Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food
Abstract
1. Introduction
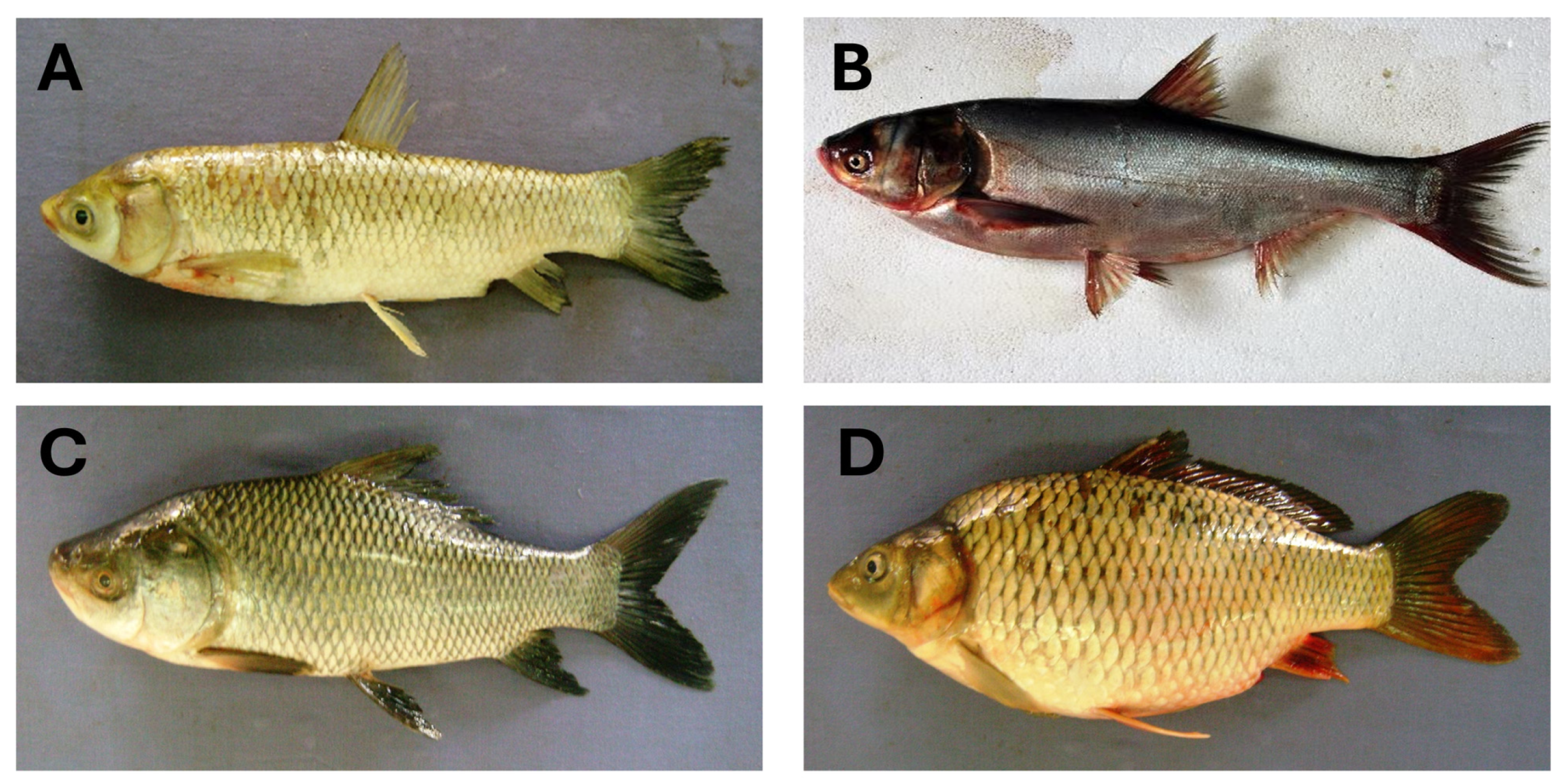
2. Carp as a Bioresource
2.1. Species Diversity and Global Production
2.2. Aquaculture Practices
2.3. Protein Composition and Suitability for Antioxidant Peptide Production
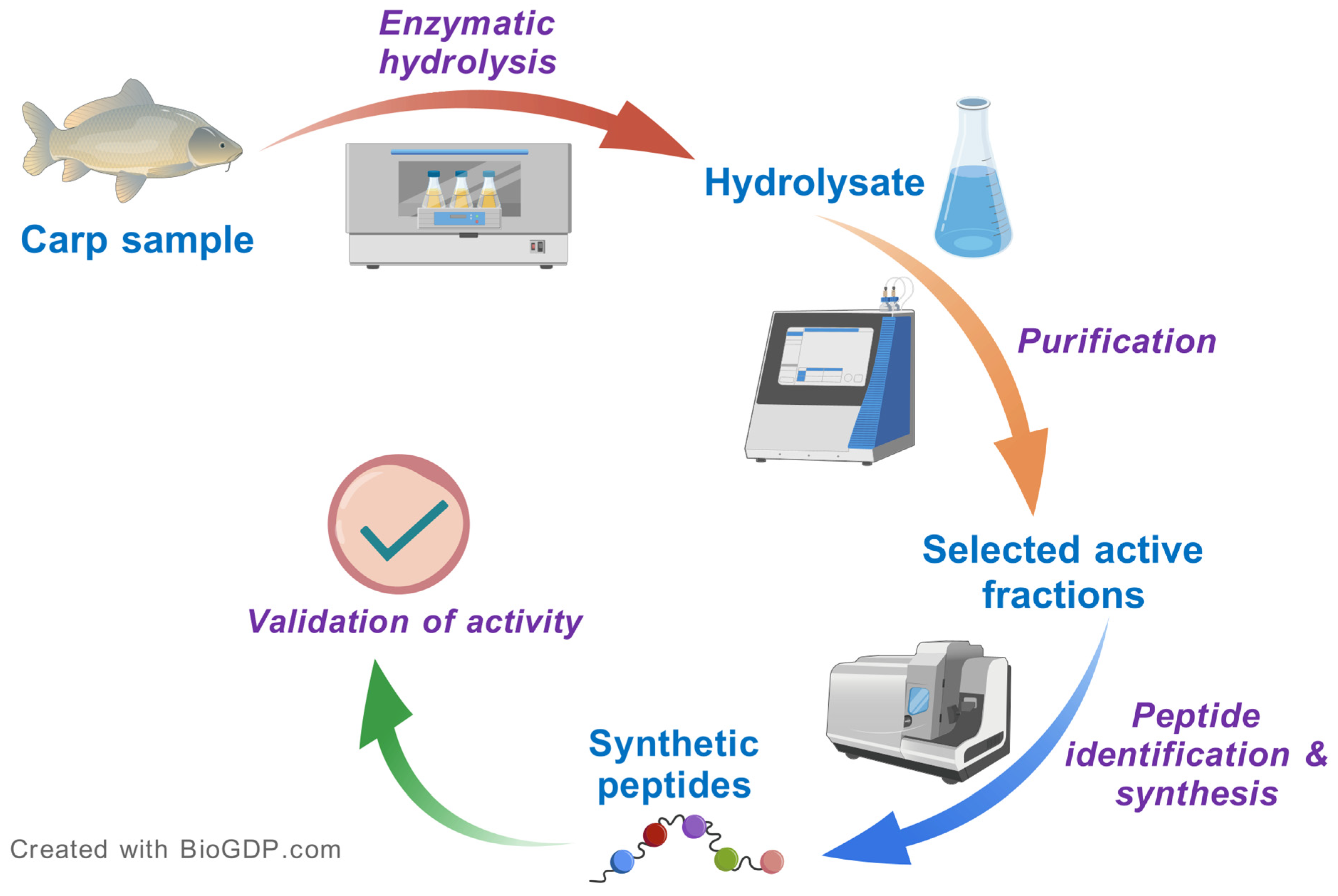
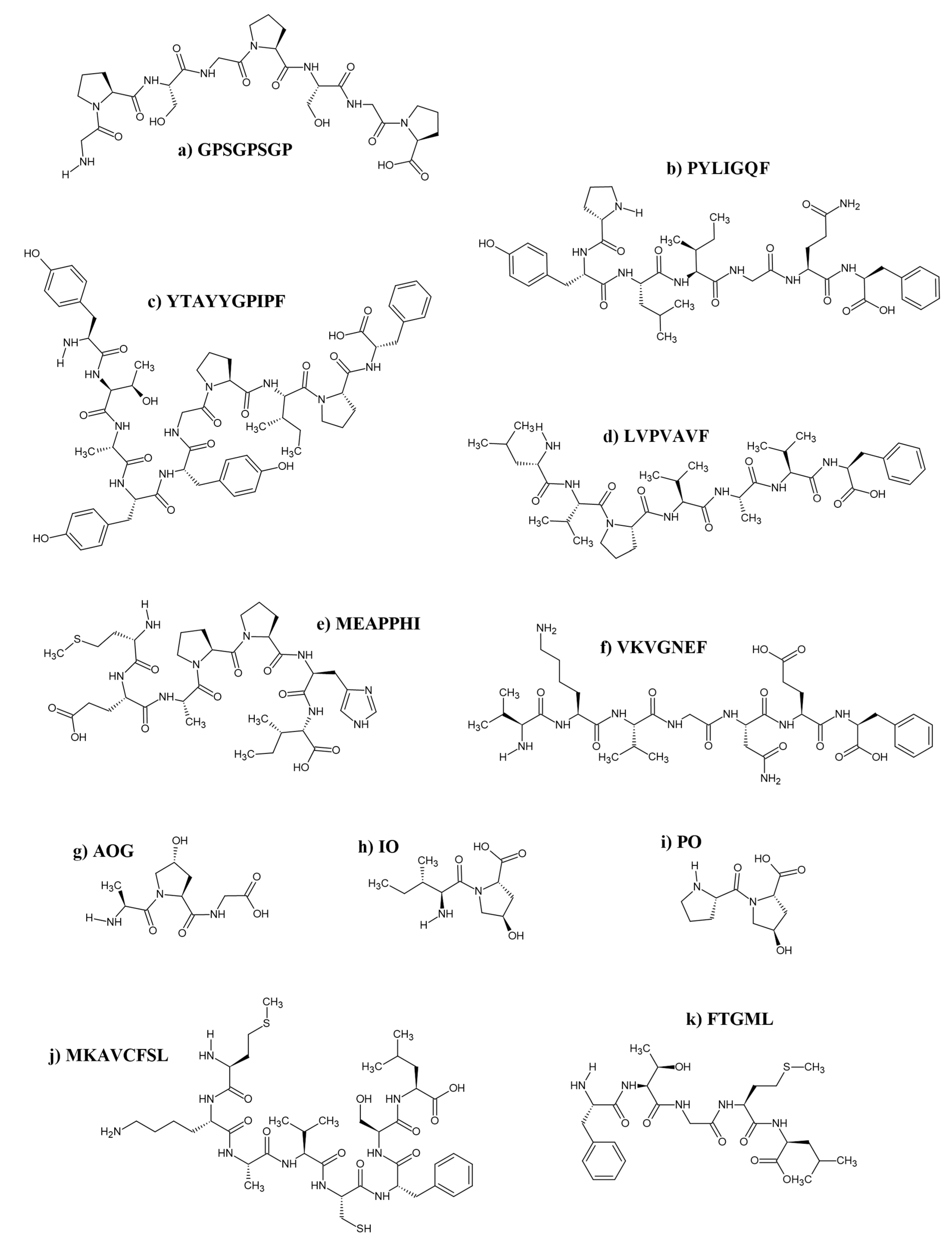
3. Production of Antioxidant Hydrolysates and Discovery of Peptides
3.1. Typical Workflow
3.2. Raw Materials and Valorization
3.3. Proteases Used and Simulated Gastrointestinal Digestion
3.4. Novel Microbial Proteases and Their Applications
3.5. Purification and Validation Pipeline
4. Antioxidant and Other Bioactivities of Carp-Derived Peptides and Hydrolysates
4.1. Cellular Models and Stressors
4.2. Cellular Antioxidant Effects of Hydrolysates
4.3. Cellular Antioxidant Effects of Peptides
4.4. Other Bioactivities of Carp Peptides and Hydrolysates
4.4.1. Wound Healing and Skin Regeneration
4.4.2. Anti-Melanogenic Effects
4.4.3. Anti-Inflammatory Activity
4.5. Bioavailability
4.6. In Vivo Evidence
5. Potential Applications in Food Preservation and Quality Improvements
5.1. Antioxidant Preservation of Food Quality
5.2. Functional and Antimicrobial Improvements
6. Potential Applications in Human Health
6.1. Skincare and Anti-Aging Applications
6.2. Internal Therapeutic and Nutritional Applications
7. Future Perspectives
7.1. Novel Microbial Proteases
7.2. Advanced Extraction
7.3. In Silico and Artificial Intelligence-Driven Discovery
7.4. Human Health Considerations and Bioavailability
- •
- How do carp-derived peptides with proven cellular antioxidant activity perform in vivo with respect to GI digestion resistance, bioavailability, and systemic potency?
- •
- Can these peptides exert in vivo antioxidant effects by downregulating pro-oxidative enzymes, such as lipoxygenase, myeloperoxidase, and xanthine oxidase?
- •
- To what extent are carp-derived antioxidant peptides multifunctional, modulating other non-antioxidant physiological pathways in vivo?
- •
- How feasible is the targeted delivery of these peptides to specific organs or tissues in vivo?
- •
- What are the long-term in vivo effects of consuming carp-derived antioxidant peptides?
- •
- What are the in vivo allergenic and toxicological risks of carp-derived peptides? Given carp’s known allergenicity, primarily due to parvalbumin [176], the allergenic potential of carp-derived antioxidant peptides must be carefully evaluated. Although enzymatic hydrolysis can reduce allergenic epitopes, a comprehensive allergenicity risk assessment, including immunoassays such as ELISA [177], is essential before their use in commercial health or food products.
7.5. Food Application Challenges and Preservation Potential
- •
- Most studies have focused on examining their food preservative potential at low temperatures. It remains unclear whether these peptides and hydrolysates will exhibit comparable preservative efficacy on food products stored at non-refrigerated temperatures. Future research should address this question, as effective preservation without refrigeration would broaden the range of food products that can be preserved using carp peptides and hydrolysates.
- •
- There is a lack of systematic comparison of the food preservation capacities of carp peptides and hydrolysates versus those from other fish species. Such comparisons could clarify whether carp have a competitive edge over other fishes as sources of food preservatives.
- •
- Most research has focused on assessing the food preservative potential of carp-derived hydrolysates, rather than specific, purified carp-derived peptides. A more in-depth characterization of the peptide profile of an effective carp-derived hydrolysate is necessary to identify the specific peptides responsible for the observed preservative effects. This is also crucial for the future development of standardized, commercially viable, carp-based preservatives with consistent, predictable preservative activity.
7.6. Impact of Aquaculture Practices and Feed Regimes
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-hydroxydopamine |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| Ca2+-ATPase | calcium-transporting ATPase |
| CAT | catalase |
| DPPH | 2,2-diphenyl-l-picrylhydrazyl |
| FRAP | Ferric Reducing Antioxidant Power |
| GI | gastrointestinal |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSG | glutathione disulfide |
| H2O2 | hydrogen peroxide |
| LDH | lactate dehydrogenase |
| LMW | low molecular weight |
| LPS | lipopolysaccharide |
| MDA | malondialdehyde |
| MMP | mitochondrial transmembrane potential |
