020, 529, 735645. [Google Scholar] [CrossRef]
- Xu, D.; Sun, L.; Liu, S.; Zhang, L.; Yang, H. Histological, Ultrastructural and Heat Shock Protein 70 (HSP70) Responses to Heat Stress in the Sea Cucumber Apostichopus japonicus. Fish Shellfish Immunol. 2015, 45, 321–326. [Google Scholar] [CrossRef]
- Lam, K.; Morton, B. Mitochondrial DNA and Morphological Identification of a New Species of Crassostrea (Bivalvia: Ostreidae) Cultured for Centuries in the Pearl River Delta, Hong Kong, China. Aquaculture 2003, 228, 1–13. [Google Scholar] [CrossRef]
- Peng, D.; Zhang, S.; Zhang, H.; Pang, D.; Yang, Q.; Jiang, R.; Lin, Y.; Mu, Y.; Zhu, Y. The Oyster Fishery in China: Trend, Concerns and Solutions. Mar. Pol. 2021, 129, 104524. [Google Scholar] [CrossRef]
- Lackie, A.M. Invertebrate Immunity. Parasitology 1980, 80, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Han, Q.; Xu, Y.; Jiang, H.; Xing, H.; Teng, X. Impaired Immune Function and Structural Integrity in the Gills of Common Carp (Cyprinus carpio L.) Caused by Chlorpyrifos Exposure: Through Oxidative Stress and Apoptosis. Fish Shellfish Immunol. 2019, 86, 239–245. [Google Scholar] [CrossRef]
- Shin, M.K.; Park, H.R.; Yeo, W.J.; Han, K.N. Effects of Thermal Stress on the MRNA Expression of SOD, HSP90, and HSP70 in the Spotted Sea Bass (Lateolabrax maculatus). Ocean Sci. J. 2018, 53, 43–52. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Mao, F.; Tong, Y.; Liu, Y.; Zhang, Y.; Yu, Z. Characterization and Identification of Differentially Expressed Genes Involved in Thermal Adaptation of the Hong Kong Oyster Crassostrea hongkongensis by Digital Gene Expression Profiling. Front. Mar. Sci. 2017, 4, 112. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Końca, K.; Lankoff, A.; Banasik, A.; Lisowska, H.; Kuszewski, T.; Góźdź, S.; Koza, Z.; Wojcik, A. A Cross-Platform Public Domain PC Image-Analysis Program for the Comet Assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 534, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; He, P.; Jiang, L.; Zhang, L.; Guan, J.; Chen, Y.; Zheng, Y.; Wei, P.; Peng, J. Transcriptional Responses of Crassostrea hongkongensis under High and Low Salinity Stress. Comp. Biochem. Physiol. D Genom. Proteom. 2024, 49, 101188. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Li, W.; Wei, P.; Jiang, L.; Guan, J.; Ma, Y.; Zhang, L.; Chen, Y.; Zheng, Y.; Zhang, X.; et al. Antioxidant Capacity, Enzyme Activities Related to Energy Metabolism, and Transcriptome Analysis of Crassostrea hongkongensis Exposed to Hypoxia. Antioxidants 2024, 13, 1063. [Google Scholar] [CrossRef]
- She, Z.; Peng, Y.; Jia, Z.; Kang, Z.; Yu, D. Molecular Mechanisms Affecting the Difference in Salinity Adaptability between Juvenile and Adult Hong Kong Oysters. Aquacult. Rep. 2022, 24, 101171. [Google Scholar] [CrossRef]
- Dettleff, P.; Zuloaga, R.; Fuentes, M.; Gonzalez, P.; Aedo, J.; Estrada, J.M.; Molina, A.; Valdés, J.A. Physiological and Molecular Responses to Thermal Stress in Red Cusk-Eel (Genypterus chilensis) Juveniles Reveals Atrophy and Oxidative Damage in Skeletal Muscle. J. Therm. Biol. 2020, 94, 102750. [Google Scholar] [CrossRef]
- Yuan, Q.; Wang, Q.; Zhang, T.; Li, Z.; Liu, J. Effects of Water Temperature on Growth, Feeding and Molting of Juvenile Chinese Mitten Crab Eriocheir sinensis. Aquaculture 2017, 468, 169–174. [Google Scholar] [CrossRef]
