Oxidative Stress Disrupts Gill Function in Eriocheir sinensis: Consequences for Ion Transport, Apoptosis, and Autophagy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Measurement of Antioxidant Parameters
2.3. Histological Analysis
2.4. Quantitative Real-Time PCR (qPCR)
2.5. Data Analysis
3. Results
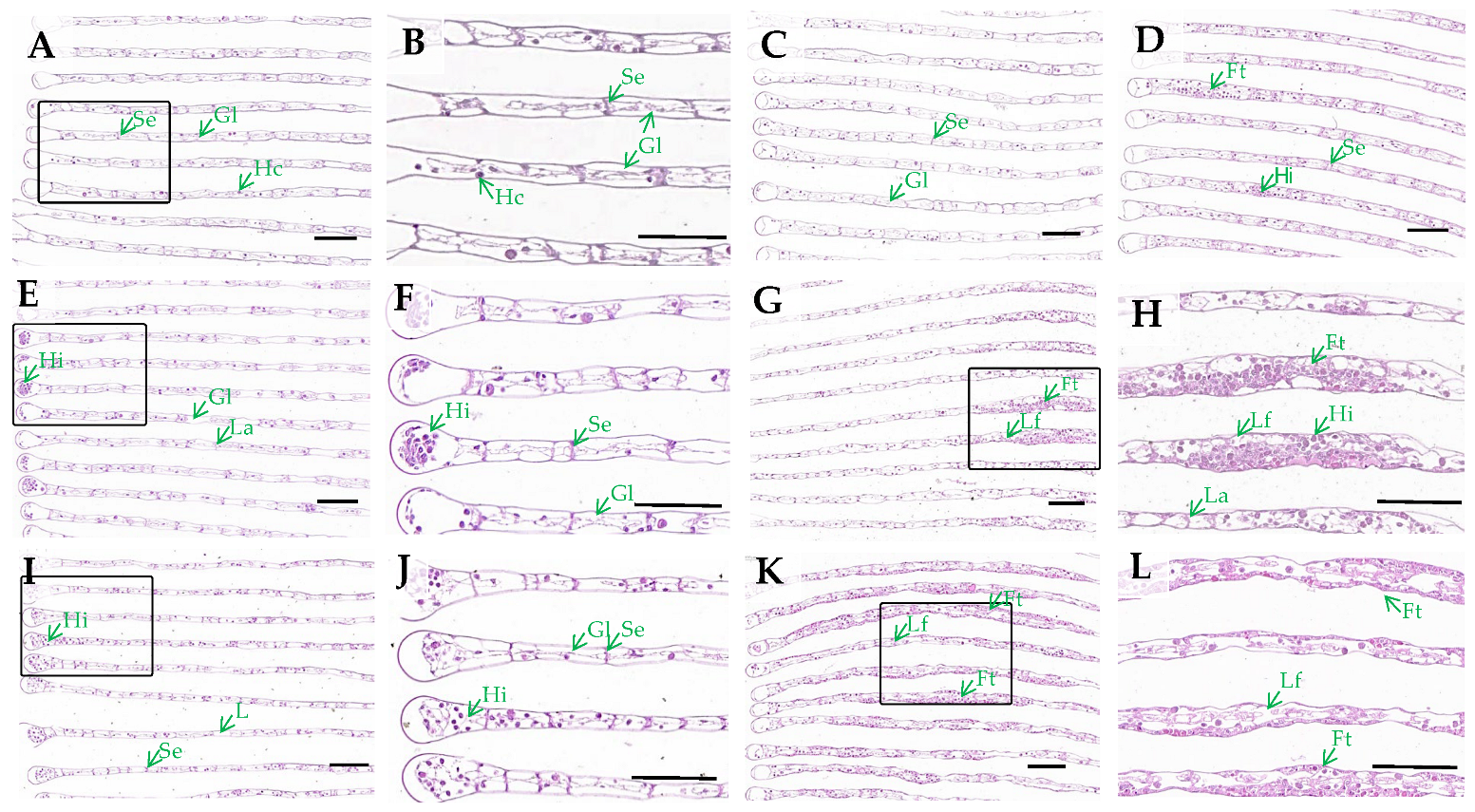
3.1. Gills Histological Observation
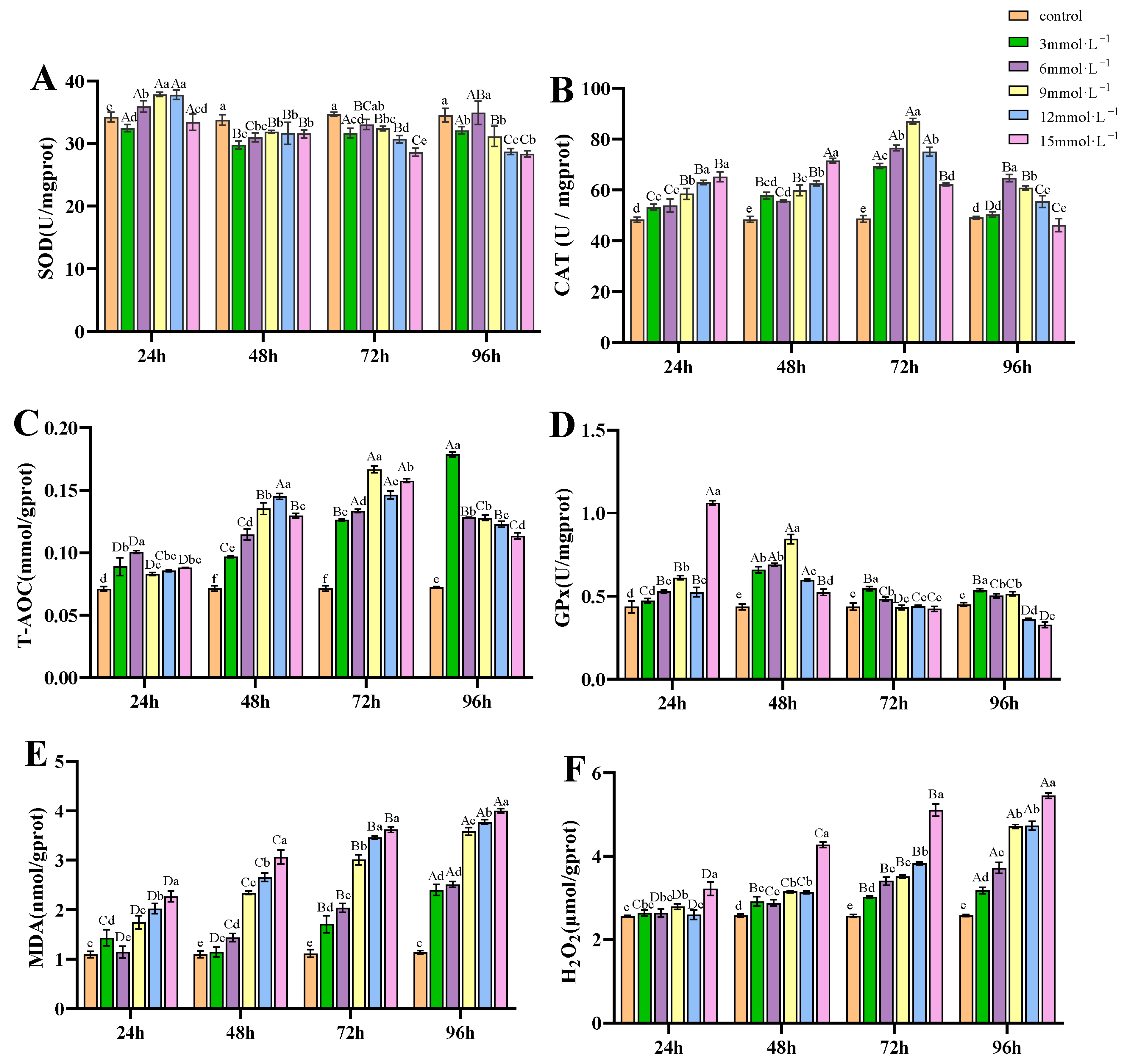
3.2. Impact of H2O2 Exposure on Antioxidant Capacity of Gills
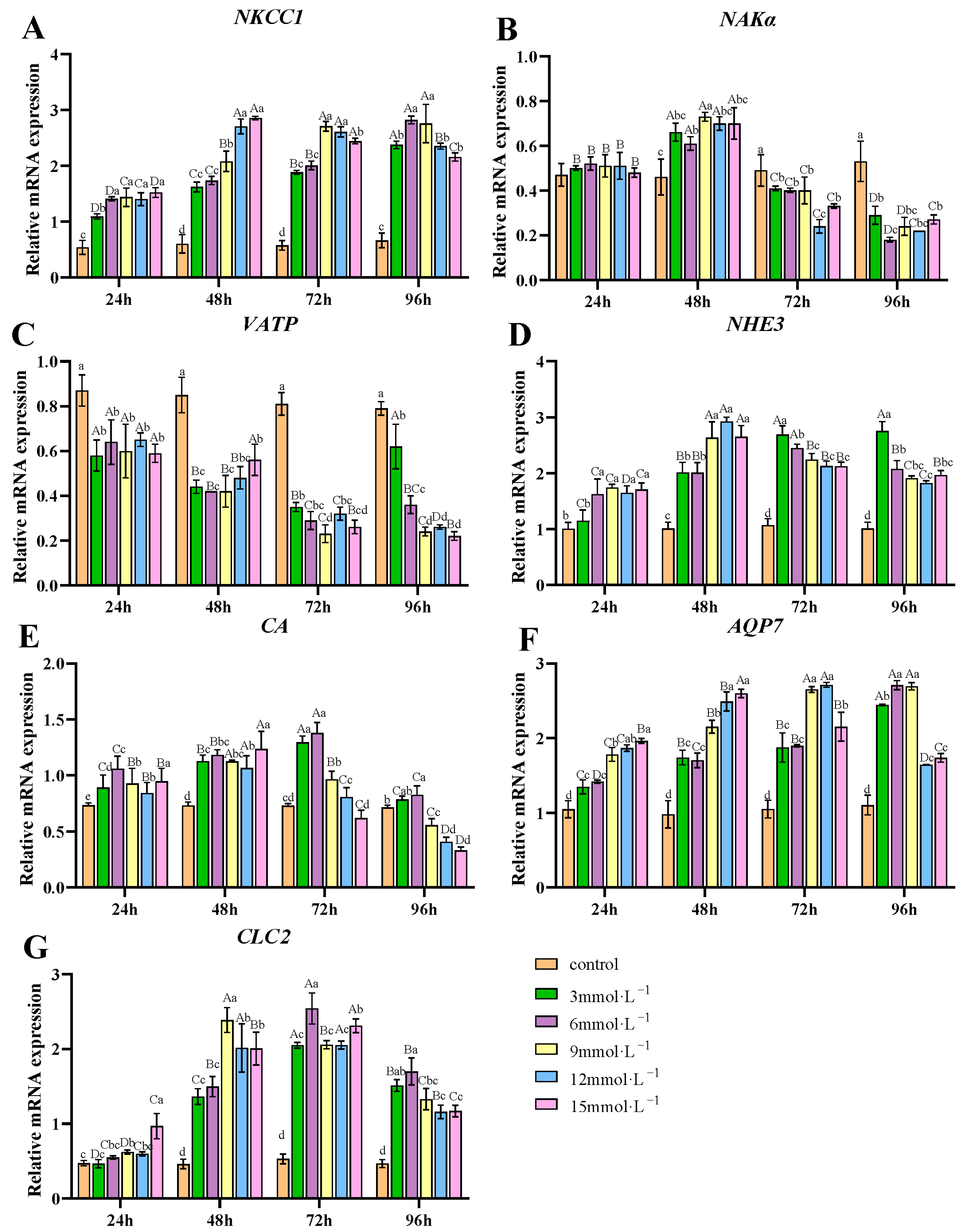
3.3. Effects of H2O2 Exposure on the mRNA Expression of Ion Transport-Related Genes in Gills
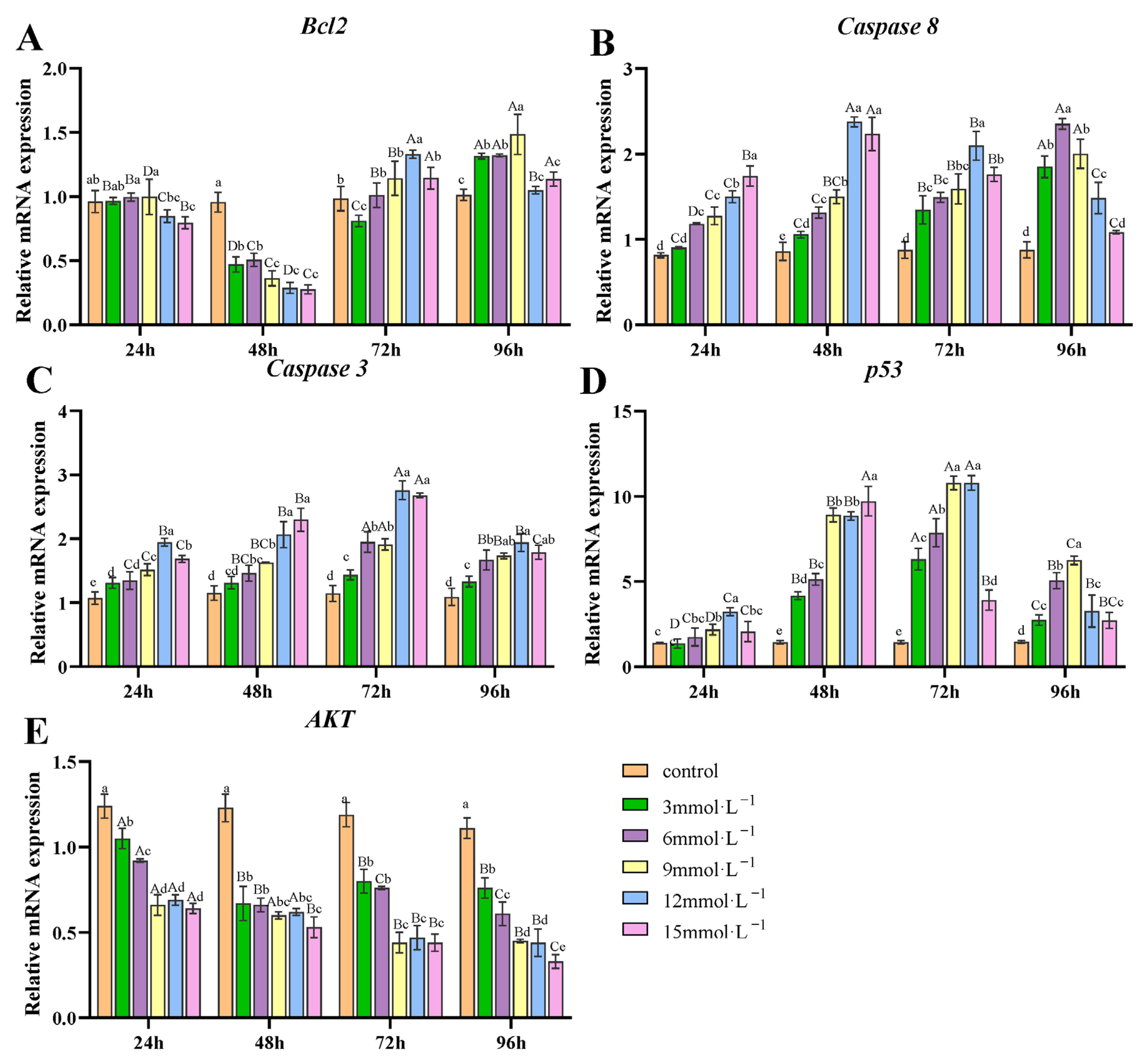
3.4. Effect of H2O2 Exposure on the Transcription of Apoptosis-Related Genes in Gills
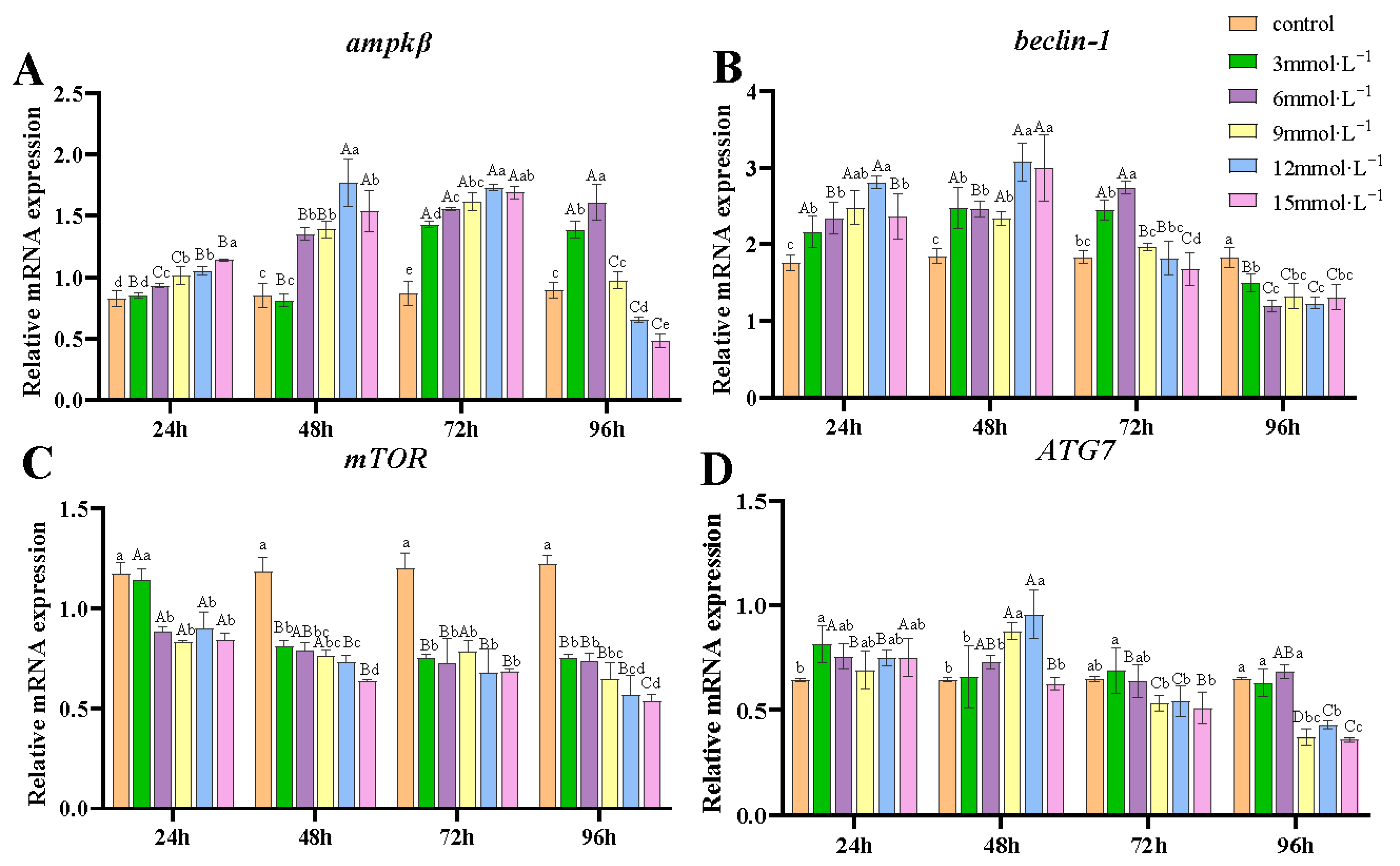
3.5. Effect of H2O2 Exposure on the Transcription of Autophagy-Related Genes in Gills
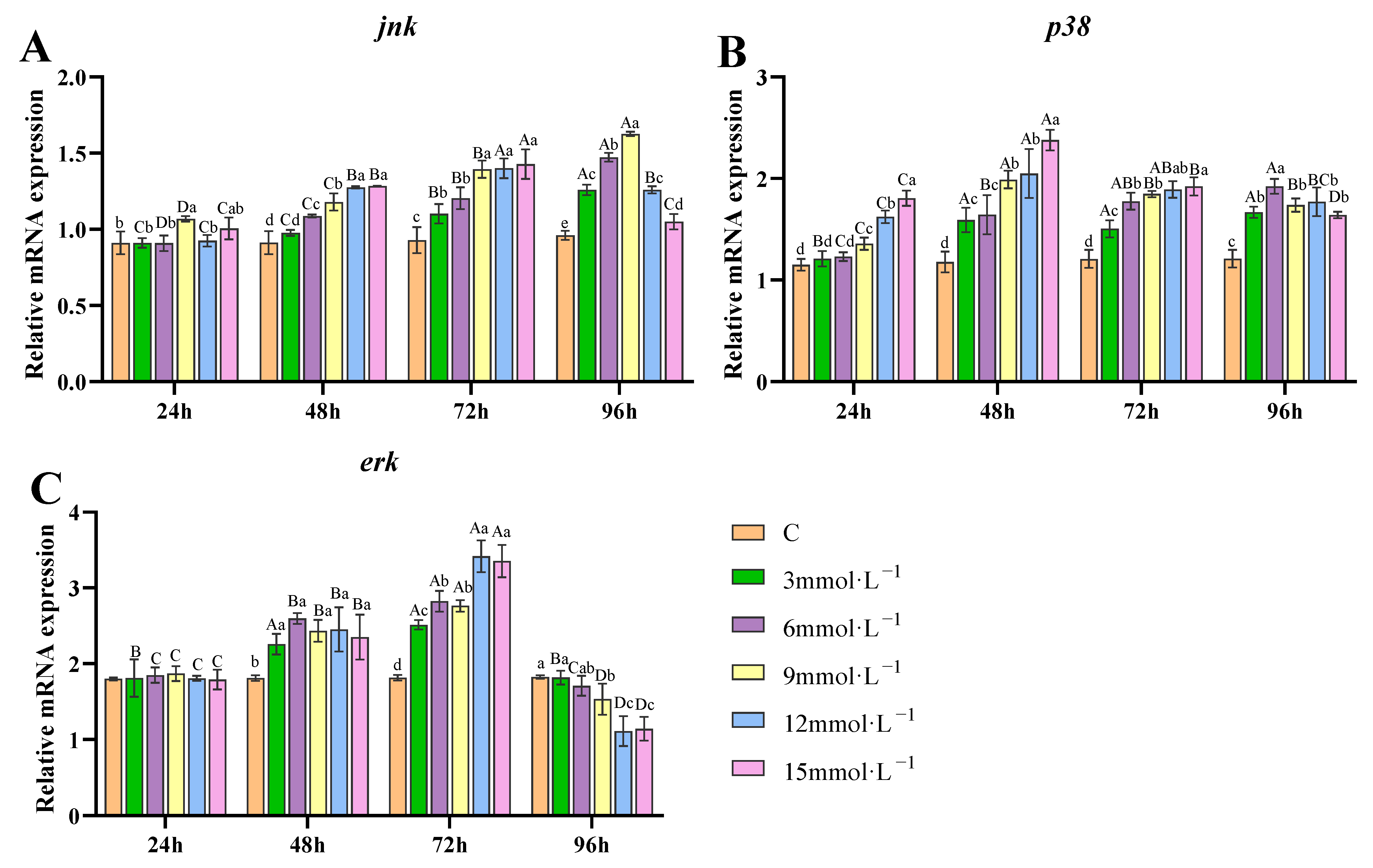
3.6. Effect of H2O2 Exposure on the Transcription of Genes Related to MAPK Pathway in Gills
4. Discussion
4.1. Effects of H2O2 on Histological Changes and Antioxidant Capacity of Gills
4.2. Effects of H2O2 Stress on the Ion Transport-Related Genes in Gills
4.3. Effects of H2O2 Exposure on Apoptosis and Autophagy of Gill Tissues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, C.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.; Lorin-Nebel, C.; Lee, T. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos Chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Wu, D.; Li, J.; Li, C.; Zheng, X.; Wang, L. Phosphorus nutrition in Songpu mirror carp (Cyprinus carpio Songpu) during chronic carbonate alkalinity stress: Effects on growth, intestinal immunity, physical barrier function, and intestinal microflora. Front. Immunol. 2022, 13, 900793. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Dong, S.; Liu, R.; Huang, M.; Dong, K.; Ge, J.; Gao, Q.; Zhou, Y. Effects of temperature, dissolved oxygen, and their interaction on the growth performance and condition of rainbow trout (Oncorhynchus mykiss). J. Therm. Biol. 2021, 98, 102928. [Google Scholar] [CrossRef]
- Singh, S.P.; Ahmad, T.; Sharma, J.; Chakrabarti, R. Effect of temperature on food consumption, immune system, antioxidant enzymes, and heat shock protein 70 of Channa punctata (Bloch, 1793). Fish Physiol. Biochem. 2021, 47, 79–91. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, X.; Feng, L.; Liu, Y.; Jiang, J. Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 2009, 288, 285–289. [Google Scholar] [CrossRef]
- Martín, D.; Salinas, M.; Fujita, N.; Tsuruo, T.; Cuadrado, A. Ceramide and reactive oxygen species generated by H2O2 induce caspase-3-independent degradation of Akt/protein kinase B. J. Biol. Chem. 2002, 277, 42943–42952. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Nair, A.R.; Munters, E.; Artois, T.J. Cadmium stress: An oxidative challenge. Biometals 2010, 23, 927–940. [Google Scholar] [CrossRef]
- Depledge, M.H.; Rainbow, P.S. Models of regulation and accumulation of trace metals in marine invertebrates. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1990, 97, 1–7. [Google Scholar] [CrossRef]
