Application of Ferric–Graphene Quantum Dot Complex for Evaluation and Imaging of Antioxidants in Foods Based on Fluorescence Turn-Off–On Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Solutions
2.2. Instrumentation and Materials
2.3. Spectrofluorometric Measurement of Antioxidants Using Fe3+-GQD Composite
2.4. Procedure for the Paper-Based Fluorescence Image Assay of l-Ascorbic Acid
2.5. Antioxidant Distribution Imaging in Vegetable Sections
3. Results
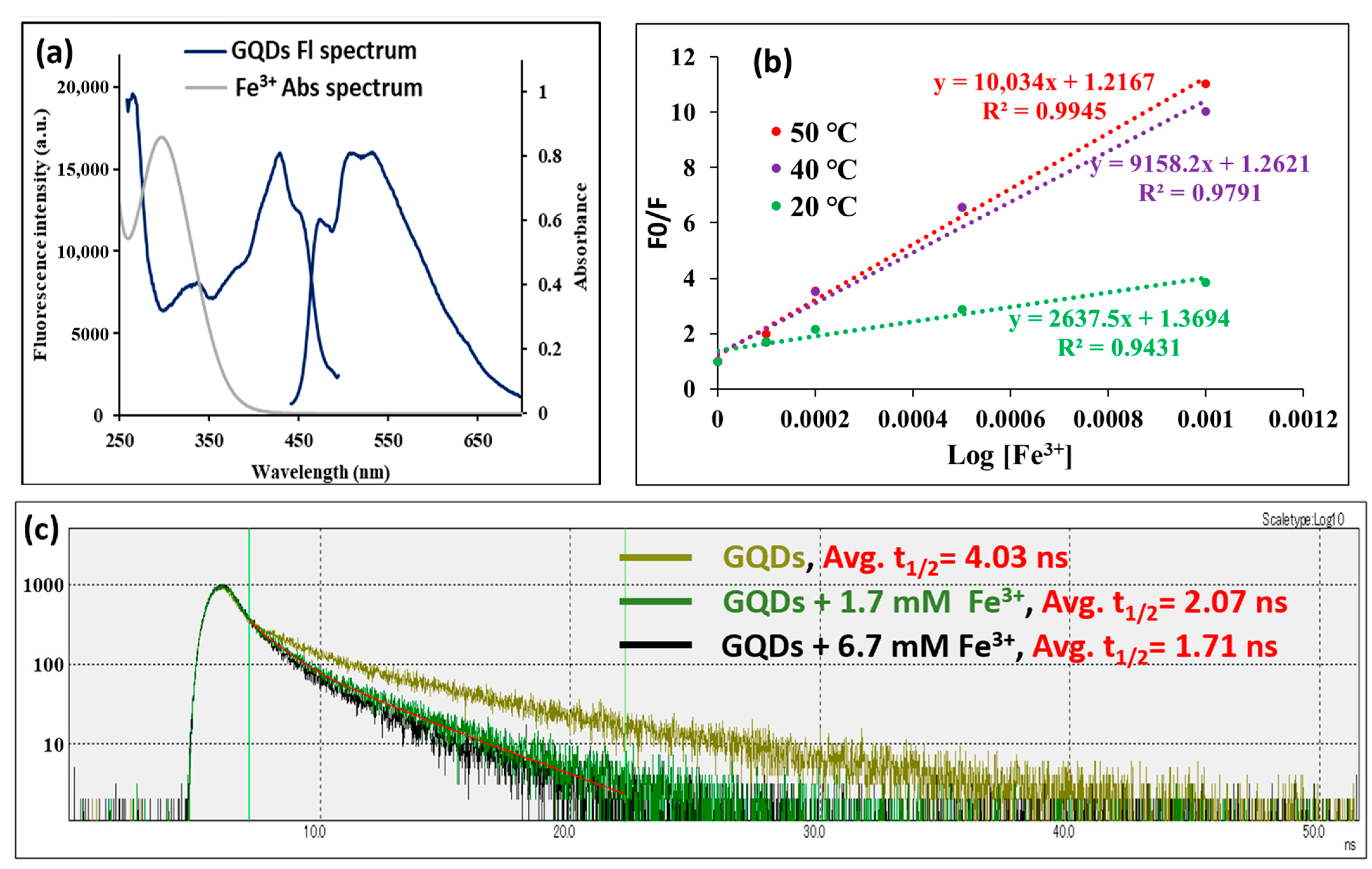
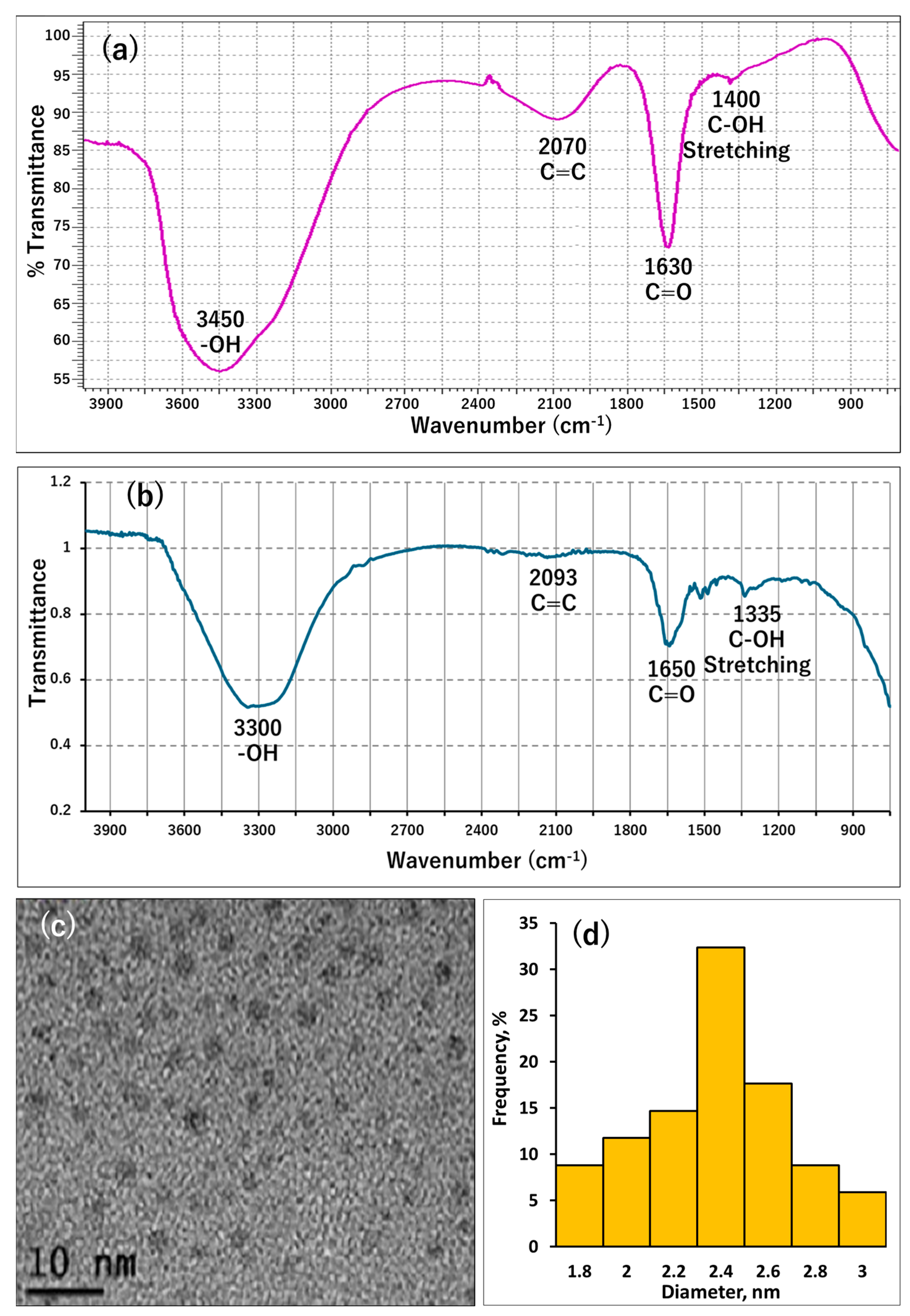
3.1. Characterization of the Used GQDs
3.2. Fluorescence Monitoring of Fe3+ Interaction with GQDs
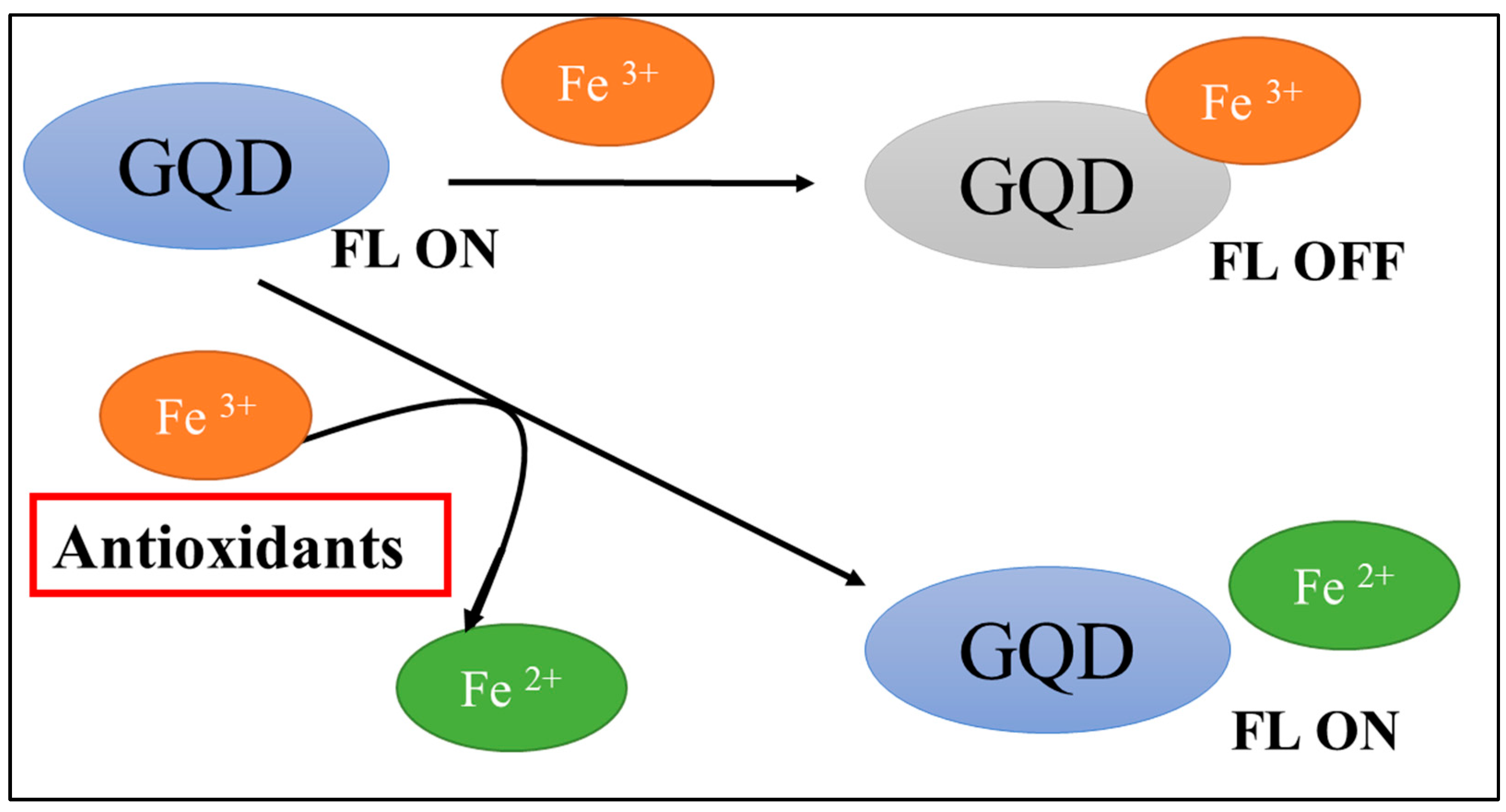
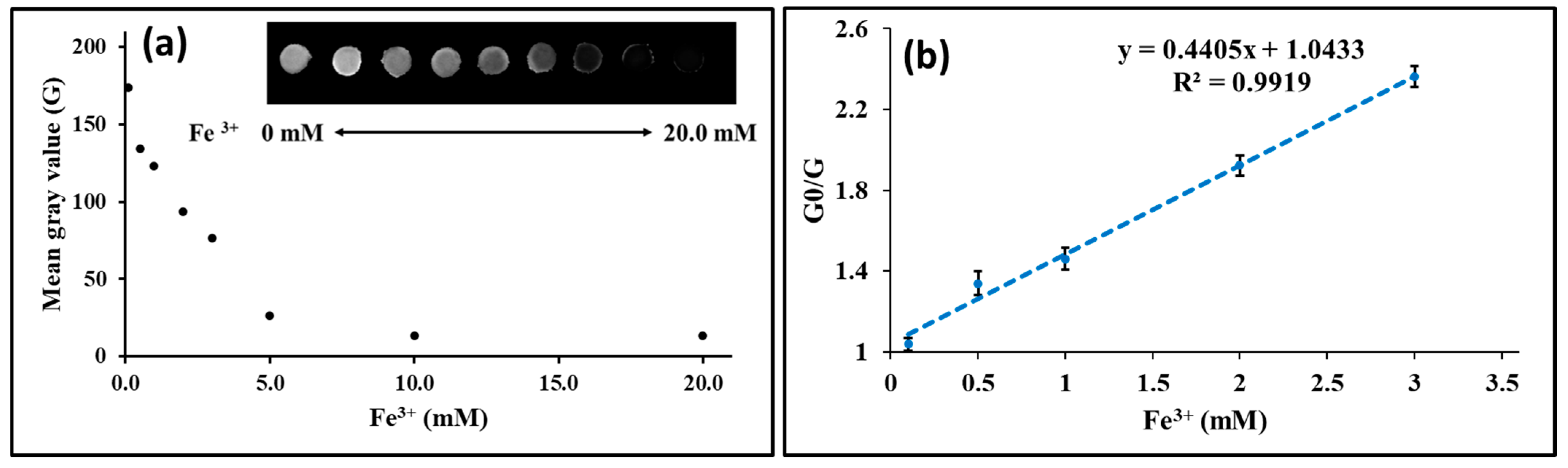
3.3. Study of the Mechanism of Fluorescence Quenching of GQDs After Interaction with Fe3+
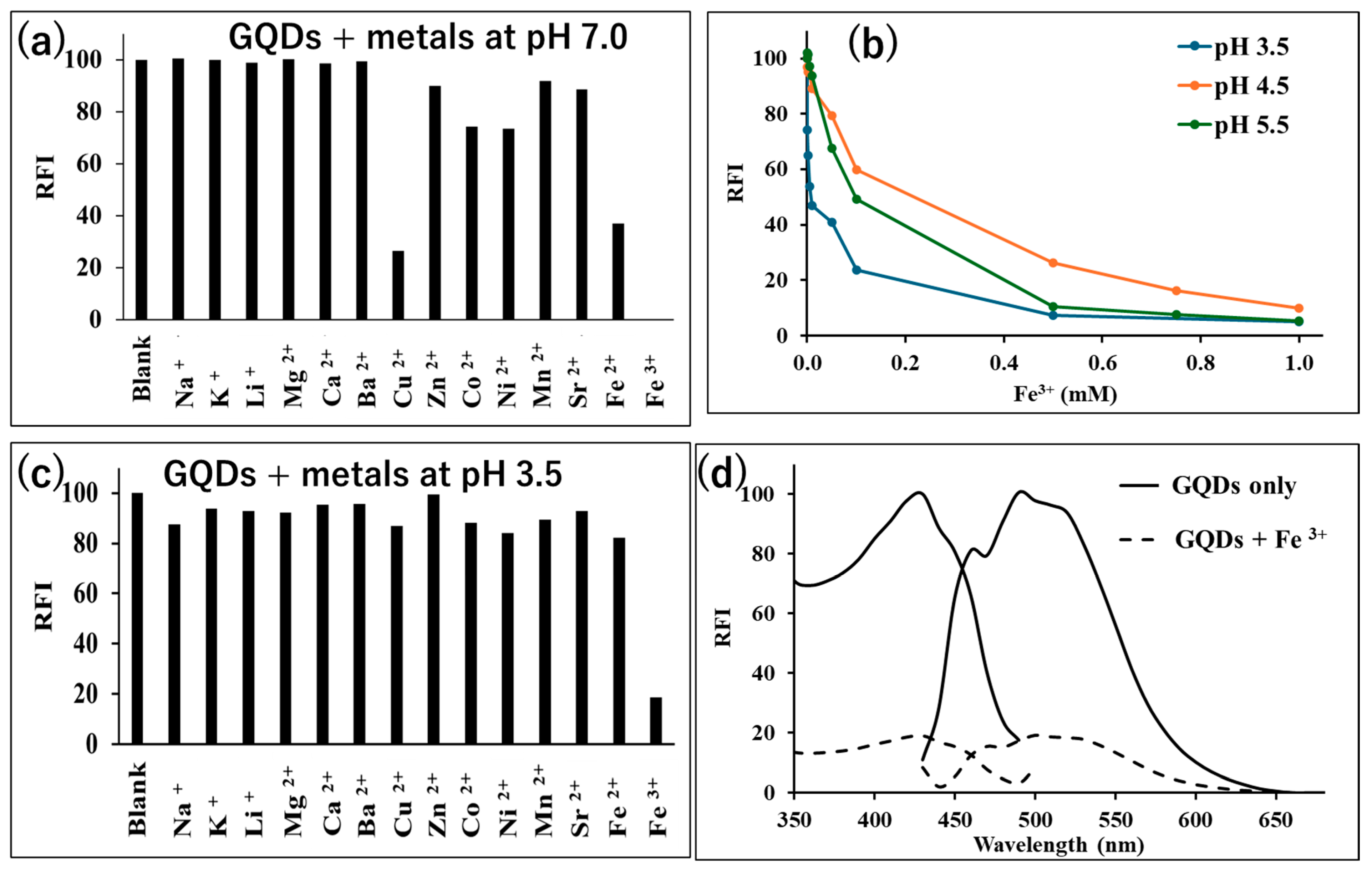
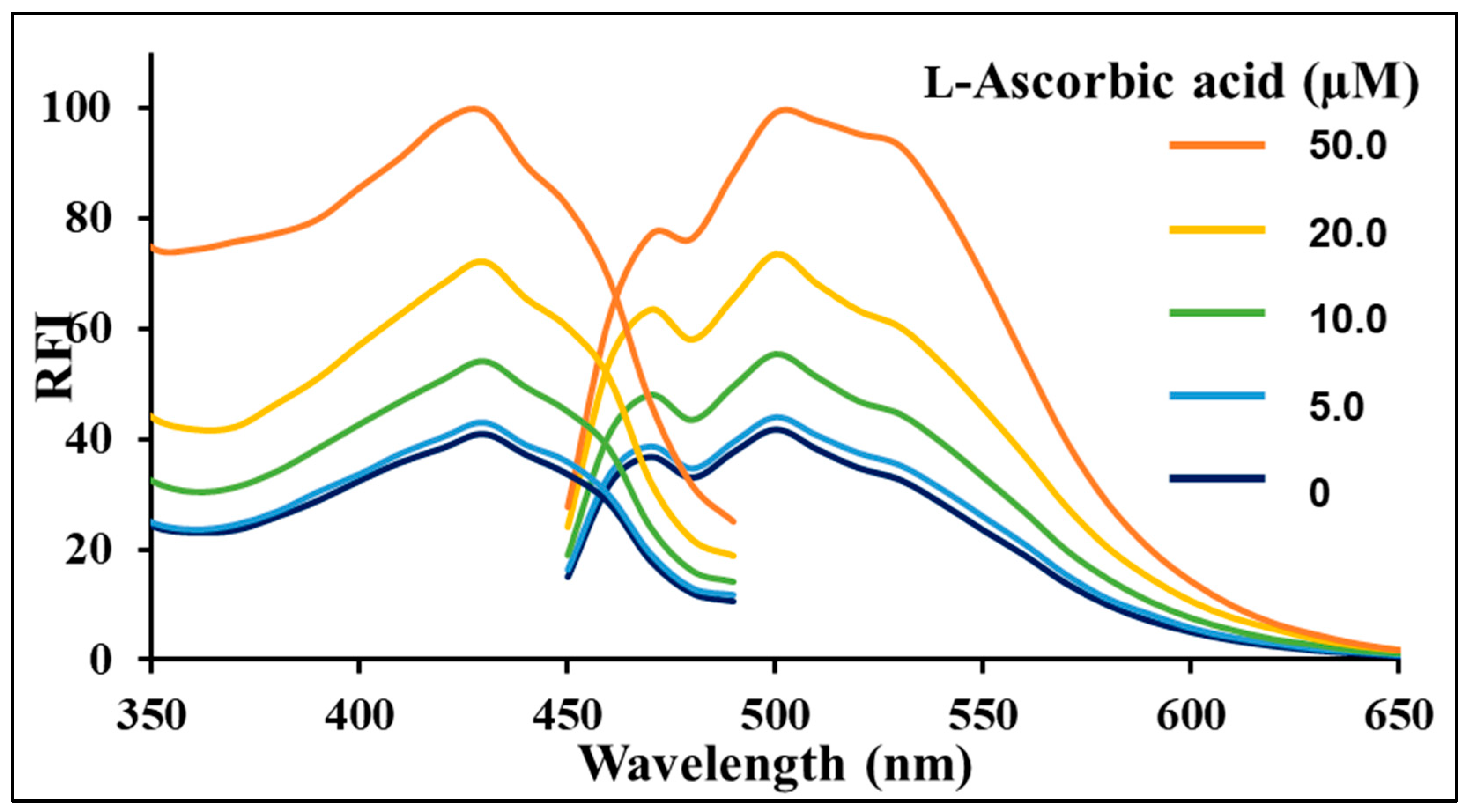
3.4. Measurement of the Antioxidants Using the Spectrofluorometry Method
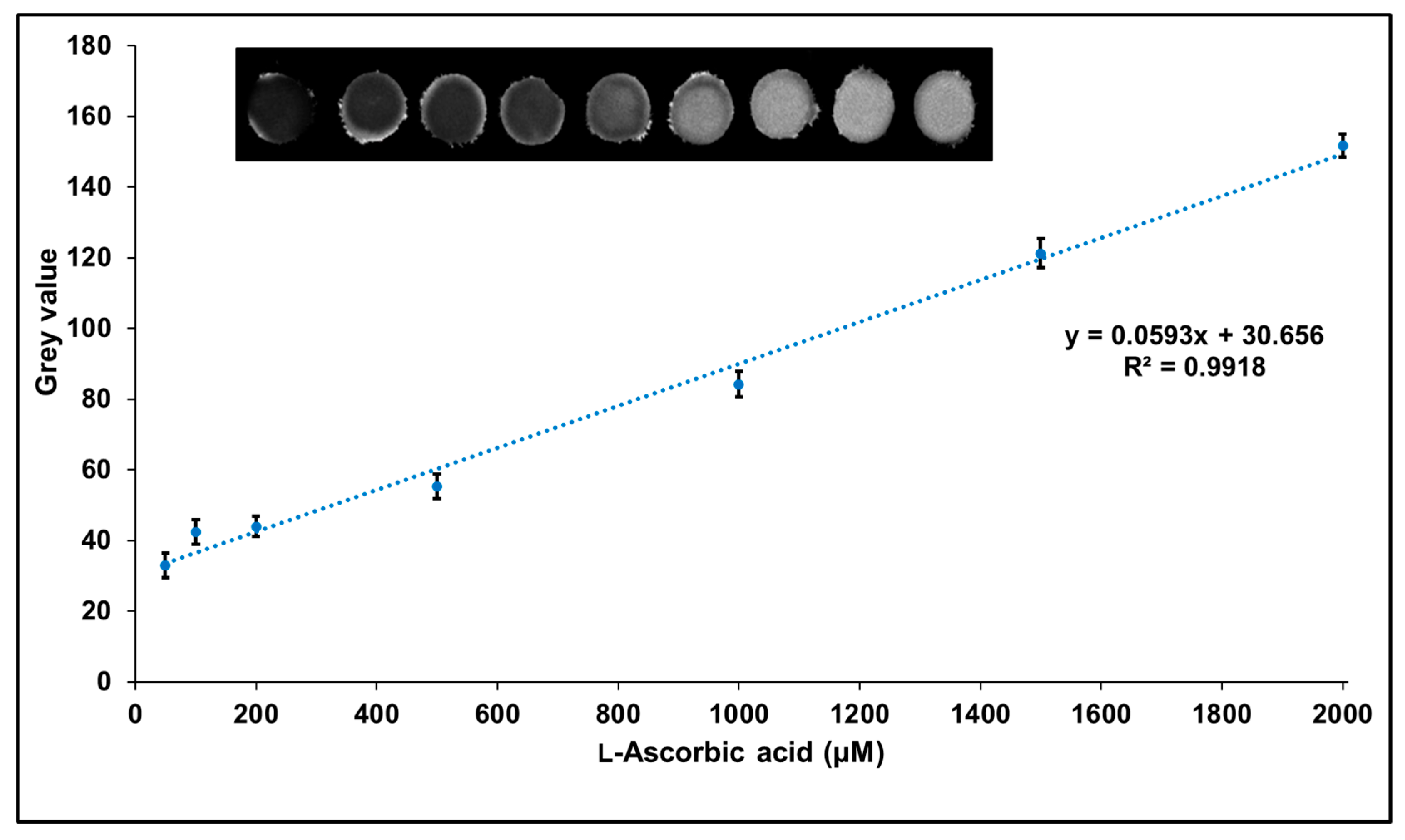
3.5. Paper-Based Assay of l-Ascorbic Acid Standard
3.6. Paper-Based Assay of l-Ascorbic Acid in Vitamin C Drink
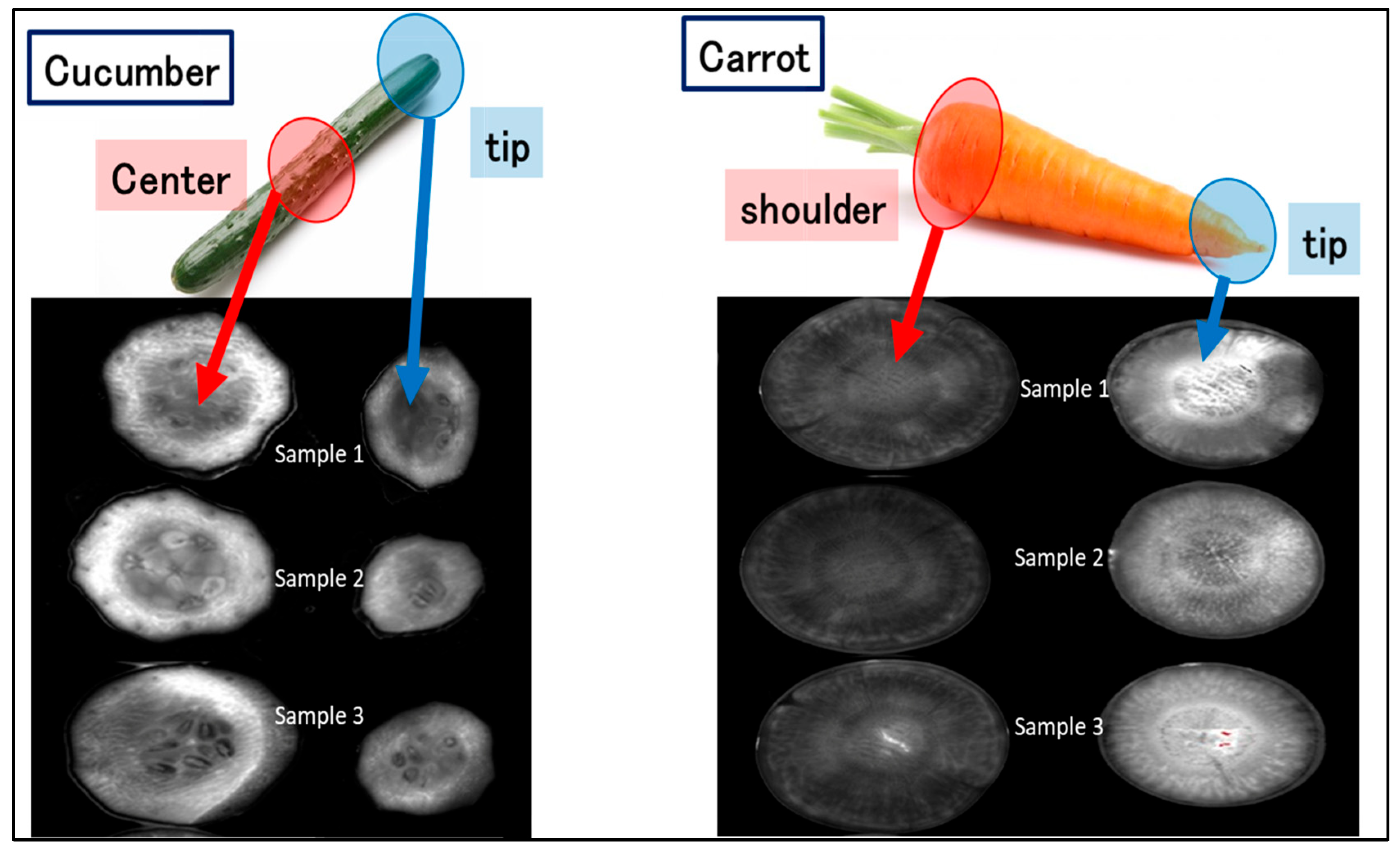
3.7. Fluorescence Imaging of Antioxidants in Food Sections and Their Aqueous Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moon, J.-K.; Shibamoto, T. Antioxidant Assays for Plant and Food Components. J. Agric. Food Chem. 2009, 57, 1655–1666. [Google Scholar] [CrossRef]
- Paz-Elizur, T.; Sevilya, Z.; Leitner-Dagan, Y.; Elinger, D.; Roisman, L.C.; Livneh, Z. DNA Repair of Oxidative DNA Damage in Human Carcinogenesis: Potential Application for Cancer Risk Assessment and Prevention. Cancer Lett. 2008, 266, 60–72. [Google Scholar] [CrossRef]
- Cichoż-Lach, H. Oxidative Stress as a Crucial Factor in Liver Diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef]