| PEF | pulsed electric field |
| ROS | reactive oxygen species |
| SOD | superoxide dismutase |
| TAS | total antioxidant status |
| TBARS | thiobarbituric acid-reactive species |
| t-BHP | tert-butyl hydroperoxide |
| TVB-N | Total Volatile Basic Nitrogen |
| UVB | ultraviolet B |
References
- Arnhold, J. Oxidant-based cytotoxic agents during aging: From disturbed energy metabolism to chronic inflammation and disease progression. Biomolecules 2025, 15, 547. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Pereira, A.G.; Echave, J.; Jorge, A.O.S.; Nogueira-Marques, R.; Nur Yuksek, E.; Barciela, P.; Perez-Vazquez, A.; Chamorro, F.; Oliveira, M.B.P.P.; Carpena, M.; et al. Therapeutic and preventive potential of plant-derived antioxidant nutraceuticals. Foods 2025, 14, 1749. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, X.; Huang, F.; Wu, Y.; Liu, J.; Liu, J. Design and advances in antioxidant hydrogels for ROS-induced oxidative disease. Acta Biomater. 2025, 194, 80–97. [Google Scholar] [CrossRef]
- Monroy-Ramirez, H.C.; Salto-Sevilla, J.; Arceo-Orozco, S.; Caloca-Camarena, F.; Flores-Peña, R.; Lopez-Mena, E.; Galicia-Moreno, M.; Armendariz-Borunda, J. Cerium oxide nanoparticles: A promising nanotherapy approach for chronic degenerative diseases. J. Mater. Sci. Mater. Eng. 2025, 20, 69. [Google Scholar] [CrossRef]
- Bilski, R.; Nuszkiewicz, J. Antioxidant therapies as emerging adjuncts in rheumatoid arthritis: Targeting oxidative stress to enhance treatment outcomes. Int. J. Mol. Sci. 2025, 26, 2873. [Google Scholar] [CrossRef]
- Budzianowska, A.; Banaś, K.; Budzianowski, J.; Kikowska, M. Antioxidants to defend healthy and youthful skin—Current trends and future directions in cosmetology. Appl. Sci. 2025, 15, 2571. [Google Scholar] [CrossRef]
- Yildiz, A.Y.; Öztekin, S.; Anaya, K. Effects of plant-derived antioxidants to the oxidative stability of edible oils under thermal and storage conditions: Benefits, challenges and sustainable solutions. Food Chem. 2025, 479, 143752. [Google Scholar] [CrossRef]
- Periyasamy, T.; Asrafali, S.P.; Lee, J. Recent advances in functional biopolymer films with antimicrobial and antioxidant properties for enhanced food packaging. Polymers 2025, 17, 1257. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Wang, S.; Ee, K.-Y.; Chai, T.-T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Ng, W.-J.; Wong, F.-C.; Abd Manan, F.; Chow, Y.-L.; Ooi, A.-L.; Ong, M.-K.; Zhang, X.; Chai, T.-T. Antioxidant peptides and protein hydrolysates from tilapia: Cellular and in vivo evidences for human health benefits. Foods 2024, 13, 2945. [Google Scholar] [CrossRef]
- Xiang, Z.; Xue, Q.; Gao, P.; Yu, H.; Wu, M.; Zhao, Z.; Li, Y.; Wang, S.; Zhang, J.; Dai, L. Antioxidant peptides from edible aquatic animals: Preparation method, mechanism of action, and structure-activity relationships. Food Chem. 2023, 404, 134701. [Google Scholar] [CrossRef] [PubMed]
- Belton, B.; Bush, S.R.; Little, D.C. Not just for the wealthy: Rethinking farmed fish consumption in the Global South. Glob. Food Secur. 2018, 16, 85–92. [Google Scholar] [CrossRef]
- Belton, B.; Little, D.C.; Zhang, W.; Edwards, P.; Skladany, M.; Thilsted, S.H. Farming fish in the sea will not nourish the world. Nat. Commun. 2020, 11, 5804. [Google Scholar] [CrossRef]
- Hua, K.; Cobcroft, J.M.; Cole, A.; Condon, K.; Jerry, D.R.; Mangott, A.; Praeger, C.; Vucko, M.J.; Zeng, C.; Zenger, K.; et al. The future of aquatic protein: Implications for protein sources in aquaculture diets. One Earth 2019, 1, 316–329. [Google Scholar] [CrossRef]
- Mihály-Karnai, L.; Fehér, M.; Bársony, P.; Szűcs, I.; Mihály, T.; Fróna, D.; Szőllősi, L. Sustainability in intensive aquaculture—Profitability of common carp (Cyprinus carpio) production in recirculating aquaculture systems based on a Hungarian case study. Animals 2025, 15, 1055. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture 2024 Blue Transformation in Action; FAO: Rome, Italy, 2024. [Google Scholar]
- Southeast Asian Fisheries Development Center. Fisheries Statistics Summary 2022; Southeast Asian Fisheries Development Center (SEAFDEC): Bangkok, Thailand, 2022. [Google Scholar]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Howes, G.J. Systematics and biogeography: An overview. In Cyprinid Fishes: Systematics, Biology and Exploitation; Winfield, I.J., Nelson, J.S., Eds.; Chapman & Hall: London, UK, 1991; pp. 1–33. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Fishery and aquaculture statistics: Global aquaculture production 1950–2022 (FishStatJ). Available online: https://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 16 November 2024).
