A Review of the Potential Use of Antioxidants in Spinal Cord Injuries
Abstract
1. Introduction
2. Mechanisms of Oxidative Stress in Spinal Cord Injury
2.1. Mitochondrial Dysfunction and Primary ROS Generation
2.2. Electron Transport Chain Disruption and Energy Crisis
2.3. Lipid Peroxidation and Membrane Damage
2.4. Cellular Sources of ROS Production
2.5. Protein Oxidation and Functional Impairment
2.6. DNA Oxidation and Genomic Instability
2.7. Antioxidant System Depletion and Defense Mechanisms
2.8. Signal Transduction Pathways and Oxidative Stress Response
3. Antioxidants in Spinal Cord Injuries
3.1. A91 Peptide
3.2. Allicin
3.3. Asiatic Acid
3.4. Curcumin
3.5. Edaravone
3.6. Epigallocatechin
3.7. Estrogen
3.8. Gastrodin
3.9. Ginko Biloba Extract 761
3.10. Ginsenosides
3.11. Glutathione
3.12. Ligustilide
3.13. Lycopene
3.14. Melatonin
3.15. Metformin
3.16. Omega-3 Fatty Acids
3.17. Quercetin
3.18. Resveratrol
3.19. Tetramethylpyrazine
| Compound | Mechanism of Action | Therapeutic Effects | References |
|---|---|---|---|
| A91 peptide | Immunomodulatory properties; reduces nitric oxide production; downregulates iNOS gene expression; enhances neurotrophic factor production (BDNF, NT-3) | Neuroprotective effects; anti-inflammatory action; enhanced functional recovery in moderate SCI (injury severity-dependent) | [229,230,231,232,233] |
| Allicin | Antioxidant properties; anti-inflammatory effects via NF-κB and TNF-α reduction; upregulates HSP70/Akt/iNOS signaling; attenuates glutamate-induced excitotoxicity | Enhanced functional recovery; reduced spinal cord edema; neuroprotection against oxidative stress and excitotoxicity | [234,235] |
| Asiatic acid/asiaticoside | Anti-inflammatory and antioxidant properties; reduces lipid peroxidation; suppresses pro-inflammatory cytokines; modulates apoptotic cascades | Improved motor function recovery; reduced tissue damage; enhanced neuronal survival and structural preservation | [236,237] |
| Curcumin | Modulates Nrf2, NF-κB, and TGF-β pathways; enhances autophagy; inhibits Akt/mTOR signaling; activates ERK1/2 pathway; epigenetic regulation via miR-137-3p/NeuroD1 | Comprehensive neuroprotection; reduced inflammation and apoptosis; enhanced tissue integrity and functional recovery; superior long-term efficacy | [238,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253] |
| Edaravone | Ferroptosis pathway regulation; upregulates anti-ferroptosis proteins (GPX4); anti-inflammatory effects; enhances BMSC differentiation into neurons | Enhanced neuronal survival; improved tissue sparing; superior functional recovery and neural regeneration | [255,256,257,258,259] |
| Epigallocatechin gallate (EGCG) | Antioxidant effects; inhibits p38MAPK/NF-κB/AQP4 signaling; reduces inflammatory mediators; anti-edema properties | Reduced oxidative stress; effective anti-inflammatory and anti-edema effects; tissue preservation | [260,261,262] |
| Estrogen | Dose-dependent neuroprotection; modulates inflammatory and apoptotic pathways; preserves neuronal survival; anti-inflammatory via COX-2 inhibition | Enhanced survival rates; superior locomotor function; reduced tissue damage and inflammatory response | [263,264,265,266] |
| Gastrodin | Antioxidant and anti-inflammatory properties; preserves blood–spinal cord barrier; upregulates Nrf2-GCLc/GCLm signaling | Improved locomotor function; reduced inflammatory cytokines; enhanced antioxidant capacity | [267] |
| Ginkgo biloba extract 761 (EGb761) | Antioxidant activity; modulates apoptotic genes (Bcl-2/Bax ratio); inhibits cPLA2 and ERK1/2 signaling; reduces free radical generation | Functional and histopathological improvements; reduced apoptosis and tissue necrosis; neuroprotection against oxidative damage | [268,269,270,271,272] |
| Ginsenosides | Immunomodulatory via miR-130b-5p/TLR4 pathway; autophagy inhibition; anti-inflammatory and antioxidant effects; MAPK pathway inhibition | Reduced neuronal apoptosis; enhanced motor function recovery; tissue preservation, and reduced inflammatory response | [273,274,275,276,277] |
| Glutathione | Antioxidant defense; chirality-dependent effects (D-GSH superior); MAPK pathway modulation; age-related therapeutic variations | Enhanced axon regeneration; improved motor function recovery; age-specific therapeutic efficacy | [36,194,278,279] |
| Ligustilide | Anti-inflammatory and antioxidative effects; suppresses inflammatory mediators (iROS, PGE2, IL-1β, TNF-α); downregulates iNOS | Enhanced motor function recovery; reduced inflammatory and oxidative damage | [280,281] |
| Lycopene | Blood–spinal cord barrier stabilization; anti-inflammatory via TNF-α and NF-κB suppression; upregulates tight junction proteins; antioxidative properties | Improved motor function; reduced spinal cord edema; enhanced barrier integrity and tissue preservation | [282,283] |
| Melatonin | Antioxidant effects; activates Nrf2/ARE pathway; suppresses NLRP3 inflammasome; modulates Wnt/β-catenin signaling; anti-apoptotic mechanisms | Enhanced motor function recovery; increased neuronal survival; reduced oxidative stress and inflammation | [284,285,286,287,288,289,290,291] |
| Metformin | PI3K/Akt pathway activation; Nrf2/ARE signaling; microtubule stabilization; antiapoptotic effects; antioxidative mechanisms | Comprehensive neuroprotection; enhanced axonal regeneration; improved functional recovery and cellular preservation | [292,293] |
| Omega-3 fatty acids | Antioxidant effects via endogenous antioxidant production; anti-inflammatory action; neuronal membrane stabilization; activates protective transcription pathways (RXR, PPAR-α, Akt, CREB) | Neuroprotective and antioxidant effects; enhanced functional recovery; reduced inflammatory response and apoptosis | [294,295,296,297,298] |
| Quercetin | Antioxidant and anti-inflammatory properties; modulates Akt/mTOR/p70S6K signaling; prevents oligodendrocyte necroptosis; induces autophagy | Enhanced motor function recovery; preserved neural tissue; improved myelin integrity and axonal regeneration | [299,300,301,302,303,304,305,306,307] |
| Resveratrol | Inhibits ferroptosis via Nrf2/GPX4 pathway; suppresses NF-κB signaling; activates LKB1/AMPK/mTOR autophagy pathway; anti-inflammatory effects | Improved locomotor recovery; reduced neuronal apoptosis; enhanced autophagy and tissue preservation | [88,308,309,310,311,312,313] |
| Tetramethylpyrazine | Anti-inflammatory, anti-apoptotic, and antioxidant properties; upregulates PGC-1α; reduces glial scar formation; preserves neuronal structure | Enhanced motor function recovery; reduced inflammation and glial scarring; improved neuronal survival | [314,315,316,317,318] |
4. Novel Formulations and Delivery Systems
| Compound | Delivery System | Key Properties | Mechanisms of Action | In Vitro Results | In Vivo Results | References |
|---|---|---|---|---|---|---|
| Curcumin | Nanocomposite with resveratrol in calcium alginate hydrogel | Ionotropic gelation-based platform; sustained release kinetics | Downregulation of NF-κB and TNF-α gene expression; anti-inflammatory effects | Complete absence of cytotoxicity against PC-12 neuronal cells; sustained release of both compounds | Superior healing outcomes in rat SCI model; effective modulation of inflammatory signaling cascades | [319] |
| Curcumin | Curcumin nanoconjugate (PA-C) | Dose-dependent enhancement above 10 µM without cytotoxicity | Prevention of H2O2-induced cytotoxicity; reduction of LPS-induced NF-κB translocation | Enhanced iPSC-derived neural stem cell viability; promoted neurite elongation in β-III tubulin-positive cells | No significant BBB scale improvements, but reduced glial scar area; enhanced β-III tubulin preservation; promoted M2 microglial polarization | [320] |
| EGCG | EGCG-selenium nanoparticles (EGCG-Se NP) | Rapid ROS scavenging capacity | Dual antioxidant and anti-inflammatory mechanisms | Protected PC12 cells from H2O2-induced oxidative damage | Significant locomotor capacity improvements; substantial reduction in injury area; protection of neuronal cell bodies and myelin sheaths | [261] |
| Estrogen | Estrogen nanoparticles with engineered release kinetics | Fast-release and slow-release variants; enhanced tissue distribution | Modulation of inflammatory responses, apoptotic signaling, and tissue preservation | Reduced ROS production and calpain activity in microglia, astroglia, macrophages, and fibroblasts | Fast release: reduced Bax/Bcl-2 ratio; slow release: prevented gliosis and penumbral demyelination | [321] |
| Metformin | Glutathione-modified macrophage-derived cell membrane-encapsulated nanogels (Met-CNG-GSH) | Biomimetic cell membrane coating; glutathione modification for BSCB penetration | Addresses oxidative stress, neuroinflammation, and apoptotic cell death | Optimal sustained-release characteristics | Significant accumulation at injury sites; amelioration of oxidative stress, neuroinflammation, and apoptosis | [322] |
| Resveratrol | Chitosan-modified hollow manganese dioxide nanoparticles (CMR) | ~130 nm particle size; 21.39 ± 2.53% drug loading efficiency | Antioxidative, anti-inflammatory, and anti-apoptotic effects | 87% sustained release over 36 h | Reduced ROS, MDA, and SOD levels; increased GPx activity; reduced iNOS, IL-1β expression; downregulated Cl caspase-3, Bax; upregulated Bcl-2 | [323] |
| Resveratrol + Puerarin | Polymeric nanoparticles (RES-PUE) | 238–274 nm particle size; −12.6 ± 2.1 mV zeta potential; 74.85% encapsulation efficiency | Addresses inflammation and neuronal apoptosis | 72–79% sustained release over 36 h vs. 96–98% rapid release of native drugs within 6 h | Decreased MDA and AOPP levels; reduced plasma nitrite/nitrate; normalized iNOS expression; increased SOD and catalase activity | [324] |
| Tetramethylpyrazine (TMP) | HIV TAT-modified nanoparticles (TAT-TMP-NPs) | 163.93 ± 0.38 nm size; −30 mV surface charge; 77.27 ± 1.99% encapsulation efficiency | Enhanced blood–spinal cord barrier penetration and targeting | 80–82% sustained release over 96 h; >80% cell viability at high concentrations; <5% hemolysis rate | Enhanced targeting to spinal cord tissue; improved bioavailability and extended circulation time | [325] |
| Tetramethylpyrazine (TMP) | Electroconductive hydrogel | Integrated approach combining drug delivery with tissue engineering | Targets microvascular dysfunction and neural regeneration simultaneously | Enhanced pharmacological effectiveness | Supports synergistic tissue repair environment; addresses vascular stabilization and neural regeneration | [326] |
4.1. Curcumin
4.2. Epigallocatechin-3-Gallate
4.3. Estrogen
4.4. Metformin
4.5. Resveratrol
4.6. Tetramethylpyrazine
5. Limitations of Antioxidant Therapies in SCI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quadri, S.A.; Farooqui, M.; Ikram, A.; Zafar, A.; Khan, M.A.; Suriya, S.; Claus, C.F.; Fiani, B.; Rahman, M.; Ramachandran, A.; et al. Recent Update on Basic Mechanisms of Spinal Cord Injury. Neurosurg. Rev. 2018, 43, 425. [Google Scholar] [CrossRef]
- Badhiwala, J.H.; Wilson, J.R.; Fehlings, M.G. Global Burden of Traumatic Brain and Spinal Cord Injury. Lancet Neurol. 2018, 18, 24. [Google Scholar] [CrossRef]
