Potential Impact of Sclerocarya birrea on Cardiovascular Health and Related Risk Factors: Review of Existing Evidence
Abstract
1. Introduction
2. Methodology
3. Results
3.1. Effect of S. birrea in In Vitro Studies
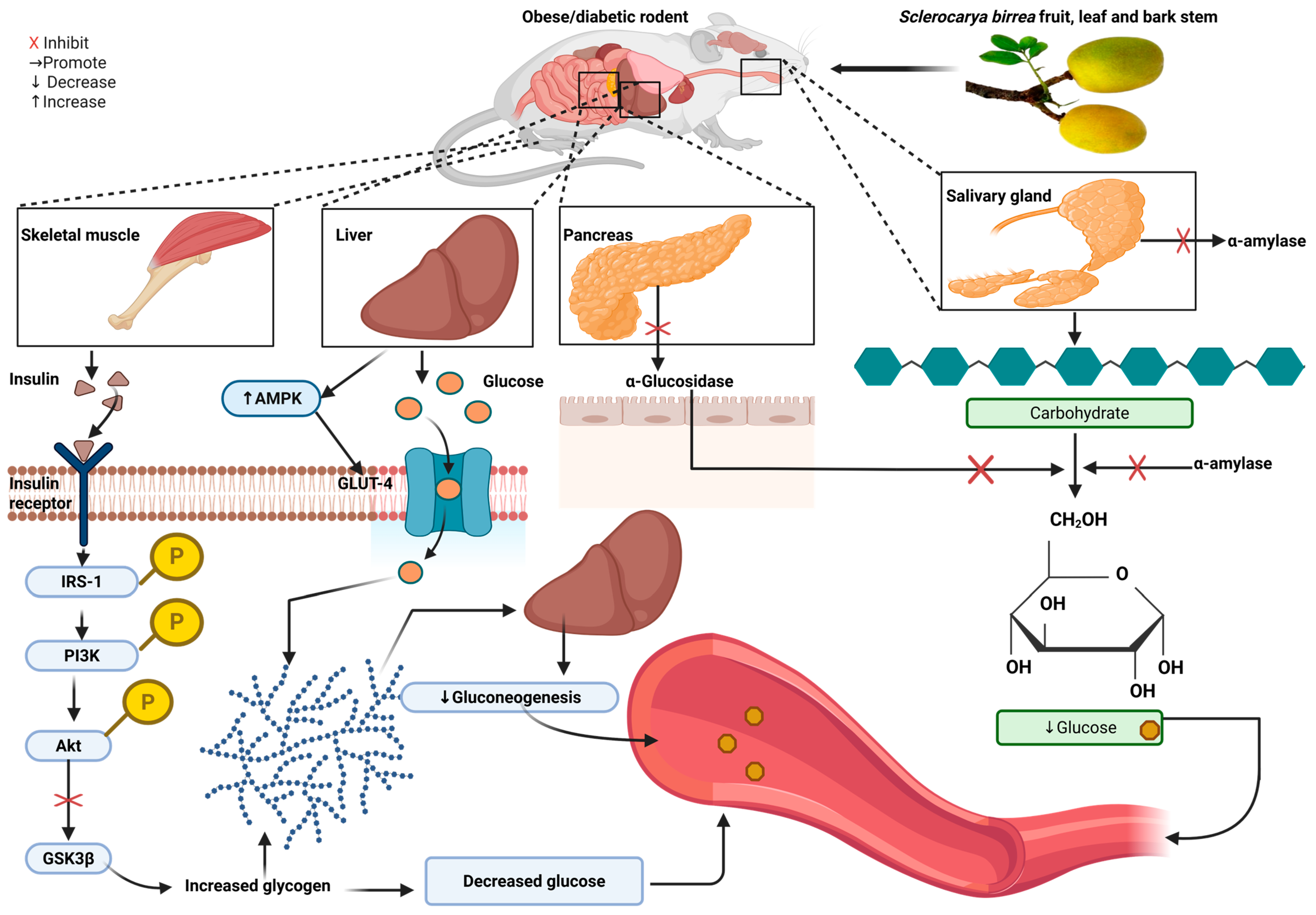
3.2. In Vivo Evidence Exploring the Effect of S. birrea in Obese and Diabetic Models
3.3. Antidiabetic Potential of S. birrea Supplementation in Rodents
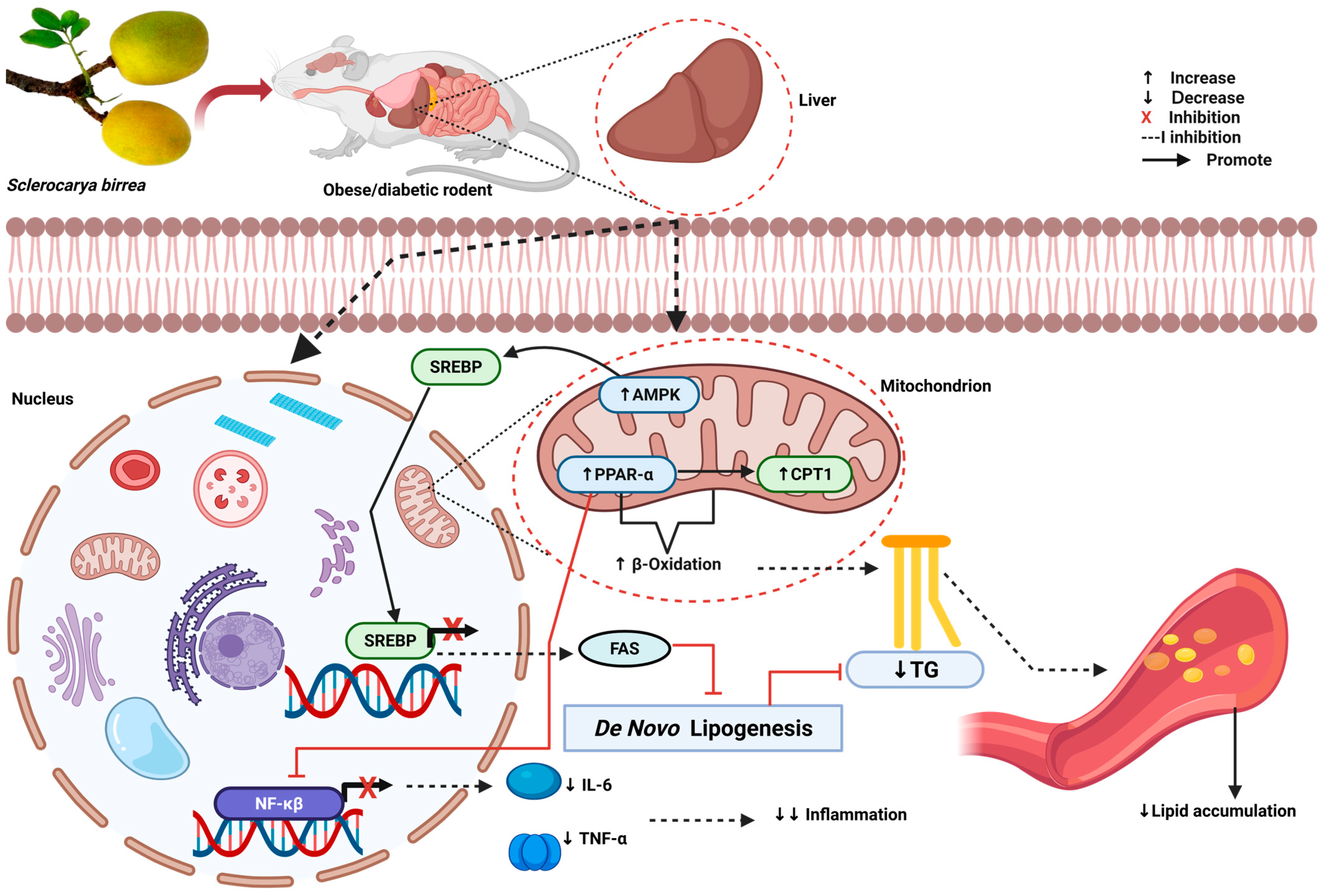
3.4. Hypolipidemic Potential of S. birrea Supplementation in Rodents
3.5. Antioxidative and Anti-Inflammatory Potential of S. birrea Supplementation in Rodents
3.6. Antihypertensive Potential of S. birrea Supplementation in Rodents
3.7. Overall Evidence from Clinical Studies
3.8. Antidiabetic Potential of S. birrea Supplementation in Humans
3.9. Hypolipidemic Potential of S. birrea Supplementation in Humans
3.10. Anti-Inflammatory Potential of S. birrea Supplementation in Humans
3.11. The Antioxidative and Antihypertensive Potency of S. birrea in Humans
3.12. Cytotoxicity and Safety Profile of S. birrea
4. Discussion
Limitations of the Studies
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 30 June 2025).