- Miao, W.; Wang, W. Trends of aquaculture production and trade: Carp, tilapia, and shrimp. Asian Fish. Sci. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Billard, R. Carp Biology and Culture; Praxis Publishers Ltd: Chichester, UK, 1999. [Google Scholar]
- Rahman, M.M.; Varga, I.; Chowdhury, S.N. Manual on Polyculture and Integrated Fish Farming in Bangladesh; Document BGD/87/045/91/11; FAO: Rome, Italy, 1992. [Google Scholar]
- Jhingran, V.G.; Pullin, R.S.V. A Hatchery Manual for the Common, Chinese and Indian Major Carps. ICLARM Studies and Review 11; Asian Development Bank and International Center for Living Aquatic Resources Management: Manila, Philippines, 1985. [Google Scholar]
- Ashraf, M.; Zafar, A.; Rauf, A.; Mehboob, S.; Qureshi, N.A. Nutritional values of wild and cultivated silver carp (Hypophthalmichthys molitrix) and grass carp (Ctenopharyngodon idella). Int. J. Agric. Biol. 2011, 13, 210–214. [Google Scholar]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Biofloc: A sustainable alternative for improving the production of farmed cyprinid species. Aquac. Rep. 2023, 33, 101748. [Google Scholar] [CrossRef]
- Szücs, I.; Stündl, L.; Váradi, L. Carp farming in Central and Eastern Europe and a case study in multifunctional aquaculture. In Species and System Selection for Sustainable Aquaculture; Wiley Online Library: Hoboken, NJ, USA, 2007; pp. 389–414. [Google Scholar]
- Yue, G.H.; Tay, Y.X.; Wong, J.; Shen, Y.; Xia, J. Aquaculture species diversification in China. Aquac. Fish. 2024, 9, 206–217. [Google Scholar] [CrossRef]
- Rossignoli, C.M.; Lozano Lazo, D.P.; Barman, B.K.; Dompreh, E.B.; Manyise, T.; Wang, Q.; Dam Lam, R.; Moruzzo, R.; Paz Mendez, A.; Gasparatos, A. Multi-stakeholder perception analysis of the status, characteristics, and factors affecting small-scale carp aquaculture systems in Bangladesh. Front. Sustain. Food Syst. 2023, 7, 1121434. [Google Scholar] [CrossRef]
- Skelton, P.H. A Complete Guide to the Freshwater Fishes of Southern Africa; Struik Publishers: Cape Town, South Africa, 2001. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Cultured Aquatic Species Information Programme: Ctenopharyngodon idella. Available online: https://www.fao.org/fishery/fr/culturedspecies/ctenopharyngodon_idella?lang=en (accessed on 22 December 2024).
- Aloden, E.T. Broodstock management: An integral part of hatchery techniques. Aqua. Farm. News. 1996, 14, 11–17. [Google Scholar]
- Froese, R.; Pauly, D.; “Cyprinidae” in FishBase. August 2011 Version. Available online: https://www.fishbase.org (accessed on 1 December 2024).
- Food and Agriculture Organization of the United Nations. Cyprinus carpio. Cultured Aquatic Species Information Programme. Text by Peteri, A. In Fisheries and Aquaculture. Updated 2005-07-12. Available online: https://www.fao.org/fishery/en/culturedspecies/cyprinus_carpio/en (accessed on 2 December 2024).
- Dong, M.; Zhang, L.; Wu, P.; Feng, L.; Jiang, W.; Liu, Y.; Kuang, S.; Li, S.; Mi, H.; Tang, L.; et al. Dietary protein levels changed the hardness of muscle by acting on muscle fiber growth and the metabolism of collagen in sub-adult grass carp (Ctenopharyngodon idella). J. Anim. Sci. Biotechnol. 2022, 13, 109. [Google Scholar] [CrossRef]
- Fan, Z.; Li, C.; Wu, D.; Li, J.; Wang, L.; Cao, D.; Miao, L.; Xie, S. Evaluation of four novel protein sources as alternatives to soybean meal for two specifications of cage-farmed grass carp (Ctenopharyngodon idellus) deeds: Effect on growth performance, flesh quality, and expressions of muscle-related genes. Front. Mar. Sci. 2022, 9, 935651. [Google Scholar] [CrossRef]
- Feng, R.; Feng, D.; Wang, L.; Zhang, L.; Liu, C.; Ma, F.; Zhang, M.; Yu, M.; Jiang, H.; Qiao, Z.; et al. Comparative analysis of nutritional quality, serum biochemical indices, and visceral peritoneum of grass carp (Ctenopharyngodon idellus) fed with two distinct aquaculture systems. Foods 2024, 13, 1248. [Google Scholar] [CrossRef]
- Khalid, M.; Naeem, M. Proximate analysis of grass carp (Ctenopharyngodon idella) from Southern Punjab, Pakistan. Sarhad J. Agric. 2018, 34, 632–639. [Google Scholar] [CrossRef]
- Matos, A.P.; Matos, A.C.; Moecke, E.H.S. Polyunsaturated fatty acids and nutritional quality of five freshwater fish species cultivated in the western region of Santa Catarina, Brazil. Braz. J. Food Technol. 2019, 22, e2018193. [Google Scholar] [CrossRef]
- Mustafa, G.; Hoque, F.; Pavel, S.; Jahan, S.S. An assessment on the nutritional value of grass carp, Ctenopharyngodon idellus (Valenciennes, 1844). Bangladesh J. Zool. 1999, 27, 37–42. [Google Scholar]
- Al-Humairi, K.O.; Al-Noor, S.S.; Al-Tameemi, R.A. Comparative study of amino and fatty acids synthesis in two different groups of common carp (Cyprinus carpio L.) cultured in floated cages. Indian J. Ecol. 2021, 48, 513–518. [Google Scholar]
- Jiang, X.; Li, Y.; Tian, J.; Li, C.; Ge, Y.; Hu, X.; Cheng, L.; Shi, X.; Shi, L.; Jia, Z. Nutritional components, biochemical characteristics, enzyme activities, and growth differences of five freshwater fish species. Fishes 2022, 7, 285. [Google Scholar] [CrossRef]
- Kaliniak-Dziura, A.; Skałecki, P.; Florek, M.; Kędzierska-Matysek, M.; Sobczak, P. Chemical composition and elements concentration of fillet, spine and bones of common carp (Cyprinus carpio) in relation to nutrient requirements for minerals. Animals 2024, 14, 1311. [Google Scholar] [CrossRef] [PubMed]
- Kłobukowski, J.; Skibniewska, K.; Janowicz, K.; Kłobukowski, F.; Siemianowska, E.; Terech-Majewska, E.; Szarek, J. Selected parameters of nutritional and pro-health value in the common carp (Cyprinus carpio L.) muscle tissue. J. Food Qual. 2018, 2018, 6082164. [Google Scholar] [CrossRef]
- Linhartová, Z.; Krejsa, J.; Zajíc, T.; Másílko, J.; Sampels, S.; Mráz, J. Proximate and fatty acid composition of 13 important freshwater fish species in central Europe. Aquac. Int. 2018, 26, 695–711. [Google Scholar] [CrossRef]
- Ljubojević, D.; Đorđević, V.; Ćirković, M. Evaluation of nutritive quality of common carp, Cyprinus carpio L. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012013. [Google Scholar] [CrossRef]
- Trbović, D.; Marković, Z.; Milojković-Opsenica, D.; Petronijević, R.; Spirić, D.; Djinović-Stojanović, J.; Spirić, A. Influence of diet on proximate composition and fatty acid profile in common carp (Cyprinus carpio). J. Food Compos. Anal. 2013, 31, 75–81. [Google Scholar] [CrossRef]
- Peng, J.; Lu, X.; Fan, R.; Ren, Y.; Sun, H.; Yu, Y.; Cheng, B. Analysis and evaluation of muscle quality in different parts of the bighead carp (Aristichthys nobilis). Foods 2023, 12, 4430. [Google Scholar] [CrossRef]
- Desta, D.T.; Zello, G.A.; Alemayehu, F.; Estfanos, T.K.; Zatti, K.; Drew, M. Proximate analysis of Nile Tilapia, (Oreochromis niloticus), fish fillet harvested from farmers pond and lake Hawassa, Southern Ethiopia. Int. J. Res. Dev. Technol. 2019, 11, 94–99. [Google Scholar]
- Ayanda, I.O.; Ekhator, U.I.; Bello, O.A. Determination of selected heavy metal and analysis of proximate composition in some fish species from Ogun River, Southwestern Nigeria. Heliyon 2019, 5, e02512. [Google Scholar] [CrossRef]
- Ghassem, M.; Khoo, T.C.; Feni, H.S.; Babji, A.S.; Tengku, T.M. Proximate composition, fatty acid and amino acid profiles of selected Malaysian freshwater fish. Malays. Fish. J. 2009, 8, 7–16. [Google Scholar]
- Geremew, H.; Abdisa, M.; Goshu, G. Proximate composition of commercially important fish species in southern Gulf of Lake Tana, Ethiopia. Ethiop. J. Sci. Technol. 2020, 13, 53–63. [Google Scholar] [CrossRef]
- Teame, T.; Natarajan, P.; Tesfay, Z. The proximate and mineral composition of some commercially important fish species of tekeze reservoir and lake Hashenge, Ethiopia. Int. J. Fish. Aquat. Stud. 2016, 4, 160–164. [Google Scholar]