- Rainbow, P.S.; Luoma, S.N. Metal toxicity, uptake and bioaccumulation in aquatic invertebrates—Modelling zinc in crustaceans. Aquat. Toxicol. 2011, 105, 455–465. [Google Scholar] [CrossRef]
- Boonsanit, P.; Pairohakul, S. Effects of salinity on haemolymph osmolality, gill Na+/K+ ATPase and antioxidant enzyme activities in the male mud crab Scylla olivacea (Herbst, 1796). Mar. Biol. Res. 2021, 17, 86–97. [Google Scholar] [CrossRef]
- Wang, X.; Yao, Q.; Zhang, D.; Lei, X.; Wang, S.; Wan, J.; Liu, H.; Chen, Y.; Zhao, Y.; Wang, G. Effects of acute salinity stress on osmoregulation, antioxidant capacity and physiological metabolism of female Chinese mitten crabs (Eriocheir sinensis). Aquaculture 2022, 552, 737989. [Google Scholar] [CrossRef]
- Issartel, J.; Boulo, V.; Wallon, S.; Geffard, O.; Charmantier, G. Cellular and molecular osmoregulatory responses to cadmium exposure in Gammarus fossarum (Crustacea, Amphipoda). Chemosphere 2010, 81, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Shen, Q.; Wang, Q.; Liu, D.; Li, J.; Wang, L. The effects of cadmium exposure on the oxidative state and cell death in the gill of freshwater crab Sinopotamon henanense. PLoS ONE 2013, 8, e64020. [Google Scholar] [CrossRef] [PubMed]
- Negro, L.; Senkman, E.; Montagna, M.; Collins, P. Freshwater decapods and pesticides: An unavoidable relation in the modern world. In Pesticides in the Modern World-Risks and Benefits; IntechOpen: London, UK, 2011. [Google Scholar]
- Silvestre, F.; Trausch, G.; Spano, L.; Devos, P. Effects of atrazine on osmoregulation in the Chinese mitten crab, Eriocheir sinensis. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 132, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Xian, J.; Miao, Y.; Li, B.; Guo, H.; Wang, A. Apoptosis of tiger shrimp (Penaeus monodon) haemocytes induced by Escherichia coli lipopolysaccharide. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 164, 301–306. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, N.; Wang, Z.; Abdallah, G.; Zhang, B.; Wang, S.; Yao, Q.; Chen, Y.; Wang, Q.; Zhang, D. Effect of infection with Aeromonas hydrophila on antioxidant capacity, inflammation response, and apoptosis proteins in Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109220. [Google Scholar] [CrossRef]
- Fan, T.; Han, L.; Cong, R.; Liang, J. Caspase family proteases and apoptosis. Acta Biochim. Et Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I. Apoptotic p53 Gene Expression in the Regulation of Persistent Organic Pollutant (POP)-Induced Oxidative Stress in the Intertidal Crab Macrophthalmus japonicus. Antioxidants 2022, 11, 771. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- She, C.; Zhu, L.; Zhen, Y.; Wang, X.; Dong, Q. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: New implications for osteonecrosis treatment? Cell. Signal. 2014, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Feng, W.; Chen, X.; Xu, Y.; Zhou, J.; Li, J.; Xu, P.; Tang, Y. H2O2-induced oxidative stress responses in Eriocheir sinensis: Antioxidant defense and immune gene expression dynamics. Antioxidants 2024, 13, 524. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Li, Y.; Johnson, O.; Gu, Z.; Jeney, G.; Xu, P.; Yin, G. Antioxidative, inflammatory and immune responses in hydrogen peroxide-induced liver injury of tilapia (GIFT, Oreochromis niloticus). Fish Shellfish Immunol. 2019, 84, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Liu, J.; Zhao, P.; Ge, B.; Liu, Q.; Jiang, S.; Wang, Z.; Zhang, H.; Tang, B.; Ding, G. A novel modulation of physiological regulation in cultured Chinese mitten crab (Eriocheir japonica sinensis) in response to consistent salinity changes. Gene 2020, 756, 144914. [Google Scholar] [CrossRef]