- Moreira, P.I.; Smith, M.A.; Zhu, X.; Honda, K.; Lee, H.G.; Aliev, G.; Perry, G. Oxidative Damage and Alzheimer’s Disease: Are Antioxidant Therapies Useful? Drug News Perspect. 2005, 18, 13. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Čolak, E. New Markers of Oxidative Damage to Macromolecules. J. Med. Biochem. 2008, 27, 1–16. [Google Scholar] [CrossRef]
- Mukherjee, A.B.; Zhang, Z.; Chilton, B.S. Uteroglobin: A Steroid-Inducible Immunomodulatory Protein That Founded the Secretoglobin Superfamily. Endocr. Rev. 2007, 28, 707–725. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.K. Superoxide Dismutase Overexpression and Cellular Oxidative Damage in Diabetes. Free Radic. Biol. Med. 2006, 41, 1187–1190. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondria, Oxidative Damage, and Inflammation in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2003, 991, 120–131. [Google Scholar] [CrossRef]
- Chaturvedi, R.K.; Beal, M.F. PPAR: A Therapeutic Target in Parkinson’s Disease. J. Neurochem. 2008, 106, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Mechanisms of Oxidative Damage of Low Density Lipoprotein in Human Atherosclerosis. Curr. Opin. Lipidol. 1997, 8, 268–274. [Google Scholar] [CrossRef]
- Sepulveda, R.; Watson, R. Treatment of Antioxidant Deficiencies in AIDS Patients. Nutr. Res. 2002, 22, 27–37. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of Assay Conditions for the Determination of Antioxidant Capacity and Polyphenols in Cereal Food Components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Singh, N.; Rajini, P.S. Free Radical Scavenging Activity of an Aqueous Extract of Potato Peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of Antioxidant Activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Benzie, I.F.; Szeto, Y.T. Total Antioxidant Capacity of Teas by the Ferric Reducing/Antioxidant Power Assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-Radical