- Golestani, A.; Shobeiri, P.; Sadeghi-Naini, M.; Jazayeri, S.B.; Maroufi, S.F.; Ghodsi, Z.; Ohadi, M.A.D.; Mohammadi, E.; Rahimi-Movaghar, V.; Ghodsi, S.M. Epidemiology of Traumatic Spinal Cord Injury in Developing Countries from 2009 to 2020: A Systematic Review and Meta-Analysis. Neuroepidemiology 2022, 56, 219. [Google Scholar] [CrossRef]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global Incidence and Characteristics of Spinal Cord Injury since 2000–2021: A Systematic Review and Meta-Analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.S.; Abraha, H.N.; Adsuar, J.C.; et al. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Safdarian, M.; Trinka, E.; Rahimi-Movaghar, V.; Thomschewski, A.; Aali, A.; Gebreheat, G.; Abate, S.M.; Abd-Allah, F.; Abedi, A.; Eshetie, D.; et al. Global, Regional, and National Burden of Spinal Cord Injury, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2023, 22, 1026–1047. [Google Scholar] [CrossRef]
- Engel-Haber, E.; Botticello, A.; Snider, B.; Kirshblum, S. Incomplete Spinal Cord Syndromes: Current Incidence and Quantifiable Criteria for Classification. J. Neurotrauma 2022, 39, 1687. [Google Scholar] [CrossRef] [PubMed]
- Bárbara-Bataller, E.; Méndez-Suárez, J.L.; Alemán-Sánchez, C.; Sánchez-Enríquez, J.; Henríquez, M.S. Change in the Profile of Traumatic Spinal Cord Injury over 15 Years in Spain. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 27. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef]
- Lima, R.; Monteiro, A.; Salgado, A.J.; Monteiro, S.; Silva, N.A. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 13833. [Google Scholar] [CrossRef]
- Li, S.; Dinh, H.T.P.; Matsuyama, Y.; Sato, K.; Yamagishi, S. Molecular Mechanisms in the Vascular and Nervous Systems Following Traumatic Spinal Cord Injury. Life 2022, 13, 9. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Aidemise, O.C. Secondary Injury Mechanisms in Traumatic Spinal Cord Injury: A Nugget of This Multiply Cascade. Acta Neurobiol. Exp. 2011, 71, 281. [Google Scholar] [CrossRef] [PubMed]
- Srikandarajah, N.; Alvi, M.A.; Fehlings, M.G. Current Insights into the Management of Spinal Cord Injury. J. Orthop. 2023, 41, 8–13. [Google Scholar] [CrossRef]
- Mautes, A.; Weinzierl, M.R.; Donovan, F.; Noble-Haeusslein, L.J. Vascular Events After Spinal Cord Injury: Contribution to Secondary Pathogenesis. Phys. Ther. 2000, 80, 673. [Google Scholar] [CrossRef]
- Benton, R.; Hagg, T. Vascular Pathology as a Potential Therapeutic Target in SCI. Transl. Stroke Res. 2011, 2, 556–574. [Google Scholar] [CrossRef]
- Zavvarian, M.-M.; Hong, J.; Chio, J.C.T.; Toossi, A.; Fehlings, M.G. Neurovascular Pathology Following Traumatic Spinal Cord Injury. In Cellular, Molecular, Physiological, and Behavioral Aspects of Spinal Cord Injury; Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2022; p. 119. [Google Scholar]
- Kumar, H.; Ropper, A.E.; Lee, S.; Han, I. Propitious Therapeutic Modulators to Prevent Blood-Spinal Cord Barrier Disruption in Spinal Cord Injury. Mol. Neurobiol. 2016, 54, 3578–3590. [Google Scholar] [CrossRef]
- Jiang, T.; Qin, T.; Gao, P.; Tao, Z.; Wang, X.; Wu, M.; Gu, J.; Chu, B.; Zheng, Z.; Jiang, Y.; et al. SIRT1 Attenuates Blood-Spinal Cord Barrier Disruption after Spinal Cord Injury by Deacetylating p66Shc. Redox Biol. 2023, 60, 102615. [Google Scholar] [CrossRef]
- Pan, Y.; Guo, Y.; Ma, Y.; Wang, L.; Zheng, S.; Liu, M.; Huang, G. Aquaporin-4 Expression Dynamically Varies after Acute Spinal Cord Injury-induced Disruption of Blood Spinal Cord Barrier in Rats. Neuropathology 2019, 39, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Li, J.; Chen, Z.; Wang, R.; Shen, Y.; Zhang, R.; Zhou, F.; Zhang, Y. Pathological Hemodynamic Changes and Leukocyte Transmigration Disrupt the Blood–Spinal Cord Barrier after Spinal Cord Injury. J. Neuroinflamm. 2023, 20, 118. [Google Scholar] [CrossRef]
- García, E.; Aguilar-Cevallos, J.; Silva-García, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Min, L.; Liang, L.; Chen, B.; Chen, H.; Zhou, Y.; Deng, W.; Liu, H.; Hou, J. Neutrophil Extracellular Traps Exacerbate Secondary Injury via Promoting Neuroinflammation and Blood–Spinal Cord Barrier Disruption in Spinal Cord Injury. Front. Immunol. 2021, 12, 698249. [Google Scholar] [CrossRef] [PubMed]
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Calcium-Dependent Excitotoxicity. J. Mol. Med. 2000, 78, 3–13. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Kim, A.H.; Choi, D.W. Glutamate-Mediated Excitotoxicity. In Handbook of Experimental Pharmacology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1999; p. 443. [Google Scholar]
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Glutamate Receptor-Mediated Excitotoxic Neuronal Cell Death. Mol. Neurobiol. 2001, 24, 107–130. [Google Scholar] [CrossRef]
- Κritis, A.; Stamoula, Ε.; Paniskaki, K.A.; Vavilis, T. Researching Glutamate €” Induced Cytotoxicity in Different Cell Lines: A Comparative/Collective Analysis/Study. Front. Cell. Neurosci. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ying, Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef]
- Neves, D.; Salazar, I.L.; Almeida, R.D.; Silva, R.M. Molecular Mechanisms of Ischemia and Glutamate Excitotoxicity. Life Sci. 2023, 328, 121814. [Google Scholar] [CrossRef] [PubMed]
- Mira, R.G.; Cerpa, W. Building a Bridge Between NMDAR-Mediated Excitotoxicity and Mitochondrial Dysfunction in Chronic and Acute Diseases. Cell. Mol. Neurobiol. 2020, 41, 1413–1430. [Google Scholar] [CrossRef]
- Hernández, D.E.; Salvadores, N.; Moya-Alvarado, G.; Catalán, R.J.; Bronfman, F.C.; Court, F.A. Axonal Degeneration Induced by Glutamate Excitotoxicity Is Mediated by Necroptosis. J. Cell Sci. 2018, 131, jcs214684. [Google Scholar] [CrossRef]
- Maiorov, S.A.; Kairat, B.K.; Berezhnov, A.V.; Зинченкo, B.П.; Gaidin, S.G.; Kosenkov, A.M. Peculiarities of Ion Homeostasis in Neurons Containing Calcium-Permeable AMPA Receptors. Arch. Biochem. Biophys. 2024, 754, 109951. [Google Scholar] [CrossRef]
- Scheijen, E.E.M.; Hendrix, S.; Wilson, D.M. Oxidative DNA Damage in the Pathophysiology of Spinal Cord Injury: Seems Obvious, but Where Is the Evidence? Antioxidants 2022, 11, 1728. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative Stress in Spinal Cord Injury and Antioxidant-Based Intervention. Spinal Cord 2011, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Carrico, K.M.; Vaishnav, R.; Hall, E.D. Temporal and Spatial Dynamics of Peroxynitrite-Induced Oxidative Damage After Spinal Cord Contusion Injury. J. Neurotrauma 2009, 26, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and Subcellular Oxidative Stress Parameters Following Severe Spinal Cord Injury. Redox Biol. 2015, 8, 59–67. [Google Scholar] [CrossRef]
- Mandelker, L. Introduction to Oxidative Stress and Mitochondrial Dysfunction. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, V.; Kaushik, S.; Saxena, J.; Jyoti, A. Free Radicals, Mitochondrial Dysfunction and Sepsis-Induced Organ Dysfunction: A Mechanistic Insight. Curr. Pharm. Des. 2024, 30, 161–168. [Google Scholar] [CrossRef]
- Miao, X.; Lin, J.; Zheng, X. Advances of the Role of Mitochondrial Dysfunction in the Spinal Cord Injury and Its Relevant Treatments. J. Reparative Reconstr. Surg. 2022, 36, 902–907. [Google Scholar]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Lund, M.C.; Ellman, D.G.; Nissen, M.; Nielsen, P.S.; Nielsen, P.V.; Jørgensen, C.; Andersen, D.C.; Gao, H.; Brambilla, R.; Degn, M.; et al. The Inflammatory Response after Moderate Contusion Spinal Cord Injury: A Time Study. Biology 2022, 11, 939. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in Tissue Injury and Repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Beck, K.D.; Nguyen, H.X.; Galvan, M.; Salazar, D.L.; Woodruff, T.M.; Anderson, A.J. Quantitative Analysis of Cellular Inflammation after Traumatic Spinal Cord Injury: Evidence for a Multiphasic Inflammatory Response in the Acute to Chronic Environment. Brain 2010, 133, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Rizo-Téllez, S.A.; Filep, J.G. Beyond Host Defense and Tissue Injury: The Emerging Role of Neutrophils in Tissue Repair. AJP Cell Physiol. 2024, 326, C661–C683. [Google Scholar] [CrossRef]
- Bouchery, T.; Harris, N.L. Neutrophil–Macrophage Cooperation and Its Impact on Tissue Repair. Immunol. Cell Biol. 2019, 97, 289–298. [Google Scholar] [CrossRef]
- Quraishe, S.; Forbes, L.H.; Andrews, M.R. The Extracellular Environment of the CNS: Influence on Plasticity, Sprouting, and Axonal Regeneration after Spinal Cord Injury. Neural Plast. 2018, 2018, 2952386. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.L.; Sajed, D.; Tuszynski, M.H. Axonal Regeneration through Regions of Chondroitin Sulfate Proteoglycan Deposition after Spinal Cord Injury: A Balance of Permissiveness and Inhibition. J. Neurosci. 2003, 23, 9276–9288. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, M.; Zeitler, C.; Paulat, M.; Gobrecht, P.; Hilla, A.; Andreadaki, A.; Guthoff, R.; Fischer, D. Inhibition of Microtubule Detyrosination by Parthenolide Facilitates Functional CNS Axon Regeneration. eLife 2023, 12, RP88279. [Google Scholar] [CrossRef]
- Pires, L.R.; Pêgo, A.P. Bridging the Lesion—Engineering a Permissive Substrate for Nerve Regeneration. Regen. Biomater. 2015, 2, 203–214. [Google Scholar] [CrossRef]
- Cafferty, W.B.J.; Duffy, P.; Huebner, E.A.; Strittmatter, S.M. MAG and OMgp Synergize with Nogo-A to Restrict Axonal Growth and Neurological Recovery after Spinal Cord Trauma. J. Neurosci. 2010, 30, 6825–6837. [Google Scholar] [CrossRef]
- Baldwin, K.T.; Giger, R.J. Insights into the Physiological Role of CNS Regeneration Inhibitors. Front. Mol. Neurosci. 2015, 8, 23. [Google Scholar] [CrossRef]
- Wang, K.; Koprivica, V.; Kim, J.A.; Sivasankaran, R.; Guo, Y.; Neve, R.L.; He, Z. Oligodendrocyte-Myelin Glycoprotein Is a Nogo Receptor Ligand That Inhibits Neurite Outgrowth. Nature 2002, 417, 941–944. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Schwab, M.E.; Montani, L.; Brazda, N.; Müller, H.W. Defeating Inhibition of Regeneration by Scar and Myelin Components. Handb. Clin. Neurol. 2012, 109, 503–522. [Google Scholar] [CrossRef]
- Hussein, R.K.; Mencio, C.P.; Katagiri, Y.; Brake, A.; Geller, H.M. Role of Chondroitin Sulfation Following Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 208. [Google Scholar] [CrossRef]
- Galindo, L.T.; Mundim, M.T.; Pinto, A.S.; Chiarantin, G.M.; Almeida, M.E.; Lamers, M.L.; Horwitz, A.R.; Santos, M.F.; Porcionatto, M. Chondroitin Sulfate Impairs Neural Stem Cell Migration Through ROCK Activation. Mol. Neurobiol. 2017, 55, 3185–3195. [Google Scholar] [CrossRef]
- Alizadeh, J.; Kochan, M.M.; Stewart, V.D.; Drewnik, D.A.; Hannila, S.S.; Ghavami, S. Inhibition of Autophagy Flux Promotes Secretion of Chondroitin Sulfate Proteoglycans in Primary Rat Astrocytes. Mol. Neurobiol. 2021, 58, 6077–6091. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Y.; Kang, J.; Zhang, C.; Ning, B. Chondroitin Sulfate Proteoglycans Revisited: Its Mechanism of Generation and Action for Spinal Cord Injury. Aging Dis. 2024, 15, 153–168. [Google Scholar] [CrossRef]