- Widdows, J. Effect of Temperature and Food on the Heart Beat, Ventilation Rate and Oxygen Uptake of Mytilus Edulis. Mar. Biol. 1973, 20, 269–276. [Google Scholar] [CrossRef]
- Widdows, J. The Effects of Temperature on the Metabolism and Activity of Mytilus edulis. Neth. J. Sea Res. 1973, 7, 387–398. [Google Scholar] [CrossRef]
- Wang, J.; Dong, B.; Yu, Z.X.; Yao, C.L. The Impact of Acute Thermal Stress on Green Mussel Perna viridis: Oxidative Damage and Responses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2018, 222, 7–15. [Google Scholar] [CrossRef]
- Zhang, G.; Li, L.; Meng, J.; Qi, H.; Qu, T.; Xu, F.; Zhang, L. Molecular Basis for Adaptation of Oysters to Stressful Marine Intertidal Environments. Annu. Rev. Anim. Biosci. 2016, 4, 357–381. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Henderson, S.; Miller-Ezzy, P.; Li, X.X.; Qin, J.G. Immune Response to Temperature Stress in Three Bivalve Species: Pacific Oyster Crassostrea gigas, Mediterranean Mussel Mytilus galloprovincialis and Mud Cockle Katelysia rhytiphora. Fish Shellfish Immunol. 2019, 86, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Van Der Velde, G.; Jansen, J.; Van Der Gaag, M.; Atsma, G.; Janssen-Mommen, J.P.M.; Polman, H.; Jenner, H.A. Thermal Tolerance of the Invasive Oyster Crassostrea gigas: Feasibility of Heat Treatment as an Antifouling Option. Water Res. 2005, 39, 4335–4342. [Google Scholar] [CrossRef] [PubMed]
- Hamdoun, A.M.; Cheney, D.P.; Cherr, G.N. Phenotypic Plasticity of HSP70 and HSP70 Gene Expression in the Pacific Oyster (Crassostrea gigas): Implications for Thermal Limits and Induction of Thermal Tolerance. Biol. Bull. 2003, 205, 160–169. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, X.; Guo, X.; Li, L.; Luo, R.; Xu, F.; Yang, P.; Zhang, L.; Wang, X.; Qi, H.; et al. The Oyster Genome Reveals Stress Adaptation and Complexity of Shell Formation. Nature 2012, 490, 49–54. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Mao, F.; Lin, Y.; Xiao, S.; Xiang, Z.; Ma, H.; Zhang, Y.; Yu, Z. The First Morphologic and Functional Characterization of Hemocytes in Hong Kong Oyster, Crassostrea hongkongensis. Fish Shellfish Immunol. 2018, 81, 423–429. [Google Scholar] [CrossRef]
- Beninger, P.G.; Le Pennec, G.; Le Pennec, M. Demonstration of Nutrient Pathway from the Digestive System to Oocytes in the Gonad Intestinal Loop of the Scallop Pecten maximus L. Biol. Bull. 2003, 205, 83–92. [Google Scholar] [CrossRef]
- Serpentini, A.; Ghayor, C.; Poncet, J.M.; Hebert, V.; Galéra, P.; Pujol, J.P.; Boucaud-Camou, E.; Lebel, J.M. Collagen Study and Regulation of the de Novo Synthesis by IGF-I in Hemocytes from the Gastropod Mollusc, Haliotis tuberculata. J. Exp. Zool. 2000, 287, 275–284. [Google Scholar] [CrossRef]
- Mount, A.S.; Wheeler, A.P.; Paradkar, R.P.; Snider, D. Hemocyte-Mediated Shell Mineralization in the Eastern Oyster. Science 2004, 304, 297–300. [Google Scholar] [CrossRef]
- Malev, O.; Šrut, M.; Maguire, I.; Štambuk, A.; Ferrero, E.A.; Lorenzon, S.; Klobučar, G.I.V. Genotoxic, Physiological and Immunological Effects Caused by Temperature Increase, Air Exposure or Food Deprivation in Freshwater Crayfish Astacus leptodactylus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 433–443. [Google Scholar] [CrossRef]
