Prenatal Melatonin Therapy Enhances Postnatal Lung Development in a Mouse Model of Inflammation-Induced Preterm Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of Mouse Models of Intrauterine Inflammation-Induced Preterm Birth
2.3. RNA Preparation, RT-PCR, and Real-Time RT-PCR
2.4. Western Blot Analysis
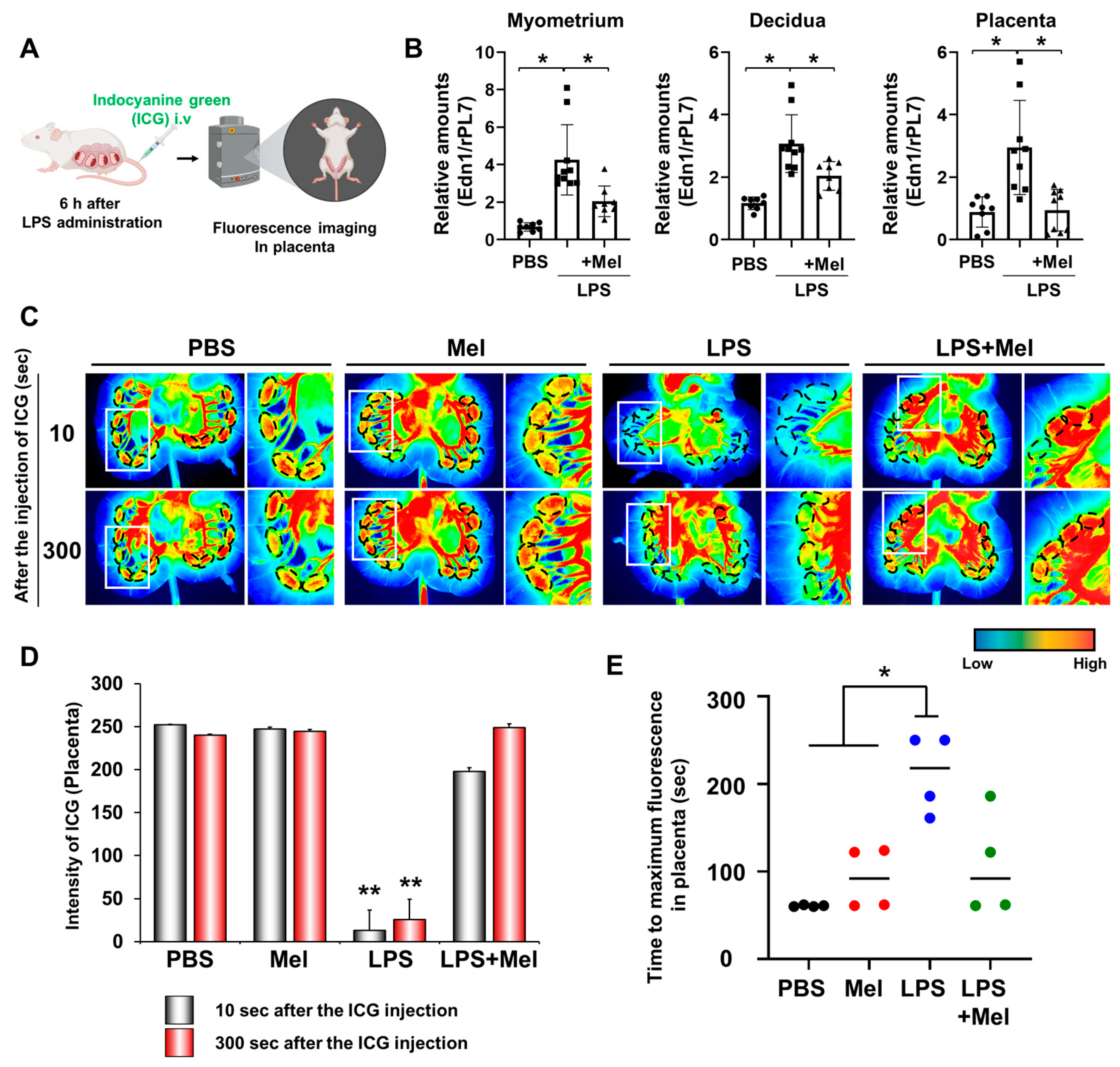
2.5. In Vivo Imaging of Placental Blood Flow
2.6. Histochemistry and Immunofluorescence Staining
2.7. Transmission Electron Microscopy (TEM)
2.8. Statistical Analyses
3. Results
3.1. Melatonin Prevents Preterm Birth and Reduces the Inflammatory Response
3.2. Melatonin Restores Reduced LPS-Induced Blood Flow in Inflammation-Induced Preterm Birth in Mice
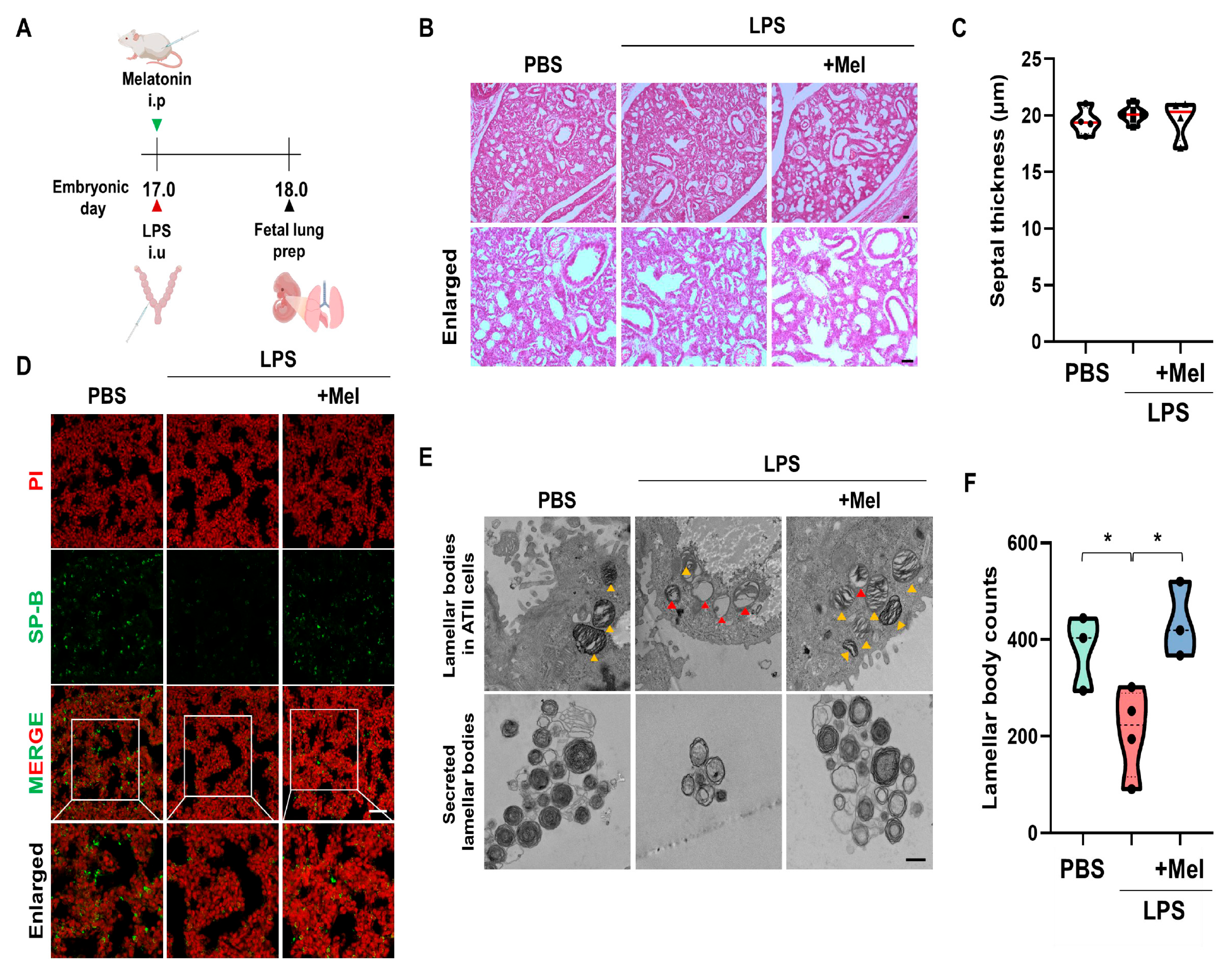
3.3. Melatonin Improves Fetal Lung Immaturity in Inflammation-Induced Preterm Birth in Mice
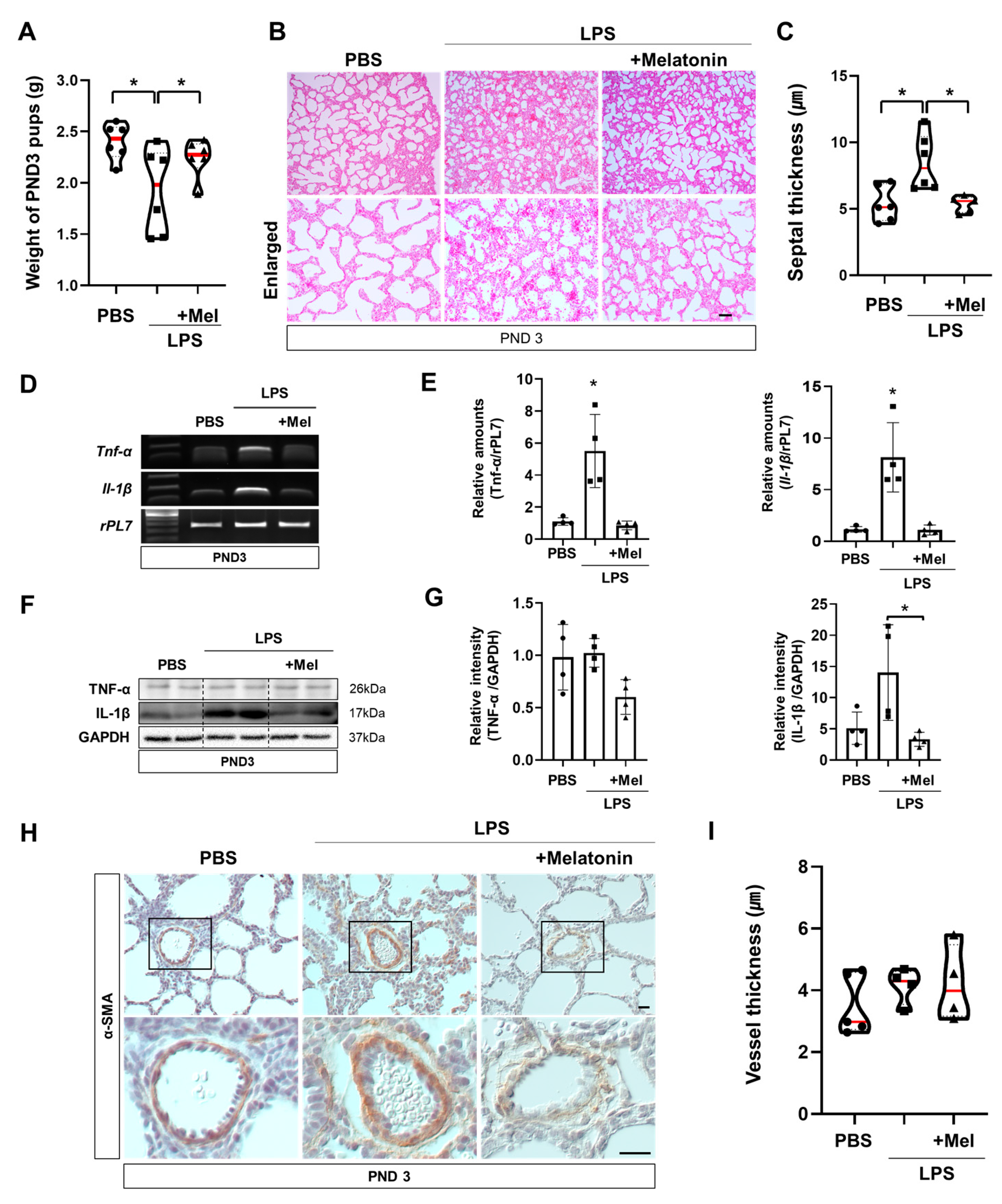
3.4. Melatonin Improves Postnatal Lung Development in Inflammation-Induced Preterm Birth in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vivekanand, K.; Jyotsana, P.; Akshunna, K. Preterm Birth: An Overview. Cureus 2022, 14, e33006. [Google Scholar] [CrossRef]
- Vrachnis, N.; Vitoratos, N.; Iliodromiti, Z.; Sifakis, S.; Deligeoroglou, E.; Creatsas, G. Intrauterine inflammation and preterm delivery. Ann. N. Y. Acad. Sci. 2010, 1205, 118–122. [Google Scholar] [CrossRef]
- Fraser, J.; Walls, M.; McGuire, W. Respiratory complications of preterm birth. BMJ 2004, 329, 962–965. [Google Scholar] [CrossRef]
- Cannavo, L.; Perrone, S.; Viola, V.; Marseglia, L.; Di Rosa, G.; Gitto, E. Oxidative Stress and Respiratory Diseases in Preterm Newborns. Int. J. Mol. Sci. 2021, 22, 12504. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, J.W. Virchow’s triad: Kussmaul, Quincke and von Recklinghausen. J. Med. Biogr. 2016, 24, 89–100. [Google Scholar] [CrossRef]