- Jiang, S.; Li, H.; Zhang, L.; Mu, W.; Zhang, Y.; Chen, T.; Wu, J.; Tang, H.; Zheng, S.; Liu, Y.; et al. Generic Diagramming Platform (GDP): A comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. 2024, 53, D1670–D1676. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, H.; Zhang, G.; Kong, F.; Hu, Y.; Xiong, S.; Zhao, S. Identification and characterization of novel antioxidant peptides from crucian carp (Carassius auratus) cooking juice released in simulated gastrointestinal digestion by UPLC-MS/MS and in silico analysis. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2020, 1136, 121893. [Google Scholar] [CrossRef]
- Ali, Z.; Yousafzai, A.M.; Sher, N.; Muhammad, I.; Nayab, G.E.; Aqeel, S.A.M.; Shah, S.T.; Aschner, M.; Khan, I.; Khan, H. Toxicity and bioaccumulation of manganese and chromium in different organs of common carp (Cyprinus carpio) fish. Toxicol. Rep. 2021, 8, 343–348. [Google Scholar] [CrossRef]
- Kareem, S.; Husain, R.H.; Rasheed, R.O. Bioaccumulation of heavy metals in common carp fish (Cyprinus carpio) and its relationship with the protein content. Iraqi J. Vet. Sci. 2022, 36, 173–178. [Google Scholar] [CrossRef]
- Rostami, H.A.; Motamedzadegan, A.; Hosseini, S.E.; Rezaei, M.; Kamali, A. A comparative study on some properties and antioxidant activity of Hypophthalmichthys molitrix (Valenciennes, 1844) protein hydrolysates produced by different hydrolysis methods. Iran. J. Fish. Sci. 2022, 21, 1204–1221. [Google Scholar] [CrossRef]
- Rostami, A.H.; Motamedzadegan, A.; Hosseini, S.E.; Rezaei, M.; Kamali, A. Evaluation of plasticizing and antioxidant properties of silver carp protein hydrolysates in fish gelatin film. J. Aquat. Food Prod. Technol. 2017, 26, 457–467. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, S.; Dong, S. Effects of silver carp antioxidant peptide on the lipid oxidation of sierra fish fillets (Scomberomorus niphonius) during frozen storage. J. Food Biochem. 2014, 38, 167–174. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, N.; Zhang, Y.; Zhang, H.; Hong, H.; Luo, Y.; Tan, Y. Plastein reaction augments the metal chelating capabilities of silver carp (Hypophthalmichthys molitrix) hydrolysates: Unlocking the chemical modification mechanism. Food Chem. 2024, 438, 138030. [Google Scholar] [CrossRef]
- Shen, X.; Li, T.; Li, X.; Wang, F.; Liu, Y.; Wu, J. Dual cryoprotective and antioxidant effects of silver carp (Hypophthalmichthys molitrix) protein hydrolysates on unwashed surimi stored at conventional and ultra-low frozen temperatures. LWT-Food Sci. Technol. 2022, 153, 112563. [Google Scholar] [CrossRef]
- Malaypally, S.P.; Liceaga, A.M.; Kim, K.H.; Ferruzzi, M.; Martin, F.S.; Goforth, R.R. Influence of molecular weight on intracellular antioxidant activity of invasive silver carp (Hypophthalmichthys molitrix) protein hydrolysates. J. Funct. Foods 2015, 18, 1158–1166. [Google Scholar] [CrossRef]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, L.; Tan, Y.; Hong, H.; Luo, Y. Generation of novel antioxidant peptides from silver carp muscle hydrolysate: Gastrointestinal digestion stability and transepithelial absorption property. Food Chem. 2023, 403, 134136. [Google Scholar] [CrossRef]
- Leng, W.; Wu, X.; Qi, X.; Liu, H.; Yuan, L.; Gao, R. Systematic functional analysis and potential application of a serine protease from cold-adapted Planococcus bacterium. Food Sci. Hum. Wellness 2023, 12, 1751–1761. [Google Scholar] [CrossRef]
- Zu, X.Y.; Zhao, Y.J.; Fu, S.M.; Liao, T.; Li, H.L.; Xiong, G.Q. Physicochemical properties and biological activities of silver carp scale peptide and its nanofiltration fractions. Front. Nutr. 2022, 8, 812443. [Google Scholar] [CrossRef]
- Lu, H.; Luo, Y.; Feng, L. Effects of hydrolysates from silver carp (Hypophthalmichthys molitrix) scales on rancidity stability and gel properties of fish products. Food Bioprocess Technol. 2014, 7, 2178–2188. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Cheng, X.; Meng, M.; Luo, Y.; Li, B. The anti-photoaging effect of antioxidant collagen peptides from silver carp (Hypophthalmichthys molitrix) skin is preferable to tea polyphenols and casein peptides. Food Funct. 2017, 8, 1698–1707. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.; Song, H.; He, J.; Li, G.; Zheng, Y.; Li, B. Collagen peptides promote photoaging skin cell repair by activating the TGF-β/Smad pathway and depressing collagen degradation. Food Funct. 2019, 10, 6121–6134. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Song, H.; Li, B. Effect of collagen hydrolysates from silver carp skin (Hypophthalmichthys molitrix) on osteoporosis in chronologically aged mice: Increasing bone remodeling. Nutrients 2018, 10, 1434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Javed, M.; Cheng, M.; Xiong, S.; Liu, Y. Gelatin hydrolysates from sliver carp (Hypophthalmichthys molitrix) improve the antioxidant and cryoprotective properties of unwashed frozen fish mince. Int. J. Food Sci. Technol. 2022, 57, 2619–2627. [Google Scholar] [CrossRef]
- Ilie, D.; Iosageanu, A.; Craciunescu, O.; Seciu-Grama, A.M.; Sanda, C.; Oancea, F. Free radical scavenging, redox balance and wound healing activity of bioactive peptides derived from proteinase K-assisted hydrolysis of Hypophthalmichthys molitrix skin collagen. Food Technol. Biotechnol. 2022, 60, 281–292. [Google Scholar] [CrossRef]
- Iosageanu, A.; Ilie, D.; Craciunescu, O.; Seciu-Grama, A.M.; Oancea, A.; Zarnescu, O.; Moraru, I.; Oancea, F. Effect of fish bone bioactive peptides on oxidative, inflammatory and pigmentation processes triggered by UVB irradiation in skin cells. Molecules 2021, 26, 2691. [Google Scholar] [CrossRef] [PubMed]
- Grigore-Gurgu, L.; Crăciunescu, O.; Aprodu, I.; Bolea, C.A.; Iosăgeanu, A.; Petre, B.A.; Bahrim, G.E.; Oancea, A.; Stănciuc, N. Tailoring the health-promoting potential of protein hydrolysate derived from fish wastes and flavonoids from yellow onion skins: From binding mechanisms to microencapsulated functional ingredients. Biomolecules 2020, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shan, Y.; Hong, H.; Luo, Y.; Hong, X.; Ye, W. Prevention of protein and lipid oxidation in freeze-thawed bighead carp (Hypophthalmichthys nobilis) fillets using silver carp (Hypophthalmichthys molitrix) fin hydrolysates. LWT-Food Sci. Technol. 2020, 123, 109050. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Q.; Hong, H.; Luo, Y. Prevention of protein oxidation and enhancement of gel properties of silver carp (Hypophthalmichthys molitrix) surimi by addition of protein hydrolysates derived from surimi processing by-products. Food Chem. 2020, 316, 126343. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Niu, L. Antilisterial and antioxidant activities of neutrase-treated grass carp proteins and their effects on the storage and quality properties of fresh noodle. J. Food Process. Preserv. 2016, 40, 1421–1428. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Jiang, Q.; Xu, Y.; Xia, W. Improvement of antioxidant activity of grass carp (Ctenopharyngodon idella) protein hydrolysate by washing and membrane removal pretreatments and ultrasonic treatment. J. Aquat. Food Prod. Technol. 2018, 27, 580–591. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, H.; Xiong, S.; Manyande, A.; Du, H. Comparative study on hydrolysis, physicochemical and antioxidant properties in simulated digestion system between cooked pork and fish meat. Foods 2023, 12, 1757. [Google Scholar] [CrossRef]