Absorbance Capacity Assay for Antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Harada, S.; Ohyama, K.; Kuroda, N. Quinone-Based Antibody Labeling Reagent for Enzyme-Free Chemiluminescent Immunoassays. Application to Avidin and Biotinylated Anti-Rabbit IgG Labeling. Biosens. Bioelectron. 2020, 160, 112215. [Google Scholar] [CrossRef]
- Fukuda, M.; El-Maghrabey, M.H.; Kishikawa, N.; Ikemoto, K.; Kuroda, N. Ultrasensitive Determination of Pyrroloquinoline Quinone in Human Plasma by HPLC with Chemiluminescence Detection Using the Redox Cycle of Quinone. J. Pharm. Biomed. Anal. 2017, 145, 814–820. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. Novel Isotope-Coded Derivatization Method for Aldehydes Using 14 N/ 15 N-Ammonium Acetate and 9,10-Phenanthrenequinone. Anal. Chem. 2018, 90, 13867–13875. [Google Scholar] [CrossRef]
- El-Maghrabey, M.; Kishikawa, N.; Kuroda, N. 9,10-Phenanthrenequinone as a Mass-Tagging Reagent for Ultra-Sensitive Liquid Chromatography-Tandem Mass Spectrometry Assay of Aliphatic Aldehydes in Human Serum. J. Chromatogr. A 2016, 1462, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Walash, M.I.; Belal, F.F.; El-Enany, N.M.; El-Maghrabey, M.H. Utility of Certain Nucleophilic Aromatic Substitution Reactions for the Assay of Pregabalin in Capsules. Chem. Cent. J. 2011, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Kishikawa, N.; El-Maghrabey, M.; Nagamune, Y.; Nagai, K.; Ohyama, K.; Kuroda, N. A Smart Advanced Chemiluminescence-Sensing Platform for Determination and Imaging of the Tissue Distribution of Natural Antioxidants. Anal. Chem. 2020, 92, 6984–6992. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hakim, A.; Belal, F.; Hammad, M.A.; Kishikawa, N.; El-Maghrabey, M. Adoption of Self-Exothermic Reaction for Synthesis of Multifunctional Carbon Quantum Dots: Applications to Vincristine Sensing and Cell Imaging. Talanta 2025, 282, 126971. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Al-Khateeb, L.A.; El-Maghrabey, M. Dual-Excitation in-Lab-Made Device Based on a Handy UV Lamp and GQDs-Modified PADs for Simultaneous Determination of Acetaminophen and Its Endocrine Disrupting Impurity 4-Nitrophenol. Sens. Actuators B Chem. 2021, 348, 130657. [Google Scholar] [CrossRef]
- El-Shaheny, R.; Al-Khateeb, L.A.; El Hamd, M.A.; El-Maghrabey, M. Correction Pen as a Hydrophobic/Lipophobic Barrier Plotter Integrated with Paper-Based Chips and a Mini UV-Torch to Implement All-in-One Device for Determination of Carbazochrome. Anal. Chim. Acta 2021, 1172, 338684. [Google Scholar] [CrossRef]
- Walash, M.I.; Belal, F.; El-Enany, N.; El-Maghrabey, M.H. Spectrofluorimetric Determination of Oseltamivir Phosphate through Derivatization with O-Phthalaldehyde. Application to Pharmaceutical Preparations with a Preliminary Study on Spiked Plasma Samples. Luminescence 2012, 27, 511–518. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-Graphene Oxide for Cellular Imaging and Drug Delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Cao, J.; Zhu, J.; Fan, L.; Li, X. Sulfur-Doped Graphene Quantum Dots as a Novel Fluorescent Probe for Highly Selective and Sensitive Detection of Fe 3+. Anal. Chem. 2014, 86, 10201–10207. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; El-Maghrabey, M.; El-Shaheny, R. Sensitive Determination of Naftazone Using Carbon Quantum Dots Nanoprobe by Fluorimetry and Smartphone-Based Techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 302, 123109. [Google Scholar] [CrossRef]
- Karami, M.H.; Abdouss, M.; Rahdar, A.; Pandey, S. Graphene Quantum Dots: Background, Synthesis Methods, and Applications as Nanocarrier in Drug Delivery and Cancer Treatment: An Updated Review. Inorg. Chem. Commun. 2024, 161, 112032. [Google Scholar] [CrossRef]