- Kamyab, E.; Kühnhold, H.; Novais, S.C.; Alves, L.M.F.; Indriana, L.; Kunzmann, A.; Slater, M.; Lemos, M.F.L. Effects of Thermal Stress on the Immune and Oxidative Stress Responses of Juvenile Sea Cucumber Holothuria scabra. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2017, 187, 51–61. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive Species and Antioxidants. Redox Biology Is a Fundamental Theme of Aerobic Life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Rahman, M.S.; Rahman, M.S. Effects of Elevated Temperature on Prooxidant-Antioxidant Homeostasis and Redox Status in the American Oyster: Signaling Pathways of Cellular Apoptosis during Heat Stress. Environ. Res. 2021, 196, 110428. [Google Scholar] [CrossRef]
- SIES, H. Strategies of Antioxidant Defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.X.; Dou, W.; Hu, F.; Wang, J.J. Effects of Thermal Stress on Lipid Peroxidation and Antioxidant Enzyme Activities of Oriental Fruit Fly, Bactrocera dorsalis (Diptera: Tephritidae). Fla. Entomol. 2011, 94, 956–963. [Google Scholar] [CrossRef]
- Bensaad, K.; Tsuruta, A.; Selak, M.A.; Vidal, M.N.C.; Nakano, K.; Bartrons, R.; Gottlieb, E.; Vousden, K.H. TIGAR, a P53-Inducible Regulator of Glycolysis and Apoptosis. Cell 2006, 126, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Bensaad, K.; Vousden, K.H. P53: New Roles in Metabolism. Trends Cell Biol. 2007, 17, 286–291. [Google Scholar] [CrossRef]
- Matheu, A.; Maraver, A.; Klatt, P.; Flores, I.; Garcia-Cao, I.; Borras, C.; Flores, J.M.; Viña, J.; Blasco, M.A.; Serrano, M. Delayed Ageing through Damage Protection by the Arf/P53 Pathway. Nature 2007, 448, 375–379. [Google Scholar] [CrossRef]
- Donald, S.P.; Sun, X.Y.; Hu, C.A.A.; Yu, J.; Mei, J.M.; Valle, D.; Phang, J.M. Proline Oxidase, Encoded by P53-Induced Gene-6, Catalyzes the Generation of Proline-Dependent Reactive Oxygen Species. Cancer Res. 2001, 61, 1810–1815. [Google Scholar]
- Vogelstein, B.; Lane, D.; Levine, A.J. Surfing the P53 Network. Nature 2000, 408, 307–310. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Letai, A. Control of Mitochondrial Apoptosis by the Bcl-2 Family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef]
- Vakifahmetoglu-Norberg, H.; Zhivotovsky, B. The Unpredictable Caspase-2: What Can It Do? Trends Cell Biol. 2010, 20, 150–159. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Caspase-2 Function in Response to DNA Damage. Biochem. Biophys. Res. Commun. 2005, 331, 859–867. [Google Scholar] [CrossRef]
- Pang, J.; Vince, J.E. The Role of Caspase-8 in Inflammatory Signalling and Pyroptotic Cell Death. Semin. Immunol. 2023, 70, 101832. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Q.; Liu, C.; Wang, L.; Zhou, Z.; Gong, C.; Zhang, A.; Zhang, H.; Qiu, L.; Song, L. The Transcriptional Response of the Pacific Oyster Crassostrea gigas against Acute Heat Stress. Fish Shellfish Immunol. 2017, 68, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Yang, F.F.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L.; Tan, J.W.; Chen, X.Y. High Temperature Induces Apoptosis and Oxidative Stress in Pufferfish (Takifugu obscurus) Blood Cells. J. Therm. Biol. 2015, 53, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New Regulators of NF-ΚB in Inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; dos Santos Neto, A.P.; Maia, S.M.A.S.; dos Santos Guimarães, C.; Quidute, I.L.; Carvalho, A.d.A.T.; Júnior, S.A.; Leão, J.C. The Role of TNF-α as a Proinflammatory Cytokine in Pathological Processes. Open Dent. J. 2019, 13, 332–338. [Google Scholar] [CrossRef]