- Alam, S.; Aijaz, M. Complications of Cardiovascular Diseases: The Impact of Diabetes, Dyslipidemia, and Metabolic Disorders. World J. Pharm. Res. 2024, 13, 321–356. [Google Scholar] [CrossRef]
- Peng, W.; Chen, S.; Chen, X.; Ma, Y.; Wang, T.; Sun, X.; Wang, Y.; Ding, G.; Wang, Y. Trends in Major Non-Communicable Diseases and Related Risk Factors in China 2002–2019: An Analysis of Nationally Representative Survey Data. Lancet Reg. Health West Pac. 2024, 43, 100809. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J.; Genitsaridi, I.; Piemonte, L.; Riley, P.; Salpea, P. (Eds.) IDF Diabetes Atlas, 11th ed.; the International Diabetes Federation (IDF): Brussels, Belgium, 2025; ISBN 978-2-930229-96-6. [Google Scholar]
- Sarki, A.M.; Nduka, C.U.; Stranges, S.; Kandala, N.B.; Uthman, O.A. Prevalence of Hypertension in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis. Medicine 2015, 94, 1959. [Google Scholar] [CrossRef]
- Beagley, J.; Guariguata, L.; Weil, C.; Motala, A.A. Global Estimates of Undiagnosed Diabetes in Adults. Diabetes Res. Clin. Pract. 2014, 103, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Ochmann, S.; von Polenz, I.; Marcus, M.E.; Theilmann, M.; Flood, D.; Agoudavi, K.; Aryal, K.K.; Bahendeka, S.; Bicaba, B.; Bovet, P.; et al. Diagnostic Testing for Hypertension, Diabetes, and Hypercholesterolaemia in Low-Income and Middle-Income Countries: A Cross-Sectional Study of Data for 994 185 Individuals from 57 Nationally Representative Surveys. Lancet Glob. Health 2023, 11, e1363–e1371. [Google Scholar] [CrossRef]
- Narkhede, M.; Pardeshi, A.; Bhagat, R.; Dharme, G. Review on Emerging Therapeutic Strategies for Managing Cardiovascular Disease. Curr. Cardiol. Rev. 2024, 20, 30. [Google Scholar] [CrossRef]
- Wirtz, V.J.; Kaplan, W.A.; Kwan, G.F.; Laing, R.O. Access to Medications for Cardiovascular Diseases in Low- and Middle-Income Countries. Circulation 2016, 133, 2076–2085. [Google Scholar] [CrossRef]
- Oana, S. Improving Access to Essential Medicines for Circulatory Diseases: A Call to Action; World Heart Federation: Geneva, Switzerland, 2019. [Google Scholar]
- Oyebode, O.; Kandala, N.B.; Chilton, P.J.; Lilford, R.J. Use of Traditional Medicine in Middle-Income Countries: A WHO-SAGE Study. Health Policy Plan 2016, 31, 984–991. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Lebelo, L.S.; Modjadji, P.; Ghaffary, S. Okra Ameliorates Hyperglycaemia in Pre-Diabetic and Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of the Clinical Evidence. Front. Pharmacol. 2023, 14, 1132650. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Phoswa, W.N. Corchorus olitorius Extract Exhibit Anti-Hyperglycemic and Anti-Inflammatory Properties in Rodent Models of Obesity and Diabetes Mellitus. Front. Nutr. 2023, 10, 1099880. [Google Scholar] [CrossRef] [PubMed]
- Sinthumule, N.I.; Mzamani, L.C.M. Communities and Conservation: Marula Trees (Sclerocarya birrea subsp. caffra) Under Communal Management at Matiyane Village, Limpopo Province, South Africa. Trop. Conserv. Sci. 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Mashau, M.E.; Kgatla, T.E.; Makhado, M.V.; Mikasi, M.S.; Ramashia, S.E. Nutritional Composition, Polyphenolic Compounds and Biological Activities of Marula Fruit (Sclerocarya birrea) with Its Potential Food Applications: A Review. Int. J. Food Prop. 2022, 25, 1549–1575. [Google Scholar] [CrossRef]
- Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P.B.; Valentão, P.; Milella, L.; Armentano, M.F. A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts. Int. J. Mol. Sci. 2018, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Kenny, O.; Smyth, T.J.; Milella, L.; Hossain, M.B.; Diop, M.S.; Rai, D.K.; Brunton, N.P. Profiling of Phytochemicals in Tissues from Sclerocarya birrea by HPLC-MS and Their Link with Antioxidant Activity. ISRN Chromatogr. 2013, 2013, 283462. [Google Scholar] [CrossRef]
- Kamanula, M.; Munthali, C.Y.; Kamanula, J.F. Nutritional and Phytochemical Variation of Marula (Sclerocarya birrea) (Subspecies Caffra and Birrea) Fruit among Nine International Provenances Tested in Malawi. Int. J. Food Sci. 2022, 2022, 4686368. [Google Scholar] [CrossRef]
- Konaré, M.A.; Maïga, A.D.; Togola, I.; Diarra, N. Antioxidant and Antidiabetic Potential of Extracts from Anacardium occidentale and Sclerocarya birrea. J. Biochem. Technol. 2024, 15, 15–24. [Google Scholar] [CrossRef]
- Coulidiaty, A.G.V.; Youl, E.N.H.; Yaméogo, T.M.; Oladoja, F.A.; Odejobi, T.A.; Ouedraogo, R.; Awodele, O. Antidiabetic Effect of Hydro-Ethanolic Leaf Extract of Sclerocarya birrea (A. Rich.) Hochst in Wistar Rats. J. Exp. Pharmacol. 2025, 17, 223–237. [Google Scholar] [CrossRef]
- Sewani-Rusike, C.; Azeh Engwa, G.; Tafadzwa Musarurwa, H.; Nkeh-Chungag, B. Sclerocarya birrea Fruit Peel Ameliorates Diet-Induced Obesity and Selected Parameters of Metabolic Syndrome in Female Wistar Rats. Pharmacogn. Mag. 2021, 17, 482–491. [Google Scholar] [CrossRef]
- Mogale, A.M.; Lebelo, L.S.; Thovhogi, N.; de Freitas, A.N.; Shai, L.J. α-Amylase and α-Glucosidase Inhibitory Effects of Sclerocarya birrea [(A. Rich.) Hochst.] Subspecies Caffra (Sond) Kokwaro (Anacardiaceae) Stem-Bark Extracts. Afr. J. Biotechnol. 2011, 10, 15033–15039. [Google Scholar] [CrossRef]
- Djientcheu Tientcheu, J.P.; Ngueguim Tsofack, F.; Gounoue, R.K.; Fifen, R.N.; Dzeufiet, P.D.D.; Dimo, T. The Aqueous Extract of Sclerocarya birrea, Nauclea latifolia, and Piper longum Mixture Protects Striatal Neurons and Movement-Associated Functionalities in a Rat Model of Diabetes-Induced Locomotion Dysfunction. Evid.-Based Complement. Altern. Med. 2023, 2023, 7865919. [Google Scholar] [CrossRef]
- Ngueguim, F.T.; Esse, E.C.; Dzeufiet, P.D.D.; Gounoue, R.K.; Bilanda, D.C.; Kamtchouing, P.; Dimo, T. Oxidised Palm Oil and Sucrose Induced Hyperglycemia in Normal Rats: Effects of Sclerocarya birrea Stem Barks Aqueous Extract. BMC Complement. Altern. Med. 2015, 16, 47. [Google Scholar] [CrossRef]
- Zhao, C. Cell Culture: In Vitro Model System and a Promising Path to In Vivo Applications. J. Histotechnol. 2023, 46, 2170772. [Google Scholar] [CrossRef]
- Kgopa, A.H.; Shai, L.J.; Mogale, M.A. Effects of Sclerocarya birrea Stem-Bark Extracts on Glucose Uptake, Insulin Synthesis and Expression of Selected Genes Involved in the Synthesis and Secretion of Insulin in Rat Insulinoma Pancreatic Beta Cells. Asian J. Chem. 2020, 32, 2195–2202. [Google Scholar] [CrossRef]
- Matsabisa, M.G.; Chukwuma, C.I.; Chaudhary, S.K. South African Traditional Herbal Formulation Inhibits α-Glucosidase, DPP-IV and Glycation Activities, and Modulates Glucose Utilisation in Chang Liver Cells and 3T3-L1 Adipocytes. S. Afr. J. Bot. 2019, 121, 121–127. [Google Scholar] [CrossRef]