- Chien, P.N.; Ryu, S.E. Protein Tyrosine Phosphatase σ in Proteoglycan-Mediated Neural Regeneration Regulation. Mol. Neurobiol. 2012, 47, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tenney, A.P.; Busch, S.A.; Horn, K.P.; Cuascut, F.X.; Liu, K.; He, Z.; Silver, J.; Flanagan, J.G. PTPσ Is a Receptor for Chondroitin Sulfate Proteoglycan, an Inhibitor of Neural Regeneration. Science 2009, 326, 592–596. [Google Scholar] [CrossRef]
- Dyck, S.M.; Alizadeh, A.; Santhosh, K.T.; Proulx, E.H.; Wu, C.; Karimi-Abdolrezaee, S. Chondroitin Sulfate Proteoglycans Negatively Modulate Spinal Cord Neural Precursor Cells by Signaling Through LAR and RPTPσ and Modulation of the Rho/ROCK Pathway. Stem Cells 2015, 33, 2550–2563. [Google Scholar] [CrossRef]
- Wang, H.; Song, G.; Chuang, H.-Y.; Chiu, C.; Abd-Elmaksoud, A.; Ye, Y.; Zhao, L. Portrait of Glial Scar in Neurological Diseases. Int. J. Immunopathol. Pharmacol. 2018, 31, 2058738418801406. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Gallo, V. The Diversity and Disparity of the Glial Scar. Nat. Neurosci. 2017, 21, 9–15. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the Glial Scar for Spinal Cord Repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef]
- Anjum, A.; Yazid, M.D.; Daud, M.; Idris, J.; Ng, M.H.; Naicker, A.S.; Ismail, O.H.R.; Kumar, R.; Lokanathan, Y. Spinal Cord Injury: Pathophysiology, Multimolecular Interactions, and Underlying Recovery Mechanisms. Int. J. Mol. Sci. 2020, 21, 7533. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, Z.; Pang, M.; Luo, Q.; Huang, C.; He, W.; Liu, B.; Rong, L. Wallerian Degeneration Assessed by Multi-Modal Magnetic Resonance Imaging of Cervical Spinal Cord Is Associated With Neurological Impairment After Spinal Cord Injury. J. Neurotrauma 2024, 41, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, B.; Wong, W.M.; Lu, P.; Wu, W.; Xu, X. Glial and Axonal Responses in Areas of Wallerian Degeneration of the Corticospinal and Dorsal Ascending Tracts after Spinal Cord Dorsal Funiculotomy. Neuropathology 2009, 29, 230–241. [Google Scholar] [CrossRef]
- García, E.; Rodríguez-Barrera, R.; Mondragón-Caso, J.; Carvajal, H.G.; Ibarra, A. Pharmacological and Nonpharmacological Therapeutic Strategies Based on the Pathophysiology of Acute and Chronic Spinal Cord Injury. In Essentials of Spinal Cord Injury Medicine; InTech eBooks; Intech Open: London, UK, 2018. [Google Scholar]
- Fehlings, M.G.; Evaniew, N.; Kurpad, S.N.; Skelly, A.C.; Tetreault, L.; Kwon, B.K. In Reply: AO Spine & Praxis Spinal Cord Institute Clinical Practice Guidelines for the Management of Acute Spinal Cord Injury. Neurosurgery 2025, 96, e58–e60. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.; Hachem, L.D.; Evaniew, N.; Ganau, M.; McKenna, S.L.; Neal, C.J.; Nagoshi, N.; Rahimi-Movaghar, V.; Aarabi, B.; et al. An Update of a Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury: Recommendations on the Role and Timing of Decompressive Surgery. Glob. Spine J. 2024, 14, 174S–186S. [Google Scholar] [CrossRef]
- Weinberg, J.A.; Farber, S.H.; Kalamchi, L.D.; Brigeman, S.; Bohl, M.A.; Varda, B.; Sioda, N.A.; Radosevich, J.; Chapple, K.; Snyder, L.A. Mean Arterial Pressure Maintenance Following Spinal Cord Injury: Does Meeting the Target Matter? J. Trauma Acute Care Surg. 2020, 90, 97–106. [Google Scholar] [CrossRef]
- Caruso, M.; Daugherty, M.; Moody, S.; Falcone, R.A.; Bierbrauer, K.S.; Geis, G.L. Lessons Learned from Administration of High-Dose Methylprednisolone Sodium Succinate for Acute Pediatric Spinal Cord Injuries. J. Neurosurg. Pediatr. 2017, 20, 567–574. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Wilson, J.R.; Tetreault, L.; Aarabi, B.; Anderson, P.A.; Arnold, P.M.; Brodke, D.S.; Burns, A.S.; Chiba, K.; Dettori, J.R.; et al. A Clinical Practice Guideline for the Management of Patients with Acute Spinal Cord Injury: Recommendations on the Use of Methylprednisolone Sodium Succinate. Glob. Spine J. 2017, 7, 203S–211S. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Imajo, Y.; Funaba, M.; Ikeda, H.; Nishida, N.; Sakai, T. Current Concepts of Biomaterial Scaffolds and Regenerative Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 2528. [Google Scholar]
- Costăchescu, B.; Niculescu, A.-G.; Dabija, M.G.; Teleanu, R.I.; Grumezescu, A.M.; Eva, L. Novel Strategies for Spinal Cord Regeneration. Int. J. Mol. Sci. 2022, 23, 4552. [Google Scholar] [CrossRef]
- Fischer, G.; Bättig, L.; Stienen, M.N.; Curt, A.; Fehlings, M.G.; Hejrati, N. Advancements in Neuroregenerative and Neuroprotective Therapies for Traumatic Spinal Cord Injury. Front. Neurosci. 2024, 18, 1372920. [Google Scholar] [CrossRef]
- Nguyen, A.; Chow, D.S.; Wu, L.; Teng, Y.; Sarkar, M.; Toups, E.G.; Harrop, J.S.; Schmitt, K.M.; Johnson, M.M.; Guest, J.D.; et al. Longitudinal Impact of Acute Spinal Cord Injury on Clinical Pharmacokinetics of Riluzole, a Potential Neuroprotective Agent. J. Clin. Pharmacol. 2021, 61, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Cotinat, M.; Boquet, I.; Ursino, M.; Brocard, C.; Jouve, É.; Alberti, C.; Bensoussan, L.; Viton, J.; Brocard, F.; Blin, O. Riluzole for Treating Spasticity in Patients with Chronic Traumatic Spinal Cord Injury: Study Protocol in the Phase Ib/Iib Adaptive Multicenter Randomized Controlled RILUSCI Trial. PLoS ONE 2023, 18, e0276892. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Moghaddamjou, A.; Harrop, J.S.; Stanford, R.; Ball, J.; Aarabi, B.; Freeman, B.D.; Guest, J.D.; Kurpad, S.N.; Schuster, J.M.; et al. 186 A Multi-Center, Randomized, Placebo-Controlled, Double-Blinded Trial of Efficacy and Safety of Riluzole in Acute Spinal Cord Injury Study (RISCIS). Neurosurgery 2023, 69, 28–29. [Google Scholar] [CrossRef]
- Chow, D.S.-L.; Nguyen, A.; Park, J.; Wu, L.; Toups, E.G.; Harrop, J.S.; Guest, J.D.; Schmitt, K.M.; Aarabi, B.; Fehlings, M.G.; et al. Riluzole in Spinal Cord Injury Study (RISCIS)–Pharmacokinetic (PK) Sub-Study: An Analysis of Pharmacokinetics, Pharmacodynamics, and Impact on Axonal Degradation of Riluzole in Patients With Traumatic Cervical Spinal Cord Injury Enrolled in the RISCIS Phase III Randomized Controlled Trial. J. Neurotrauma 2023, 40, 1889–1906. [Google Scholar] [CrossRef]
- Kim, H.N.; McCrea, M.R.; Li, S. Advances in Molecular Therapies for Targeting Pathophysiology in Spinal Cord Injury. Expert Opin. Ther. Targets 2023, 27, 171–187. [Google Scholar] [CrossRef]
- Nagoshi, N.; Nakashima, H.; Fehlings, M.G. Riluzole as a Neuroprotective Drug for Spinal Cord Injury: From Bench to Bedside. Molecules 2015, 20, 7775–7789. [Google Scholar] [CrossRef]
- Bassani, T.B.; Bartolomeo, C.S.; Oliveira, R.B.; Ureshino, R.P. Progestogen-Mediated Neuroprotection in Central Nervous System Disorders. Neuroendocrinology 2022, 113, 14–35. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.J.; Salgado-Ceballos, H.; Castillo-Mendieta, T.; Sánchez-Torres, S.; Freyermuth-Trujillo, X.; Orozco-Barrios, C.E.; Orozco-Suárez, S.; Feria-Romero, I.A.; Pinto-Almazán, R.; et al. Evaluating Sex Steroid Hormone Neuroprotection in Spinal Cord Injury in Animal Models: Is It Promising in the Clinic? Biomedicines 2024, 12, 1478. [Google Scholar] [CrossRef]
- Figueroa, J.D.; Cordero, K.; llán, M.S.; León, M.D. Dietary Omega-3 Polyunsaturated Fatty Acids Improve the Neurolipidome and Restore the DHA Status While Promoting Functional Recovery after Experimental Spinal Cord Injury. J. Neurotrauma 2013, 30, 853–868. [Google Scholar] [CrossRef]
- He, N.; Shen, G.; Jin, X.; Li, H.; Wang, J.; Xu, L.; Chen, J.; Cao, X.; Fu, C.; Shi, D.; et al. Resveratrol Suppresses Microglial Activation and Promotes Functional Recovery of Traumatic Spinal Cord via Improving Intestinal Microbiota. Pharmacol. Res. 2022, 183, 106377. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Shao, D.; Li, H.; Huang, X.; Yang, G.; Xu, B.; Niu, H. Resveratrol Improves Neurological Outcome and Neuroinflammation Following Spinal Cord Injury through Enhancing Autophagy Involving the AMPK/mTOR Pathway. Mol. Med. Rep. 2018, 18, 2237–2244. [Google Scholar] [CrossRef] [PubMed]
- Darehbagh, R.R.; Seyedoshohadaei, S.A.; Ramezani, R.; Rezaei, N. Stem Cell Therapies for Neurological Disorders: Current Progress, Challenges, and Future Perspectives. Eur. J. Med. Res. 2024, 29, 1–20. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.J.; Khazaei, M.R.; Badhiwala, J.H.; Gilbert, E.; Kooy, D.; Morshead, C.M.; Tator, C.H.; Fehlings, M.G. The Leading Edge: Emerging Neuroprotective and Neuroregenerative Cell-Based Therapies for Spinal Cord Injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.F.; Coelho-Cruz, B.; de Sousa, B.M.; Correia, P.D.; David, N.; Rocha, C.; Almeida, R.D.; Cunha, M.; Baptista, A.A.M.; Vieira, S.I. Cell Therapies for Spinal Cord Injury: A Review of the Clinical Trials and Cell-Type Therapeutic Potential. Brain 2023, 146, 2672–2693. [Google Scholar] [CrossRef] [PubMed]
- Ghane, N.; Beigi, M.; Labbaf, S.; Nasr-Esfahani, M.H.; Kiani, A. Design of Hydrogel-Based Scaffolds for the Treatment of Spinal Cord Injuries. J. Mater. Chem. B 2020, 8, 10712–10738. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, H.; Chao, X.; Xu, W.; Liu, Y.; Ling, G.; Zhang, P. Multimodal Therapy Strategies Based on Hydrogels for the Repair of Spinal Cord Injury. Mil. Med. Res. 2022, 9, 16. [Google Scholar] [CrossRef]
- Cai, M.; Chen, L.; Wang, T.; Liang, Y.; Zhao, J.; Zhang, X.; Li, Z.; Wu, H. Hydrogel Scaffolds in the Treatment of Spinal Cord Injury: A Review. Front. Neurosci. 2023, 17, 1211066. [Google Scholar] [CrossRef]
- Minassian, K.; Perret, I.; Hofstoetter, U.S. Epidural and Transcutaneous Spinal Cord Stimulation Strategies for Motor Recovery After Spinal Cord Injury. In Neuroprosthetics and Brain-Computer Interfaces in Spinal Cord Injury; Springer eBooks; Springer Nature: Berlin/Heidelberg, Germany, 2021; p. 167. [Google Scholar]
- Porceban, M.M.; Angelin, L.G.; Alonso, A.C.; Prota, C.; de Abreu, C.P.C.; Ribeiro, L.H.S.; Santos, J.G.B.; de Souza, J.C.; de Biase, M.E.M.; Sitthinamsuwan, B.; et al. 481 Spinal Cord Stimulator Promotes Neuroplasticity in Patients with Severe Spinal Cord Injury. Neurosurgery 2024, 70, 147. [Google Scholar] [CrossRef]
- Beck, L.A.; Veith, D.D.; Linde, M.; Gill, M.L.; Calvert, J.S.; Grahn, P.J.; Garlanger, K.; Husmann, D.A.; Lavrov, I.; Sayenko, D.G.; et al. Impact of Long-Term Epidural Electrical Stimulation Enabled Task-Specific Training on Secondary Conditions of Chronic Paraplegia in Two Humans. J. Spinal Cord Med. 2020, 44, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Rowald, A.; Komi, S.; Demesmaeker, R.; Baaklini, E.; Hernandez-Charpak, S.D.; Paoles, E.; Montanaro, H.; Cassarà, A.M.; Becce, F.; Lloyd, B.; et al. Activity-Dependent Spinal Cord Neuromodulation Rapidly Restores Trunk and Leg Motor Functions after Complete Paralysis. Nat. Med. 2022, 28, 260–271. [Google Scholar] [CrossRef]