- Xu, H.; Lyu, S.; Li, Y.; Xu, J.; Lu, B.; Zhao, J. Identification, characterization and expression analysis of ERK2 in Chinese mitten crab Eriocheir sinensis after challenge with LPS and Aeromonas hydrophila. Fish Shellfish Immunol. 2016, 52, 325–333. [Google Scholar] [CrossRef]
- Wang, Y.; Nan, X.; Hao, S.; Zhao, K.; Guo, Y.; Wang, Q.; Li, W. AKT regulates hemocyte proliferation via glucose metabolism in Eriocheir sinensis. Fish Shellfish Immunol. 2022, 127, 247–255. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Li, X.; Tao, S.; Wang, F.; Shi, Y.; Guan, H.; Yang, Y.; Zhao, Z. Alkali exposure induces autophagy through activation of the MAPKpathway by ROS and inhibition of mTOR in Eriocheir sinensis. Aquat. Toxicol. 2023, 258, 106481. [Google Scholar] [CrossRef]
- Soegianto, A.; Charmantier-Daures, M.; Trilles, J.; Charmantier, G. Impact of cadmium on the structure of gills and epipodites of the shrimp Penaeus japonicas (Crustacea: Decapoda). Aquat. Living Resour. 1999, 12, 57–70. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Ontogenetic changes in tolerance to acute ammonia exposure and associated gill histological alterations during early juvenile development of the blue swimmer crab, Portunus pelagicus. Aquaculture 2007, 266, 246–254. [Google Scholar] [CrossRef]
- Victor, B. Gill tissue pathogenicity and hemocyte behavior in the crab Paratelphusa hydrodromous exposed to lead chloride. J. Environ. Sci. Health Part A 1994, 29, 1011–1034. [Google Scholar]
- Wang, L.; Xu, T.; Lei, W.; Liu, D.; Li, Y.; Xuan, R.; Ma, J. Cadmium-induced oxidative stress and apoptotic changes in the testis of freshwater crab, Sinopotamon henanense. PLoS ONE 2011, 6, e27853. [Google Scholar] [CrossRef]
- Birnie Gauvin, K.S.S.; Costantini, D.S.S.; Cooke, S.J.S.S.; Willmore, W.G.S.S. A comparative and evolutionary approach to oxidative stress in fish: A review. Fish Fish. 2017, 18, 928–942. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, D.; Wang, F.; Dong, S. Immune responses of L itopenaeus vannamei to non-ionic ammonia stress: A comparative study on shrimps in freshwater and seawater conditions. Aquac. Res. 2017, 48, 177–188. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, G.; Feng, Y.; Kong, C.; Ayisi, C.L.; Huang, X.; Hua, X. Dietary soybean antigen impairs growth and health through stress-induced non-specific immune responses in Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 84, 124–129. [Google Scholar] [CrossRef]