- Da Costa Mousinho, N.M.H.; Van Tonder, J.J.; Steenkamp, V. In Vitro Anti-Diabetic Activity of Sclerocarya birrea and Ziziphus mucronata. Nat. Prod. Commun. 2013, 8, 1279–1284. [Google Scholar] [CrossRef]
- Ndifossap, I.G.M.; Frigerio, F.; Casimir, M.; Tsofack, F.N.; Dongo, E.; Kamtchouing, P.; Dimo, T.; Maechler, P. Sclerocarya birrea (Anacardiaceae) Stem-Bark Extract Corrects Glycaemia in Diabetic Rats and Acts on β-Cells by Enhancing Glucose-Stimulated Insulin Secretion. J. Endocrinol. 2010, 205, 79–86. [Google Scholar] [CrossRef]
- Noviana, D.; Estuningsih, S.; Ulum, M.F. Animal Study and Pre-Clinical Trials of Biomaterials. In Advanced Structured Materials; Mahyudin, F., Hermawan, H., Eds.; Springer International: Cham, Switzerland, 2016; Volume 58, pp. 67–101. [Google Scholar]
- Singh, V.; Ashish, R.; Editors, R. Preclinical In Vivo Drug Development Studies: Limitations, Model Organisms, and Techniques. In Drugs and a Methodological Compendium; Rajput, V.S., Runthala, A., Eds.; Springer: Singapore, 2023; pp. 149–171. [Google Scholar]
- Mabasa, L.; Kotze, A.; Shabalala, S.; Kimani, C.; Gabuza, K.; Johnson, R.; Sangweni, N.F.; Maharaj, V.; Muller, C.J.F. Sclerocarya birrea (Marula) Extract Inhibits Hepatic Steatosis in Db/Db Mice. Int. J. Environ. Res. Public Health 2022, 19, 3782. [Google Scholar] [CrossRef]
- Gondwe, M.; Kamadyaapa, D.R.; Tufts, M.; Chuturgoon, A.A.; Musabayane, C.T. Sclerocarya birrea [(A. Rich.) Hochst.] [Anacardiaceae] Stem-Bark Ethanolic Extract (SBE) Modulates Blood Glucose, Glomerular Filtration Rate (GFR) and Mean Arterial Blood Pressure (MAP) of STZ-Induced Diabetic Rats. Phytomedicine 2008, 15, 699–709. [Google Scholar] [CrossRef]
- Dimo, T.; Rakotonirina, S.V.; Tan, P.V.; Azay, J.; Dongo, E.; Kamtchouing, P.; Cros, G. Effect of Sclerocarya birrea (Anacardiaceae) Stem Bark Methylene Chloride/Methanol Extract on Streptozotocin-Diabetic Rats. J. Ethnopharmacol. 2007, 110, 434–438. [Google Scholar] [CrossRef]
- Ojewole, J.A.O. Evaluation of the Anti-Inflammatory Properties of Sclerocarya birrea (A. Rich.) Hochst. (Family: Anacardiaceae) Stem-Bark Extracts in Rats. J. Ethnopharmacol. 2003, 85, 217–220. [Google Scholar] [CrossRef]
- Traoré, I.V.; Belemnaba, L.; Ouedraogo, W.R.; Kaboré, B.; Bationo, R.K.; Belemlilga, B.M.; Compaoré, S.; Goumbri, W.B.F.; Ouedraogo, S.; Koala, M.; et al. Preclinical Evaluation of the Antidiabetic Effect and Phytochemical HPLC-MS ESI-QTOF Analysis of Sclerocarya birrea (A. Rich) Hoscht Bark of Trunk Aqueous Extract in Alloxan-Induced Diabetic Wistar Rat. Pharmacol. Amp. Pharm. 2024, 15, 364–387. [Google Scholar] [CrossRef]
- Attakpa, E.S.; Sezan, A.; Baba-Moussa, L.; Khan, N. Sclerocarya birrea (Anacardiaceae) Stem-Bark Extract Stimulates Protein Kinase Akt and AMPK Pathways in the Liver in a Diet-Induced Obesity Mouse Model. Indian J. Appl. Res. 2015, 5, 710–716. [Google Scholar]
- Ebrahimi, M.; Thompson, P.M.; Kafashan, Z.; Ceriello, A.; Kolko, M.; Grauslund, J. Association between Cerebral Lesions and the Severity of Diabetic Cardiovascular Disease, Retinopathy, and Nephropathy-New Lessons to Learn from Neuroimaging. J. Endocrinol. Investig. 2025. [Google Scholar] [CrossRef]