- El Hamd, M.A.; El-Maghrabey, M.; Almawash, S.; Radwan, A.S.; El-Shaheny, R.; Magdy, G. Citrus/Urea Nitrogen-doped Carbon Quantum Dots as Nanosensors for Vanillin Determination in Infant Formula and Food Products via Factorial Experimental Design Fluorimetry and Smartphone. Luminescence 2024, 39, e4643. [Google Scholar] [CrossRef]
- Biswas, M.C.; Islam, M.T.; Nandy, P.K.; Hossain, M.M. Graphene Quantum Dots (GQDs) for Bioimaging and Drug Delivery Applications: A Review. ACS Mater. Lett. 2021, 3, 889–911. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, Y.; Song, L.; Liu, X.; Hu, Z. Microwave Assisted One-Pot Synthesis of Graphene Quantum Dots as Highly Sensitive Fluorescent Probes for Detection of Iron Ions and PH Value. Talanta 2016, 150, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Dianoczki, C.; Recseg, K.; Karlovits, G.; Szłyk, E. Determination of Antioxidant Capacities of Vegetable Oils by Ferric-Ion Spectrophotometric Methods. Talanta 2008, 76, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97. [Google Scholar] [CrossRef]
- Lu, F.; Zhou, Y.; Wu, L.; Qian, J.; Cao, S.; Deng, Y.; Chen, Y. Highly Fluorescent Nitrogen-Doped Graphene Quantum Dots’ Synthesis and Their Applications as Fe(III) Ions Sensor. Int. J. Opt. 2019, 2019, 8724320. [Google Scholar] [CrossRef]
- Wu, Y.; Combs, C.; Okosun, B.O.; Tayutivutikul, K.; Darland, D.C.; Zhao, J.X. Fe3+-Doped Graphene Quantum Dots-Based Nanozyme for H2O2 Detection in Cellular Metabolic Distress. ACS Appl. Nano Mater. 2025, 8, 2774–2784. [Google Scholar] [CrossRef]
- Stan, C.S.; Coroabă, A.; Ursu, E.L.; Secula, M.S.; Simionescu, B.C. Fe(III) Doped Carbon Nanodots with Intense Green Photoluminescence and Dispersion Medium Dependent Emission. Sci. Rep. 2019, 9, 18893. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Z.; Xue, Z.; Huang, C.; Shan, Y.; Liu, C.; Qin, X.; Yang, W.; Chen, X.; Wang, T. Understanding the Selective Detection of Fe 3+ Based on Graphene Quantum Dots as Fluorescent Probes: The K Sp of a Metal Hydroxide-Assisted Mechanism. Anal. Chem. 2017, 89, 12054–12058. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, S.; Li, D.; Chen, G.; Ho, S.-H. Detecting Ferric Iron by Microalgal Residue-Derived Fluorescent Nanosensor with an Advanced Kinetic Model. iScience 2020, 23, 101174. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Cheregi, M.C.; Danet, A.F. Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches. Molecules 2009, 14, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Suktham, T.; Jones, A.; Soliven, A.; Dennis, G.R.; Shalliker, R.A. A Comparison of the Performance of the Cupric Reducing Antioxidant Potential Assay and the Ferric Reducing Antioxidant Power Assay for the Analysis of Antioxidants Using Reaction Flow Chromatography. Microchem. J. 2019, 149, 104046. [Google Scholar] [CrossRef]

| Concentration of Fe3+ (mM) | l-Ascorbic Acid Range (µM) | Calibration Equation for l-Ascorbic Acid * | Coefficient of Determination (r2) | LOD of l-Ascorbic Acid (µM) ** |
|---|---|---|---|---|
| 0.06 | 2.0–5.0 | Y = 18.5X + 466 | 0.986 | 0.222 |
| 0.12 | 2.0–15.0 | Y = 11.9X + 449 | 0.968 | 0.512 |
| 0.3 | 5.0–50.0 | Y = 6.18X + 395 | 0.998 | 0.805 |
| 0.6 | 5.0–100 | Y = 4.04X + 383 | 0.990 | 1.60 |
| Antioxidant | Linear Range (µM) | Calibration Equation * | r2 | LOD ** (µM) |
|---|---|---|---|---|
| l-Ascorbic acid | 5.0–50.0 | Y = 6.18X + 395 | 0.998 | 0.805 |
| Gallic acid | 2.0–20.0 | Y = 10.8X + 429 | 0.992 | 0.460 |
| Pyrogallol | 5.0–20.0 | Y= 8.24X + 414 | 0.995 | 0.603 |
| DTT | 5.0–50.0 | Y = 3.54X + 448 | 0.999 | 1.40 |
| (+)-Catechin | 10.0–50.0 | Y= 2.74X + 474 | 0.996 | 1.81 |
| Caffeic acid | 20.0–100 | Y = 0.604X + 485 | 0.998 | 8.23 |
| Trolox | 5.0–50.0 | Y = 5.92X + 566 | 0.991 | 2.49 |
| Antioxidant | Concentration (µM) | Accuracy | Precision (%RSD) | |