- Jia, X.Y.; Zhu, M.F.; Zhang, L.; Ma, T.X.; Li, Y.H.; Sheng, W.S.; Tu, Z.C. Extraction optimization and screening of antioxidant peptides from grass carp meat and synergistic–antagonistic effect. Food Sci. Nutr. 2022, 10, 1481–1493. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Xia, W.; Xiong, Y.L.; Ye, R.; Wang, H. Grass carp peptides hydrolysed by the combination of Alcalase and Neutrase: Angiotensin-I converting enzyme (ACE) inhibitory activity, antioxidant activities and physicochemical profiles. Int. J. Food Sci. Technol. 2016, 51, 499–508. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, Q.; Xu, Y.; Xia, W. Effects of washing and membrane removal pretreatments on the antioxidant properties of grass carp (Ctenopharyngodon idella) protein hydrolysates produced by in vitro digestion. Int. J. Food Sci. Technol. 2017, 52, 1260–1268. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Lv, Y.; Xu, Y.; Mi, G.; Li, J. The neuroprotective and antioxidant activities of protein hydrolysates from grass carp (Ctenopharyngodon idella) skin. J. Food Sci. Technol. 2015, 52, 3750–3755. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yi, J.; De Gobba, C.; Skibsted, L.H.; Otte, J. Angiotensin-I converting enzyme inhibitory and antioxidant activity of bioactive peptides produced by enzymatic hydrolysis of skin from grass carp (Ctenopharyngodon idella). Int. J. Food Prop. 2017, 20, 1129–1144. [Google Scholar] [CrossRef]
- Rao, H.; Huan, R.; Chen, Y.; Xiao, X.; Li, W.; He, H. Characteristics and application of a novel cold-adapted and salt-tolerant protease EK4-1 produced by an Arctic bacterium Mesonia algae K4-1. Int. J. Mol. Sci. 2023, 24, 7985. [Google Scholar] [CrossRef]
- Li, S.; Tian, Q.; Meng, T.; Guan, Z.; Cai, Y.; Liao, X. Production, purification and activity evaluation of three novel antioxidant peptides obtained from grass carp (Ctenopharyngodon idella) scale waste by microbial protease BaApr1 hydrolysis. Syst. Microbiol. Biomanufacturing 2022, 2, 568–579. [Google Scholar] [CrossRef]
- Chen, K.; Yang, X.; Huang, Z.; Jia, S.; Zhang, Y.; Shi, J.; Hong, H.; Feng, L.; Luo, Y. Modification of gelatin hydrolysates from grass carp (Ctenopharyngodon idellus) scales by Maillard reaction: Antioxidant activity and volatile compounds. Food Chem. 2019, 295, 569–578. [Google Scholar] [CrossRef]
- Hu, Y.M.; Lu, S.Z.; Li, Y.S.; Wang, H.; Shi, Y.; Zhang, L.; Tu, Z.C. Protective effect of antioxidant peptides from grass carp scale gelatin on the H2O2-mediated oxidative injured HepG2 cells. Food Chem. 2022, 373, 131539. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gu, J.; Zhong, B.; Feng, R.; Pan, H.; Liu, Y.; Shi, W. Isolation and purification of antioxidant peptides from swim bladder of grass carp (Ctenopharyngodon idella). Aquac. Fish. 2025, 10, 485–493. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xue, Y.; Ruan, S.; Zhou, A.; Huang, S.; Ma, H. The preparation and identification of characteristic flavour compounds of maillard reaction products of protein hydrolysate from grass carp (Ctenopharyngodon idella) bone. J. Food Qual. 2021, 2021, 8394152. [Google Scholar] [CrossRef]
- Du, Y.J.; Zhao, Y.Q.; Cai, W.W. Hydroxyl radical scavenging activity of peptide from fish intestine protein by hydrolysis with complex enzyme. Adv. J. Food Sci. Technol. 2014, 6, 126–129. [Google Scholar] [CrossRef]
- Martínez-Pérez, R.B.; Rodríguez, J.A.; Cira-Chávez, L.A.; Dendooven, L.; Viniegra-González, G.; Estrada-Alvarado, I. Exoenzyme-producing halophilic bacteria from the former Lake Texcoco: Identification and production of n-butyl oleate and bioactive peptides. Folia Microbiol. 2020, 65, 835–847. [Google Scholar] [CrossRef]
- Delgado-García, M.; Flores-Gallegos, A.C.; Kirchmayr, M.; Rodríguez, J.A.; Mateos-Díaz, J.C.; Aguilar, C.N.; Muller, M.; Camacho-Ruíz, R.M. Bioprospection of proteases from Halobacillus andaensis for bioactive peptide production from fish muscle protein. Electron. J. Biotechnol. 2019, 39, 52–60. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Iwaniak, A.; Minkiewicz, P. Ex vivo digestion of carp muscle tissue—ACE inhibitory and antioxidant activities of the obtained hydrolysates. Food Funct. 2015, 6, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J.; Borczak, B.; Piątkowska, E.; Kapusta-Duch, J.; Morawska, M.; Czech, T. Effect of protein hydrolysates from carp (Cyprinus carpio) skin gelatine on oxidative stress biomarkers and other blood parameters in healthy rats. J. Funct. Foods 2019, 60, 103411. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Kulawik, P.; Morawska, M.; Szczurowska, K. Evaluation of the potential use of a carp (Cyprinus carpio) skin gelatine hydrolysate as an antioxidant component. Food Funct. 2019, 10, 1038–1048. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Piątkowska, E.; Borczak, B.; Kapusta-Duch, J.; Morawska, M. Furcellaran-coated microcapsules as carriers of Cyprinus carpio skin-derived antioxidant hydrolysate: An in vitro and in vivo study. Nutrients 2019, 11, 2502. [Google Scholar] [CrossRef] [PubMed]
- Tkaczewska, J.; Borawska-Dziadkiewicz, J.; Kulawik, P.; Duda, I.; Morawska, M.; Mickowska, B. The effects of hydrolysis condition on the antioxidant activity of protein hydrolysate from Cyprinus carpio skin gelatin. LWT-Food Sci. Technol. 2020, 117, 108616. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Li, L.; He, J.; Cheng, W.; Ren, G. In vitro antioxidant activity of peptides from simulated gastro-intestinal digestion products of Cyprinus carpio haematopterus scale gelatin. Foods 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Ghelichi, S.; Sørensen, A.D.M.; García-Moreno, P.J.; Hajfathalian, M.; Jacobsen, C. Physical and oxidative stability of fish oil-in-water emulsions fortified with enzymatic hydrolysates from common carp (Cyprinus carpio) roe. Food Chem. 2017, 237, 1048–1057. [Google Scholar] [CrossRef][Green Version]
- Ghelichi, S.; Shabanpour, B.; Pourashouri, P.; Hajfathalian, M.; Jacobsen, C. Extraction of unsaturated fatty acid-rich oil from common carp (Cyprinus carpio) roe and production of defatted roe hydrolysates with functional, antioxidant, and antibacterial properties. J. Sci. Food Agric. 2018, 98, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Tavares Kütter, M.; Borges Tesser, M.; Monserrat, J.M.; Prentice, C. Effect of dietary common carp by-product protein hydrolysates on antioxidant status in different organs of zebrafish (Danio rerio). Aquac. Nutr. 2019, 25, 110–118. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Ramos, P.; Monserrat, J.M.; Prentice, C. Evaluation of the antioxidant activity in vitro and in hippocampal HT-22 cells system of protein hydrolysates of common carp (Cyprinus carpio) by-product. J. Aquat. Food Prod. Technol. 2018, 27, 21–34. [Google Scholar] [CrossRef]
- González-Serrano, D.J.; Hadidi, M.; Varcheh, M.; Jelyani, A.Z.; Moreno, A.; Lorenzo, J.M. Bioactive peptide fractions from collagen hydrolysate of common carp fish byproduct: Antioxidant and functional properties. Antioxidants 2022, 11, 509. [Google Scholar] [CrossRef]
- Borawska, J.; Darewicz, M.; Vegarud, G.E.; Minkiewicz, P. Antioxidant properties of carp (Cyprinus carpio L.) protein ex vivo and in vitro hydrolysates. Food Chem. 2016, 194, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, Y.; Wang, Z.; Chen, S.; Luo, Y. Production and identification of antioxidant and angiotensin-converting enzyme inhibition and dipeptidyl peptidase IV inhibitory peptides from bighead carp (Hypophthalmichthys nobilis) muscle hydrolysate. J. Funct. Foods 2017, 35, 224–235. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Xia, S.; Zhu, S.; Li, H.; Liu, X. Evaluating the effects of MKAVCFSL derived from bighead carp (Hypophthalmichthys nobilis) flesh on antioxidant activity in Caco-2 cells in vitro. J. Food Qual. 2021, 2021, 9975586. [Google Scholar] [CrossRef]
- Lin, J.; Hong, H.; Zhang, L.; Zhang, C.; Luo, Y. Antioxidant and cryoprotective effects of hydrolysate from gill protein of bighead carp (Hypophthalmichthys nobilis) in preventing denaturation of frozen surimi. Food Chem. 2019, 298, 124868. [Google Scholar] [CrossRef]