- Frías-Espericueta, M.G.; Bautista-Covarrubias, J.C.; Osuna-Martínez, C.C.; Delgado-Alvarez, C.; Bojórquez, C.; Aguilar-Juárez, M.; Roos-Muñoz, S.; Osuna-López, I.; Páez-Osuna, F. Metals and oxidative stress in aquatic decapod crustaceans: A review with special reference to shrimp and crabs. Aquat. Toxicol. 2022, 242, 106024. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, D.C.; Mejiba, S.E.G.; Mason, R.P. Mechanism of hydrogen peroxide-induced Cu, Zn-superoxide dismutase-centered radical formation as explored by immuno-spin trapping: The role of copper-and carbonate radical anion-mediated oxidations. Free Radic. Biol. Med. 2005, 38, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, O. Manganese complexes displaying superoxide dismutase activity: A balance between different factors. Bioorganic Chem. 2011, 39, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Koh, Y.H.; Park, Y.S.; Fujiwara, N.; Sakiyama, H.; Misonou, Y.; Ookawara, T.; Suzuki, K.; Honke, K.; Taniguchi, N. Oxidative stress caused by inactivation of glutathione peroxidase and adaptive responses. Biol. Chem. 2003, 384, 567–574. [Google Scholar] [CrossRef]
- Jia, R.; Du, J.; Cao, L.; Feng, W.; He, Q.; Xu, P.; Yin, G. Chronic exposure of hydrogen peroxide alters redox state, apoptosis and endoplasmic reticulum stress in common carp (Cyprinus carpio). Aquat. Toxicol. 2020, 229, 105657. [Google Scholar] [CrossRef]
- Yang, X.; Shi, X.; Wu, M.; Pang, Y.; Song, X.; Shi, A.; Niu, C.; Cheng, Y. Effects of melatonin feed on histology and antioxidant ability of the gills and oxygen consumption of Chinese mitten crab (Eriocheir sinensis), exposed to acute hypoxia stress. Aquaculture 2021, 544, 737015. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, H. Metallothionein, antioxidant enzymes and DNA strand breaks as biomarkers of Cd exposure in a marine crab, Charybdis japonica. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Wang, J.; Liu, L.; Li, Y.; Xun, A.; Zeng, W.; Jia, C.; Wei, X.; Feng, J.; Zhao, L.I. Anti-oxidant effects of resveratrol on mice with DSS-induced ulcerative colitis. Arch. Med. Res. 2010, 41, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Nordgren, M.; Fransen, M. Peroxisomal metabolism and oxidative stress. Biochimie 2014, 98, 56–62. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G. Effects of temperature on complexes I and II mediated respiration, ROS generation and oxidative stress status in isolated gill mitochondria of the mud crab Scylla serrata. J. Therm. Biol. 2014, 41, 104–111. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B.N. Antioxidant defenses and oxidative stress parameters in tissues of mud crab (Scylla serrata) with reference to changing salinity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 151, 142–151. [Google Scholar] [CrossRef]
- Xing, Y.; Feng, Y.; Tian, J.; Zhang, Y.; Han, C.; Liu, X.; Yang, J. Nitrite and sulfide stress affects physiological and metabolic functions of gills in crab Eriocheir sinensis. Aquaculture 2025, 594, 741437. [Google Scholar] [CrossRef]
- McNamara, J.C.; Faria, S.C. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: A review. J. Comp. Physiol. B 2012, 182, 997–1014. [Google Scholar] [CrossRef]
- Faleiros, R.O.; Goldman, M.H.S.; Furriel, R.P.; McNamara, J.C. Differential adjustment in gill Na+/K+-and V-ATPase activities and transporter mRNA expression during osmoregulatory acclimation in the cinnamon shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). J. Exp. Biol. 2010, 213, 3894–3905. [Google Scholar] [CrossRef]