|---|---|---|---|---|
| Within Day (n = 5) | Between Days (n = 5) | |||
| l-Ascorbic acid | 5.0 | 90.6 | 5.4 | 3.5 |
| 20.0 | 88.47 | 2.6 | 2.9 | |
| 50.0 | 86.08 | 2.3 | 1.8 | |
| Gallic acid | 5.0 | 103.26 | 4.9 | 4.2 |
| 10.0 | 100.33 | 2.4 | 2.9 | |
| 20.0 | 98.63 | 6.7 | 2.0 | |
| Pyrogallol | 5.0 | 108.97 | 4.8 | 2.0 |
| 10.0 | 103.5 | 3.9 | 2.0 | |
| 20.0 | 108.55 | 3.5 | 1.7 | |
| DTT | 5.0 | 115.8 | 8.1 | 3.0 |
| 20.0 | 113.34 | 7.7 | 1.9 | |
| 50.0 | 110.76 | 4.7 | 2.3 | |
| (+)-Catechin | 10.0 | 111.5 | 5.3 | 2.9 |
| 20.0 | 113.8 | 8.3 | 3.6 | |
| 50.0 | 113.75 | 6.6 | 2.0 | |
| Caffeic acid | 30.0 | 105.87 | 3.3 | 3.1 |
| 50.0 | 113.8 | 5.5 | 1.4 | |
| 100.0 | 111.8 | 7.3 | 2.5 | |
| Trolox | 5.0 | 93.37 | 6.5 | 3.6 |
| 20.0 | 102.94 | 5.4 | 2.5 | |
| 50.0 | 92.6 | 4.1 | 3.9 | |
| Sample | Parameter | Value |
|---|---|---|
| Lemon juice | Antioxidant content (mM ASC; mean found ± SD) | |
| Proposed method | 12.59 ± 0.52 | |
| Comparison method [44] | 12.65 ± 0.29 | |
| N | 3 | |
| t-test (2.77) | 0.15 | |
| F-test (19.00) | 3.21 | |
| Apple juice | Antioxidant content (mM ASC; mean found ± SD) | |
| Proposed method | 0.544 ± 0.041 | |
| Comparison method [44] | 0.529 ± 0.035 | |
| N | 3 | |
| t-test (2.77) | 0.49 | |
| F-test (19.00) | 1.37 |
| Antioxidant | LOD (µM) | ||
|---|---|---|---|
| Proposed Method | FRAP Method [45] | Chemiluminescence Sensing [25] | |
| l-Ascorbic acid | 0.805 | 14.2 | 0.18 |
| Gallic acid | 0.460 | 77.6 | 1.19 |
| Pyrogallol | 0.603 | 0.28 | |
| DTT | 1.40 | 0.61 | |
| (+)-Catechin | 1.81 | 0.517 | |
| Trolox | 2.49 | 12.3 | |
| l-Ascorbic Acid Concentration (µM) | Accuracy | Precision (%RSD) | |
|---|---|---|---|
| Within Day (n = 5) | Between Days (n = 5) | ||
| 100.0 | 115.62 | 8.2 | 7.9 |
| 1000.0 | 93.63 | 3.0 | 3.4 |
| 2000.0 | 101.62 | 7.9 | 2.1 |
| Parameter | Reported (μM) | Found (μM) | Found (%) * |
