Abstract
Erythrina caffra is a traditional plant used to treat cancer and inflammation. The study aimed to assess and isolate anticancer compounds from E. caffra bark. The plant material was extracted sequentially in n-hexane, dichloromethane, ethyl acetate and methanol. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging and 3-(4,5-di methyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were used to evaluate the crude extracts’ antioxidant and anticancer activities, respectively. Column chromatography was used to purify the potent extracts of the stem bark in order to isolate the bioactive compounds. The crude extracts of the E. caffra bark demonstrated antioxidant and anticancer activity, with the dichloromethane (DCM) extract producing the most favorable activity. Three compounds, namely Hexacosanyl isoferulate, Tetradecyl isoferulate, and 1-Heneicosanol, were detected in fractions from the DCM extract. All the isolated compounds showed significant anticancer potential, with the hydroxycinnamic acid compounds showing better anticancer effects in the cervical (HeLa) and breast cancer (MCF-7) cells. The compounds showed greater activity than even the standard drug, 5-fluorouracil, in the MCF-7 cells, with the tetradecyl isoferulate and hexacosanyl isoferulate fractions having IC50 values of 123.62 and 58.84 µg/mL, respectively. The compounds were observed to be capable of triggering caspase cascade events, leading to apoptotic cell death. Overall, E. caffra extracts contained important bioactive compounds that induced apoptotic cell death in HeLa and MCF-7 tumor cells, warranting further investigations in vitro and in vivo.
Keywords:
Erythrina caffra; anticancer; antioxidant; bioactivity; phytochemicals; ferulate; apoptosis 1. Introduction
Since the mid-20th century, there has been a surge in the incidence of cancer. This situation has been linked to a variety of unhealthy changes, such as lifestyle, diet, environment, and diseases [1]. According to GLOBOCAN estimates for 2022, about one in five individuals, both men and women, will be diagnosed with cancer during their lifetime, while approximately one in nine men and one in twelve women will die from the disease [2]. The problem of cancer is far from solved, despite the many successful treatments that have been developed over the years. As the incidence of cancer continues to rise, finding effective and safe treatments for this disease remains a priority. Among the available cancer treatments, chemotherapy is still one of the most widely used and successful approaches. Chemotherapy has proved to be a useful procedure in the fight against cancer since it helps to reduce tumor size and prevent cancer from spreading to other parts of the body [3]. Despite the advances made in chemotherapy, the search for improved treatments and better outcomes for cancer patients continues. The use of medicinal plants as a source of biologically active compounds, including antioxidants and anticancer drugs, is a promising area of research. Many plant-derived compounds, such as paclitaxel and vincristine, have already made significant contributions to cancer therapy [4]. With the increasing success of this approach, wider research is needed to develop more effective treatments and to better understand the potential of plants in disease management.
One of the interesting medicinal plants with therapeutic potential is Erythrina caffra (E. caffra. Commonly known as the coastal coral tree, it is an ethnomedicinal plant belonging to the family Fabaceae. Traditional healers in Southern Africa have long utilized various parts of the plants for the treatment of cancer, inflammation and wounds [5]. E. caffra contains diverse bioactive compounds such as alkaloids, flavonoids, terpenoids, phenolics and saponins [6]. The extracts from E. caffra have been found to have a number of biological and pharmacological activities. They have been shown to possess antioxidant, anti-inflammatory, and antimicrobial properties, linked to the presence of several bioactive constituents in the plant [7].
In recent years, a number of experimental studies have been conducted to examine the biomedical properties of extracts from E. caffra [8,9]. The findings of these studies have been promising, indicating that E. caffra extracts could be a potential source of natural therapeutic and anticancer agents [8,9]. Moreover, some researchers were able to identify several phytoalexins from the root bark of E. caffra, including caffraisoflavan I and caffraisoflavan II, erystagallin A, erythrabyssin I, erythrabyssin II, abyssinone II, abyssinone IV and abyssinone V [9]. These studies point to the therapeutic and anticancer prospects of E. caffra. Despite the significance of these studies, there are currently no reports on bioactive anticancer compounds from the stem bark of E. caffra. In addition, the underlying mechanism implicated in the anticancer effects of E. caffra remains unclear. Considering that phytochemical profiles can vary based on geographical origin, plant part, and environmental conditions, further investigation is warranted [10].
Taking these conditions into account, the current study aims to evaluate the anticancer potential of E. caffra from South Africa and purify bioactive anticancer compounds that could be developed as drug candidates for cancer treatment.
2. Materials and Methods
2.1. Materials
Hexane (>99%, CAS 110-54-3), dichloromethane (>99%, CAS 75-09-2), ethyl acetate (>99%, CAS 141-78-6), methanol (>99%, CAS 67-56-1), ascorbic acid, phosphate-buffered saline (PBS) tablets, sodium carbonate, dimethyl sulfoxide (DMSO), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were procured from Merck (Darmstadt, Germany). The 2,2-diphenyl-1-picrylhydrazyl (DPPH), acridine orange, and ethidium bromide were obtained from Sigma Aldrich (St Louis, MO, USA). Cell culture reagents: Eagle’s minimum essential medium (EMEM), fetal bovine serum (FBS), trypsin and antibiotics (Penicillin (5000 units/mL)/Streptomycin (5000 µg/mL)) were purchased from Lonza Bio-Whittaker (Verviers, Belgium). All sterile tissue culture plasticware was supplied by Corning Inc. (New York, NY, USA). The human embryonic kidney (HEK293, CRL-1573), cervical cancer (HeLa, CCL-2) and breast cancer (MCF-7, HTB-22) cells were originally sourced from the American Type Culture Collection (Manassas, VA, USA), with mycoplasma testing performed routinely before in vitro studies. All other reagents were of analytical grade and were locally sourced. The experiments were conducted as presented in the workflow in Supplementary Figure S1.
2.2. Plant Collection
The E. caffra stem bark was collected from a tree growing within the University of KwaZulu-Natal, Westville (29.8674° S, 30.9807° E). The plant was authenticated at the Department of Biology, University of KwaZulu-Natal, and a voucher specimen (F. Olawale 1) was deposited in the Ward Herbarium of the university.
2.3. Sample Extraction
The harvested bark was air-dried and pulverized. Subsequently, about 1 kg of the sample was subjected to sequential solvent extraction in n-hexane, dichloromethane, ethyl acetate and methanol by continuous stirring in a mechanical shaker at 80 rpm for 72 h. The filtrate was concentrated using a rotary evaporator, air-dried to a constant weight and the crude extracts obtained were analyzed for their antioxidant and anticancer activities.
2.4. Antioxidant Study by 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
The antioxidant potential of the crude extracts was evaluated by measuring their DPPH radical scavenging activity following the protocol described by Bukhari et al. [11]. Approximately 50 µL of a 0.3 mM DPPH solution (in methanol) was incubated with 100 µL of the extracts or ascorbic acid standard (with varying concentrations from 50 to 250 µg/mL) in 96-well plates. A control well containing 5% dimethyl sulfoxide (DMSO) and DPPH was run concurrently. The samples were incubated in the dark for 30 min at room temperature. The absorbance was read against a DMSO blank at a wavelength of 517 nm using a microplate reader. DPPH scavenging activity was evaluated using Equation (1).
where Ac = absorbance of control, and As = sample absorbance
The IC50 values were calculated directly using GraphPad Prism version 9.0, where the data were used to fit a linear or non-linear regression model.