- Wagner, F.B.; Mignardot, J.-B.; Goff-Mignardot, C.G.L.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted Neurotechnology Restores Walking in Humans with Spinal Cord Injury. Nature 2018, 563, 65–71. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Straaten, M.G.V.; Drubach, D.I.; et al. Neuromodulation of Lumbosacral Spinal Networks Enables Independent Stepping after Complete Paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Velumian, A.A. The Impact of Spinal Cord Neuromodulation on Restoration of Walking Ability After Spinal Cord Injury. Neurospine 2022, 19, 244–245. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Z.; Wang, D.; Aierxi, M.; Ma, Z.; Wang, Y. Oxidative Stress Following Spinal Cord Injury: From Molecular Mechanisms to Therapeutic Targets. J. Neurosci. Res. 2023, 101, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial Formation of Reactive Oxygen Species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Zhao, X.; Lu, M.; Yuan, D.; Xu, D.-E.; Yao, P.-P.; Ji, W.; Chen, H.; Liu, W.; Yan, C.-X.; Xia, Y.; et al. Mitochondrial Dysfunction in Neural Injury. Front. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Sawhney, S.K. Oxidative Stress, Mitochondrial Dysfunction and Neuro-Degenerative Diseases: A Review. Int. J. Res. Dev. Pharm. Life Sci. 2019, 8, 1–5. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. de ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxidative Med. Cell. Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Bailey, W.M.; McVicar, A.L.; Gensel, J.C. Age Increases Reactive Oxygen Species Production in Macrophages and Potentiates Oxidative Damage after Spinal Cord Injury. Neurobiol. Aging 2016, 47, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species—Sources, Functions, Oxidative Damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Imlay, J.A. Pathways of Oxidative Damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef]
- Cohen, G. Enzymatic/Nonenzymatic Sources of Oxyradicals and Regulation of Antioxidant Defensesa. Ann. N. Y. Acad. Sci. 1994, 738, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Salgado, P.; Melín, V.; Contreras, D.; Moreno, Y.; Mansilla, H.D. Fenton Reaction Driven By Iron Ligands. J. Chil. Chem. Soc. 2013, 58, 2096–2101. [Google Scholar] [CrossRef]
- Urbański, N.K.; Beresewicz, A. Generation of *OH Initiated by Interaction of Fe2+ and Cu+ with Dioxygen; Comparison with the Fenton Chemistry. Acta Biochim. Pol. 2000, 47, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Kheya, I.; Asaduzzaman, S.I.; Maniha, S.; Fayz, A.; Zakaria, A.D.; Fayz, A.; Zakaria, A.D.; Noor, R. A Review on the Possible Leakage of Electrons through the Electron Transport Chain within Mitochondria. J. Biomed. Res. Environ. Sci. 2020, 1, 105–113. [Google Scholar] [CrossRef]
- Dröse, S.; Brandt, U. Molecular Mechanisms of Superoxide Production by the Mitochondrial Respiratory Chain. Adv. Exp. Med. Biol. 2012, 748, 145–169. [Google Scholar] [PubMed]
- Tian, H.; Huang, B.; Nie, H.; Chen, X.; Zhou, Y.; Yang, T.; Cheng, S.; Mei, Z.; Ge, J. The Interplay between Mitochondrial Dysfunction and Ferroptosis during Ischemia-Associated Central Nervous System Diseases. Brain Sci. 2023, 13, 1367. [Google Scholar] [CrossRef]
- Lebiedzińska, M.; Suski, J.M.; Bonora, M.; Pakuła, B.; Pinton, P.; Duszyński, J.; Jakubek-Olszewska, P.; Więckowski, M.R. The Relation Between Mitochondrial Membrane Potential and Reactive Oxygen Species Formation. In Methods and Protocols; Springer: New York, NY, USA, 2024; p. 133. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Shang, Z.; Zhao, L. Mechanism and Prospects of Mitochondrial Transplantation for Spinal Cord Injury Treatment. Stem Cell Res. Ther. 2024, 15, 457. [Google Scholar] [CrossRef]
- O’Brien, L.C.; Gorgey, A.S. Skeletal Muscle Mitochondrial Health and Spinal Cord Injury. World J. Orthop. 2016, 7, 628–637. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, A.; Sheline, C.T.; Hyrc, K.; Yang, A.; Goldberg, M.P.; Choi, D.W.; Yu, S. Apoptotic Insults Impair Na+, K+-ATPase Activity as a Mechanism of Neuronal Death Mediated by Concurrent ATP Deficiency and Oxidant Stress. J. Cell Sci. 2003, 116, 2099–2110. [Google Scholar] [CrossRef]
- Castro, J.; Ruminot, I.; Porras, O.; Flores, C.M.; Hermosilla, T.; Verdugo, E.; Venegas, F.; Härtel, S.; Michea, L.; Barros, L.F. ATP Steal between Cation Pumps: A Mechanism Linking Na+ Influx to the Onset of Necrotic Ca2+ Overload. Cell Death Differ. 2006, 13, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Mitochondria, Sodium, and Calcium in Neuronal Dysfunction. In Mitochondrial Dysfunction in Neurodegenerative Disorders; Springer eBooks; Springer Nature: Berlin/Heidelberg, Germany, 2011; p. 113. [Google Scholar]
- Bogdanova, A.; Petrushanko, I.; Boldyrev, A.; Gassmann, M. Oxygen- and Redox-Induced Regulation of the Na/K ATPase. Curr. Enzym. Inhib. 2006, 2, 37–59. [Google Scholar] [CrossRef]
- Brookes, P.S.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S. Calcium, ATP, and ROS: A Mitochondrial Love-Hate Triangle. AJP Cell Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Mironova, G.D.; Pavlov, E.V. Mitochondrial Cyclosporine A-Independent Palmitate/Ca2+-Induced Permeability Transition Pore (PA-mPT Pore) and Its Role in Mitochondrial Function and Protection against Calcium Overload and Glutamate Toxicity. Cells 2021, 10, 125. [Google Scholar] [CrossRef]
- Zhou, Y.; Jing, S.; Liu, S.; Shen, X.; Cai, L.; Zhu, C.; Zhao, Y.; Pang, M. Double-Activation of Mitochondrial Permeability Transition Pore Opening via Calcium Overload and Reactive Oxygen Species for Cancer Therapy. J. Nanobiotechnology 2022, 20, 188. [Google Scholar] [CrossRef]
- Peng, T.; Jou, M. Oxidative Stress Caused by Mitochondrial Calcium Overload. Ann. N. Y. Acad. Sci. 2010, 1201, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ferdinándy, P. Peroxynitrite: Just an Oxidative/Nitrosative Stressor or a Physiological Regulator as Well? Br. J. Pharmacol. 2006, 148, 1–3. [Google Scholar] [CrossRef]
- Möller, M.N.; Denicola, A. Diffusion of Peroxynitrite, Its Precursors, and Derived Reactive Species, and the Effect of Cell Membranes. Redox Biochem. Chem. 2024, 9, 100033. [Google Scholar] [CrossRef]
- de la Lastra, J.M.P.; Andrés, C.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Catalá, Á.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Wang, J.A.; Bosken, J.M.; Singh, I.N. Lipid Peroxidation in Brain or Spinal Cord Mitochondria after Injury. J. Bioenerg. Biomembr. 2015, 48, 169–174. [Google Scholar] [CrossRef]
- Perluigi, M.; Coccia, R.; Butterfield, D.A. 4-Hydroxy-2-Nonenal, a Reactive Product of Lipid Peroxidation, and Neurodegenerative Diseases: A Toxic Combination Illuminated by Redox Proteomics Studies. Antioxid. Redox Signal. 2011, 17, 1590–1609. [Google Scholar] [CrossRef] [PubMed]
- Pizzimenti, S.; Ciamporcero, E.; Daga, M.; Pettazzoni, P.; Arcaro, A.; Cetrangolo, G.; Minelli, R.; Dianzani, C.; Lepore, A.; Gentile, F.; et al. Interaction of Aldehydes Derived from Lipid Peroxidation and Membrane Proteins. Front. Physiol. 2013, 4, 242. [Google Scholar] [CrossRef]
- Taso, O.V.; Philippou, A.; Moustogiannis, A.; Zevolis, E.; Koutsilieris, M. Lipid Peroxidation Products and Their Role in Neurodegenerative Diseases. Ann. Res. Hosp. 2019, 3, 2. [Google Scholar] [CrossRef]
- Allowitz, K.V.; Taylor, J.; Harames, K.; Yoo, J.H.; Baloch, O.A.; Ramana, K.V. Oxidative Stress-Mediated Lipid Peroxidation-Derived Lipid Aldehydes in the Pathophysiology of Neurodegenerative Diseases. Curr. Neuropharmacol. 2024, 23, 671–685. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.H.; Anderson, D.K.; Panter, S.S.; Hallaway, P.E.; Eaton, J.W. Hemoglobin Potentiates Central Nervous System Damage. J. Clin. Investig. 1987, 79, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Nasudivar, S.L.; Pedrera, L.; García-Sáez, A.J. Iron-Induced Lipid Oxidation Alters Membrane Mechanics Favoring Permeabilization. Langmuir 2024, 40, 25061–25068. [Google Scholar] [CrossRef]
- Recknagel, R.O.; Glende, E.A.; Britton, R.S. Free Radical Damage and Lipid Peroxidation. In Hepatotoxicology; CRC Press eBooks; CRC Press: Boca Raton, FL, USA, 2020; p. 401. [Google Scholar]
- Winkler, E.A.; Sengillo, J.D.; Sagare, A.P.; Zhao, Z.; Ma, Q.; Zúñiga, E.; Wang, Y.; Zhong, Z.; Sullivan, J.S.; Griffin, J.H.; et al. Blood–Spinal Cord Barrier Disruption Contributes to Early Motor-Neuron Degeneration in ALS-Model Mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1035–E1042. [Google Scholar] [CrossRef]
- Couillard-Després, S.; Bieler, L.; Vogl, M. Pathophysiology of Traumatic Spinal Cord Injury. In Neurological Aspects of Spinal Cord Injury; Springer eBooks; Springer Nature: Berlin/Heidelberg, Germany, 2017; p. 503. [Google Scholar]
- Jin, L.; Li, J.; Wang, K.; Xia, W.; Zhu, Z.; Wang, C.; Li, X.; Liu, H. Blood–Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2020, 38, 1203–1224. [Google Scholar] [CrossRef]
- Kumar, S.; Theis, T.; Tschang, M.; Nagaraj, V.; Berthiaume, F. Reactive Oxygen Species and Pressure Ulcer Formation after Traumatic Injury to Spinal Cord and Brain. Antioxidants 2021, 10, 1013. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, B.; Gong, G.; Yin, J. ROS: Executioner of Regulating Cell Death in Spinal Cord Injury. Front. Immunol. 2024, 15, 1330678. [Google Scholar] [CrossRef]
- von Leden, R.E.; Khayrullina, G.; Moritz, K.E.; Byrnes, K.R. Age Exacerbates Microglial Activation, Oxidative Stress, Inflammatory and NOX2 Gene Expression, and Delays Functional Recovery in a Middle-Aged Rodent Model of Spinal Cord Injury. J. Neuroinflammation 2017, 14, 161. [Google Scholar] [CrossRef]
- Khayrullina, G.; Bermudez, S.; Hopkins, D.; Yauger, Y.J.; Byrnes, K.R. Differential Effects of NOX2 and NOX4 Inhibition after Rodent Spinal Cord Injury. PLoS ONE 2023, 18, e0281045. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced Oxidation Protein Products Induce Microglia-Mediated Neuroinflammation via MAPKs-NF-κB Signaling Pathway and Pyroptosis after Secondary Spinal Cord Injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar] [CrossRef]
- Smith, A.N.; Shaughness, M.; Collier, S.; Hopkins, D.; Byrnes, K.R. Therapeutic Targeting of Microglia Mediated Oxidative Stress after Neurotrauma. Front. Med. 2022, 9, 1034692. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive Oxygen Species in Macrophages: Sources and Targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Haupt, M.; Gerner, S.T.; Doeppner, T.R. The Dual Role of Microglia in Ischemic Stroke and Its Modulation via Extracellular Vesicles and Stem Cells. Neuroprotection 2024, 2, 4–15. [Google Scholar] [CrossRef]
- Nauseef, W.M. Myeloperoxidase in Human Neutrophil Host Defence. Cell. Microbiol. 2014, 16, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A Key Regulator of Neutrophil Oxidant Production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase. Proc. Assoc. Am. Physicians 1999, 111, 383. [Google Scholar] [CrossRef]
- Anaya-Fernández, R.; Anaya-Prado, R.; Anaya-Fernandez, M.M.; Guerrero-Palomera, M.A.; Garcia-Ramirez, I.F.; Gonzalez-Martinez, D.; Azcona-Ramirez, C.C.; Guerrero-Palomera, C.S.; Garcia-Perez, C.; Tenorio-Gonzalez, B.; et al. Oxidative Stress in Cerebral Ischemia/Reperfusion Injury. OBM Neurobiol. 2024, 8, 1–15. [Google Scholar] [CrossRef]
- Douzinas, E.E.; Apeiranthitis, A. Basic Mechanisms of Ischemia/Reperfusion Injury Leading to Cellular and Tissue Damage: Therapeutic Implications. In Modulation of Oxidative Stress in Heart Disease; Springer eBooks; Springer Nature: Berlin/Heidelberg, Germany, 2019; p. 645. [Google Scholar]