- Islam, K.; Islam, R.; Nguyen, I.; Malik, H.; Pirzadah, H.; Shrestha, B.; Lentz, I.B.; Shekoohi, S.; Kaye, A.D. Diabetes Mellitus and Associated Vascular Disease: Pathogenesis, Complications, and Evolving Treatments. Adv. Ther. 2025, 42, 2659–2678. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Martínez-Rodríguez, J.; González-Lucán, M.; Fernández-Fernández, C.; Castro-Quintela, E. Insulin Resistance Is a Cardiovascular Risk Factor in Humans. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1449–1455. [Google Scholar] [CrossRef]
- Ojewole, J.A.O.; Mawoza, T.; Chiwororo, W.D.H.; Owira, P.M.O. Sclerocarya birrea (A. Rich) Hochst. [‘Marula’] (Anacardiaceae): A Review of Its Phytochemistry, Pharmacology and Toxicology and Its Ethnomedicinal Uses. Phytother. Res. 2010, 24, 633–639. [Google Scholar] [CrossRef]
- Fotio, A.L.; Dimo, T.; Nguelefack, T.B.; Dzeufiet, P.D.D.; Ngo Lemba, E.; Temdie, R.J.; Ngueguim, F.; Olleros, M.L.; Vesin, D.; Dongo, E.; et al. Acute and Chronic Anti-Inflammatory Properties of the Stem Bark Aqueous and Methanol Extracts of Sclerocarya birrea (Anacardiaceae). Inflammopharmacology 2009, 17, 229–237. [Google Scholar] [CrossRef]
- Victoria-Montesinos, D.; Sánchez-Macarro, M.; Gabaldón-Hernández, J.A.; Abellán-Ruiz, M.S.; Querol-Calderón, M.; Luque-Rubia, A.J.; Bernal-Morell, E.; Ávila-Gandía, V.; López-Román, F.J. Effect of Dietary Supplementation with a Natural Extract of Sclerocarya birrea on Glycemic Metabolism in Subjects with Prediabetes: A Randomized Double-Blind Placebo-Controlled Study. Nutrients 2021, 13, 1948. [Google Scholar] [CrossRef]
- Borochov-Neori, H.; Judeinstein, S.; Greenberg, A.; Fuhrman, B.; Attias, J.; Volkova, N.; Hayek, T.; Aviram, M. Phenolic Antioxidants and Antiatherogenic Effects of Marula (Sclerocarrya birrea subsp. caffra) Fruit Juice in Healthy Humans. J. Agric. Food Chem. 2008, 56, 9884–9891. [Google Scholar] [CrossRef]
- Coulidiaty, A.G.V.; Yaméogo, T.M.; Ouedraogo, R.; Clark, K.; Dakuyo, V.M.; Youl, E.N.H. Evaluation of Sclerocarya birrea for Type 2 Diabetes Management: Phase I Safety and Preliminary Efficacy Study in Healthy Volunteers. Clin. Tradit. Med. Pharmacol. 2025, 6, 200212. [Google Scholar] [CrossRef]
- Coulidiaty, A.G.V.; Boni, S.I.; Ouedraogo, R.; Koama, B.K.; Soré, H.; Meda, R.N.T.; Yaméogo, T.M.; Youl, E.N.H. Acute and Chronic Oral Toxicity of Hydroethanolic Extract of Sclerocarya birrea (Anacardiaceae) in Wistar Rats. J. Exp. Pharmacol. 2024, 16, 231–242. [Google Scholar] [CrossRef]
- Hu, R.; Yan, H.; Fei, X.; Liu, H.; Wu, J. Modulation of Glucose Metabolism by a Natural Compound from Chloranthus Japonicus via Activation of AMP-Activated Protein Kinase. Sci. Rep. 2017, 7, 778. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPARa Action and Its Impact on Lipid Metabolism, Inflammation and Fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Yilgor, A.; Demir, C. Determination of Oxidative Stress Level and Some Antioxidant Activities in Refractory Epilepsy Patients. Sci. Rep. 2024, 14, 6688. [Google Scholar] [CrossRef] [PubMed]