2.5. MTT Cytotoxicity Assay
Cytotoxicity was evaluated using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay to measure cell viability after treatment with the plant extracts. This assay measures the percentage viability of a cell population by monitoring the ability of the NAD(P)H-dependent oxidoreductase enzyme system in live cells to reduce MTT to formazan, an insoluble purple form of the dye [12]. Confluent MCF-7 cells were seeded in 96-well plates at 2.0 × 104 cells/well and incubated for 24 h at 37 °C in 5% CO2. The medium was replaced with fresh medium, and the extracts were added in varying concentrations (50–250 µg/mL). The plates were incubated at 37 °C in 5% CO2 for 48 h. At the end of the incubation period, the spent medium was discarded and replaced with 100 µL fresh medium containing 10 µL (5 mg/mL in PBS) MTT reagent. The cells were then incubated for an additional 4 h. The MTT/medium was subsequently removed, and 100 μL DMSO was added to solubilize the formazan dye product. Absorbance at 570 nm was assessed using a microplate reader (Vacutec, Hamburg, Germany). Cell viability was calculated as in Equation (2).
where At = absorbance of test or treated cells and Ac = absorbance of control or untreated cells.
2.6. Acridine Orange/Ethidium Bromide Dual Staining for Apoptosis
A qualitative assessment of apoptosis induction in cancer cells was conducted by the acridine orange-ethidium bromide (AO/EB) dual staining method, as described previously [13,14]. Confluent cells (4.0 × 103 cells/well) were trypsinized and seeded into 48-well plates and incubated for 24 h at 37 °C in 5% CO2. Subsequently, the spent medium was replaced, and cells were incubated for a further 48 h with the extracts or standard drug (5-fluorouracil) at a concentration of 250 µg/mL. The cells were rinsed twice with cold PBS, and 15 µL of the AO/EB dye mixture (0.1 mg/mL: 0.1 mg/mL) was added, and the cells were incubated for 5 min at ambient temperature. The cells were washed with PBS to remove excess dye and then visualized under an Olympus fluorescence microscope (Wirsam Scientific and Precision Equipment Ltd., Johannesburg, South Africa). Images were captured using a CC12 fluorescence camera and Analysis Five Software (Olympus Soft Imaging Solutions, Olympus, Tokyo, Japan) at 200× magnification.
2.7. Isolation of Bioactive Fractions of E. caffra and NMR Characterization
Column chromatography (CC) was conducted by the wet packing method using silica gel 60 (0.040–0.063 mm, Merck, Darmstadt, Germany) and n-hexane. A 4 g portion of the crude dichloromethane (DCM) extract was subjected to column chromatography using hexane/DCM as the mobile phase. The column was eluted using a gradient solvent system starting with a mixture of 100% n-hexane, n-hexane/DCM, and 100% DCM. From the column chromatography, a total of 19 aliquots (about 100 mL) were collected, which were later spotted on thin-layer chromatography (TLC) plates (silica gel 60 F254). The plates were first subjected to UV irradiation at 254 nm and 366 nm. This was followed by spraying with 10% sulfuric acid in methanol (MeOH) solution, and heating to visualize the fractions on the plates. Similar aliquots were combined into four fractions: fraction 1 (1–8), fraction 2 (9–13), fraction 3 (14–16), and fraction 4 (17–19).
2.8. Qualitative Analysis
Fraction 1 yielded compound 3, fraction 3 yielded compound 2, and fraction 4 yielded compound 1, as determined by the 1H and 13C NMR and HRMS analysis.
2.8.1. NMR Characterization
The fractions were later analyzed via NMR spectroscopy and mass spectrometry. 1H and 13C NMR spectra were recorded in deuterated chloroform (CDCl3) at room temperature on a Bruker Avance III 400 MHz spectrometer (Bruker Corporation, Johannesburg, South Africa).
2.8.2. GC–MS Characterization
A single quadrupole Shimadzu GC–MS GC–MS-QP2010SE gas chromatograph–mass spectrometer (Shimadzu, Shiga, Japan) was used to analyse the phytochemicals from the plant extract, by direct injection of the sample. The data interpretation and peak identification were carried out by comparing the molecular spectra to those of substances with related spectra in the National Institute of Standards and Technology (NIST) database.
2.8.3. High-Resolution Mass Spectrometry (HRMS)
High-resolution electrospray ionisation mass spectra of the samples were obtained on a Waters Micromass LCT Premier TOF-MS instrument (Waters Corporation, Milford, MA, USA).
2.9. MultiCaspase Analysis
The multicaspase assay was performed using the Muse MultiCaspase kit, which can detect the presence of multiple caspases (caspase-1, 3, 4, 5, 6, 7, 8, and 9). The kit utilizes a derivatized VAD peptide that can detect the activity of multiple caspases and a dead cell dye that provides information on membrane integrity or cell death. Cells were seeded into 48-well plates at a density of 1.0 × 105 cells/well and allowed to attach over 24 h. Subsequently, 100 µg/mL of the isolated fractions were added, and the cells were incubated for 48 h. The cells were then trypsinized and reconstituted in 1 × caspase buffer. Approximately 5 μL of the Muse™ MultiCaspase working solution was added to 50 μL of cell suspension and incubated at 37 °C for 30 min. Thereafter, 150 μL of 7-AAD working solution was added, mixed thoroughly, and analyzed using the Muse™ cell analyzer (Luminex Corporation, Austin, TX, USA).
2.10. Statistical Analysis
The data in this study are presented as mean ± standard deviation (SD) from three independent replicates (n = 3). Statistical analysis was performed using GraphPad Prism version 9.0 (GraphPad Software Inc., San Diego, CA, USA). One-way analysis of variance (ANOVA) was used to assess statistical differences, and Tukey’s post hoc test was applied when the ANOVA results were statistically significant (p < 0.05).