- Granger, D.N. Role of Xanthine Oxidase and Granulocytes in Ischemia-Reperfusion Injury. AJP Heart Circ. Physiol. 1988, 255, H1269–H1275. [Google Scholar] [CrossRef]
- Nishino, T. The Conversion of Xanthine Dehydrogenase to Xanthine Oxidase and the Role of the Enzyme in Reperfusion Injury1. J. Biochem. 1994, 116, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Reeg, S.; Grune, T. Protein Oxidation in Toxicology. In Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; p. 81. [Google Scholar]
- Davies, M.J. Protein Oxidation and Peroxidation. Biochem. J. 2016, 473, 805. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free Radical-Mediated Oxidation of Free Amino Acids and Amino Acid Residues in Proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, V.B. Superoxide Dismutase Dysregulation Undermines Endogenous Antioxidant System and Promote Retinal Neurodegenerative Pathology. J. Biochem. Mol. Biol. Res. 2016, 2, 131–133. [Google Scholar] [CrossRef][Green Version]
- Skoryk, O.D.; Horila, M.V. Oxidative Stress and Disruption of the Antioxidant Defense System as Triggers of Diseases. Regul. Mech. Biosyst. 2023, 14, 665–672. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Babu, K.N.; Periyasamy, L.; Stanley, J.A.; Ramachandran, I.; Kumaran, R.; Muthusami, S. Targeting the Antioxidant Enzymes for the Treatment of Reactive Oxygen Species (ROS)-Induced Cancer. In Handbook of Oxidative Stress in Cancer: Therapeutic Aspects; Springer: Singapore, 2022; p. 3857. [Google Scholar]
- Bodnar, Y.; Lillig, C.H. Cysteinyl and Methionyl Redox Switches: Structural Prerequisites and Consequences. Redox Biol. 2023, 65, 102832. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Moskovitz, J.; Levine, R.L. Oxidation of Methionine Residues of Proteins: Biological Consequences. Antioxid. Redox Signal. 2003, 5, 577–582. [Google Scholar] [CrossRef]
- Levine, R.L.; Moskovitz, J.; Stadtman, E.R. Oxidation of Methionine in Proteins: Roles in Antioxidant Defense and Cellular Regulation. IUBMB Life 2000, 50, 301. [Google Scholar] [CrossRef]
- Lim, J.M.; Kim, G.; Levine, R.L. Methionine in Proteins: It’s Not Just for Protein Initiation Anymore. Neurochem. Res. 2018, 44, 247–257. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine Residues as Endogenous Antioxidants in Proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.L.; Petropoulos, I.; Friguet, B. The Oxidized Protein Repair Enzymes Methionine Sulfoxide Reductases and Their Roles in Protecting against Oxidative Stress, in Ageing and in Regulating Protein Function. Antioxidants 2018, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M. Protein Carbonylation: Molecular Mechanisms, Biological Implications, and Analytical Approaches. Free Radic. Res. 2020, 55, 307–320. [Google Scholar] [CrossRef]
- Pineda-Alemán, R.; Alvíz-Amador, A.; Galindo-Murillo, R.; Pérez-González, H.; Rodríguez-Cavallo, E.; Méndez-Cuadro, D. Cysteine Carbonylation with Reactive Carbonyl Species from Lipid Peroxidation Induce Local Structural Changes on Thioredoxin Active Site. J. Mol. Graph. Model. 2023, 124, 108533. [Google Scholar] [CrossRef]
- Cadet, J.; Angelov, D.; Wagner, J.R. Hydroxyl Radical Is Predominantly Involved in Oxidatively Generated Base Damage to Cellular DNA Exposed to Ionizing Radiation. Int. J. Radiat. Biol. 2022, 98, 1684–1690. [Google Scholar] [CrossRef]
- Cadet, J.; Berger, M.; Morin, B.; Raoul, S.; Wagner, J.R. Oxidative Damage to DNA. In Analysis of Free Radicals in Biological Systems; Birkhäuser Basel eBooks; Birkhäuser: Basel, Switzerland, 1995; p. 51. [Google Scholar]
- D’Errico, M.; Parlanti, E.; Dogliotti, E. Mechanism of Oxidative DNA Damage Repair and Relevance to Human Pathology. Mutat. Res./Rev. Mutat. Res. 2007, 659, 4–14. [Google Scholar] [CrossRef]
- Bryant-Friedrich, A. Fate of DNA Sugar Radicals. In Advances in Molecular Toxicology; Elsevier BV: Amsterdam, The Netherlands, 2010; p. 127. [Google Scholar]
- Fleming, A.M.; Burrows, C.J. Chemistry of ROS-Mediated Oxidation to the Guanine Base in DNA and Its Biological Consequences. Int. J. Radiat. Biol. 2021, 98, 452–460. [Google Scholar] [CrossRef]
- Altieri, F.; Grillo, C.; Maceroni, M.; Chichiarelli, S. DNA Damage and Repair: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Dizdaroğlu, M.; Jaruga, P. Oxidatively Induced DNA Damage and Cancer. J. Mol. Biomark. Diagn. 2012, S:2, 002. [Google Scholar] [CrossRef]
- Poetsch, A.R. The Genomics of Oxidative DNA Damage, Repair, and Resulting Mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The Mitochondrial Response to DNA Damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Pawłowska, E.; Szczepańska, J.; Jabłkowska, A.; Błasiak, J. Role of Mitochondrial DNA Damage in ROS-Mediated Pathogenesis of Age-Related Macular Degeneration (AMD). Int. J. Mol. Sci. 2019, 20, 2374. [Google Scholar] [CrossRef]
- Kang, D.; Hamasaki, N. Mitochondrial Oxidative Stress and Mitochondrial DNA. Clin. Chem. Lab. Med. 2003, 41, 12811288. [Google Scholar] [CrossRef]
- Akhmedov, A.; Marín-García, J. Mitochondrial DNA Maintenance: An Appraisal. Mol. Cell. Biochem. 2015, 409, 283–305. [Google Scholar] [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Grechko, A.V.; Yet, S.; Sobenin, I.A.; Orekhov, A.N. Neurodegenerative Diseases Associated with Mitochondrial DNA Mutations. Curr. Pharm. Des. 2019, 26, 103–109. [Google Scholar] [CrossRef]
- Cha, M.-Y.; Kim, D.K.; Mook-Jung, I. The Role of Mitochondrial DNA Mutation on Neurodegenerative Diseases. Exp. Mol. Med. 2015, 47, e150. [Google Scholar] [CrossRef]
- Wei, Y.; Lee, H. Oxidative Stress, Mitochondrial DNA Mutation, and Impairment of Antioxidant Enzymes in Aging. Exp. Biol. Med. 2002, 227, 671–682. [Google Scholar] [CrossRef]
- Wei, Y. Oxidative Stress and Mitochondrial DNA Mutations in Human Aging. Exp. Biol. Med. 1998, 217, 53–63. [Google Scholar] [CrossRef]
- Płoszaj, T.; Robaszkiewicz, A.; Witas, H.W. Oxidative Damage of Mitochondrial DNA: The Result or Consequence of Enhanced Generation of Reactive Oxygen Species. Postep. Biochem. 2010, 56, 139–146. [Google Scholar]
- Aguilar, T.A.F.; Navarro, B.C.H.; Pérez, J.A.M. Endogenous Antioxidants: A Review of Their Role in Oxidative Stress. In A Master Regulator of Oxidative Stress—The Transcription Factor Nrf2; InTech eBooks; Intech Open: London, UK, 2016. [Google Scholar]
- Stewart, A.N.; Glaser, E.P.; Mott, C.A.; Bailey, W.M.; Sullivan, P.G.; Patel, S.P.; Gensel, J.C. Advanced Age and Neurotrauma Diminish Glutathione and Impair Antioxidant Defense after Spinal Cord Injury. J. Neurotrauma 2022, 39, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Eddaikra, A.; Eddaikra, N. Endogenous Enzymatic Antioxidant Defense and Pathologies. In Antioxidants—Benefits, Sources, Mechanisms of Action; IntechOpen eBooks; Intech Open: London, UK, 2021. [Google Scholar]
- Lucas, J.H.; Wheeler, D.G.; Guan, Z.; Suntres, Z.E.; Stokes, B.T. Effect of Glutathione Augmentation on Lipid Peroxidation after Spinal Cord Injury. J. Neurotrauma 2002, 19, 763–775. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The Antioxidant Glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The Glutathione System: A Journey from Cyanobacteria to Higher Eukaryotes. Antioxidants 2023, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Lemus, E.; Reyes, J.S.; Figueroa, J.D.; Davies, M.J.; López-Alarcón, C. The Enzymes of the Oxidative Phase of the Pentose Phosphate Pathway as Targets of Reactive Species: Consequences for NADPH Production. Biochem. Soc. Trans. 2023, 51, 2173–2187. [Google Scholar] [CrossRef]
- Cano, M.; Datta, S.; Wang, L.; Liu, T.; Flores-Bellver, M.; Sachdeva, M.M.; Sinha, D.; Handa, J.T. Nrf2 Deficiency Decreases NADPH from Impaired IDH Shuttle and Pentose Phosphate Pathway in Retinal Pigmented Epithelial Cells to Magnify Oxidative Stress-induced Mitochondrial Dysfunction. Aging Cell 2021, 20, e13444. [Google Scholar] [CrossRef] [PubMed]
- TeSlaa, T.; Ralser, M.; Fan, J.; Rabinowitz, J.D. The Pentose Phosphate Pathway in Health and Disease. Nat. Metab. 2023, 5, 275–1289. [Google Scholar] [CrossRef]
- Cherkas, A.; Holota, S.; Mdzinarashvili, T.; Gabbianelli, R.; Žarković, N. Glucose as a Major Antioxidant: When, What for and Why It Fails? Antioxidants 2020, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Neuroprotection by Glucose-6-phosphate Dehydrogenase and the Pentose Phosphate Pathway. J. Cell. Biochem. 2019, 120, 14285–14295. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, N.; Xiong, Y.; Zhang, J.; Zhao, D.; Yin, Y.; Song, L.; Yin, Y.; Wang, J.; Luan, X.; et al. Downregulated Recycling Process but Not De Novo Synthesis of Glutathione Limits Antioxidant Capacity of Erythrocytes in Hypoxia. Oxidative Med. Cell. Longev. 2020, 2020, 7834252. [Google Scholar] [CrossRef] [PubMed]
- Boas, S.M.; Joyce, K.L.; Cowell, R.M. The NRF2-Dependent Transcriptional Regulation of Antioxidant Defense Pathways: Relevance for Cell Type-Specific Vulnerability to Neurodegeneration and Therapeutic Intervention. Antioxidants 2021, 11, 8. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE Signaling: Towards Specific Regulation. Life Sci. 2021, 291, 120111. [Google Scholar] [CrossRef] [PubMed]
- Zgórzyńska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Zhao, W.; Gasterich, N.; Clarner, T.; Voelz, C.; Behrens, V.; Beyer, C.; Fragoulis, A.; Zendedel, A. Astrocytic Nrf2 Expression Protects Spinal Cord from Oxidative Stress Following Spinal Cord Injury in a Male Mouse Model. J. Neuroinflamma. 2022, 19, 134. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative Stress: The Mitochondria-Dependent and Mitochondria-Independent Pathways of Apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef]
- McCubrey, J.A.; LaHair, M.M.; Franklin, R.A. Reactive Oxygen Species-Induced Activation of the MAP Kinase Signaling Pathways. Antioxid. Redox Signal. 2006, 8, 1775–1789. [Google Scholar] [CrossRef]
- Radnaa, E.; Richardson, L.; Goldman, B.; Burks, J.K.; Baljinnyam, T.; Vora, N.; Zhang, H.; Bonney, E.A.; Han, A.; Menon, R. Stress Signaler P38 Mitogen-Activated Kinase Activation: A Cause for Concern? Clin. Sci. 2022, 136, 1591–1614. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.-K.; Kim, N.; Chung, H.; Kang, D.G.; Pae, H. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef] [PubMed]
- Corre, I.; Paris, F.; Huot, J. The P38 Pathway, a Major Pleiotropic Cascade That Transduces Stress and Metastatic Signals in Endothelial Cells. Oncotarget 2017, 8, 55684–55714. [Google Scholar] [CrossRef]
- Christman, J.W.; Blackwell, T.S.; Juurlink, B.H.J. Redox Regulation of Nuclear Factor Kappa B: Therapeutic Potential for Attenuating Inflammatory Responses. Brain Pathol. 2000, 10, 153–162. [Google Scholar] [CrossRef]
- Kratsovnik, E.; Bromberg, Y.; Sperling, O.; Zoref-Shani, E. Oxidative Stress Activates Transcription Factor NF-kB-Mediated Protective Signaling in Primary Rat Neuronal Cultures. J. Mol. Neurosci. 2005, 26, 27. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Garcia, M.D.; Plaza-Díaz, J.; Gómez-Llorente, C. Molecular Basis of Oxidative Stress and Inflammation. In Obesity; Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2018; p. 41. [Google Scholar]