- Zelová, H.; Hošek, J. TNF-α Signalling and Inflammation: Interactions between Old Acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Sabio, G.; Davis, R.J. TNF and MAP Kinase Signalling Pathways. Semin. Immunol. 2014, 26, 237–245. [Google Scholar] [CrossRef]
- Herlaar, E.; Brown, Z. P38 MAPK Signalling Cascades in Inflammatory Disease. Mol. Med. Today 1999, 5, 439–447. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Guinea-Viniegra, J.; Wagner, E.F. Targeting Inflammation by Modulating the Jun/AP-1 Pathway. Ann. Rheum. Dis. 2011, 70, i109–i112. [Google Scholar] [CrossRef]
- Jiang, F.; Xu, X.R.; Li, W.M.; Xia, K.; Wang, L.F.; Yang, X.C. Monotropein Alleviates H2O2-Induced Inflammation, Oxidative Stress and Apoptosis via NF-ΚB/AP-1 Signaling. Mol. Med. Rep. 2020, 22, 4828–4836. [Google Scholar] [CrossRef]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of Acute Heat Stress on Liver Damage, Apoptosis and Inflammation of Pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Feidantsis, K.; Georgoulis, I.; Zachariou, A.; Campaz, B.; Christoforou, M.; Pörtner, H.O.; Michaelidis, B. Energetic, Antioxidant, Inflammatory and Cell Death Responses in the Red Muscle of Thermally Stressed Sparus Aurata. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2020, 190, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Traylor-Knowles, N.; Rose, N.H.; Palumbi, S.R. The Cell Specificity of Gene Expression in the Response to Heat Stress in Corals. J. Exp. Biol. 2017, 220, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Li, J.; Sun, J.; Zhang, W.; Li, Y.; Cui, D.; Hu, W.; Chang, Y. The Impact of Chronic Heat Stress on the Growth, Survival, Feeding, and Differential Gene Expression in the Sea Urchin Strongylocentrotus intermedius. Front. Genet. 2019, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, L.; Xin, Y.; Wang, W.N.; He, W.Y.; Wang, A.L.; Liu, Y. Effect of Temperature on Antioxidant Enzyme Gene Expression and Stress Protein Response in White Shrimp, Litopenaeus vannamei. J. Therm. Biol. 2010, 35, 284–289. [Google Scholar] [CrossRef]
- Oksala, N.K.J.; Ekmekçi, F.G.; Özsoy, E.; Kirankaya, Ş.; Kokkola, T.; Emecen, G.; Lappalainen, J.; Kaarniranta, K.; Atalay, M. Natural Thermal Adaptation Increases Heat Shock Protein Levels and Decreases Oxidative Stress. Redox Biol. 2014, 3, 25–28. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, C.J.; Yoo, H.K.; Choi, J.; Byun, S.G.; Kim, W.J.; Lim, H.J.; Park, J.S. Effect of Water Temperature on Walleye Pollock (Gadus chalcogrammus) Embryos, Larvae and Juveniles: Survival, HSP70 Expression, and Physiological Responses. Aquaculture 2022, 554, 738136. [Google Scholar] [CrossRef]
- Zou, D.; Cao, W.; Liu, G.; Ning, J.; Lu, X.; Wang, J.; Chen, M.; Liu, B.; Zhang, J.; Wang, C. Effects of Acute and Chronic Thermal Stress on Survival, Apoptosis, and Transcriptional Responses of Scapharca broughtonii. J. Oceanol. Limnol. 2023, 41, 2363–2373. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-Shock Proteins, Molecular Chaperones, and the Stress Response: Evolutionary and Ecological Physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef]