3. Results
3.1. Antioxidant and Anticancer Activity of Crude Extracts of Erythrina caffra Stem Bark
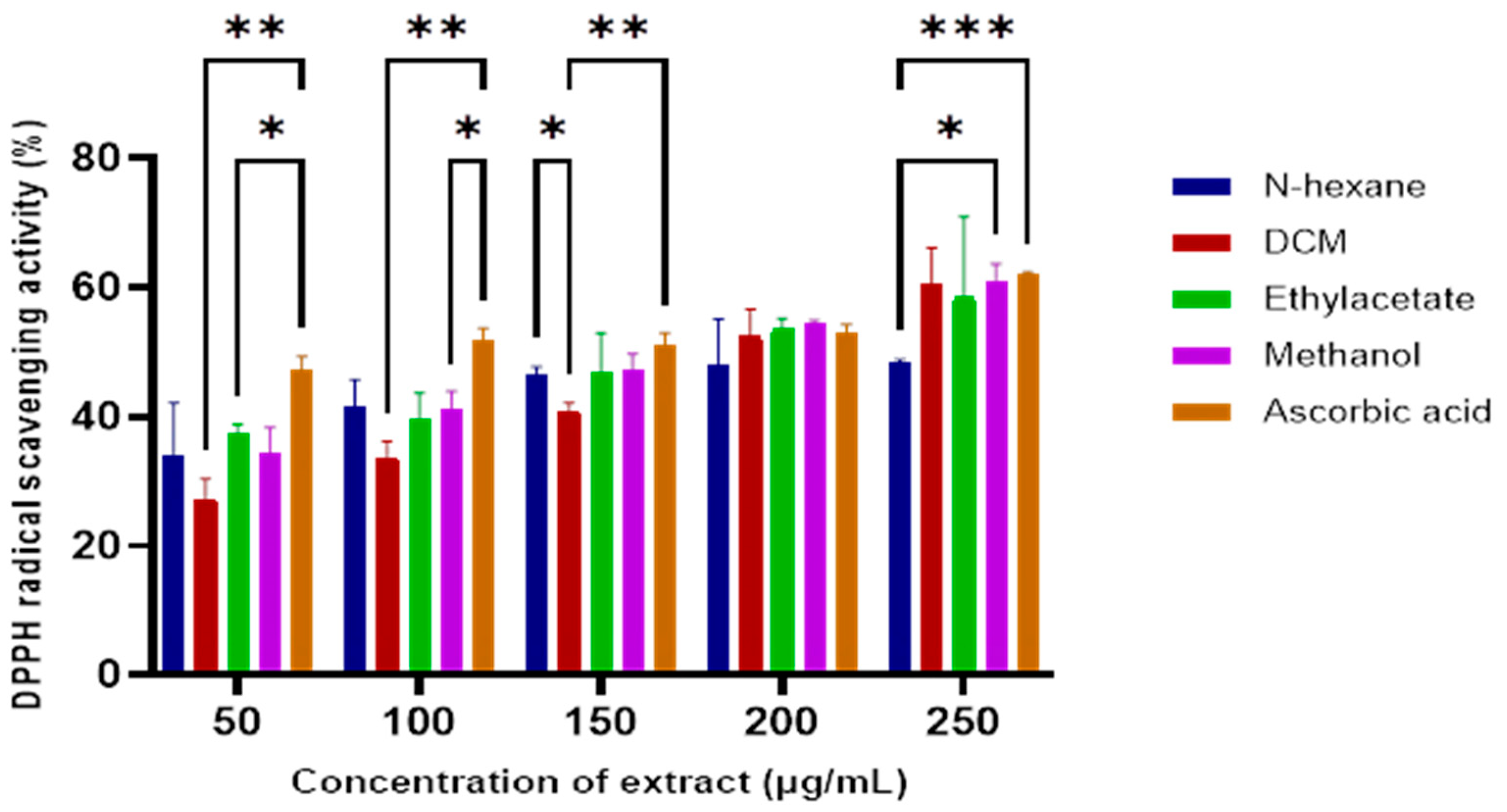
Crude extracts from the sequential extraction of the E. caffra stem bark were evaluated for antioxidant activity using the DPPH radical scavenging assay. As illustrated in Figure 1, increasing concentrations of the extracts corresponded to increased DPPH scavenging activity. The dichloromethane (DCM) extract exhibited the most potent antioxidant activity from all tested extracts, with an IC50 value of 144.17 µg/mL (Table 1). The antioxidant activity index of these extracts was comparable to that of ascorbic acid, indicating their potential in chemoprevention.

Figure 1.
The DPPH radical scavenging activity of E. caffra stem bark extracts compared to the ascorbic acid standard. Data are presented as mean ±SD (n = 3). * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001, for statistical significance.

Table 1.
IC50 values (µg/mL) of E. caffra bark extract in DPPH radical scavenging and anticancer activities.
Although no prior reports exist on the antioxidant activity of the E. caffra bark specifically, earlier studies of the Erythrina genus have shown significant antioxidant capacity both in vitro and in vivo [7,15,16]. Since oxidative stress can cause DNA damage, leading to altered signaling and cancer development, the antioxidant properties of the extracts could contribute to cancer prevention.
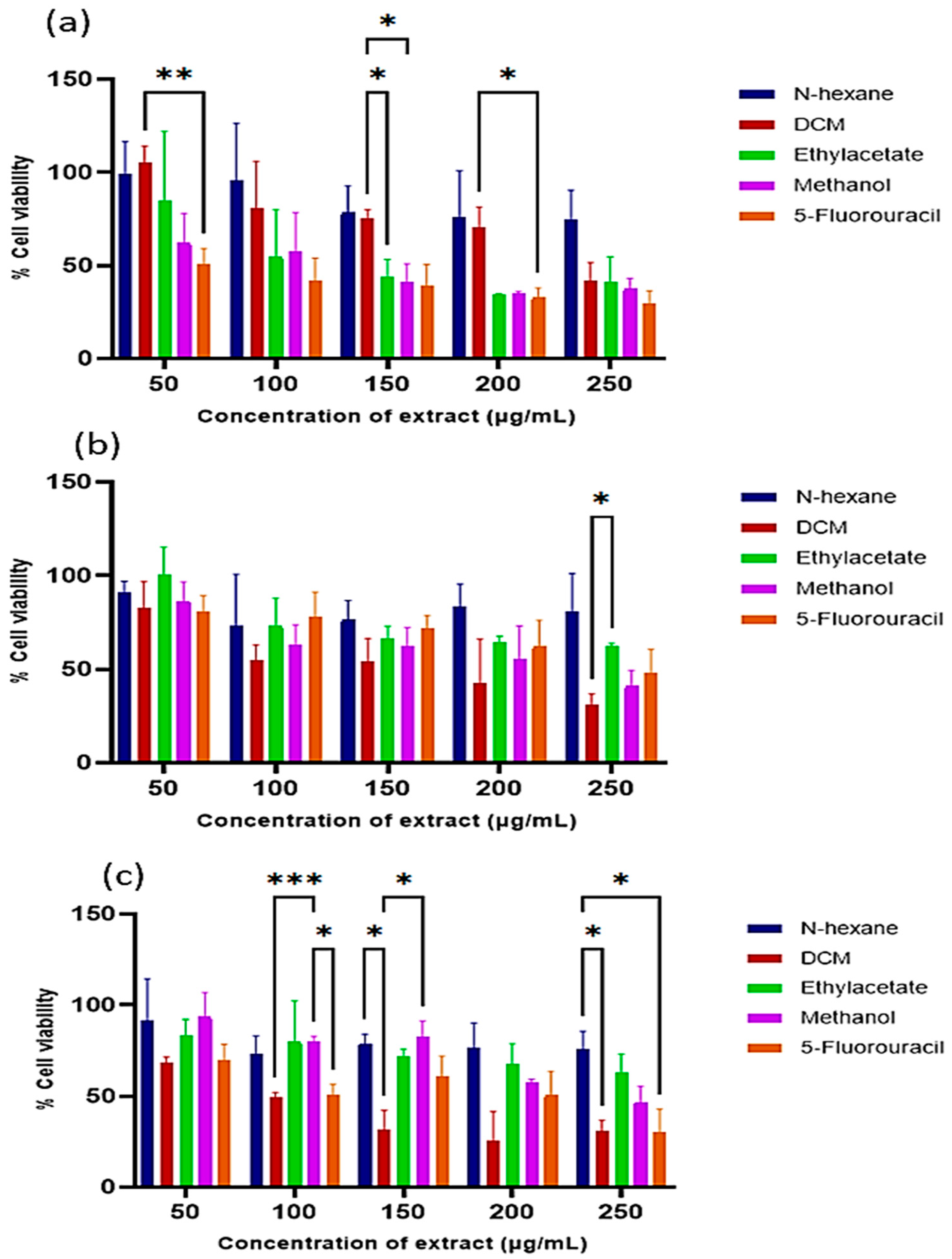
The cytotoxicity of the extracts was evaluated in the human HEK293 (served as normal cells), HeLa (cervical cancer), and MCF-7 (breast cancer) cells. The extracts exhibited the highest cytotoxicity at 250 µg/mL (Figure 2) with a dose-dependent effect. The DCM extract again showed the most potent anticancer activity, with IC50 values of 273.47 µg/mL (HEK293), 93.82 µg/mL (HeLa), and 144.17 µg/mL (MCF-7) (Table 1). Notably, the extracts exerted greater cytotoxic effects in cancer cells compared to normal cells.