- Naghdi, S.; Lorenzo, J.M.; Mirnejad, R.; Ahmadvand, M.; Moosazadeh Moghaddam, M. Bioactivity evaluation of peptide fractions from bighead carp (Hypophthalmichthys nobilis) using alcalase and hydrolytic enzymes extracted from Oncorhynchus mykiss and their potential to develop the edible coats. Food Bioprocess Technol. 2023, 16, 1128–1148. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, W.; Zhang, Y.Q. Effects of spray drying and freeze drying on physicochemical properties, antioxidant and ACE inhibitory activities of bighead carp (Aristichthys nobilis) skin hydrolysates. Foods 2022, 11, 2083. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Dai, Z. Antioxidant and cryoprotective effects of bone hydrolysates from bighead carp (Aristichthys nobilis) in freeze-thawed fish fillets. Foods 2021, 10, 1409. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, L.; Xie, B.; Ma, A.; Hu, K.; Zheng, C.; Xiong, G.; Shi, L.; Ding, A.; Li, X.; et al. Effects of high-pressure treatments (ultra-high hydrostatic pressure and high-pressure homogenization) on bighead carp (Aristichthys nobilis) myofibrillar protein native state and its hydrolysate. Food Bioprocess Technol. 2022, 15, 2252–2266. [Google Scholar] [CrossRef]
- Elavarasan, K.; Naveen Kumar, V.; Shamasundar, B.A. Antioxidant and functional properties of fish protein hydrolysates from fresh water carp (Catla catla) as influenced by the nature of enzyme. J. Food Process. Preserv. 2014, 38, 1207–1214. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Physico-chemical characteristics and fibril-forming capacity of carp swim bladder collagens and exploration of their potential bioactive peptides by in silico approaches. Int. J. Biol. Macromol. 2017, 101, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Behera, A.; Das, R.; Mahanta, S.; Akhtar, J.; Mohanty, G. Antioxidant potential of bioactive peptides derived from fish waste: A focus on Catla catla liver and intestinal tissue. Int. J. Exp. Res. Rev. 2024, 39, 39–50. [Google Scholar] [CrossRef]
- Mohanty, U.; Majumdar, R.K.; Mohanty, B.; Mehta, N.K.; Parhi, J. Influence of the extent of enzymatic hydrolysis on the functional properties of protein hydrolysates from visceral waste of Labeo rohita. J. Food Sci. Technol. 2021, 58, 4349–4358. [Google Scholar] [CrossRef]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Effects of different pretreatments and proteases on recovery, umami taste compound contents and antioxidant potentials of Labeo rohita head protein hydrolysates. J. Food Sci. Technol. 2019, 56, 1966–1977. [Google Scholar] [CrossRef]
- Kanwate, B.W.; Karkal, S.S.; Kudre, T.G. Impact of antioxidant potential of rohu (Labeo rohita) swim bladder gelatin hydrolysate on oxidative stability, textural and sensory properties of fish sausage enriched with polyunsaturated fatty acids. J. Food Sci. Technol. 2024, 61, 1083–1093. [Google Scholar] [CrossRef]
- Kanwate, B.W.; Patel, K.; Karkal, S.S.; Rajoriya, D.; Sharan, K.; Kudre, T.G. Production of antioxidant, angiotensin-converting enzyme inhibitory and osteogenic gelatin hydrolysate from Labeo rohita swim bladder. Mar. Biotechnol. 2024, 26, 404–420. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Dai, L.; Yu, F.J.; Li, X.N.; Wang, G.X.; Chen, J.; Wang, C.; Lu, Y.P. Chemical and biological characteristics of hydrolysate of crucian carp swim bladder: Focus on preventing ulcerative colitis. J. Funct. Foods 2020, 75, 104256. [Google Scholar] [CrossRef]
- Elavarasan, K.; Shamasundar, B.A. Antioxidant properties of papain mediated protein hydrolysates from fresh water carps (Catla catla, Labeo rohita and Cirrhinus mrigala) and its application on inhibition of lipid oxidation in oil sardine mince during ice storage. J. Food Sci. Technol. 2022, 59, 636–645. [Google Scholar] [CrossRef]
- Wu, X.; Cai, L.; Zhang, Y.; Mi, H.; Cheng, X.; Li, J. Compositions and antioxidant properties of protein hydrolysates from the skins of four carp species. Int. J. Food Sci. Technol. 2015, 50, 2589–2597. [Google Scholar] [CrossRef]
- Benjakul, S.; Yarnpakdee, S.; Senphan, T.; Halldorsdottir, S.M.; Kristinsson, H.G. Fish protein hydrolysates: Production, bioactivities, and applications. In Antioxidants and Functional Components in Aquatic Foods; Kristinsson, H.G., Ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2014; pp. 237–282. [Google Scholar]
- Szopa, D.; Skrzypczak, D.; Izydorczyk, G.; Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Waste valorization towards industrial products through chemo- and enzymatic-hydrolysis. Bioengineered 2023, 14, 2184480. [Google Scholar] [CrossRef]
- Mora, L.; Toldrá, F. Advanced enzymatic hydrolysis of food proteins for the production of bioactive peptides. Curr. Opin. Food Sci. 2023, 49, 100973. [Google Scholar] [CrossRef]
- Almaas, H.; Cases, A.L.; Devold, T.G.; Holm, H.; Langsrud, T.; Aabakken, L.; Aadnoey, T.; Vegarud, G.E. In vitro digestion of bovine and caprine milk by human gastric and duodenal enzymes. Int. Dairy J. 2006, 16, 961–968. [Google Scholar] [CrossRef]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Gu, J.; Liu, Y.; Qiu, W.; Shi, W. Physicochemical properties and stability of antioxidant peptides from swim bladder of grass carp (Ctenopharyngodon idella). Foods 2025, 14, 1216. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, L.; Du, Z.; Yu, Z.; Liu, J. Hydrolysis and transport of egg white-derived peptides in Caco-2 cell monolayers and everted rat sacs. J. Agric. Food Chem. 2019, 67, 4839–4848. [Google Scholar] [CrossRef]
- Zu, X.Y.; Huang, Y.Q.; Zhao, Y.J.; Xiong, G.Q.; Liao, T.; Li, H.L. Peptide extraction from silver carp (Hypophthalmichthys molitrix) scales via enzymatic hydrolysis and membrane filtration. Ital. J. Food Sci. 2023, 35, 44–53. [Google Scholar] [CrossRef]
- Hu, Z.; Sha, X.; Zhang, L.; Huang, S.; Tu, Z. Effect of grass carp scale collagen peptide FTGML on cAMP-PI3K/Akt and MAPK signaling pathways in B16F10 melanoma cells and correlation between anti-melanin and antioxidant properties. Foods 2022, 11, 391. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, Y.; Miao, X.; Cheng, L.; Qi, X. Protective effect of ferulic acid against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced oxidative stress in PC12 cells. Cell. Mol. Biol. 2016, 62, 109–116. [Google Scholar]
- Lenzen, S.; Lushchak, V.I.; Scholz, F. The pro-radical hydrogen peroxide as a stable hydroxyl radical distributor: Lessons from pancreatic beta cells. Arch. Toxicol. 2022, 96, 1915–1920. [Google Scholar] [CrossRef]
- Wedel, S.; Martic, I.; Hrapovic, N.; Fabre, S.; Madreiter-Sokolowski, C.T.; Haller, T.; Pierer, G.; Ploner, C.; Jansen-Dürr, P.; Cavinato, M. tBHP treatment as a model for cellular senescence and pollution-induced skin aging. Mech. Ageing Dev. 2020, 190, 111318. [Google Scholar] [CrossRef]
- Nishio, R.; Morioka, H.; Takeuchi, A.; Saeki, N.; Furuhata, R.; Katahira, M.; Chinenn, T.; Tamura, H.; Tamano, H.; Takeda, A. Intracellular hydrogen peroxide produced by 6-hydroxydopamine is a trigger for nigral dopaminergic degeneration of rats via rapid influx of extracellular Zn2+. NeuroToxicology 2022, 89, 1–8. [Google Scholar] [CrossRef]
- Preda, S.; Anastasescu, C.; Balint, I.; Umek, P.; Sluban, M.; Negrila, C.C.; Angelescu, D.G.; Bratan, V.; Rusu, A.; Zaharescu, M. Charge separation and ROS generation on tubular sodium titanates exposed to simulated solar light. Appl. Surf. Sci. 2019, 470, 1053–1063. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Yue, W.; Xie, J.; Ran, H.; Xiong, S.; Rong, J.; Wang, P.; Hu, Y. Antioxidant peptides from silver carp steak by alkaline protease and flavor enzyme hydrolysis: Characterization of their structure and cytoprotective effects against H2O2-induced oxidative stress. J. Food Sci. 2024, 89, 8868–8886. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Guo, L.; Zhou, Z.; Xiong, G.; Peng, L.; Liu, Q.; Fu, J.; Wang, Y.; Guo, P.; Li, H. Antioxidant and anti-blackening dual-function silver carp scale peptides: In vitro studies and molecular mechanism. LWT-Food Sci. Technol. 2025, 224, 117857. [Google Scholar] [CrossRef]