- Choe, K.P.; Kato, A.; Hirose, S.; Plata, C.; Sindic, A.; Romero, M.F.; Claiborne, J.B.; Evans, D.H. NHE3 in an ancestral vertebrate: Primary sequence, distribution, localization, and function in gills. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1520–R1534. [Google Scholar] [CrossRef]
- Rahim, S.M.; Mazlan, A.G.; Simon, K.D.; Delaunoy, J.P.; Laurent, P. Immunocytochemical localization of carbonic anhydrase in the pseudobranch tissue of the rainbow trout Oncorhynchus mykiss. J. Zhejiang Univ.Sci. B 2014, 15, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.P.; Moore, M.N.; Readman, J.W.; Mou, Z.; Langston, W.J.; Lowe, D.M.; Frickers, P.E.; Al-Moosawi, L.; Pascoe, C.; Beesley, A. Oxidative stress, lysosomal damage and dysfunctional autophagy in molluscan hepatopancreas (digestive gland) induced by chemical contaminants. Mar. Environ. Res. 2019, 152, 104825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, M.; Jayasundara, N.; Ren, X.; Gao, B.; Liu, P.; Li, J.; Meng, X. Physiological and molecular responses in the gill of the swimming crab Portunus trituberculatus during long-term ammonia Stress. Front. Mar. Sci. 2021, 8, 797241. [Google Scholar] [CrossRef]
- Nan, Y.; Xiao, M.; Duan, Y.; Yang, Y. Toxicity of ammonia stress on the physiological homeostasis in the gills of Litopenaeus vannamei under seawater and low-salinity conditions. Biology 2024, 13, 281. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Jiang, Q.; Ye, Y.; Zhao, Y. Effects of nanoplastic on cell apoptosis and ion regulation in the gills of Macrobrachium nipponense. Environ. Pollut. 2022, 300, 118989. [Google Scholar] [CrossRef]
- Tang, D.; Guo, H.; Shi, X.; Wang, Z. Comparative transcriptome analysis of the gills from the Chinese mitten crab (Eriocheir japonica sinensis) exposed to the heavy metal Cadmium. Turk. J. Fish. Aquat. Sci. 2019, 20, 467–479. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Fowden, H.; Castille, R.; Charmantier, G.; Cramb, G. Aquaporin molecular characterization in the sea-bass (Dicentrarchus labrax): The effect of salinity on AQP1 and AQP3 expression. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Wang, F.; Dong, S. Effects of periodical salinity fluctuation on the growth, molting, energy homeostasis and molting-related gene expression of Litopenaeus vannamei. J. Ocean Univ. China 2016, 15, 911–917. [Google Scholar] [CrossRef]
- Downs, C.A.; Helms, M.N. Regulation of ion transport by oxidants. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2013, 305, L595–L603. [Google Scholar] [CrossRef]
- Orfali, R.; Alwatban, A.Z.; Orfali, R.S.; Lau, L.; Chea, N.; Alotaibi, A.M.; Nam, Y.; Zhang, M. Oxidative stress and ion channels in neurodegenerative diseases. Front. Physiol. 2024, 15, 1320086. [Google Scholar] [CrossRef]
- Yin, J.; Duan, J.; Cui, Z.; Ren, W.; Li, T.; Yin, Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015, 5, 15479–15486. [Google Scholar] [CrossRef]