Figure 2.
Cytotoxicity of the E. caffra bark extracts on: (a) HEK293, (b) HeLa, and (c) MCF-7 cells. Data are presented as mean percentage cell viability ± SD (n = 3). * p < 0.05, ** p ≤ 0.01, *** p ≤ 0.001, for statistical significance.
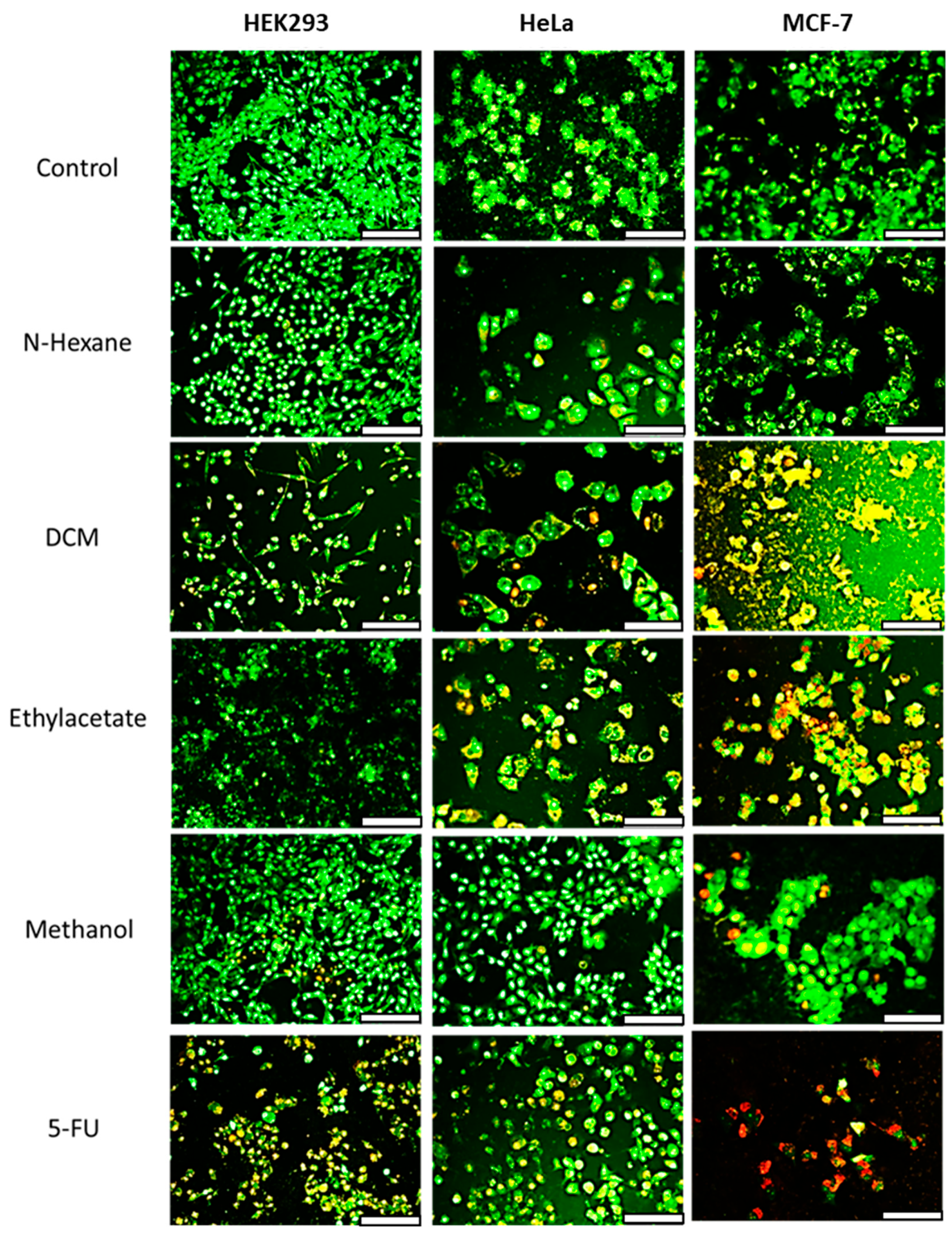
Further insights into the mechanism of cell death were obtained using acridine orange/ethidium bromide (AO/EB) dual staining (Figure 3). Cells treated with the n-hexane extract displayed green fluorescence, indicating viable cells with intact double-stranded DNA. HEK293 cells treated with extracts showed green to orange fluorescence, suggesting early apoptosis. HeLa and MCF-7 cells treated with the extracts exhibited early to late apoptotic features, including condensed green nuclei and red fluorescence indicative of fragmented DNA. The standard anticancer drug 5-FU induced red fluorescence in MCF-7 cells due to ethidium bromide binding to the DNA fragments, confirming apoptosis. Thus, treatment with the extracts induced apoptotic cell death in the cancer cells.

Figure 3.
Fluorescent images of acridine orange/ethidium bromide dual-stained HEK293, HeLa and MCF-7 cells treated with 100 µg/mL extracts of E. caffra. Scale Bar = 100 µm.