- Lee, J.Y.; Na, Q.; Shin, N.E.; Shin, H.E.; Kang, Y.; Chudnovets, A.; Lei, J.; Song, H.; Burd, I. Melatonin for prevention of fetal lung injury associated with intrauterine inflammation and for improvement of lung maturation. J. Pineal Res. 2020, 69, e12687. [Google Scholar] [CrossRef]
- Sutherland, M.R.; Bertagnolli, M.; Lukaszewski, M.-A.; Huyard, F.; Yzydorczyk, C.; Luu, T.M.; Nuyt, A.M. Preterm birth and hypertension risk: The oxidative stress paradigm. Hypertension 2014, 63, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Preston, M.; Hall, M.; Shennan, A.; Story, L. The role of placental insufficiency in spontaneous preterm birth: A literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 295, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxidative Med. Cell. Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef]
- Hentschel, R.; Bohlin, K.; van Kaam, A.; Fuchs, H.; Danhaive, O. Surfactant replacement therapy: From biological basis to current clinical practice. Pediatr. Res. 2020, 88, 176–183. [Google Scholar] [CrossRef]
- Ramaswamy, V.V.; Abiramalatha, T.; Bandyopadhyay, T.; Boyle, E.; Roehr, C.C. Surfactant therapy in late preterm and term neonates with respiratory distress syndrome: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 393–397. [Google Scholar] [CrossRef]
- Committee on Obstetric, P. Committee Opinion No. 713: Antenatal Corticosteroid Therapy for Fetal Maturation. Obstet. Gynecol. 2017, 130, e102–e109. [Google Scholar] [CrossRef] [PubMed]
- Lofberg, L.; Serenius, F.; Hellstrom-Westas, L.; Olhager, E.; Ley, D.; Farooqi, A.; Stephansson, O.; Abrahamsson, T. Postnatal betamethasone treatment in extremely preterm infants and risk of neurodevelopmental impairment: A cohort study. Arch. Dis. Child. Fetal Neonatal Ed. 2025, 110, 382–387. [Google Scholar] [CrossRef]