- Menze, M.A.; Fortner, G.; Nag, S.; Hand, S.C. Mechanisms of apoptosis in Crustacea: What conditions induce versus suppress cell death? Apoptosis 2010, 15, 293–312. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. The pathophysiology of mitochondrial cell death. Science 2004, 305, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jin, X.; Yu, A.; Zhu, Y.; Li, D.; Li, W.; Wang, Q. Caspase-mediated apoptosis in crustaceans: Cloning and functional characterization of EsCaspase-3-like protein from Eriocheir sinensis. Fish Shellfish Immunol. 2014, 41, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Q.; Liu, N.; Jing, W.; Wang, L.; Zhou, F. Hydrogen peroxide leads to cell damage and apoptosis in the gill of the freshwater crab Sinopotamon henanense (Crustacea, Decapoda). Hydrobiologia 2014, 741, 13–21. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Tong, R.; Xu, Q.; Zhang, N.; Liao, Q.; Pan, L. Mechanisms of ammonotelism, epithelium damage, cellular apoptosis, and proliferation in gill of Litopenaeus vannamei under NH4Cl exposure. Environ. Sci. Pollut. Res. 2024, 31, 15153–15171. [Google Scholar] [CrossRef]
- Alarifi, S.; Ali, H.; Alkahtani, S.; Alessia, M.S. Regulation of apoptosis through bcl-2/bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int. J. Nanomed. 2017, 12, 4541–4551. [Google Scholar] [CrossRef]
- Guan, T.; Feng, J.; Zhu, Q.; Wang, L.; Xie, P.; Wang, H.; Li, J. Effects of abamectin on nonspecific immunity, antioxidation, and apoptosis in red swamp crayfish (Procambarus clarkii). Fish Shellfish Immunol. 2023, 142, 109137. [Google Scholar] [CrossRef]
- Liu, J.; Liu, W.; Zhang, C.; Xu, C.; Zheng, X.; Zhang, D.; Chi, C. Dietary glutathione supplementation enhances antioxidant activity and protects against lipopolysaccharide-induced acute hepatopancreatic injury and cell apoptosis in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 97, 440–454. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kon, N.; Jiang, L.; Tan, M.; Ludwig, T.; Zhao, Y.; Baer, R.; Gu, W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell 2012, 149, 1269–1283. [Google Scholar] [CrossRef]
- Sun, S.; Gu, Z.; Fu, H.; Zhu, J.; Ge, X.; Xuan, F. Molecular cloning, characterization, and expression analysis of p53 from the oriental river prawn, Macrobrachium nipponense, in response to hypoxia. Fish Shellfish Immunol. 2016, 54, 68–76. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, N.; Hadden, T.J.; Rishi, A.K. Akt, FoxO and regulation of apoptosis. Biochim. Et Biophys. Acta Mol. Cell Res. 2011, 1813, 1978–1986. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, J.; Chan, S.; Wang, W. Molecular characterization and expression dynamics of three key genes in the PI3K-AKT pathway reveal its involvement in the immunotoxicological responses of the giant river prawn Macrobrachium rosenbergii to acute ammonia and nitrite stress. Ecotoxicol. Environ. Saf. 2021, 208, 111767. [Google Scholar] [CrossRef]
- Huang, P.; Du, J.; Cao, L.; Gao, J.; Li, Q.; Sun, Y.; Shao, N.; Zhang, Y.; Xu, G. Effects of prometryn on oxidative stress, immune response and apoptosis in the hepatopancreas of Eriocheir sinensis (Crustacea: Decapoda). Ecotoxicol. Environ. Saf. 2023, 262, 115159. [Google Scholar] [CrossRef]