3.2. Chemical Characterization of Isolated Compounds
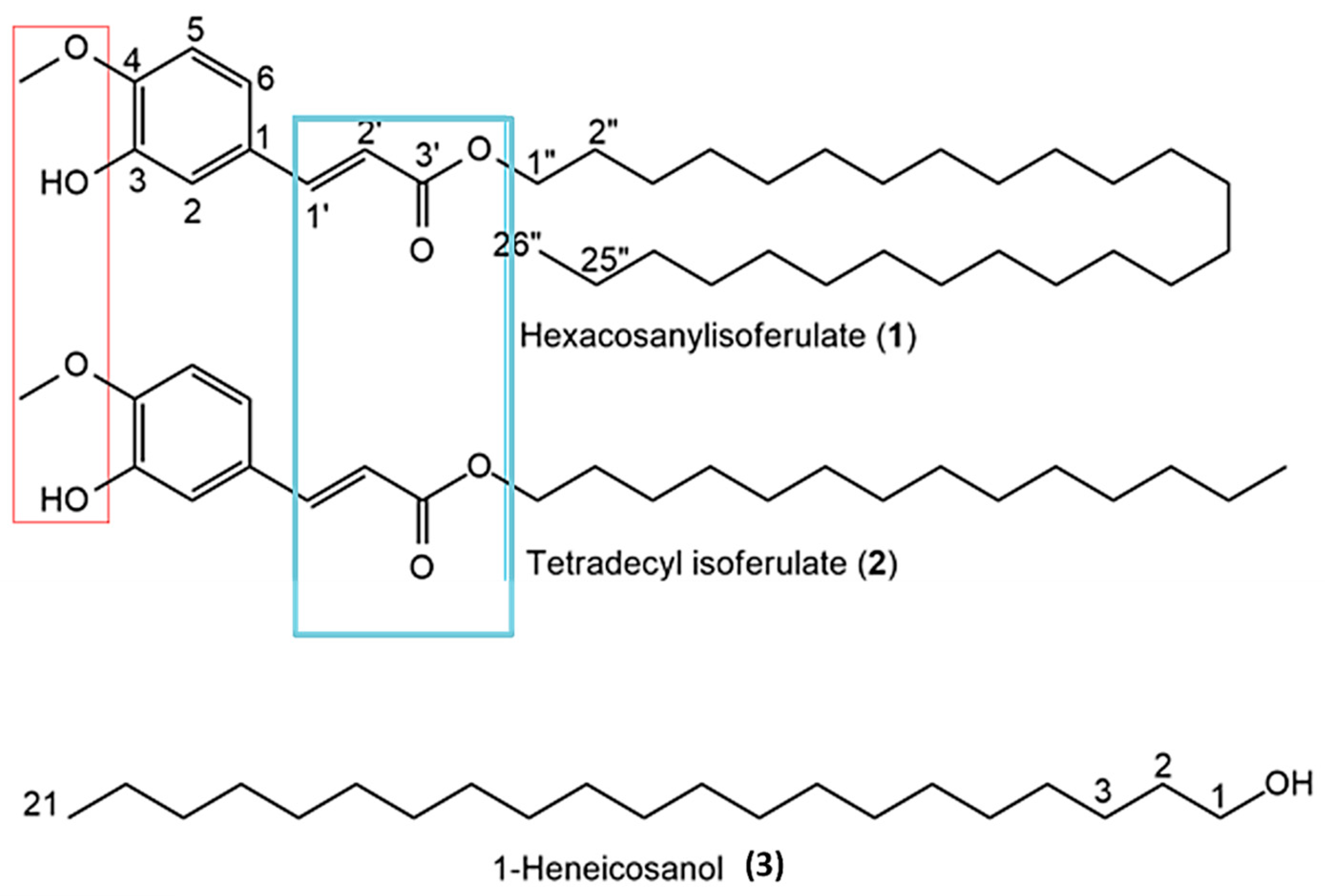
Due to the superior antioxidant and anticancer activity of the DCM extract (Table 1), it was selected for the purification of bioactive compounds via column chromatography. Three different compounds were isolated. All NMR spectra, HR-ESI-MS, and GC–MS data are provided in Supplementary Figure S2a–i. The chemical structures of the isolated compounds, including shared functional groups, are shown in Figure 4.

Figure 4.
Chemical structures of compounds isolated from Erythrina caffra. Red and blue boxes show common functionalities that are likely responsible for bioactivities.
Compound 1 (201 mg) was isolated as a yellow amorphous solid with molecular formula C36H62O4, confirmed by HR-ESI-MS (m/z 557.4571 [M–H]−; calcd 557.4570). Its 1H NMR (400 MHz, CDCl3) data: δ_H 0.85 (3H, t, J = 6.5 Hz; terminal CH3), 1.19–1.31 (–(CH2)n–), 3.90 (3H, s; 4-OMe), 4.16 (2H, t, J = 6.7 Hz; –OCH2–), 6.28 (1H, d, J = 15.9 Hz; =CH, H-2’), 6.89 (1H, d, J = 8.1 Hz; H-5), 7.01 (H-2), 7.06 (H-6), 7.60 (1H, d, J = 15.9 Hz; =CH, H-’). 13C NMR (CDCl3): δ_C 14.1, 22.7, 25.8, 28.8, 29.3–29.6, 31.9, 55.9 (4-OMe), 64.7, 109.3, 114.7, 115.6, 123.0, 125.8, 144.7, 168.2. Based on a literature comparison, compound 1 was identified as n-Hexacosanyl isoferulate [17].
Compound 2 (137 mg) was a yellow solid with molecular formula C24H38O4 from HR-ESI-MS (m/z 391.2844 [M+H]+; calcd 391.2848). Its 1H NMR data closely resembled compound 1, but with differences indicating a shorter hydrocarbon chain (δ_H 0.85 (3H, t, J = 6.6 Hz), 1.17–1.37 (–(CH2)n–), 3.90 (3H, s; 4-OMe), 4.16 (2H, t, J = 6.7 Hz), 6.28 (1H, d, J = 15.9 Hz), 6.90 (1H, d, J = 8.1 Hz), 7.01 (1H, d, J = 1.6 Hz), 7.06 (1H, dd, J = 8.1 and 1.6 Hz), 7.60 (1H, d, J = 15.9 Hz)). The compound was characterized as tetradecyl isoferulate, a derivative similar to Tetradecyl ferulate previously found in Erythrina sigmoidea [17].
Compound 3 (73 mg) was a white crystalline solid identified as 1-heneicosanol with molecular formula C21H44O (mass 312, GC–MS data). Its 1H NMR showed typical fatty alcohol signals: δ_H 0.85 (3H, t, J = 6.8 Hz; H-21), 1.23–1.56 ((CH2)19; H-2–H-20), 3.61 (2H, t, J = 6.6 Hz; H-1), and 13C NMR signals at δ_C 14.1, 22.7, 25.7, 29.3–29.7, 31.9, 32.8, 63.1, consistent with the literature for 1-Heneicosanol from Senecio coluhuapiensis [18].
3.3. Anticancer Activity of Isolated Bioactive Compounds from Erythrina caffra
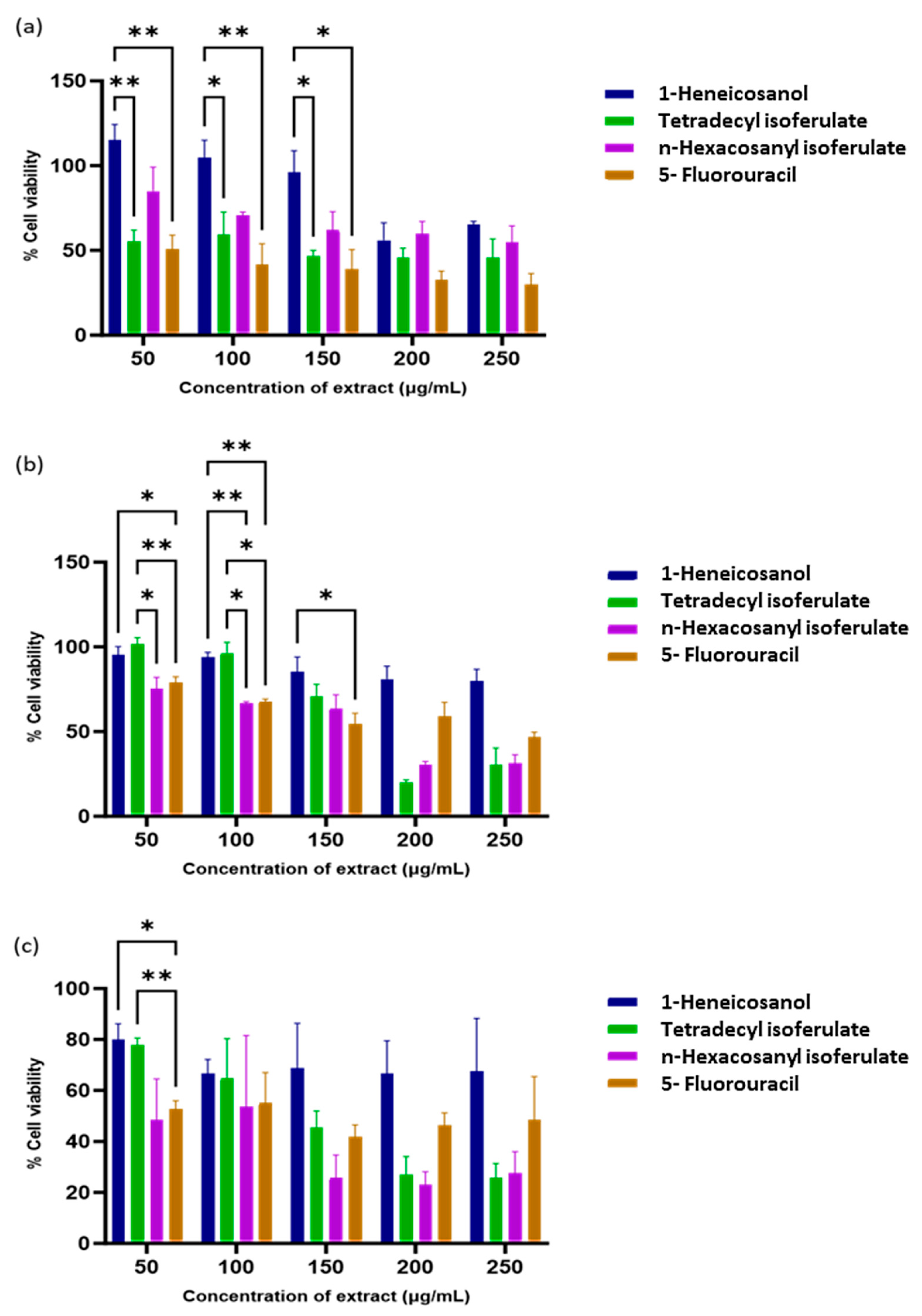
The isolated compounds were assessed for anticancer activity against normal and cancer cell lines. All fractions showed significant dose-dependent cytotoxic effects (Table 2).