- Wispriyono, B.; Jalaludin, J.; Kusnoputranto, H.; Pakpahan, S.; Aryati, G.P.; Pratama, S.; Librianty, N.; Rozaliyani, A.; Taufik, F.F.; Novirsa, R. Glutathione (GSH) and Superoxide Dismutase (SOD) Levels among Junior High School Students Induced by Indoor Particulate Matter 2.5 (PM2.5) and Nitrogen Dioxide (NO2) Exposure. J. Public Health Res. 2021, 10, 2372. [Google Scholar] [CrossRef] [PubMed]
- Fauziah, P.N.; Maskoen, A.M.; Yuliati, T.; Widiarsih, E. Optimized Steps in Determination of Malondialdehyde (MDA) Standards on Diagnostic of Lipid Peroxidation. Padjadjaran J. Dent. 2018, 30, 136–139. [Google Scholar] [CrossRef]
- Rizzo, M. Measurement of Malondialdehyde as a Biomarker of Lipid Oxidation in Fish. Am. J. Analyt. Chem. 2024, 15, 303–332. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Mncwangi, B.; Baraldo, G.; Jansen-Dürr, P.; Viljoen, A.; Stuppner, H.N.W. Sclerocarya birrea Cortex Ethanolic Extract–Chemical Characterisation and NOX4 Inhibition (Anti-Ageing Property). Planta Medica Int. Open 2017, 4, Mo-PO-211. [Google Scholar] [CrossRef]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef]
- Nowak, D.; Gośliński, M.; Wojtowicz, E.; Przygoński, K. Antioxidant Properties and Phenolic Compounds of Vitamin C-Rich Juices. J. Food Sci. 2018, 83, 2237–2246. [Google Scholar] [CrossRef] [PubMed]
- Dorothy, V.; Lucy, H.; Sunday, O.O.; Mary, O.U. The Anti-Inflammatory Activities of Twelve Nigerian Medicinal Plants: Inhibition of NfkB, Activation of Nrf2, and Antioxidant Content. Afr. J. Biochem. Res. 2022, 16, 1–10. [Google Scholar] [CrossRef]
- Moyo, M.; Finnie, J.F.; Van Staden, J. Antimicrobial and Cyclooxygenase Enzyme Inhibitory Activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) Plant Extracts. S. Afr. J. Bot. 2011, 77, 592–597. [Google Scholar] [CrossRef][Green Version]
- Harris, R.A.; Nishiyama, S.K.; Wray, D.W.; Richardson, R.S. Ultrasound Assessment of Flow-Mediated Dilation. Hypertension 2010, 55, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Soga, J.; Fujimura, N.; Idei, N.; Mikami, S.; Iwamoto, Y.; Kajikawa, M.; Matsumoto, T.; Hidaka, T.; Kihara, Y.; et al. Relationship between Flow-Mediated Vasodilation and Cardiovascular Risk Factors in a Large Community-Based Study. Heart 2013, 99, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country | Experimental Model and Interventions | Experimental Findings |
|---|---|---|---|
| Kgopa et al., 2020 [26] | South Africa | Insulinoma cells, H-4-II-E liver cells. Cells treated with 50 µg/mL of S. birrea stem bark extract. | Dose-dependent increase in glucose uptake, enhanced insulin synthesis, and glucose-stimulated insulin secretion. Upregulation of glucose synthase. |
| Matsabisa et al., 2019 [27] | South Africa | 3T3-L1 adipocytes and Chang liver cells. 50 μg/mL of stem bark S. birrea extract (methanol, hexane, and DCM). | Inhibited α-glucosidase and high phenolic content. Increased glucose uptake and AGEs. |
| Russo et al., 2018 [16] | Italy | HepG2 and HDFa cells were treated with bark and leaf methanol extracts of S. birrea (10, 50, 100, 200, and 300 μg/mL) for 24 h. | Higher ROS, polyphenol, tannin, and cytotoxic activities in S. birrea bark extract than in the leaf. Higher scavenging activity of methanol. |
| Mousinho et al., 2013 [28] | South Africa | C2C12, HepG2, and 3T3-L1 cells were treated with 1.56–6.25 μg/mL of bark of S. birrea extract (aqueous and methanol). | Methanol extract showed strong scavenging activity. Increased glucose uptake. Inhibited α-amylase and α-glucosidase |