- Vázquez-Medina, J.P. Redox Signaling and the Onset of the Inflammatory Cascade. In Immunity and Inflammation in Health and Disease; Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2017; p. 37. [Google Scholar]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ Consumption by PARP1 in Response to DNA Damage Triggers Metabolic Shift Critical for Damaged Cell Survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef]
- Martín-Guerrero, S.M.; Casado, P.; Hijazi, M.; Rajeeve, V.; Plaza-Díaz, J.; Abadía-Molina, F.; Navascués, J.; Cuadros, M.A.; Cutillas, P.R.; Martín-Oliva, D. PARP-1 Activation after Oxidative Insult Promotes Energy Stress-Dependent Phosphorylation of YAP1 and Reduces Cell Viability. Biochem. J. 2020, 477, 4491–4513. [Google Scholar] [CrossRef]
- Ying, W.; Alano, C.C.; Garnier, P.; Swanson, R.A. NAD+ as a Metabolic Link between DNA Damage and Cell Death. J. Neurosci. Res. 2004, 79, 216–223. [Google Scholar] [CrossRef]
- Huang, Q.; Shen, H. To Die or to Live: The Dual Role of Poly(ADP-Ribose) Polymerase-1 in Autophagy and Necrosis under Oxidative Stress and DNA Damage. Autophagy 2009, 5, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Strosznajder, R.P.; Jęśko, H.; Zambrzycka, A. Poly(ADP-Ribose) Polymerase: The Nuclear Target in Signal Transduction and Its Role in Brain Ischemia–Reperfusion Injury. Mol. Neurobiol. 2005, 31, 149–168. [Google Scholar] [CrossRef]
- Skaper, S.D. Poly(ADP-Ribose) Polymerase-1 in Acute Neuronal Death and Inflammation. Ann. N. Y. Acad. Sci. 2003, 993, 217. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.K.; Thiemermann, C. Polymerase and Ischemia-Reperfusion Injury. In Poly(ADP-Ribose); Springer eBooks; Springer Nature: Berlin/Heidelberg, Germany, 2008; p. 164. [Google Scholar]
- Chio, J.C.T.; Punjani, N.; Hejrati, N.; Zavvarian, M.-M.; Hong, J.; Fehlings, M.G. Extracellular Matrix and Oxidative Stress Following Traumatic Spinal Cord Injury: Physiological and Pathophysiological Roles and Opportunities for Therapeutic Intervention. Antioxid. Redox Signal. 2021, 37, 184–207. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, T.; Wang, Y.; Meng, J.; Tan, G.; Zhao, Q.; Feng, S.; Xu, L.; Pei, Q. Oxidative Stress Disrupts the Cytoskeleton of Spinal Motor Neurons. Brain Behav. 2022, 13, e2870. [Google Scholar] [CrossRef]
- Farooqui, A.A. Molecular Aspects of Spinal Cord Injury. In Neurochemical Aspects of Neurotraumatic and Neurodegenerative Diseases; Elsevier eBooks; Elsevier BV: Amsterdam, The Netherlands, 2018; p. 155. [Google Scholar]
- Villa, J.V.; Villamar, D.M.P.; Zapien, J.A.T.; Espinoza, L.B.; García, J.H.; García, R.S. Current Developments in Antioxidant Therapies for Spinal Cord Injury. In Spinal Cord Injury Therapy; Intech Open eBooks; Intech Open: London, UK, 2019. [Google Scholar]
- Garrido, M.d.R.; Silva-García, R.; García, E.; Martiñón, S.; Morales, M.; Mestre, H.; Flores-Domínguez, C.; Flores, A.; Ibarra, A. Therapeutic Window for Combination Therapy of A91 Peptide and Glutathione Allows Delayed Treatment After Spinal Cord Injury. Basic Clin. Pharmacol. Toxicol. 2012, 112, 314–318. [Google Scholar] [CrossRef]
- García, E.; Silva-García, R.; Mestre, H.; Flores, N.; Martiñón, S.; Calderón-Aranda, E.S.; Ibarra, A. Immunization with A91 Peptide or Copolymer-1 Reduces the Production of Nitric Oxide and Inducible Nitric Oxide Synthase Gene Expression after Spinal Cord Injury. J. Neurosci. Res. 2011, 90, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Martiñón, S.; García, E.; Toscano-Tejeida, D.; Flores-Romero, A.; Rodríguez-Barrera, R.; Ferrusquia, M.; Hernández-Muñoz, R.; Ibarra, A. Long-Term Production of BDNF and NT-3 Induced by A91-Immunization after Spinal Cord Injury. BMC Neurosci. 2016, 17, 42. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Kong, P.; Zhao, S.; Yang, H.; Chen, C.; Yan, J. Enhanced Expression of Neurotrophic Factors in the Injured Spinal Cord Through Vaccination With Myelin Basic Protein-Derived Peptide Pulsed Dendritic Cells. Spine 2015, 40, 95–101. [Google Scholar] [CrossRef]
- García, E.; Silva-García, R.; Flores-Romero, A.; Blancas-Espinoza, L.; Rodríguez-Barrera, R.; Ibarra, A. The Severity of Spinal Cord Injury Determines the Inflammatory Gene Expression Pattern after Immunization with Neural-Derived Peptides. J. Mol. Neurosci. 2018, 65, 190–195. [Google Scholar] [CrossRef]
- Wang, S.; Ren, D. Allicin Protects Traumatic Spinal Cord Injury through Regulating the HSP70/Akt/iNOS Pathway in Mice. Mol. Med. Rep. 2016, 14, 3086–3092. [Google Scholar] [CrossRef]
- Liu, S.; Ren, P.; Wang, G.; Yao, S.; He, X. Allicin Protects Spinal Cord Neurons from Glutamate-Induced Oxidative Stress through Regulating the Heat Shock Protein 70/Inducible Nitric Oxide Synthase Pathway. Food Funct. 2014, 6, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, B.; Liu, T. Asiaticoside Inhibits Neuronal Apoptosis and Promotes Functional Recovery After Spinal Cord Injury in Rats. J. Mol. Neurosci. 2020, 70, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Gürcan, O.; Gürçay, A.G.; Kazancı, A.; Şentürk, S.; Bodur, E.; Karaca, E.U.; Türkoğlu, Ö.F. Efficiency of an Eastern Traditional Medicine- Asiatic Acid on Traumatic Spinal Cord Injury: An Experimental Study. Turk. Neurosurg. 2017, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Kahuripour, M.; Behroozi, Z.; Rahimi, B.; Hamblin, M.R.; Ramezani, F. The Potential of Curcumin for Treating Spinal Cord Injury: A Meta-Analysis Study. Nutr. Neurosci. 2022, 26, 560–571. [Google Scholar] [CrossRef]
- Lee, S.; Cho, D.-C.; Han, I.; Kim, K. Curcumin as a Promising Neuroprotective Agent for the Treatment of Spinal Cord Injury: A Review of the Literature. Neurospine 2022, 19, 249–261. [Google Scholar] [CrossRef]
- Razavi, S.M.; Khayatan, D.; Arab, Z.N.; Hosseini, Y.; Khanahmadi, M.; Momtaz, S.; Jamialahmadi, T.; Johnston, T.P.; Abdolghaffari, A.H.; Sahebkar, A. Protective Effects of Curcumin against Spinal Cord Injury. JOR Spine 2024, 7, e1364. [Google Scholar] [CrossRef]
- Nowacka, A.; Ziółkowska, E.; Smuczyński, W.; Bożiłow, D.; Śniegocki, M. Potential of Curcumin and Its Analogs in Glioblastoma Therapy. Antioxidants 2025, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huo, X.; Liu, X. “mTOR Signaling Pathway”: A Potential Target of Curcumin in the Treatment of Spinal Cord Injury. BioMed Res. Int. 2017, 2017, 1634801. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yao, S.; Li, H.-R.; Meng, Z.; Sun, X. Curcumin Promotes Functional Recovery and Inhibits Neuronal Apoptosis after Spinal Cord Injury through the Modulation of Autophagy. J. Spinal Cord Med. 2019, 44, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Botchway, B.O.A.; Liu, X. Curcumin Can Activate the Nrf2/HO-1 Signaling Pathway and Scavenge Free Radicals in Spinal Cord Injury Treatment. Neurorehabilit. Neural Repair 2021, 35, 576–584. [Google Scholar] [CrossRef]
- Yuan, J.; Botchway, B.O.A.; Zhang, Y.; Tan, X.; Wang, X.; Liu, X. Curcumin Can Improve Spinal Cord Injury by Inhibiting TGF-β-SOX9 Signaling Pathway. Cell. Mol. Neurobiol. 2019, 39, 569–575. [Google Scholar] [CrossRef]
- Bang, W.-S.; Kim, K.; Seo, Y.J.; Cho, D.-C.; Sung, J.-K.; Kim, C.H. Curcumin Increase the Expression of Neural Stem/Progenitor Cells and Improves Functional Recovery after Spinal Cord Injury. J. Korean Neurosurg. Soc. 2017, 61, 10–18. [Google Scholar] [CrossRef]
- Wanjiang, W.; Chen, X.; Chen, Y.; Wang, J.; Zhang, H.; Fei, N.; Chengmin, L.; Feng, C.; Yuan, J.; Lin, J. Curcumin Improves Human Umbilical Cord-Derived Mesenchymal Stem Cell Survival via ERK1/2 Signaling and Promotes Motor Outcomes After Spinal Cord Injury. Cell. Mol. Neurobiol. 2020, 42, 1241–1252. [Google Scholar] [CrossRef]
- Gökçe, E.C.; Kahveci, R.; Gökçe, A.; Sargon, M.F.; Kısa, Ü.; Aksoy, N.; Cemil, B.; Erdoğan, B. Curcumin Attenuates Inflammation, Oxidative Stress, and Ultrastructural Damage Induced by Spinal Cord Ischemia–Reperfusion Injury in Rats. J. Stroke Cerebrovasc. Dis. 2016, 25, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Sanchez, B.G.; Salgado-Ceballos, H.; Torres-Castillo, S.; Rodríguez-Silverio, J.; Lopez-Hernandez, M.E.; Quiróz-González, S.; Sánchez-Torres, S.; Mondragón-Lozano, R.; Fabela-Sánchez, O. Electroacupuncture and Curcumin Promote Oxidative Balance and Motor Function Recovery in Rats Following Traumatic Spinal Cord Injury. Neurochem. Res. 2019, 44, 498–506. [Google Scholar] [CrossRef]
- Gao, F.; Lei, J.; Zhang, Z.; Yang, Y.; You, H. Curcumin Alleviates LPS-Induced Inflammation and Oxidative Stress in Mouse Microglial BV2 Cells by Targeting miR-137-3p/NeuroD1. RSC Adv. 2019, 9, 38397–38406. [Google Scholar] [CrossRef]
- Cemil, B.; Topuz, K.; Demircan, M.; Kurt, G.; Tun, K.; Kutlay, M.; İpçioğlu, O.M.; Küçükodacı, Z. Curcumin Improves Early Functional Results after Experimental Spinal Cord Injury. Acta Neurochir. 2010, 152, 1583–1590. [Google Scholar] [CrossRef]
- Gao, F.; Shen, J.; Zhao, L.; Hao, Q.; Yang, Y. Curcumin Alleviates Lipopolysaccharide (LPS)-Activated Neuroinflammation via Modulation of miR-199b-5p/IκB Kinase β (IKKβ)/Nuclear Factor Kappa B (NF-κB) Pathway in Microglia. Med. Sci. Monit. 2019, 25, 9801–9810. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Yang, Y.; Lin, J.; Huo, X.; Du, X.; Botchway, B.O.A.; Fang, M. Therapeutic Effect of Curcumin and Methylprednisolone in the Rat Spinal Cord Injury. Anat. Rec. 2017, 301, 686–696. [Google Scholar] [CrossRef]
- Xi, J.; Luo, X.; Wang, Y.; Li, J.; Guo, L.; Wu, G.; Li, Q. Tetrahydrocurcumin Protects against Spinal Cord Injury and Inhibits the Oxidative Stress Response by Regulating FOXO4 in Model Rats. Exp. Ther. Med. 2019, 18, 3681–3687. [Google Scholar] [CrossRef]
- Magaki, T.; Kurisu, K.; Yamaguchi, S.; Okazaki, T.; Takeda, M. Effect of Free Radical Scavenger Edaravone on Experimental Spinal Cord Injury. Spinal Surg. 2005, 19, 315–320. [Google Scholar] [CrossRef][Green Version]
- Aoyama, T.; Hida, K.; Kuroda, S.; Seki, T.; Yano, S.; Shichinohe, H.; Iwasaki, Y. Edaravone (MCI-186) Scavenges Reactive Oxygen Species and Ameliorates Tissue Damage in the Murine Spinal Cord Injury Model. Neurol. Med. Chir. 2008, 48, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Yu, Z.; Yu, Y.; Sun, B.; Xiao, C.; Luo, S.-P.; Li, L. Effects of Edaravone on Functional Recovery of a Rat Model with Spinal Cord Injury Through Induced Differentiation of Bone Marrow Mesenchymal Stem Cells into Neuron-Like Cells. Cell. Reprogram. 2021, 23, 47–56. [Google Scholar] [CrossRef]
- Pang, Y.; Liu, X.; Wang, X.; Shi, X.; Ma, L.; Zhang, Y.; Zhou, T.; Zhao, C.; Zhang, X.; Fan, B.; et al. Edaravone Modulates Neuronal GPX4/ACSL4/5-LOX to Promote Recovery After Spinal Cord Injury. Front. Cell Dev. Biol. 2022, 10, 849854. [Google Scholar] [CrossRef]
- Song, Y.; Peng, C.; Ye, X.B. Combination of Edaravone and Neural Stem Cell Transplantation Repairs Injured Spinal Cord in Rats. Genet. Mol. Res. 2015, 14, 19136–19143. [Google Scholar] [CrossRef] [PubMed]