Table 2.
The IC50 values of isolated compounds from Erythrina caffra in the HEK293, MCF-7, and HeLa cells.
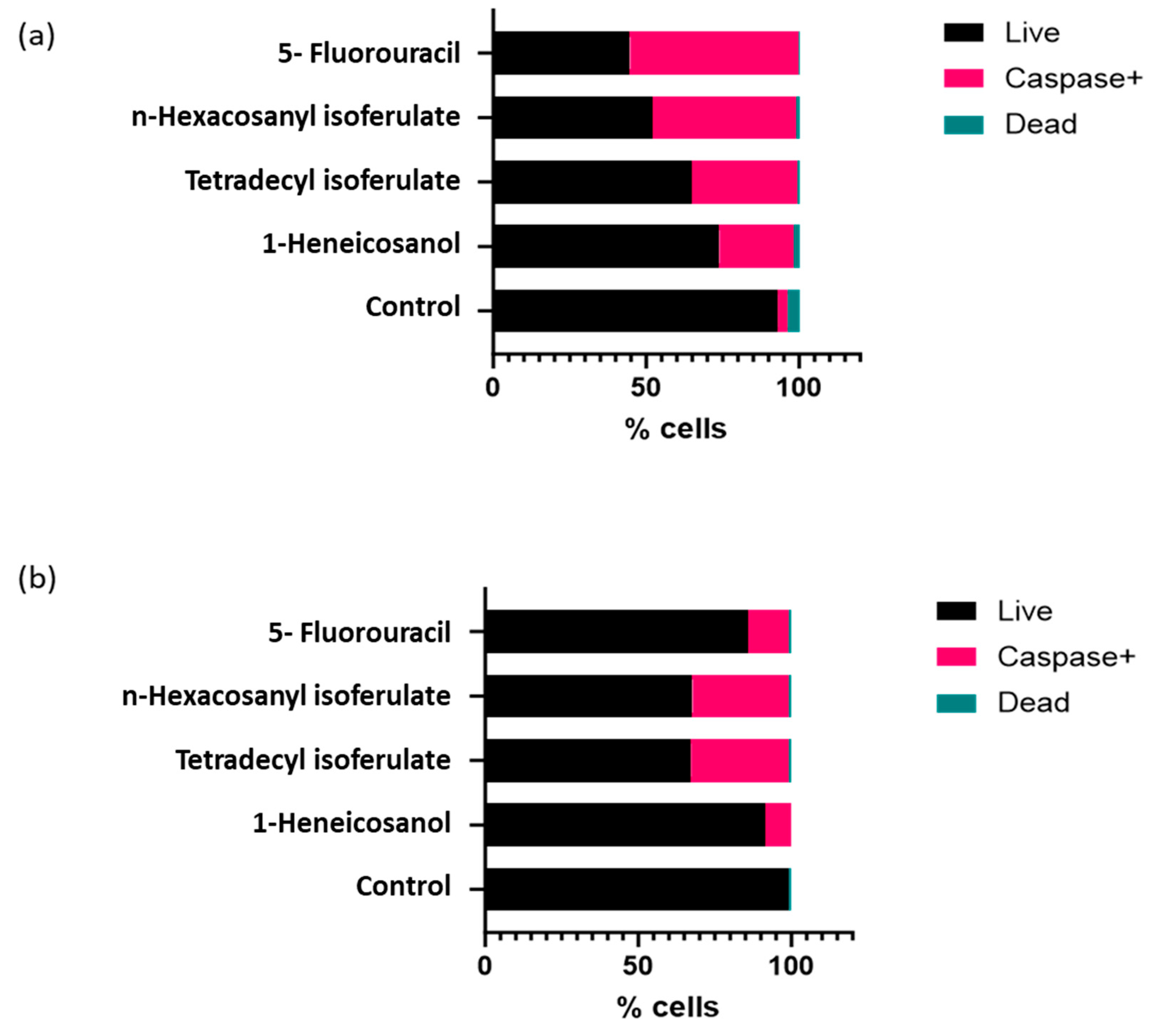
From the isolated compounds, 1-heneicosanol exhibited the weakest cytotoxicity, while the ferulate derivatives—n-hexacosanyl isoferulate and tetradecyl isoferulate—demonstrated comparable and significant anticancer effects. Notably, n-hexacosanyl isoferulate demonstrated the most potent anticancer activity, with IC50 values of 58.8 µg/mL and 146.6 µg/mL in the MCF-7 and HeLa cells (Table 2). The ferulic acid derivatives, tetradecyl isoferulate and n-hexacosanyl isoferulate, demonstrated pronounced caspase activation potential (Figure 5 and Figure 6, Supplementary Figure S3).

Figure 5.
Image showing the effects of the identified E. caffra fractions on caspase expression in the (a) HeLa and (b) MCF-7 cells.

Figure 6.
Cytotoxicity of E. caffra fractions on: (a) HEK293, (b) HeLa, and (c) MCF-7 cells. Data are presented as mean percentage cell viability ± SD (n = 3). * p < 0.05 and ** p ≤ 0.01 for statistical significance.
4. Discussion
This study presents significant evidence that the stem bark of Erythrina caffra contains bioactive compounds with antioxidant and anticancer properties. These findings extend previous investigations on other Erythrina species and support the growing body of evidence suggesting that this genus possesses considerable antioxidant and anticancer potential [9,19,20].