|---|---|---|---|
| 500 | 479.6 | 95.9 | |
| 1000 | 921.0 | 92.1 | |
| 1500 | 1444 | 96.3 | |
| Mean ± SE (%) | 94.8 ± 5.83 | ||
| Nominal (mM) | 38.45 ± 2.36 | ||
| Sample | Antioxidant Capacity (mg ASC/100 g Sample) Mean ± SD Using FL Method | Antioxidant Capacity (mg ASC/100 g Sample) Using the Reference Method [25] |
|---|---|---|
| Cucumber | ||
| Central part | 252.5 ± 13.1 | 254.6 ± 10.4 |
| Tip | 256.9 ± 26.9 | 260.6 ± 27.5 |
| Carrot | ||
| Near shoulder | 227.2 ± 9.2 | 225.1 ± 8.1 |
| Tip | 276.2 ± 17.8 | 279.3 ± 21.2 |
| Paired t-test p-value | 0.2848 | |
| r | 0.9984 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Maghrabey, M.; Yamamichi, A.; Abdel-Hakim, A.; Kishikawa, N.; Kuroda, N. Application of Ferric–Graphene Quantum Dot Complex for Evaluation and Imaging of Antioxidants in Foods Based on Fluorescence Turn-Off–On Strategy. Antioxidants 2025, 14, 1034. https://doi.org/10.3390/antiox14091034
El-Maghrabey M, Yamamichi A, Abdel-Hakim A, Kishikawa N, Kuroda N. Application of Ferric–Graphene Quantum Dot Complex for Evaluation and Imaging of Antioxidants in Foods Based on Fluorescence Turn-Off–On Strategy. Antioxidants. 2025; 14(9):1034. https://doi.org/10.3390/antiox14091034
Chicago/Turabian StyleEl-Maghrabey, Mahmoud, Aya Yamamichi, Ali Abdel-Hakim, Naoya Kishikawa, and Naotaka Kuroda. 2025. "Application of Ferric–Graphene Quantum Dot Complex for Evaluation and Imaging of Antioxidants in Foods Based on Fluorescence Turn-Off–On Strategy" Antioxidants 14, no. 9: 1034. https://doi.org/10.3390/antiox14091034
APA StyleEl-Maghrabey, M., Yamamichi, A., Abdel-Hakim, A., Kishikawa, N., & Kuroda, N. (2025). Application of Ferric–Graphene Quantum Dot Complex for Evaluation and Imaging of Antioxidants in Foods Based on Fluorescence Turn-Off–On Strategy. Antioxidants, 14(9), 1034. https://doi.org/10.3390/antiox14091034