- Hellstrom, A.; Ley, D.; Hallberg, B.; Lofqvist, C.; Hansen-Pupp, I.; Ramenghi, L.A.; Borg, J.; Smith, L.E.H.; Hard, A.L. IGF-1 as a Drug for Preterm Infants: A Step-Wise Clinical Development. Curr. Pharm. Des. 2017, 23, 5964–5970. [Google Scholar] [CrossRef] [PubMed]
- De Rose, D.U.; Landolfo, F.; Pugnaloni, F.; Giliberti, P.; Santisi, A.; Columbo, C.; Martini, L.; Ronchetti, M.P.; Schingo, P.M.; Salvatori, G.; et al. Use of N-Acetylcysteine in Preterm Neonates with Enteral Feeding Intolerance and Intestinal Obstruction: A Case Series and Review of the Literature. Children 2024, 11, 873. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The placental antioxidant and anti-inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef] [PubMed]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Shi, D.; Xiao, X.; Wang, J.; Liu, L.; Chen, W.; Fu, L.; Xie, F.; Huang, W.; Deng, W. Melatonin suppresses proinflammatory mediators in lipopolysaccharide-stimulated CRL1999 cells via targeting MAPK, NF-kappaB, c/EBPbeta, and p300 signaling. J. Pineal Res. 2012, 53, 154–165. [Google Scholar] [CrossRef]
- Lee, J.Y.; Song, H.; Dash, O.; Park, M.; Shin, N.E.; McLane, M.W.; Lei, J.; Hwang, J.Y.; Burd, I. Administration of melatonin for prevention of preterm birth and fetal brain injury associated with premature birth in a mouse model. Am. J. Reprod. Immunol. 2019, 82, e13151. [Google Scholar] [CrossRef]
- He, F.; Wang, Q.F.; Li, L.; Yu, C.; Liu, C.Z.; Wei, W.C.; Chen, L.P.; Li, H.Y. Melatonin Protects Against Hyperoxia-Induced Apoptosis in Alveolar Epithelial type II Cells by Activating the MT2/PI3K/AKT/ETS1 Signaling Pathway. Lung 2023, 201, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Su, X.; Hu, X.; Zhou, Y.; Huang, L.; Fan, Y.; Li, J.; Lu, L. Melatonin improves hypoxic-ischemic brain damage through the Akt/Nrf2/Gpx4 signaling pathway. Brain Res. Bull. 2020, 163, 40–48. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Romero, R.; Arenas-Hernandez, M.; Panaitescu, B.; Garcia-Flores, V.; Mial, T.N.; Sahi, A.; Hassan, S.S. Intra-amniotic administration of lipopolysaccharide induces spontaneous preterm labor and birth in the absence of a body temperature change. J. Matern.-Fetal Neonatal Med. 2018, 31, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Motomura, K.; Romero, R.; Galaz, J.; Tao, L.; Garcia-Flores, V.; Xu, Y.; Done, B.; Arenas-Hernandez, M.; Miller, D.; Gutierrez-Contreras, P. Fetal and maternal NLRP3 signaling is required for preterm labor and birth. JCI Insight 2022, 7, e158238. [Google Scholar] [CrossRef]
- Chen, Z.; Shan, Y.; You, X.; Gu, H.; Xu, C.; Long, J.; Ni, X. NLRP3 inflammasome is involved in uterine activation for labor at term and preterm. Reproduction 2021, 162, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Xiao, F.; Wu, L.; Guo, Y.; Zhang, Z.; Xiao, Y.; Sun, G.; Yang, Q.; Guo, H. Melatonin-Driven NLRP3 Inflammation Inhibition Via Regulation of NF-kappaB Nucleocytoplasmic Transport: Implications for Postoperative Cognitive Dysfunction. Inflammation 2023, 46, 1471–1492. [Google Scholar] [CrossRef]
- Arioz, B.I.; Tarakcioglu, E.; Olcum, M.; Genc, S. The Role of Melatonin on NLRP3 Inflammasome Activation in Diseases. Antioxidants 2021, 10, 1020. [Google Scholar] [CrossRef]
- Hudalla, H.; Karenberg, K.; Kuon, R.J.; Poschl, J.; Tschada, R.; Frommhold, D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr. Res. 2018, 84, 757–764. [Google Scholar] [CrossRef]
- Jackson, C.M.; Demmert, M.; Mukherjee, S.; Isaacs, T.; Thompson, R.; Chastain, C.; Gray, J.; Senthamaraikannan, P.; Presicce, P.; Chetal, K.; et al. A potent myeloid response is rapidly activated in the lungs of premature Rhesus macaques exposed to intra-uterine inflammation. Mucosal Immunol. 2022, 15, 730–744. [Google Scholar] [CrossRef]