The DCM extract of E. caffra showed the highest antioxidant activity from all tested fractions, with an IC50 of 144.17 µg/mL. While antioxidant and anticancer activities have previously been reported in other Erythrina species such as E. abyssinica and E. variegata, the current study is the first to our knowledge to specifically examine the stem bark of E. caffra [21,22]. The observed antioxidant effects are likely due to phenolic compounds, especially ferulic acid derivatives, which are known for their ability to neutralize reactive oxygen species and protect biomolecules from oxidative damage [23]. The hydrogen of the hydroxyl group is known to react with free radicals. The antioxidant activity further depends on the groups of the carbon side chain and the positioning of the methoxyl and hydroxyl groups on the aromatic ring [24]. Free radicals are reported to play a crucial role in cancer development. Since ferulic acid and its derivatives can scavenge ROS, enhance the activity of cytoprotective enzymes such as UDP-glucuronosyl transferases in the liver, and detoxification of carcinogenic compounds [25], they have the potential to act as anticancer agents. Since oxidative stress plays a major role in cancer development, these findings suggest that E. caffra could have significant potential in cancer prevention.
In addition to the favorable antioxidant effects, the DCM extract of E. caffra exhibited selective cytotoxicity in the HeLa and MCF-7 cancer cells, while exerting significantly lower toxicity on the non-cancer HEK293 cells. This selective effect is a desirable property in the development of anticancer agents, as many conventional chemotherapeutics lack specificity and damage both malignant and healthy cells, leading to adverse side effects [26]. A comparable pattern of selective toxicity has been reported for compounds isolated from Erythrina excelsa and Erythrina senegalensis on sensitive and drug-resistant cancer cell lines [27]. Apoptotic cell death due to plant extracts has been reported previously using acridine orange/ethidium bromide (AO/EB) staining to identify classic apoptotic features, such as red-orange fluorescence, chromatin condensation and membrane blebbing, while viable cells emitted a green fluorescence [28]. These results are favorable compared to those reported using the anticancer drug doxorubicin alone and complexed to bimetallic [29] and magnetic [30] nanoparticles. Furthermore, these observations also align with known bioactivities of Erythrina-derived compounds, including alkaloids and flavonoids. It was reported that 4′-methoxy licoflavanone and alpinumisoflavone, flavonoids isolated from Erythrina suberosa, can induce apoptosis in leukemia HL-60 cells by disrupting mitochondrial membrane potential and activating the caspase cascade [19].
A significant outcome of this study was the isolation of the three key compounds (n-hexacosanyl isoferulate, tetradecyl isoferulate, and 1-heneicosanol) from the DCM extract, which showed significant antioxidant and anticancer effects. Among them, the isoferulate derivatives showed more potent anticancer activity. Ferulic acid has been previously reported to exhibit anticancer effects, which are mediated through modulation of key signalling pathways, including PI3K/Akt and MAPK, and the induction of p53-dependent apoptosis [31]. Interestingly, the n-hexacosanyl isoferulate was more cytotoxic than tetradecyl isoferulate, possibly due to its longer aliphatic chain, which might enhance its ability to penetrate cell membranes [32].
Of note was the activation of caspases by the isoferulate compounds, which are also shown to be good antioxidants. This suggests that apoptosis is triggered by activating the caspase cascade, thereby enhancing its cytotoxic efficacy against cancer cells. Similar caspase-driven apoptosis has been reported in studies on ferulic acid, highlighting its potential as a multitargeted cancer therapy. Overall, this research underscores the promising role of plant-derived phenolics in cancer treatment. Compared to synthetic compounds, natural isoferulates like those found in E. caffra offer a safer and potentially more effective option for developing new cancer therapeutics.
5. Conclusions
The results have thus far shown that the E. caffra bark extract contains bioactive constituents such as 1-heneicosanol, tetradecyl isoferrulate and n-hexacosanyl isoferrulate, with significant biomedical potential. Of these, n-hexacosanyl isoferrulate was found to have the most potent anticancer effects, with the ability to induce apoptosis and cell death in cervical cancer (HeLa) and breast cancer (MCF-7) cells by triggering the caspase protein cascade. Hence, this lead compound warrants further investigation in more cell lines and in an in vivo model. Further mechanistic studies could also unravel more information on its mechanism of action. This could entail investigating specific signalling pathways, such as the PI3K/Akt and MAPK pathways, and examining the expression or regulation of important pro-apoptotic and anti-apoptotic genes. Based on the promising in vitro results obtained, selected cancer mouse models can be utilized for in vivo studies to identify cancer type specificity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14091035/s1. Supplementary Figure S1: Flow chart for the purification of anticancer compounds from Erythrina caffra; Supplementary Figure S2: 1H and 13C NMR spectrum of compounds 1–3; Supplementary Figure S3: Quantitative analysis of multicaspase expression in HeLa and MCF7 cells using flow cytometry.
Author Contributions
Conceptualization, F.O., M.A. and M.S.; methodology, F.O. and O.B.; software, F.O.; validation, F.O., O.B., M.A. and M.S.; formal analysis, F.O. and O.B.; investigation, F.O. and O.B.; resources, M.A. and M.S.; data curation, F.O. and O.B.; writing—original draft preparation, F.O.; writing—review and editing, M.S. and O.B.; visualization, F.O.; supervision, M.A. and M.S.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Research Foundation, South Africa (Grant numbers 129263 and 120455).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and contributions presented in the study are included in the article and Supplementary File. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors acknowledge members of the Nano-Gene and Drug Delivery group for advice and technical support.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| DCM | Dichloromethane |
| MeOH | Methanol |
| HEK293 | Human Embryonic Kidney 293 cells |
| HeLa | Human cervical cancer cell line |
| MCF-7 | Human breast cancer cell line |
| IC50 | Half-maximal inhibitory concentration |
| AO/EB | Acridine Orange/Ethidium Bromide |
| 5FU | 5-Fluorouracil |
| CC | Column Chromatography |
| TLC | Thin-Layer Chromatography |
| NMR | Nuclear Magnetic Resonance |
| 1H NMR | Proton Nuclear Magnetic Resonance |
| 13C NMR | Carbon-13 Nuclear Magnetic Resonance |
| HRMS | High-Resolution Mass Spectrometry |