- Khalatbary, A.R.; Ahmadvand, H. Effects of Epigallocatechin Gallate on Tissue Lipid Peroxide Levels in Traumatized Spinal Cord of Rat. Iran. J. Basic Med. Sci. 2010, 13, 239–242. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, W.; Lin, F.; Liu, W.; Gu, R. Epigallocatechin-3-Gallate Selenium Nanoparticles for Neuroprotection by Scavenging Reactive Oxygen Species and Reducing Inflammation. Front. Bioeng. Biotechnol. 2022, 10, 989602. [Google Scholar] [CrossRef]
- Zhu, L. Anti-Edema Effect of Epigallocatechin against Acute Spinal Cord Injury in Rats and Its Correlation with the P38MAPK/NF-Κb Signaling Pathway. J. Spine 2013, S4, 006. [Google Scholar] [CrossRef]
- Brotfain, E.; Gruenbaum, S.E.; Boyko, M.; Kutz, R.; Zlotnik, A.; Klein, M. Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Curr. Neuropharmacol. 2016, 14, 641–653. [Google Scholar] [CrossRef]
- Zendedel, A.; Mönnink, F.; Hassanzadeh, G.; Zaminy, A.; Ansar, M.; Habib, P.; Slowik, A.; Kipp, M.; Beyer, C. Estrogen Attenuates Local Inflammasome Expression and Activation after Spinal Cord Injury. Mol. Neurobiol. 2017, 55, 1364–1375. [Google Scholar] [CrossRef]
- Samantaray, S.; Das, A.; Matzelle, D.C.; Yu, S.P.; Wei, L.; Varma, A.K.; Ray, S.K.; Banik, N.L. Administration of Low Dose Estrogen Attenuates Gliosis and Protects Neurons in Acute Spinal Cord Injury in Rats. J. Neurochem. 2015, 136, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Sribnick, E.A.; Samantaray, S.; Das, A.; Smith, J.A.; Matzelle, D.; Ray, S.K.; Banik, N.L. Postinjury Estrogen Treatment of Chronic Spinal Cord Injury Improves Locomotor Function in Rats. J. Neurosci. Res. 2010, 88, 1738–1750. [Google Scholar] [CrossRef]
- Du, F.; Wang, X.; Shang, B.; Fang, J.; Xi, Y.; Li, A.; Diao, Y. Gastrodin Ameliorates Spinal Cord Injury via Antioxidant and Anti-Inflammatory Effects. Acta Biochim. Pol. 2016, 63, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Ao, Q.; Sun, X.-H.; Wang, A.; Fu, P.-F.; Gong, K.; Zuo, H.-Z.; Gong, Y.-D.; Zhang, X.-F. Protective Effects of Extract of Ginkgo Biloba (EGb 761) on Nerve Cells after Spinal Cord Injury in Rats. Spinal Cord 2006, 44, 662–667. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jadhav, R.S.; Patil, N.N.; Saha, B. Neuroprotective activities and repair mechanisms of ginkgo biloba extract egb 761 on cns injuries and neurodegenerative disorders. Innovare J. Med. Sci. 2023, 11, 1–6. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.-P.; Shao, W.; Mao, X.; Yang, M.; Ye, Z.; Liang, W.; Luo, Z. EGb761 Improves Histological and Functional Recovery in Rats with Acute Spinal Cord Contusion Injury. Spinal Cord 2015, 54, 259–265. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, B.; Fu, S.; Hu, J.; Yin, L.; Lin, L.; Wang, X.; Lu, P.; Xu, X. EGb761 Protects Hydrogen Peroxide-Induced Death of Spinal Cord Neurons through Inhibition of Intracellular ROS Production and Modulation of Apoptotic Regulating Genes. J. Mol. Neurosci. 2009, 38, 103–113. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, N.; Huang, J.; Lu, P.; Xu, X. Inhibition of cPLA2 Activation by Ginkgo Biloba Extract Protects Spinal Cord Neurons from Glutamate Excitotoxicity and Oxidative Stress-induced Cell Death. J. Neurochem. 2010, 116, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Zhang, J.; Wang, J.; An, J.; Xue, W.; Liu, Q.; Zhang, Y. Mechanisms of Ginsenosides Exert Neuroprotective Effects on Spinal Cord Injury: A Promising Traditional Chinese Medicine. Front. Neurosci. 2022, 16, 969056. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, S.; Pan, J.; Wang, Z.; Li, Y.; Xu, X.; Yang, J.; Zhang, X.; Wang, Y.; Liu, M. Ginsenoside Rb1 Attenuates Microglia Activation to Improve Spinal Cord Injury via microRNA-130b-5p/TLR4/NF-κB Axis. J. Cell. Physiol. 2020, 236, 2144–2155. [Google Scholar] [CrossRef]
- Wang, P.; Lin, C.; Wu, S.; Huang, K.; Wang, Y.; Bao, X.; Zhang, F.; Huang, Z.; Teng, H. Inhibition of Autophagy Is Involved in the Protective Effects of Ginsenoside Rb1 on Spinal Cord Injury. Cell. Mol. Neurobiol. 2017, 38, 679–690. [Google Scholar] [CrossRef]
- Kim, D.; Kweon, K.-J.; Kim, P.; Kim, H.; Kim, S.S.; Sohn, N.; Maeng, S.; Shin, J.-W. Ginsenoside Rg3 Improves Recovery from Spinal Cord Injury in Rats via Suppression of Neuronal Apoptosis, Pro-Inflammatory Mediators, and Microglial Activation. Molecules 2017, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Chen, W. Neuroprotective Effect of Ginsenoside Rd in Spinal Cord Injury Rats. Basic Clin. Pharmacol. Toxicol. 2016, 119, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wyss, P.O.; Richter, J.; Zweers, P.; Brust, A.K.; Funk, C.; Zoelch, N.; Vallesi, V.; Verma, R.; Hock, A.; Berger, M.F.; et al. Glutathione in the Pons Is Associated With Clinical Status Improvements in Subacute Spinal Cord Injury. Investig. Radiol. 2022, 58, 131–138. [Google Scholar] [CrossRef]
- Kim, S.; Ko, W.; Han, G.-H.; Lee, D.; Lee, Y.; Sheen, S.H.; Hong, J.B.; Sohn, S. Chirality-Dependent Anti-Inflammatory Effect of Glutathione after Spinal Cord Injury in an Animal Model. Pharmaceuticals 2021, 14, 792. [Google Scholar] [CrossRef]
- Shi, L.; Jiang, C.-C.; Lu, J.-J.; Li, Z.-X.; Li, W.T.; Yin, X.; Chen, Z.; Zhao, X.-Y.; Zhang, H.; Hu, H.; et al. Molecular Mechanism of Ligustilide Attenuating OGD/R Injury in PC12 Cells by Inhibiting Ferroptosis. Zhongguo Zhong Yao Za Zhi 2023, 48, 3046–3054. [Google Scholar] [CrossRef]
- Xiao, W.; Yu, A.; Liu, D.; Shen, J.; Xu, Z. Ligustilide Treatment Promotes Functional Recovery in a Rat Model of Spinal Cord Injury via Preventing ROS Production. Int. J. Clin. Exp. Pathol. 2015, 8, 12005. [Google Scholar] [PubMed]
- Zhang, Q.; Wang, J.; Gu, Z.; Zhang, Q.; Zheng, H. Effect of Lycopene on the Blood-Spinal Cord Barrier after Spinal Cord Injury in Mice. Biosci. Trends 2016, 10, 288–293. [Google Scholar] [CrossRef][Green Version]
- Çelik, H.; Küçükler, S.; Özdemir, S.; Çomaklı, S.; Gür, C.; Kandemir, F.M.; Yardım, A. Lycopene Protects against Central and Peripheral Neuropathy by Inhibiting Oxaliplatin-Induced ATF-6 Pathway, Apoptosis, Inflammation and Oxidative Stress in Brains and Sciatic Tissues of Rats. NeuroToxicology 2020, 80, 29–40. [Google Scholar] [CrossRef]
- Taşkıran, D.; Tanyalcin, T.; Sözmen, E.Y.; Peker, G.; Gülmen, V.; Çağlı, S.; Kanıt, L.; Tekeli, G.; Barçın, E.; Zileli, M.; et al. The Effects of Melatonin on the Antioxidant Systems in Experimental Spinal Injury. Int. J. Neurosci. 2000, 104, 63–73. [Google Scholar] [CrossRef]
- Koff, M.A.E.; Ajiboye, L.O.; LISBOA, N.D.; Falavigna, A. Systematic review of recovery of spinal cord injury with antioxidant therapy. Coluna/Columna 2017, 16, 67–73. [Google Scholar] [CrossRef]
- Cocchi, M.A.; Borsani, E.; Bonazza, V.; Brunelli, G.; Monini, L.; Rezzani, R. H2O2 Stress Damage Is Reversed by Melatonin in a Spinal Cord Organotypic Model. Ital. J. Anat. Embryol. 2016, 121, 182. [Google Scholar] [CrossRef]
- Cocchi, M.A.; Brunelli, G.; Agazzi, G.M.; Borsani, E. Effect of Melatonin on SOD Expression in Spinal Cord Injury. Ital. J. Anat. Embryol. 2015, 120, 163. [Google Scholar] [CrossRef]
- Naseem, M.; Parvez, S. Role of Melatonin in Traumatic Brain Injury and Spinal Cord Injury. Sci. World J. 2014, 2014, 586270. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Huang, H.; Qu, Z.; Ma, D.; Dang, X.; Dong, Q. Melatonin Attenuates Spinal Cord Injury in Mice by Activating the Nrf2/ARE Signaling Pathway to Inhibit the NLRP3 Inflammasome. Cells 2022, 11, 2809. [Google Scholar] [CrossRef]
- Bi, J.; Shen, J.; Chen, C.; Li, Z.; Tan, H.; Sun, P.; Lin, Y. Role of Melatonin in the Dynamics of Acute Spinal Cord Injury in Rats. J. Cell. Mol. Med. 2021, 25, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhou, Z.; Gao, S.; Guo, Y.; Gao, K.; Wang, H.; Dang, X. Melatonin Inhibits Neural Cell Apoptosis and Promotes Locomotor Recovery via Activation of the Wnt/β-Catenin Signaling Pathway After Spinal Cord Injury. Neurochem. Res. 2017, 42, 2336–2343. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, X.; Yu, B.; Pan, M.; Lü, F.; Li, J.; Cui, X.; Yao, M.; Lu, X. The Effect of Metformin on Ameliorating Neurological Function Deficits and Tissue Damage in Rats Following Spinal Cord Injury: A Systematic Review and Network Meta-Analysis. Front. Neurosci. 2022, 16, 946879. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Z.; Han, W.; Yuan, Y.; Li, Y.; Zhou, K.; Wang, Q.; Xie, L.; Xu, K.; Zhang, H.; et al. Metformin Promotes Axon Regeneration after Spinal Cord Injury through Inhibiting Oxidative Stress and Stabilizing Microtubule. Oxidative Med. Cell. Longev. 2020, 2020, 9741369. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh-Smith, W.; Abdallah, A.; Cunningham, C. The Potential Effects of Polyunsaturated ω-3 Fatty Acids on Spinal Cord Injury: A Systematic Review & Meta-Analysis of Preclinical Evidence. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102554. [Google Scholar]
- Bi, J.; Chen, C.; Sun, P.; Tan, H.; Feng, F.; Jianxiong, S. Neuroprotective Effect of Omega-3 Fatty Acids on Spinal Cord Injury Induced Rats. Brain Behav. 2019, 9, e01339. [Google Scholar] [CrossRef]
- Figueroa, J.D.; León, M.D. Neurorestorative Targets of Dietary Long-Chain Omega-3 Fatty Acids in Neurological Injury. Mol. Neurobiol. 2014, 50, 197–213. [Google Scholar] [CrossRef]
- King, V.R.; Huang, W.; Dyall, S.C.; Curran, O.E.; Priestley, J.V.; Michael-Titus, A.T. Omega-3 Fatty Acids Improve Recovery, Whereas Omega-6 Fatty Acids Worsen Outcome, after Spinal Cord Injury in the Adult Rat. J. Neurosci. 2006, 26, 4672–4680. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Turczyn, P.; Frasuńska, J.; Paradowska-Gorycka, A.; Tarnacka, B. Significance of Omega-3 Fatty Acids in the Prophylaxis and Treatment after Spinal Cord Injury in Rodent Models. Mediat. Inflamm. 2020, 2020, 3164260. [Google Scholar] [CrossRef] [PubMed]
- Schültke, E.; Griebel, R.; Juurlink, B.H.J. Quercetin Attenuates Inflammatory Processes after Spinal Cord Injury in an Animal Model. Spinal Cord 2010, 48, 857–861. [Google Scholar] [CrossRef]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Moradi, S.Z.; Echeverría, J. Quercetin Derivatives in Combating Spinal Cord Injury: A Mechanistic and Systematic Review. Life 2022, 12, 1960. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Botchway, B.O.A.; Zhang, Y.; Zhang, Y.; Jin, T.; Liu, X. Quercetin Can Improve Spinal Cord Injury by Regulating the mTOR Signaling Pathway. Front. Neurol. 2022, 13, 905640. [Google Scholar] [CrossRef] [PubMed]
- Fideles, S.O.M.; de Cássia Ortiz, A.; Buchaim, D.V.; de Souza Bastos Mazuqueli Pereira, E.; Parreira, M.J.B.M.; de Oliveira Rossi, J.; da Cunha, M.R.; de Souza, A.T.; Soares, W.C.; Buchaim, R.L. Influence of the Neuroprotective Properties of Quercetin on Regeneration and Functional Recovery of the Nervous System. Antioxidants 2023, 12, 149. [Google Scholar] [CrossRef]
- Öcal, Ö.; Börcek, A.Ö.; Paşaoğlu, Ö.T.; Gündoğdu, A.Ç.; Kaplanoğlu, G.T.; Baykaner, M.K. Can Quercetin Be an Option for Treatment of Spinal Cord Injury?: An Experimental Study. Turk. Neurosurg. 2018, 29, 247–253. [Google Scholar] [CrossRef]