- Zhu, H.L.; Shi, X.T.; Xu, X.F.; Zhou, G.X.; Xiong, Y.W.; Yi, S.J.; Liu, W.B.; Dai, L.M.; Cao, X.L.; Xu, D.X.; et al. Melatonin protects against environmental stress-induced fetal growth restriction via suppressing ROS-mediated GCN2/ATF4/BNIP3-dependent mitophagy in placental trophoblasts. Redox Biol. 2021, 40, 101854. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, A.; Sezik, M.; Eris Yalcin, S.; Asci, H.; Ozmen, O. Immunohistochemical analysis of protective effects of maternal fingolimod on the placenta and fetal lung and brain in chorioamnionitis-induced preterm birth rat model. Immunopharmacol. Immunotoxicol. 2020, 42, 564–571. [Google Scholar] [CrossRef]
- Bayes-Genis, A. Hypertrophy and inflammation: Too much for one heart. Eur. Heart J. 2007, 28, 661–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cañadas, O.; Olmeda, B.; Alonso, A.; Pérez-Gil, J. Lipid–protein and protein–protein interactions in the pulmonary surfactant system and their role in lung homeostasis. Int. J. Mol. Sci. 2020, 21, 3708. [Google Scholar] [CrossRef]
- Beers, M.F.; Shuman, H.; Liley, H.G.; Floros, J.; Gonzales, L.W.; Yue, N.; Ballard, P.L. Surfactant protein B in human fetal lung: Developmental and glucocorticoid regulation. Pediatr. Res. 1995, 38, 668–675. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Jin, J.-Y.; Choi, J.-I.; Choi, I.S.; Kim, S.-J. DHA suppresses Prevotella intermedia lipopolysaccharide-induced production of proinflammatory mediators in murine macrophages. Br. J. Nutr. 2014, 111, 1221–1230. [Google Scholar] [CrossRef]
- Deng, W.-G.; Tang, S.-T.; Tseng, H.-P.; Wu, K.K. Melatonin suppresses macrophage cyclooxygenase-2 and inducible nitric oxide synthase expression by inhibiting p52 acetylation and binding. Blood 2006, 108, 518–524. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Collin, F. Melatonin: Action as antioxidant and potential applications in human disease and aging. Toxicology 2010, 278, 55–67. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Su, S.