| HR-ESI-MS | High-Resolution Electrospray Ionization Mass Spectrometry |
| GC–MS | Gas Chromatography–Mass Spectrometry |
| CDCl3 | Deuterated Chloroform |
| PBS | Phosphate-Buffered Saline |
| DMSO | Dimethyl Sulfoxide |
| NAD(P)H | Nicotinamide Adenine Dinucleotide (Phosphate) |
| 7-AAD | 7-Aminoactinomycin D |
| MAPK | Mitogen-Activated Protein Kinase |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B |
References
- Peto, J. Cancer Epidemiology in the Last Century and the next Decade. Nature 2001, 411, 390–395. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Moraes, D.F.C.; de Mesquita, L.S.S.; do Amaral, F.M.M.; de Sousa Ribeiro, M.N.; Malik, S. Anticancer Drugs from Plants. In Biotechnology and Production of Anti-Cancer Compounds; Springer: Berlin/Heidelberg, Germany, 2017; pp. 121–142. [Google Scholar]
- Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. [Google Scholar] [CrossRef]
- Chingwaru, C.; Bagar, T.; Maroyi, A.; Kapewangolo, P.T.; Chingwaru, W. Wound Healing Potential of Selected Southern African Medicinal Plants: A Review. J. Herb. Med. 2019, 17, 100263. [Google Scholar] [CrossRef]
- Olajuyigbe, O.O.; Afolayan, A.J. In Vitro Antibacterial and Time-kill Evaluation of the Erythrina Caffra Thunb. Extract against Bacteria Associated with Diarrhoea. Sci. World J. 2012, 2012, 738314. [Google Scholar] [CrossRef]
- Wintola, O.A.; Olajuyigbe, A.A.; Afolayan, A.J.; Coopoosamy, R.M.; Olajuyigbe, O.O. Chemical Composition, Antioxidant Activities and Antibacterial Activities of Essential Oil from Erythrina Caffra Thunb. Growing in South Africa. Heliyon 2021, 7, e07244. [Google Scholar] [CrossRef] [PubMed]
- Desta, Z.Y.; Sewald, N.; Majinda, R.R. Cytotoxic Flavonoids from Erythrina Caffra Thunb. Bull. Chem. Soc. Ethiop. 2016, 30, 427–435. [Google Scholar] [CrossRef]
- El-Masry, S.; Amer, M.; Dawood, H.; Radwan, M.; ElSohly, M.; Abou-Karam, M.; Shier, W. Bioassay-Guided Isolation of Cytotoxic Agents from Erythrina Caffra Root Bark. Planta Medica 2014, 80, PD138. [Google Scholar] [CrossRef]
- Gololo, S.S. Effects of Environmental Factors on the Accumulation of Phytochemicals in Plants. In Phytochemistry; Apple Academic Press: Palm Bay, FL, USA, 2018; pp. 267–278. [Google Scholar]
- Bukhari, S.M.; Simic, N.; Siddiqui, H.L.; Ahmad, V.U. Determination of Antioxidant Activity of Crambe Cordifolia. World Appl. Sci. J. 2013, 22, 1561–1565. [Google Scholar]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of Cellular 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium Bromide (MTT) Reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef]
- Olawale, F.; Ariatti, M.; Singh, M. Biogenic Synthesis of Silver-Core Selenium-Shell Nanoparticles Using Ocimum Tenuiflorum L.: Response Surface Methodology-Based Optimization and Biological Activity. Nanomaterials 2021, 11, 2516. [Google Scholar] [CrossRef]
- Zenze, M.; Singh, M. Receptor targeting using copolymer-modified gold nanoparticles for pCMV-Luc gene delivery to liver cancer cells in vitro. Int. J. Mol. Sci. 2024, 25, 5016. [Google Scholar] [CrossRef]
- Jiménez-Cabrera, T.; Bautista, M.; Velázquez-González, C.; Jaramillo-Morales, O.A.; Guerrero-Solano, J.A.; Urrutia-Hernández, T.A.; De la O-Arciniega, M. Promising Antioxidant Activity of Erythrina Genus: An Alternative Treatment for Inflammatory Pain? Int. J. Mol. Sci. 2020, 22, 248. [Google Scholar] [CrossRef]
- Gabr, S.; Bakr, R.; Mostafa, E.; El-Fishawy, A.; El-Alfy, T. Antioxidant Activity and Molecular Docking Study of Erythrina × Neillii Polyphenolics. South Afr. J. Bot. 2019, 121, 470–477. [Google Scholar] [CrossRef]
- Nkengfack, A.E.; Vouffo, T.W.; Vardamides, J.C.; Kouam, J.; Fomum, Z.T.; Meyer, M.; Sterner, O. Phenolic Metabolites from Erythrina Species. Phytochemistry 1997, 46, 573–578. [Google Scholar] [CrossRef]
- Arancibia, L.; Naspi, C.; Pucci, G.; Arce, M.; Colloca, C. Biological Activity of 1-Heneicosanol Isolated from Senecio Coluhuapiensis, an Endemic Species from Patagonia, Argentina. Pharm. Chem. J. 2016, 3, 73–77. [Google Scholar]
- Kumar, S.; Pathania, A.S.; Saxena, A.; Vishwakarma, R.; Ali, A.; Bhushan, S. The Anticancer Potential of Flavonoids Isolated from the Stem Bark of Erythrina Suberosa through Induction of Apoptosis and Inhibition of STAT Signaling Pathway in Human Leukemia HL-60 Cells. Chem.-Biol. Interact. 2013, 205, 128–137. [Google Scholar] [CrossRef]
- Priya, R.; Mani, P.; Maneemegalai, S. Anti-Oxidant and Anticancer Properties of Ethanol Leaves Extract of Erythrina Indica. Eur. J. Biomed. 2018, 5, 438–443. [Google Scholar]
- Obakiro, S.B.; Kiprop, A.; Kigondu, E.; K’Owino, I.; Odero, M.P.; Manyim, S.; Omara, T.; Namukobe, J.; Owor, R.O.; Gavamukulya, Y. Traditional Medicinal Uses, Phytoconstituents, Bioactivities, and Toxicities of Erythrina Abyssinica Lam. Ex DC. (Fabaceae): A Systematic Review. Evid.-Based Complement. Altern. Med. 2021, 2021, 5513484. [Google Scholar] [CrossRef]
- Baskar, N.; Devi, B.P.; Kumar, R.M. Anti-Cancer Activity of Methanol Extract of Root Bark of Erythrina Variegata Linn. Int. J. Toxicol. Pharmacol. Res. 2010, 2, 4–76. [Google Scholar]
- Karamać, M.; Koleva, L.; Kancheva, V.D.; Amarowicz, R. The Structure–Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef]
- Van der Logt, E.M.J.; Roelofs, H.M.J.; Nagengast, F.M.; Peters, W.H.M. Induction of rat hepatic and intestinal UDP-glucuronosyltransferases by naturally occurring dietary anticarcinogens. Carcinogenesis 2003, 24, 1651–1657. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Sandjo, L.P.; Kwamou, G.M.; Wiench, B.; Nkengfack, A.E.; Efferth, T. Activity of Three Cytotoxic Isoflavonoids from Erythrina Excelsa and Erythrina Senegalensis (Neobavaisoflavone, Sigmoidin H and Isoneorautenol) toward Multi-Factorial Drug Resistant Cancer Cells. Phytomedicine 2014, 21, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Maiyo, F.; Moodley, R.; Singh, M. Cytotoxicity, antioxidant and apoptosis studies of Quercetin-3-O-glucoside and 4-(β-D-Glucopyranosyl-1→4-α-L-Rhamnopyranosyloxy)-benzyl isothiocyanate from Moringa oleifera. Anti-Cancer Agents Med. Chem. 2016, 16, 648–656. [Google Scholar] [CrossRef]
- Maney, V.; Singh, M. An in vitro assessment of Chitosan/Bimetallic PtAu nanocomposites as delivery vehicles for Doxorubicin. Nanomedicine 2017, 12, 2625–2640. [Google Scholar] [CrossRef]
- Ramnandan, D.; Mokhosi, S.; Daniels, A.; Singh, M. Chitosan, Polyethylene glycol and Polyvinyl alcohol modified MgFe2O4 ferrite magnetic nanoparticles in Doxorubicin delivery: A comparative study in vitro. Molecules 2021, 26, 3893. [Google Scholar] [CrossRef]
- Bao, X.; Li, W.; Jia, R.; Meng, D.; Zhang, H.; Xia, L. Molecular Mechanism of Ferulic Acid and Its Derivatives in Tumor Progression. Pharmacol. Rep. 2023, 75, 891–906. [Google Scholar] [CrossRef]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving Cellular Uptake of Therapeutic Entities through Interaction with Components of Cell Membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).