- Sanaei, M.; Razi, S.; Pourbagheri-Sigaroodi, A.; Bashash, D. The PI3K/Akt/mTOR pathway in lung cancer; oncogenic alterations, therapeutic opportunities, challenges, and a glance at the application of nanoparticles. Transl. Oncol. 2022, 18, 101364. [Google Scholar] [CrossRef]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and autophagy: Interactions and molecular regulatory mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Suwansa-ard, S.; Kankuan, W.; Thongbuakaew, T.; Saetan, J.; Kornthong, N.; Kruangkum, T.; Khornchatri, K.; Cummins, S.F.; Isidoro, C.; Sobhon, P. Transcriptomic analysis of the autophagy machinery in crustaceans. BMC Genom. 2016, 17, 587. [Google Scholar] [CrossRef]
- Funderburk, S.F.; Wang, Q.J.; Yue, Z. The Beclin 1-VPS34 complex--at the crossroads of autophagy and beyond. Trends Cell Biol. 2010, 20, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, X.; Zheng, C.; Xue, C.; Jin, Y.; Zhou, N.; Sun, S. The evolutionarily conserved hif-1/bnip3 pathway promotes mitophagy and mitochondrial fission in crustacean testes during hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2023, 324, R128–R142. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wu, Y.; Fu, H.; Yang, M.; Ge, X.; Zhu, J.; Xuan, F.; Wu, X. Evaluating expression of autophagy-related genes in oriental river prawn Macrobrachium nipponense as potential biomarkers for hypoxia exposure. Ecotoxicol. Environ. Saf. 2019, 171, 484–492. [Google Scholar] [CrossRef]
- Yao, Y.; Meng, X.; Fu, H.; Lu, S.; Yang, J.; Geng, C.; Li, X.; Gu, W.; Zhou, J.; Meng, Q. Two autophagy-related proteins, ATG5 and ATG7, from Eriocheir sinensis involved into Spiroplasma eriocheiris infection. Aquaculture 2024, 582, 740556. [Google Scholar] [CrossRef]
- Davis, R.J. Signal transduction by the JNK group of MAP kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef]
- Wagner, E.F.; Nebreda, Á.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef]
- Shi, Y.; Nikulenkov, F.; Zawacka-Pankau, J.; Li, H.; Gabdoulline, R.; Xu, J.; Eriksson, S.; Hedström, E.; Issaeva, N.; Kel, A. ROS-dependent activation of JNK converts p53 into an efficient inhibitor of oncogenes leading to robust apoptosis. Cell Death Differ. 2014, 21, 612–623. [Google Scholar] [CrossRef]
- Guyton, K.Z.; Liu, Y.; Gorospe, M.; Xu, Q.; Holbrook, N.J. Activation of mitogen-activated protein kinase by H2O2: Role in cell survival following oxidant injury. J. Biol. Chem. 1996, 271, 4138–4142. [Google Scholar] [CrossRef]
- Peng, T.; Wang, W.; Gu, M.; Xie, C.; Xiao, Y.; Liu, Y.; Wang, L. Essential roles of Cdc42 and MAPK in cadmium-induced apoptosis in Litopenaeus vannamei. Aquat. Toxicol. 2015, 163, 89–96. [Google Scholar] [CrossRef]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of copper exposure on oxidative stress, apoptosis, endoplasmic reticulum stress, autophagy and immune response in different tissues of Chinese mitten crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Yan, B.; Liu, X.; Zhou, Y.; Zhang, M.; Fang, P.; Jiang, M.; Yuan, R.; Hu, X.; Cao, G.; Xue, R. Transcriptomic analysis reveals that hepatopancreatic necrosis disease in Eriocheir sinensis (Chinese mitten crabs) may be the result of autophagy and apoptosis. Aquaculture 2020, 515, 734579. [Google Scholar] [CrossRef]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef]
| Gene | Description | Primer Sequence (5′-3′) | Product Length (bp) | GenBank Accession Number |
|---|---|---|---|---|
| NHE3 | Na+/H+ exchanger 3 | CGCAACCCACCGTAGCTCAT TGCTCCGTCTACCGCATCCT | 113 | SRR769751 |