- De Marco, A.; Baldassarro, V.A.; Calzà, L.; Giardino, L.; Dondi, F.; Ferrari, M.G.; Bignami, G.; Parma, L.; Bonaldo, A. Prolonged Heat Waves Reduce the Condition Index and Alter the Molecular Parameters in the Pacific Oyster Crassostrea gigas. Fish Shellfish Immunol. 2023, 133, 108518. [Google Scholar] [CrossRef]
- Chiappori, F.; Merelli, I.; Milanesi, L.; Colombo, G.; Morra, G. An Atomistic View of Hsp70 Allosteric Crosstalk: From the Nucleotide to the Substrate Binding Domain and Back. Sci. Rep. 2016, 6, 23474. [Google Scholar] [CrossRef]






| Primer Name | Primer Sequence (5′-3′) | Expected Size (bp) | Efficiency (%) | Gene Accession Number or Source |
|---|---|---|---|---|
| Hsp70-F | CACCACATACTCCGATAACCA | 255 | 97.3 | FJ157365.1 |
| Hsp70-R | GCCTTACTCAGACGACCTTTA | |||
| Hsp90-F | TTGAAAAGGTGGTGGTATCTAAC | 298 | 98.1 | HM171376.1 |
| Hsp90-R | CCTGGCTCCTCCAAACTAAA | |||
| Hsp68-F | GGACGCAGGTTTACTGACGA | 229 | 96.2 | GU586491.1 |
| Hsp68-R | TGAAATAGGCTGGCACGGT | |||
| Hsp27-F | ACAAAGACCTATCTGCTTCCTG | 128 | 99.5 | Li et al. [33] |
| Hsp27-R | ATCACTCTCTCCTTCGGGG | |||
| Caspase-2-F | AGATAAAATCCACAGGAGCAAT | 164 | 97.6 | ON803398.1 |
| Caspase-2-R | CAGAAACCAGGACCCGTAT | |||
| Caspase-8-F | GATGGACCAAACTATCTTACCG | 217 | 98.5 | KC822928.1 |
| Caspase-8-R | GCTGTTCGAGTGTCTTCACG | |||
| Bax-F | CACACCCACAGGTCCTCCAC | 185 | 99.1 | KM262836.1 |
| Bax-R | CCCAGTTGTAAACACCATCAGC | |||
| P53-F | GAGTCAACGCCAACCACC | 152 | 97.8 | MZ420636.1 |
| P53-R | TCACCAAATGAGTCGGAGC | |||
| TNF-F | GAGACATCGCCTTCATCAGC | 130 | 98.7 | KX698405.1 |
| TNF-R | TGCGTGCCTCCACTACTTC | |||
| p38-MAPK-F | GGAACCCCTAACCAGGCACTT | 220 | 97.5 | KX698406.1 |
| p38-MAPK-R | CAGCATACTGAGCCAAATACGG | |||
| AP-1-F | CGGGTTCGTGGAGGCAT | 158 | 99.2 | KC890768.1 |
| AP-1-R | CGTCAGTGTTGGAATAGGAGGA | |||
| GAPDH-F | GGATTGGCGTGGTGGTAGAG | 184 | 99.3 | He et al. [34] |
| GAPDH-R | GTATGATGCCCCTTTGTTGAGTC | |||
| β-actin-F | CTGTGCTACGTTGCCCTGGACTT | 129 | 99.5 | She et al. [35] |
| β-actin-R | TGGGCACCTGAATCGCTCGTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, T.; Wang, X.; Lu, J.; Fu, S.; Cheng, C.; Ye, L. Heat Tolerance in Magallana hongkongensis: Integrative Analysis of DNA Damage, Antioxidant Defense, and Stress Gene Regulation. Antioxidants 2025, 14, 1075. https://doi.org/10.3390/antiox14091075
Yao T, Wang X, Lu J, Fu S, Cheng C, Ye L. Heat Tolerance in Magallana hongkongensis: Integrative Analysis of DNA Damage, Antioxidant Defense, and Stress Gene Regulation. Antioxidants. 2025; 14(9):1075. https://doi.org/10.3390/antiox14091075
Chicago/Turabian StyleYao, Tuo, Xiaodi Wang, Jie Lu, Shengli Fu, Changhong Cheng, and Lingtong Ye. 2025. "Heat Tolerance in Magallana hongkongensis: Integrative Analysis of DNA Damage, Antioxidant Defense, and Stress Gene Regulation" Antioxidants 14, no. 9: 1075. https://doi.org/10.3390/antiox14091075
APA StyleYao, T., Wang, X., Lu, J., Fu, S., Cheng, C., & Ye, L. (2025). Heat Tolerance in Magallana hongkongensis: Integrative Analysis of DNA Damage, Antioxidant Defense, and Stress Gene Regulation. Antioxidants, 14(9), 1075. https://doi.org/10.3390/antiox14091075