- Çiftçi, U. Efficiacy Of Resveratrol And Quercetin After Experimental Spinal Cord İnjury. Turk. J. Trauma Emerg. Surg. 2016, 22, 423–431. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Xiong, M.; Wang, M.; Chen, H.; Li, W.; Zhou, X. Quercetin Promotes Locomotor Function Recovery and Axonal Regeneration through Induction of Autophagy after Spinal Cord Injury. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1642–1652. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wang, M.; Lin, C.; Li, G.; Zhou, X.; Luo, J.; Jin, D. Quercetin Reduces Neural Tissue Damage and Promotes Astrocyte Activation after Spinal Cord Injury in Rats. J. Cell. Biochem. 2017, 119, 2298–2306. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.; Shan, L.; Liu, S.; Huang, D.; Chen, X.; Chen, Z.; Yang, M.; Yin, X.; Yang, H.; et al. Quercetin Prevents Necroptosis of Oligodendrocytes by Inhibiting Macrophages/Microglia Polarization to M1 Phenotype after Spinal Cord Injury in Rats. J. Neuroinflamm. 2019, 16, 206. [Google Scholar] [CrossRef]
- Nowacka, A.; Śniegocka, M.; Smuczyński, W.; Liss, S.; Ziółkowska, E.; Bożiłow, D.; Śniegocki, M.; Wiciński, M. The Potential Application of Resveratrol and Its Derivatives in Central Nervous System Tumors. Int. J. Mol. Sci. 2024, 25, 13338. [Google Scholar] [CrossRef]
- Fan, R.; Zhang, Y.; Botchway, B.O.A.; Liu, X. Resveratrol Can Attenuate Astrocyte Activation to Treat Spinal Cord Injury by Inhibiting Inflammatory Responses. Mol. Neurobiol. 2021, 58, 5799–5813. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Ye, Q.; Mi, X.; Jiao, D.; Zhang, S.; Cheng, R.; Fang, Z.; Fang, M.; Ye, X. Resveratrol Inhibits Ferroptosis via Activating NRF2/GPX4 Pathway in Mice with Spinal Cord Injury. Microsc. Res. Tech. 2023, 86, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Botchway, B.O.A.; Zhang, Y.; Liu, X. Resveratrol Can Inhibit Notch Signaling Pathway to Improve Spinal Cord Injury. Ann. Anat. Anat. Anz. 2019, 223, 100–107. [Google Scholar] [CrossRef]
- Wang, P.; Jiang, L.; Zhou, N.; Zhou, H.; Liu, H.; Zhao, W.; Zhang, H.; Zhang, X.; Hu, Z. Resveratrol Ameliorates Autophagic Flux to Promote Functional Recovery in Rats after Spinal Cord Injury. Oncotarget 2018, 9, 8427–8440. [Google Scholar] [CrossRef]
- Xu, L.; Botchway, B.O.A.; Zhang, S.; Zhou, J.; Liu, X. Inhibition of NF-κB Signaling Pathway by Resveratrol Improves Spinal Cord Injury. Front. Neurosci. 2018, 12, 690. [Google Scholar] [CrossRef]
- Hu, J.; Lang, Y.; Cao, Y.; Zhang, T.; Lü, H. The Neuroprotective Effect of Tetramethylpyrazine Against Contusive Spinal Cord Injury by Activating PGC-1α in Rats. Neurochem. Res. 2015, 40, 1393–1401. [Google Scholar] [CrossRef]
- Shin, J.-W.; Moon, J.-Y.; Seong, J.-W.; Song, S.; Cheong, Y.-J.; Kang, C.; Sohn, N. Effects of Tetramethylpyrazine on Microglia Activation in Spinal Cord Compression Injury of Mice. Am. J. Chin. Med. 2013, 41, 1361–1376. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Sng, K.S.; Shu, B.; Wang, Y.; Yao, M.; Cui, X. Effects of Tetramethylpyrazine Treatment in a Rat Model of Spinal Cord Injury: A Systematic Review and Meta-Analysis. Eur. J. Pharmacol. 2023, 945, 175524. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Cui, W.; Zhang, Y.; Liang, X. Regulatory Mechanisms of Tetramethylpyrazine on Central Nervous System Diseases: A Review. Front. Pharmacol. 2022, 13, 948600. [Google Scholar] [CrossRef]
- Li, S.; Qi, G.; Qi, W.; Yang, Z.-X.; Yu, Z.-J.; Jiang, Q. Neuroprotective Effect of Tetramethylpyrazine on Mice after Spinal Cord Injury. China J. Chin. Mater. Medica 2023, 48, 3848–3854. [Google Scholar] [CrossRef]
- Liu, X.; Ma, W.; Song, D.; Rohani, S. Co-Delivery of Curcumin and Resveratrol via a Hydrogel/Nanoparticle System Modulate NF-kB Inflammatory Signalling Pathway in Rat Model of Traumatic Spinal Cord Injury. J. Biomed. Nanotechnol. 2023, 19, 342–348. [Google Scholar] [CrossRef]
- Bonilla, P.; Hernández, J.; Giraldo, E.; González-Pérez, M.A.; Alastrue-Agudo, A.; Elkhenany, H.; Vicent, M.J.; Navarro, X.; Edel, M.J.; Moreno-Manzano, V. Human-Induced Neural and Mesenchymal Stem Cell Therapy Combined with a Curcumin Nanoconjugate as a Spinal Cord Injury Treatment. Int. J. Mol. Sci. 2021, 22, 5966. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.; Drasites, K.P.; Cox, A.; Capone, M.; Myatich, A.; Shams, R.; Matzelle, D.; Garner, D.P.; Bredikhin, M.; Shields, D.C.; et al. Protective Effects of Estrogen via Nanoparticle Delivery to Attenuate Myelin Loss and Neuronal Death after Spinal Cord Injury. Neurochem. Res. 2021, 46, 2979–2990. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, X.; Liu, X.; Shen, W.; Mei, X.; Tian, H.; Wu, C. Glutathione-Modified Macrophage-Derived Cell Membranes Encapsulated Metformin Nanogels for the Treatment of Spinal Cord Injury. Biomater. Adv. 2022, 133, 112668. [Google Scholar] [CrossRef]
- Li, Y.; Zou, Z.; An, J.; Wu, Q.; Tong, L.; Mei, X.; Tian, H.; Wu, C. Chitosan-Modified Hollow Manganese Dioxide Nanoparticles Loaded with Resveratrol for the Treatment of Spinal Cord Injury. Drug Deliv. 2022, 29, 2498–2512. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Zhao, S.; Zhang, L.; Song, Q. Resveratrol and Puerarin Loaded Polymeric Nanoparticles to Enhance the Chemotherapeutic Efficacy in Spinal Cord Injury. Biomed. Microdevices 2020, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wan, Y.; Du, X.; Li, J.; Wei, J.; Li, T.; Li, C.; Liu, Z.; Zhou, M.; Zhong, Z. TAT-Modified Serum Albumin Nanoparticles for Sustained-Release of Tetramethylpyrazine and Improved Targeting to Spinal Cord Injury. J. Nanobiotechnol. 2021, 19, 28. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, S.; Liu, G.; Li, X.; Zhao, Y.; Fan, X.; Ren, J.; Ning, C.; Xu, L.; Ji, L.; et al. Tetramethylpyrazine-Loaded Electroconductive Hydrogels Promote Tissue Repair after Spinal Cord Injury by Protecting the Blood–Spinal Cord Barrier and Neurons. J. Mater. Chem. B 2024, 12, 4409–4426. [Google Scholar] [CrossRef]
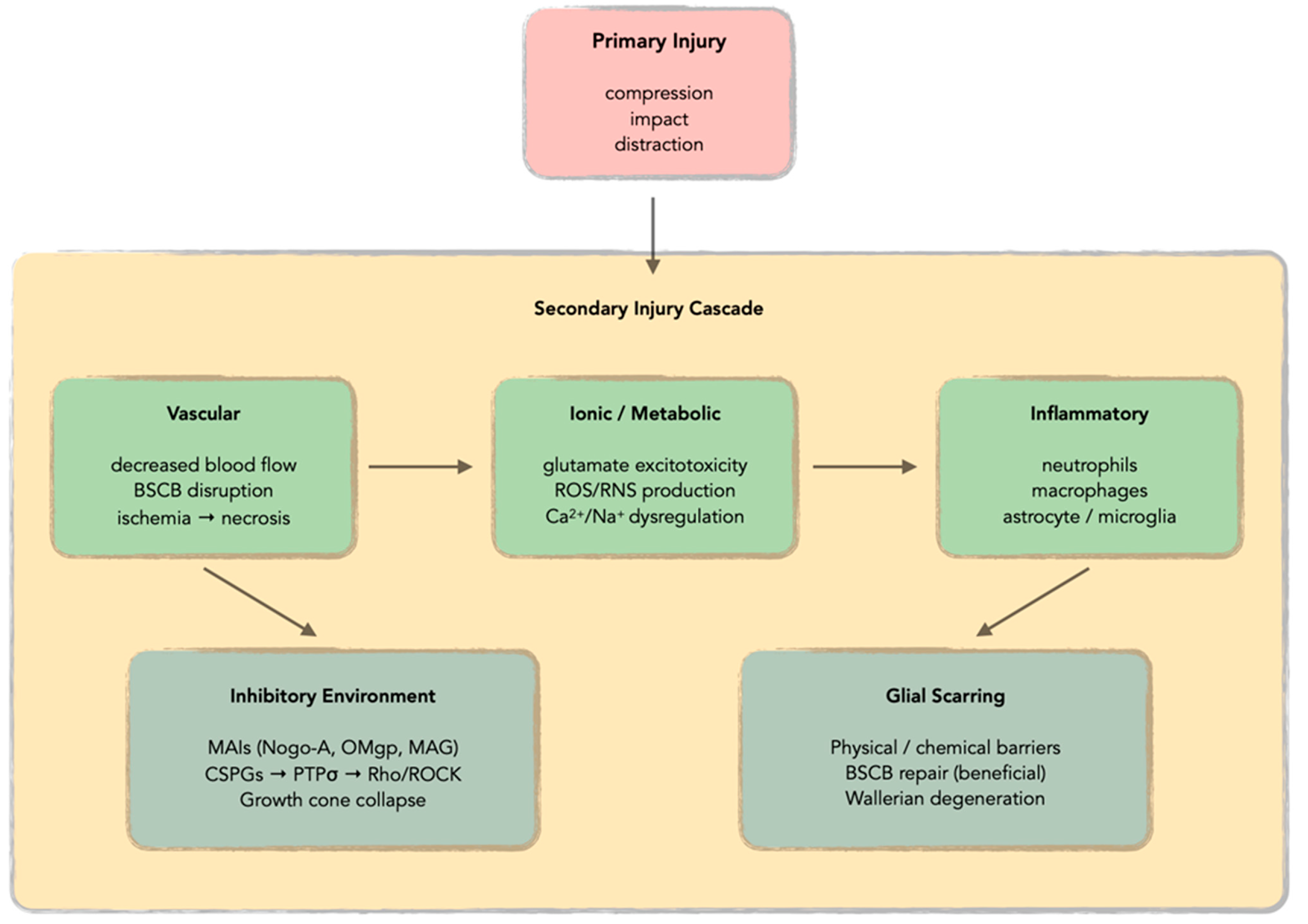
| Category | Mechanism/Source | Key Components | Molecular Details | Consequences |
|---|---|---|---|---|
| PRIMARY ROS GENERATION | Mitochondrial Dysfunction | Electron transport chain disruption Complexes I and III electron leakage Compromised energy metabolism | Superoxide (O2−•)—one-electron reduction H2O2—two-electron transfer Hydroxyl radical (•OH) Singlet oxygen (1O2) | Self-perpetuating cycle of ROS production and mitochondrial damage |
| Fenton Chemistry | Transition metal catalysis Iron from hemoglobin and ferritin BSCB breakdown | Fe2+/Cu+ + H2O2 → •OH + OH− + Fe3+/Cu2+ Highly reactive hydroxyl radicals | Enhanced lipid peroxidation and cellular damage | |
| Energy Crisis | ATP depletion Coenzyme Q10 depletion Cytochrome c loss | Na+/K+-ATPase failure Ca2+-ATPase dysfunction Mitochondrial membrane potential disruption | Ionic imbalances Calcium overload | |
| Permeability Transition | Ca2+ overload trigger Pore formation Pro-apoptotic factor release | Cytochrome c release Phospholipase activation Protease activation Endonuclease activation | Apoptotic cell death pathway activation | |
| CELLULAR ROS SOURCES | NADPH Oxidase | Activated microglia Infiltrating macrophages Inflammatory response | Superoxide generation Dual role: neuroprotection/neurodegeneration Controlled vs. excessive production | Tissue damage and secondary injury propagation |
| Neutrophil Infiltration | Early infiltration (hours) Myeloperoxidase activity Respiratory burst | Hypochlorous acid (HOCl) Chlorinated oxidants Massive superoxide and H2O2 production | Significant contribution to oxidative stress | |
| Xanthine Oxidase | Ischemia–reperfusion injury Dehydrogenase → oxidase conversion ATP breakdown products | Hypoxanthine metabolism Xanthine metabolism Superoxide production during purine catabolism | Additional ROS burden during reperfusion | |
| DAMAGE MECHANISMS | Lipid Peroxidation | PUFA in membranes Chain reaction propagation Iron-catalyzed process | •OH abstracts H atoms from PUFA 4-hydroxynonenal (4-HNE) Malondialdehyde (MDA) Covalent protein modification | Membrane disruption Neural tissue vulnerability |
| Protein Oxidation | Amino acid modification Cysteine/methionine targets Antioxidant enzyme loss | Disulfide bond formation Methionine sulfoxide Protein carbonyls Protein aggregation | Enzyme inactivation Cellular dysfunction | |
| DNA Oxidation | Base modification Strand breaks mtDNA vulnerability | 8-hydroxyguanosine Sugar–phosphate backbone damage Limited mtDNA repair mechanisms Mutation accumulation | Genomic instability Cell death pathway activation | |
| SECONDARY CASCADES | Peroxynitrite Formation | NO + O2−• combination Diffusion-limited reaction Highly reactive species | NO + O2−• → ONOO− Protein nitrosylation Lipid oxidation at diffusion limits DNA damage | Potent oxidative and nitrosative damage |
| PARP Activation | DNA damage response Energy depletion NAD+ consumption | Poly(ADP-ribose) polymerase activation NAD+ and ATP depletion Cell death promotion Additional oxidative stress source | Energy crisis Cell death acceleration | |
| ANTIOXIDANT DEPLETION | Enzymatic Systems | Direct oxidative modification Transcriptional downregulation Nrf2-ARE pathway disruption | SOD (superoxide dismutase) Catalase Glutathione peroxidase (GPx) Glutathione reductase | Compromised cellular defense Vicious cycle formation |
| Non-enzymatic Systems | Rapid consumption post-injury NADPH limitation Impaired recycling | GSH (glutathione) Ascorbic acid α-tocopherol Coenzyme Q10 | Primary defense depletion Oxidative stress amplification | |
| SIGNAL TRANSDUCTION | JNK Pathway | ROS accumulation response Mitochondrial dysfunction Pro-apoptotic activation | c-Jun N-terminal kinase Pro-apoptotic protein phosphorylation Transcription factor activation | Apoptotic cell death promotion |
| p38 MAPK | Oxidative stress activation Inflammatory gene expression Cytokine production | p38 mitogen-activated protein kinase Inflammatory mediator transcription Additional ROS generation | Tissue damage propagation and inflammation | |
| NF-κB Pathway | ROS-mediated activation Dual protective/harmful role Transcriptional regulation | Nuclear factor-κB Inflammatory mediator transcription Antioxidant gene upregulation Complex dual nature | Inflammation with some protective effects | |
| Nrf2-ARE System | Antioxidant response disruption Transcriptional downregulation Protective enzyme synthesis | Nuclear factor erythroid 2-related factor 2 Antioxidant response elements Protective enzyme transcription | Compromised antioxidant defense synthesis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, A.; Śniegocki, M.; Ziółkowska, E. A Review of the Potential Use of Antioxidants in Spinal Cord Injuries. Antioxidants 2025, 14, 1081. https://doi.org/10.3390/antiox14091081
Nowacka A, Śniegocki M, Ziółkowska E. A Review of the Potential Use of Antioxidants in Spinal Cord Injuries. Antioxidants. 2025; 14(9):1081. https://doi.org/10.3390/antiox14091081
Chicago/Turabian StyleNowacka, Agnieszka, Maciej Śniegocki, and Ewa Ziółkowska. 2025. "A Review of the Potential Use of Antioxidants in Spinal Cord Injuries" Antioxidants 14, no. 9: 1081. https://doi.org/10.3390/antiox14091081
APA StyleNowacka, A., Śniegocki, M., & Ziółkowska, E. (2025). A Review of the Potential Use of Antioxidants in Spinal Cord Injuries. Antioxidants, 14(9), 1081. https://doi.org/10.3390/antiox14091081