- Abeynayake, R.; Zhang, S.; Yang, W.; Chen, L. Development of antioxidant peptides from brewers’ spent grain proteins. LWT-Food Sci. Technol. 2022, 158, 113162. [Google Scholar] [CrossRef]
- Prakash Nirmal, N.; Singh Rajput, M.; Bhojraj Rathod, N.; Mudgil, P.; Pati, S.; Bono, G.; Nalinanon, S.; Li, L.; Maqsood, S. Structural characteristic and molecular docking simulation of fish protein-derived peptides: Recent updates on antioxidant, anti-hypertensive and anti-diabetic peptides. Food Chem. 2023, 405, 134737. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Assadpour, E.; Dima, S.; Jafari, S.M. Bioavailability and bioaccessibility of food bioactive compounds; overview and assessment by in vitro methods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2862–2884. [Google Scholar] [CrossRef]
- Wang, B.; Xie, N.; Li, B. Influence of peptide characteristics on their stability, intestinal transport, and in vitro bioavailability: A review. J. Food Biochem. 2019, 43, e12571. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Y.; Li, K.; Zhang, J.; Wei, B.; Wang, H. Absorption of food-derived peptides: Mechanisms, influencing factors, and enhancement strategies. Food Res. Int. 2024, 197, 115190. [Google Scholar] [CrossRef]
- Okagu, I.U.; Udenigwe, C.C. Transepithelial transport and cellular mechanisms of food-derived antioxidant peptides. Heliyon 2022, 8, e10861. [Google Scholar] [CrossRef]
- Liang, Q.; Chalamaiah, M.; Ren, X.; Ma, H.; Wu, J. Identification of new anti-inflammatory peptides from zein hydrolysate after simulated gastrointestinal digestion and transport in Caco-2 cells. J. Agric. Food Chem. 2018, 66, 1114–1120. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and characterization of antioxidant peptides from Alcalase-hydrolyzed soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal Caco-2 cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Jiang, B.; Miao, M.; Mu, W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Koch, R.E.; Hill, G.E. An assessment of techniques to manipulate oxidative stress in animals. Funct. Ecol. 2017, 31, 9–21. [Google Scholar] [CrossRef]
- Janik, M.; Jamróz, E.; Tkaczewska, J.; Juszczak, L.; Kulawik, P.; Szuwarzyński, M.; Khachatryan, K.; Kopel, P. Utilisation of carp skin post-production waste in binary films based on furcellaran and chitosan to obtain packaging materials for storing blueberries. Materials 2021, 14, 7848. [Google Scholar] [CrossRef]
- Malekkolaei, K.J.; Jafarian, S.; Javadian, S.R.; Mahdavi, S.K.; Bahram, S. Edible coatings of chitosan and fucoidan with crucian carp protein hydrolysate to extend shelf life of beluga sturgeon fillets. Heliyon 2025, 11, e42296. [Google Scholar] [CrossRef]
- Jamróz, E.; Cabaj, A.; Tkaczewska, J.; Kawecka, A.; Krzyściak, P.; Szuwarzyński, M.; Mazur, T.; Juszczak, L. Incorporation of curcumin extract with lemongrass essential oil into the middle layer of triple-layered films based on furcellaran/chitosan/gelatin hydrolysates—In vitro and in vivo studies on active and intelligent properties. Food Chem. 2023, 402, 134476. [Google Scholar] [CrossRef]
- Dou, P.; Wang, K.; Ding, N.; Zheng, Y.; Hong, H.; Liu, H.; Tan, Y.; Luo, Y. Sensory improvement and antioxidant enhancement in silver carp hydrolysate using prebiotic oligosaccharides: Insights from the Maillard reaction. Food Funct. 2024, 15, 9888–9902. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Kulawik, P.; Tkaczewska, J.; Guzik, P.; Zając, M.; Juszczak, L.; Krzyściak, P.; Turek, K. The effects of active double-layered furcellaran/gelatin hydrolysate film system with Ala-Tyr peptide on fresh Atlantic mackerel stored at −18 °C. Food Chem. 2021, 338, 127867. [Google Scholar] [CrossRef] [PubMed]
- Javadian, S.R.; Rasoul, S.S.; Ariaii, P. The effects of liposomal encapsulated thyme extract on the quality of fish mince and Escherichia coli O157:H7 inhibition during refrigerated storage. J. Aquat. Food Prod. Technol. 2017, 26, 115–123. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.M.; Li, L.Y.; Chi, C.F.; Wang, B. Twelve antioxidant peptides from protein hydrolysate of skipjack tuna (Katsuwonus pelamis) roe prepared by flavourzyme: Purification, sequence identification, and activity evaluation. Front. Nutr. 2022, 8, 813780. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Kulawik, P.; Jamróz, E.; Guzik, P.; Zając, M.; Szymkowiak, A.; Turek, K. One- and double-layered furcellaran/carp skin gelatin hydrolysate film system with antioxidant peptide as an innovative packaging for perishable foods products. Food Chem. 2021, 351, 129347. [Google Scholar] [CrossRef]
- Kulawik, P.; Jamróz, E.; Janik, M.; Tkaczewska, J.; Krzyściak, P.; Skóra, M.; Guzik, P.; Milosavljević, V.; Tadele, W. Antimicrobial and antioxidant properties of chitosan-furcellaran-gelatin hydrolysate coatings enhanced with bioactive peptides. Food Control 2023, 153, 109931. [Google Scholar] [CrossRef]
- Egner, P.; Pavlačková, J.; Sedlaříková, J.; Matošková, L.; Mokrejš, P.; Janalíková, M. Collagen hydrolysates from animal by-products in topical cosmetic formulations. Int. J. Mol. Sci. 2025, 26, 2776. [Google Scholar] [CrossRef] [PubMed]
- Jarzebski, M.P.; Elmqvist, T.; Gasparatos, A.; Fukushi, K.; Eckersten, S.; Haase, D.; Goodness, J.; Khoshkar, S.; Saito, O.; Takeuchi, K.; et al. Ageing and population shrinking: Implications for sustainability in the urban century. npj Urban Sustain. 2021, 1, 17. [Google Scholar] [CrossRef]
- Morawska-Tota, M.; Tota, Ł.; Tkaczewska, J. Influence of 5-week snack supplementation with the addition of gelatin hydrolysates from carp skins on pro-oxidative and antioxidant balance disturbances (TOS, TAS) in a group of athletes. Antioxidants 2022, 11, 1314. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Munekata, P.E.S.; Agregán, R.; Bermúdez, R.; López-Pedrouso, M.; Pateiro, M.; Lorenzo, J.M. Application of pulsed electric fields for obtaining antioxidant extracts from fish residues. Antioxidants 2020, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lin, J.; Chen, J.; Fang, T. Pulsed electric field-assisted enzymatic extraction of protein from abalone (Haliotis discus hannai Ino) viscera. J. Food Process Eng. 2016, 39, 702–710. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. BIOPEP-UWM database—Present and future. Curr. Opin. Food Sci. 2024, 55, 101108. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, L.; Tianyi, R.; Ping, X.; Duan, J.-A. Novel and efficient techniques in the discovery of antioxidant peptides. Crit. Rev. Food Sci. Nutr. 2024, 64, 11934–11948. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Horka, P.; Zdenkova, K.; Cermakova, E. Parvalbumin: A major fish allergen and a forensically relevant marker. Genes 2023, 14, 223. [Google Scholar] [CrossRef] [PubMed]
- Günal-Köroğlu, D.; Karabulut, G.; Ozkan, G.; Yılmaz, H.; Gültekin-Subaşı, B.; Capanoglu, E. Allergenicity of alternative proteins: Reduction mechanisms and processing strategies. J. Agric. Food Chem. 2025, 73, 7522–7546. [Google Scholar] [CrossRef] [PubMed]
| Year | |||||
|---|---|---|---|---|---|
| Carp Species | 2000 | 2005 | 2010 | 2020 | 2022 |
| Grass carp, Ctenopharyngodon idella | 2976.5 | 3396.6 | 4213.1 | 5792.0 | 6151.6 |
| Silver carp, Hypophthalmichthys molitrix | 3034.7 | 3690.0 | 3972.0 | 4896.3 | 5070.0 |
| Catla, Labeo catla | 602.3 | 1317.5 | 2526.4 | 2313.4 | 4145.1 |