| NKAα | Na+/K+-ATPase subunit α | TCTGCTTCATCGCCTACTCCA AGGACTCCATGATACGCGAAC | 153 | KC691291.1 |
| CA | carbonic anhydrase | CTCGCAGTTCCACTTCCACT CGTGTTTCAATGCCTCGTCC | 122 | XM_050884291.1 |
| NKCC1 | Na+-K+-2Cl− cotransporter-1 | TGCCTCAGGGTCTTGACTACTCC GCCTCACTGTCTGTTCCGTCT | 154 | MF062032.1 |
| VATP | V-type proton ATPase subunit d1-like | AGTTGATGCCTAAATGCC TCGTCCAAGTCCTGCTC | 154 | XM_050847128.1 [25] |
| AQP7 | aquaporin 7 | CACTCTCGTTGGTGGATGGG GTGGAGGTGTCCTGGTGC | 201 | XM_050868012 |
| CLCN2 | Chloride channel protein 2 | CAGCCCTCAAGCAAACA GGAGGCGATGGCTATTT | 192 | XM_050870418.1 [25] |
| jnk | c-Jun N-terminal Kinase | TACAGTAGAGGTGGGCGACA TAGGCTCGCTTGGCATGAG | 180 | KC900087 |
| p38 | p38 mitogen-activated protein kinase | AAGATCACCAGCGATGAGGC TGCTAGGTAGGGATGGGCAA | 183 | KF582665.1 |
| erk | extracellular signal-regulated kinase 2 | CGCGAGTTGCAGATCCAGAA CAAGGGGCGATTGGACAACA | 170 | GU002542.1 [26] |
| Caspase-8 | Cysteine-aspartic acid protease 8 | TGGAGCGTCATGGTTCAGAC CAGACAAGCCACCACTGCTA | 161 | AKS36884.1 |
| Caspase-3 | Cysteine-aspartic acid protease 3 | GCTGCTAAGCCAGTAGGCTG CATATTGCCCACGCTCTGGAA | 130 | MH183147.1 |
| Bcl2 | bcl-2-like protein1 | AAAAGGAACCTGTGGCGTCT GAGACGGCGAGCCTTGATAA | 209 | XM_050860189.1 |
| P53 | cellular tumor antigen p53-like | TCGACATGGAAGGGAAGCAC CTGACTTCAAACGGCACAGC | 139 | JQ613218.1 |
| AKT | AKT-threonine/serine protein kinase | CAAGATCCTGCGCAAAGACG CATGACGAAGCAGAGACGGT | 148 | KY412800.1 [27] |
| ATG7 | ubiquitin-like modifier-activating enzyme ATG7 | GCTCTGGGCTTTGACTCCTT TCGTGTGTGGAATTCCCTGG | 167 | MT543027.1 |
| ampkβ | 5′-AMP-activated protein kinase subunit beta-1 | CAATCGTTGACCTCCCAGAA ACTTCCCTTTCCTTCCCAGAG | 232 | MK676045.1 |
| mTOR | serine/threonine-protein kinase mTOR-like | AGAAGCTGCATGACTGGGAC CGGTCACACGACACACTGTA | 148 | XM_050855996.1 [28] |
| Beclin-1 | beclin-1-like protein | GCCCATATACTGTGGCGAGG CCAGGTCAAAGAGCCCAGTT | 176 | MH173046.1 |
| UBE | internal standard gene | TTGCGTTCACAACTCGTATCTACC GTCCGTGAGGAGGGAACAGA | 137 | HQ436509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; He, Q.; Yang, Q.; Xu, Y.; Jiang, G.; Li, J.; Zhou, J.; Jia, R.; Tang, Y. Oxidative Stress Disrupts Gill Function in Eriocheir sinensis: Consequences for Ion Transport, Apoptosis, and Autophagy. Antioxidants 2025, 14, 897. https://doi.org/10.3390/antiox14080897
Feng W, He Q, Yang Q, Xu Y, Jiang G, Li J, Zhou J, Jia R, Tang Y. Oxidative Stress Disrupts Gill Function in Eriocheir sinensis: Consequences for Ion Transport, Apoptosis, and Autophagy. Antioxidants. 2025; 14(8):897. https://doi.org/10.3390/antiox14080897
Chicago/Turabian StyleFeng, Wenrong, Qinghong He, Qiqin Yang, Yuanfeng Xu, Gang Jiang, Jianlin Li, Jun Zhou, Rui Jia, and Yongkai Tang. 2025. "Oxidative Stress Disrupts Gill Function in Eriocheir sinensis: Consequences for Ion Transport, Apoptosis, and Autophagy" Antioxidants 14, no. 8: 897. https://doi.org/10.3390/antiox14080897
APA StyleFeng, W., He, Q., Yang, Q., Xu, Y., Jiang, G., Li, J., Zhou, J., Jia, R., & Tang, Y. (2025). Oxidative Stress Disrupts Gill Function in Eriocheir sinensis: Consequences for Ion Transport, Apoptosis, and Autophagy. Antioxidants, 14(8), 897. https://doi.org/10.3390/antiox14080897