-C.; Lin, C.-W.; Yang, W.-E.; Chien, M.-H.; Reiter, R.J.; Yang, S.-F. Melatonin as a potential inhibitory agent in head and neck cancer. Oncotarget 2017, 8, 90545–90556. [Google Scholar] [CrossRef]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Li, S.; Shin, N.E.; Na, Q.; Dong, J.; Jia, B.; Jones-Beatty, K.; McLane, M.W.; Ozen, M.; Lei, J. Melatonin for prevention of placental malperfusion and fetal compromise associated with intrauterine inflammation-induced oxidative stress in a mouse model. J. Pineal Res. 2019, 67, e12591. [Google Scholar] [CrossRef]
- Chu, J.; Tu, Y.; Chen, J.; Tan, D.; Liu, X.; Pi, R. Effects of melatonin and its analogues on neural stem cells. Mol. Cell. Endocrinol. 2016, 420, 169–179. [Google Scholar] [CrossRef]
- Gharehbaghi, M.M.; Yeganedoust, S.; Shaseb, E.; Fekri, M. Evaluation of melatonin efficacy in prevention of bronchopulmonary dysplasia in preterm newborn infants. Turk. J. Pediatr. 2022, 64, 79–84. [Google Scholar] [CrossRef]
- Pan, L.; Fu, J.H.; Xue, X.D.; Xu, W.; Zhou, P.; Wei, B. Melatonin protects against oxidative damage in a neonatal rat model of bronchopulmonary dysplasia. World J. Pediatr. 2009, 5, 216–221. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y.; Sugino, N. Melatonin and female reproduction. J. Obstet. Gynaecol. Res. 2014, 40, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Domínguez Rubio, A.P.; Sordelli, M.S.; Salazar, A.I.; Aisemberg, J.; Bariani, M.V.; Cella, M.; Rosenstein, R.E.; Franchi, A.M. Melatonin prevents experimental preterm labor and increases offspring survival. J. Pineal Res. 2014, 56, 154–162. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Zhou, W.; Ji, S.; Li, X.; Li, G.; Liu, G.; Wang, F.; Hao, A. Effect of melatonin on neuronal differentiation requires CBP/p300-mediated acetylation of histone H3 lysine 14. Neuroscience 2017, 364, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Xu, D.X.; Wang, J.P.; Wang, H.; Wei, L.Z.; Sun, M.F.; Wei, W. Melatonin protects against lipopolysaccharide-induced intra-uterine fetal death and growth retardation in mice. J. Pineal Res. 2006, 40, 40–47. [Google Scholar] [CrossRef]