| Common carp, Cyprinus carpio | 2410.4 | 2666.3 | 3299.3 | 4169.0 | 4012.7 |
| Bighead carp, Hypophthalmichthys nobilis | 1438.9 | 1929.4 | 2513.6 | 3187.2 | 3320.3 |
| Amino Acid | Bighead Carp, Dorsal [50] (g/100 g) | Bighead Carp, Belly [50] (g/100 g) | Common Carp, Muscle [43] (%) | Grass Carp Fed with Chlorella Powder, Muscle [38] (% Dry Weight) | Grass Carp, Ventral, Dorsal, and Tail White Muscles [27] (%) | Silver Carp, Ventral, Dorsal, and Tail White Muscles [27] (%) |
|---|---|---|---|---|---|---|
| Alanine (Ala) | 0.98 ± 0.06 | 0.60 ± 0.03 | 7.72 ± 0.99 | 5.30 ± 0.04 | 6.17 ± 0.29 | 6.17 ± 0.29 |
| Arginine (Arg) | 1.04 ± 0.04 | 0.71 ± 0.02 | 1.22 ± 0.03 | 4.90 ± 0.06 | 1.60 ± 0.03 | 1.60 ± 0.03 |
| Aspartic acid (Asp) | 1.82 ± 0.11 | 1.04 ± 0.15 | 6.84 ± 0.53 | 9.14 ± 0.07 | 6.18 ± 0.13 | 7.01 ± 0.82 |
| Cysteine (Cys) | 0.45 ± 0.02 | 0.45 ± 0.11 | NA | 0.32 ± 0.01 | 1.13 ± 0.03 | 1.39 ± 0.04 |
| Glutamic acid (Glu) | 2.69 ± 0.13 | 1.54 ± 0.18 | 3.43 ± 1.62 | NA | 4.83 ± 0.06 | 4.83 ± 0.06 |
| Glutamine (Gln) | NA | NA | NA | 11.47 ± 0.04 | NA | NA |
| Glycine (Gly) | 0.96 ± 0.04 | 0.68 ± 0.00 | 15.83 ± 0.54 | 4.36 ± 0.03 | NA | NA |
| Histidine (His) | 0.54 ± 0.04 | 0.27 ± 0.01 | 1.28 ± 1.16 | 1.38 ± 0.01 | 2.01 ± 0.09 | 1.34 ± 0.06 |
| Isoleucine (Ile) | 0.78 ± 0.04 | 0.45 ± 0.04 | 5.74 ± 0.84 | 4.08 ± 0.03 | 7.15 ± 0.10 | 6.50 ± 0.05 |
| Leucine (Leu) | 1.47 ± 0.08 | 0.91 ± 0.06 | 3.75 ± 0.65 | 7.75 ± 0.04 | NA | NA |
| Lysine (Lys) | 1.65 ± 0.08 | 0.92 ± 0.13 | 8.73 ± 1.01 | 7.86 ± 0.08 | 6.23 ± 0.10 | 1.92 ± 0.06 |
| Methionine (Met) | 0.29 ± 0.01 | 0.08 ± 0.04 | 2.92 ± 1.07 | 2.43 ± 0.05 | 4.37 ± 0.17 | 2.48 ± 0.42 |
| Phenylalanine (Phe) | 0.84 ± 0.05 | 0.70 ± 0.06 | 3.54 ± 0.29 | 3.20 ± 0.02 | 7.41 ± 0.05 | 7.02 ± 0.05 |
| Proline (Pro) | 0.65 ± 0.03 | 0.59 ± 0.07 | 8.39 ± 0.55 | 2.30 ± 0.01 | 2.71 ± 0.13 | 2.29 ± 0.07 |
| Serine (Ser) | 0.76 ± 0.04 | 0.47 ± 0.04 | 6.39 ± 1.56 | 2.74 ± 0.26 | NA | NA |
| Threonine (Thr) | 0.80 ± 0.04 | 0.51 ± 0.01 | 2.60 ± 1.18 | 3.97 ± 0.03 | 1.91 ± 0.10 | 1.53 ± 0.08 |
| Tryptophan (Trp) | NA | NA | 2.67 ± 0.62 | NA | NA | NA |
| Tyrosine (Tyr) | 0.70 ± 0.04 | 0.47 ± 0.09 | 3.36 ± 1.68 | 2.41 ± 0.04 | 3.44 ± 0.09 | 2.45 ± 0.10 |
| Valine (Val) | 0.83 ± 0.05 | 0.52 ± 0.08 | 4.37 ± 0.05 | 4.27 ± 0.04 | 2.55 ± 0.00 | 1.59 ± 0.04 |
| Species | Part of the Fish | Proteases a | References |
|---|---|---|---|
| Silver carp | Muscle |
| [60,61,62] |
| [63] | ||
| [64] | ||
| [65] | ||
| [66,67] | ||
| [68] | ||
| Scale |
| [69] | |
| [70] | ||
| Skin gelatin |
| [71,72] | |
| [73] | ||
| [74] | ||
| Skin collagen |
| [75] | |
| Bone |
| [76,77] | |
| Fin |
| [78] | |
| By-products (head, skin, fin, scale, bone, white muscle leftover on bones, and dark muscle) |
| [79] | |
| Grass carp | Muscle |
| [80] |
| [81] | ||
| [82] | ||
| [83] | ||
| [84] | ||
| [85] | ||
| Skin |
| [86] | |
| [87] | ||
| [88] | ||
| [89] | ||
| Scale |
| [90] | |
| Scale gelatin |
| [91] | |
| [92] | ||
| Swim bladder |
| [93] | |
| Bone |
| [94] | |
| Intestine |
| [95] | |
| Common carp | Muscle |
| [96] |
| [97] | ||
| [98] | ||
| Skin gelatin |
| [99,100,101,102] | |
| [103] | ||
| Scale gelatin |
| [104] | |
| Roe |
| [105,106] | |
| [107] | ||
| By-products (head, viscera, bones and skin) |
| [108] | |
| [109] | ||
| Collagen extract of by-products (heads, skins, and skeletons) |
| [110] | |
|
| [111] | |
| Bighead carp | Muscle |
| [112,113] |
| Gill |
| [114] | |
| Head |
| [115] | |
| Skin |
| [116] | |
| Bone |
| [117] | |
| Myofibrillar proteins |
| [118] | |
| Catla | Muscle |
| [119] |
| Swim bladder collagens |
| [120] | |
|
| [121] | |
| Rohu | Viscera |
| [122] |
| Head |
| [123] | |
| Swim bladder gelatin |
| [124,125] | |
| Crucian carp | Swim bladder |
| [126] |
| Cooking juice |
| [57] | |
| Muscle |
| [127] |
| [127] | ||
| Skin |
| [128] |
| Species | Part of the Fish | Enzyme Used for Hydrolysis | Hydrolysis Conditions | Purification and Peptide Identification | Peptide Sequence | Validation of Peptide Activity | Reference |
|---|---|---|---|---|---|---|---|
| Grass carp | Muscle | Protamex followed by Alcalase |
|
|
|
| [83] |
| Skin | Alcalase |
|
|
|
| [86] | |
| Scale gelatin | Alcalase followed by trypsin |
|
|
|
| [92] | |
| Scale | Alkaline protease BaAprl from the Bacillus altitudinis W3 |
|
|
|
| [90] | |
| Swim bladder | Combination of alkaline protease and neutral protease |
|
|
|
| [93,134] | |
| Silver carp | Muscle | Hydrolysis with Alcalase or papain, followed by simulated GI digestion (pepsin and pancreatin) |
|
| From papain hydrolysate:
|
| [67] |
| Bighead carp | Muscle | Pepsin |
|
|
|
| [112,113] |
| Crucian carp | Cooking juice | Simulated GI digestion (pepsin and pancreatin) |
|
|
|
| [57] |
| Species | Part of the Fish | Sample Dose | Cell Model | Key Findings | Reference |
|---|---|---|---|---|---|
| Silver carp | Filets | 0.625–5 mg/mL | AAPH-treated Caco-2 cells |
| [65] |
| Dorsal muscle | 0.25–2 mg/mL | H2O2-treated Caco-2 cells |
| [66] | |
| Bone | 10–1500 μg/mL | UVB-irradiated L929 and HaCaT cells |
| [76] | |
| t-BHP-treated L929 and HaCaT cells |
| ||||
| LPS-treated THP-1 cells |
| ||||
| UVB- irradiated Mel-JuSo cells |
| ||||
| Skin | 10–400 μg/mL | t-BHP-treated L929 cells |
| [75] | |
| Muscle | 0.25–2 mg/mL | Ox-LDL- treated HUVECs |
| [67] | |
| Scales | 0.01–1 mg/mL | B16 cells |
| [136] | |
| Grass carp | Skin | 1 and 0.1 mg/mL | 6-OHDA-treated MES 23.5 cells |
| [87] |
| 6-OHDA-treated SH-SY5Y cells | |||||
| Scales | 0.19–3 mg/mL | H2O2-treated HepG2 cells |
| [92] | |
| Common carp | By-products (head, viscera, bones and skin) | 0.03–1.25 mg/mL | HT-22 cells |
| [109] |
| Crucian carp | Swim bladder | 50–150 μg/mL | H2O2-treated IEC-6 cells |
| [126] |
| Species | Part of the Fish | Peptide | Sample Dosage | Cell Model | Key Findings | Reference |
|---|---|---|---|---|---|---|
| Silver carp | Steak |
| 100 μg/mL | H2O2-treated HepG2 cells |
| [144] |
| Muscle |
| 50 μg/mL | H2O2-treated Caco-2 cells |
| [66] | |
| Muscle |
| 75 μg/mL | Ox-LDL-treated HUVECs |
| [67] | |
| Skin |
| 200 μM | UVA-irradiated ESF cells |
| [72] | |
| Scales |
| 0.01–0.5 mM | B16 melanoma cells |
| [145] | |
| Bighead carp | Muscle |
| 50 μg/mL | H2O2-treated Caco-2 cells |
| [112,113] |
| Grass carp | Scale |
| 0.1–1.6 mg/mL | B16F10 cells |
| [137] |
| Peptide Mixture/Hydrolysate | Source | Duration (days) | Dosage (g/kg) | In Vivo Model | Key Findings a | References |
|---|---|---|---|---|---|---|
| CSGH | Skin | 30 | 10 and 100 | Healthy male Wistar rats |
| [102] |
| Furcellaran-coated CSGH microcapsules | Skin | 35 | 14.29 | Healthy male Wistar rats |
| [99] |
| Collagen peptides from silver carp | Skin | 14 | 0.2 | Female KM mice |
| [71] |
| Carp protein hydrolysate | Head, viscera, bones, and skin | 44 | 25, 50 and 100 | Zebrafish |
| [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, F.-C.; Ng, W.-J.; Ooi, A.-L.; Lem, F.-F.; Chai, T.-T. Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food. Antioxidants 2025, 14, 1095. https://doi.org/10.3390/antiox14091095
Wong F-C, Ng W-J, Ooi A-L, Lem F-F, Chai T-T. Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food. Antioxidants. 2025; 14(9):1095. https://doi.org/10.3390/antiox14091095
Chicago/Turabian StyleWong, Fai-Chu, Wen-Jie Ng, Ai-Lin Ooi, Fui-Fui Lem, and Tsun-Thai Chai. 2025. "Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food" Antioxidants 14, no. 9: 1095. https://doi.org/10.3390/antiox14091095
APA StyleWong, F.-C., Ng, W.-J., Ooi, A.-L., Lem, F.-F., & Chai, T.-T. (2025). Carp-Derived Antioxidant Peptides and Hydrolysates: Biological Effects and Potential Applications in Health and Food. Antioxidants, 14(9), 1095. https://doi.org/10.3390/antiox14091095