- Polglase, G.R.; Barbuto, J.; Allison, B.J.; Yawno, T.; Sutherland, A.E.; Malhotra, A.; Schulze, K.E.; Wallace, E.M.; Jenkin, G.; Ricardo, S.D. Effects of antenatal melatonin therapy on lung structure in growth-restricted newborn lambs. J. Appl. Physiol. 2017, 123, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Mazzon, E.; Muia, C.; Bella, P.; Esposito, E.; Meli, R.; Cuzzocrea, S. Effects of combination of melatonin and dexamethasone on acute lung injury in a mice model of carrageenan-induced pleurisy. J. Pineal Res. 2006, 41, 228–237. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.H.; Cho, H.Y.; Jun, J.H.; Song, H.; Lee, J.Y. Prenatal Melatonin Therapy Enhances Postnatal Lung Development in a Mouse Model of Inflammation-Induced Preterm Birth. Antioxidants 2025, 14, 1094. https://doi.org/10.3390/antiox14091094
Park SH, Cho HY, Jun JH, Song H, Lee JY. Prenatal Melatonin Therapy Enhances Postnatal Lung Development in a Mouse Model of Inflammation-Induced Preterm Birth. Antioxidants. 2025; 14(9):1094. https://doi.org/10.3390/antiox14091094
Chicago/Turabian StylePark, So Hee, Hee Young Cho, Jin Hyun Jun, Haengseok Song, and Ji Yeon Lee. 2025. "Prenatal Melatonin Therapy Enhances Postnatal Lung Development in a Mouse Model of Inflammation-Induced Preterm Birth" Antioxidants 14, no. 9: 1094. https://doi.org/10.3390/antiox14091094
APA StylePark, S. H., Cho, H. Y., Jun, J. H., Song, H., & Lee, J. Y. (2025). Prenatal Melatonin Therapy Enhances Postnatal Lung Development in a Mouse Model of Inflammation-Induced Preterm Birth. Antioxidants, 14(9), 1094. https://doi.org/10.3390/antiox14091094