| Ndifossap et al., 2010 [29] | Cameroon | INS-1E cells were treated with 5 μg/mL of S. birrea stem bark extract for 24 h. | Increased glucose oxidation and ATP generation. No effect on glucokinase, GLUT2, pyruvate carboxylase, or COX1. |
| Reference | Country | Experimental Model, Dose and Duration of Intervention | Hypoglycemic Activities | Hypolipidemic Activities | Anti-Inflammatory Antioxidative Stress Activities | Hypertensive Activities |
|---|---|---|---|---|---|---|
| Coulidiaty et al., 2025 [20] | Nigeria | High fructose-fed, streptozotocin (STZ)-induced type 2 diabetes (T2D) in Wistar rats. 100, 200, and 400 mg/kg of hydroethanolic extract of S. birrea leaves, administered orally for 21 days. | All doses reduced blood glucose. No effect on HbA1c, insulin, or GLUT-4. | No effect on TC, TG, LDL, or HDL | NS | NS |
| Traoré et al., 2024 [36] | Burkina Faso | Alloxan monohydrate-induced diabetes in Wistar rats. 5 and 25 mg/kg of S. birrea bark aqueous extract for 4 weeks. | Reduced FBG. | 5 mg/kg increased TC, LDL, and HDL without effect on TG. 25 mg/kg decreased TG and LDL. | NS | NS |
| Tientcheu et al., 2023 [23] | Cameroon | Fructose-fed, STZ-induced diabetes in Wistar rats. 75, 150, and 300 mg/kg of stem barks of S. birrea and Nauclea latifolia plus fruits of Piper longum (SNP) for 21 days. | 150 and 300 mg/kg decreased glucose. All doses decreased HOMA-IR. Increased insulin and hepatic glycogen. | NS | Decreased TNF-α, IL-1β, INF-γ with 150 mg/kg. MDA decreased. CAT increased. GSH increased at 150 and 300 mg/kg. | NS |
| Mabasa et al., 2022 [32] | South Africa | Nine-week-old diabetic BKS.Cg-Dock7(m) +/+ Lepr(db)/J obese mice on standard rodent maintenance diet. 600 mg/kg of S. birrea leaves, macerated in de-ionized water, was administered orally for 29 days. | Decreased non-FBG levels, upregulated Akt. No effect on PI3K, AMPK, or GLUT2. | Decreased hepatic lipid accumulation, FAS Upregulated expression of PPARα and CPT1, enhancing β-oxidation of fatty acids. | Contains high EGCG and myricetin content. | NS |
| Sewani-Rusike et al., 2021 [21] | South Africa | High-energy diet (HED)-induced obesity in Wistar rats. 100–200 mg/kg of S. birrea fruit peel hydro-ethanolic extract administered orally for 28 days. | Decreased FBG, insulin, and HOMA-IR. Enhanced glucose tolerance. | Decreased TC, TG, and LDL at both doses. | High polyphenol, flavonoid, and TAC content in fruit peel compared to pulp. | Significant decrease in BP at both doses compared to the untreated obese. |
| Ngueguim et al., 2016 [24] | Cameroon | Oxidized palm oil and sucrose induced hyperglycemia in albino Wistar rats. 150 and 300 mg/kg of S. birrea stem bark were administered orally for 21 days. | Both doses decreased blood glucose. Increased insulin sensitivity index. | Decreased TG, LDL, TC, and AIP. Increased HDL. | Decreased lipid peroxidation, nitrite levels, and MDA in the liver. Increased SOD and GSH in the liver and kidney. | Both doses reduced SBP, DBP, and MBP. |
| Attakpa et al., 2015 [37] | Benin | High-fat-diet-fed C57BL/6J mice. 200 or 300 mg/kg stem bark extract for 10 weeks. | Both doses reduced non-FBG. 300 mg/kg reduced insulin. Increased Akt, AMPK. | Decreased TG, FFA, and SREBP-1. Increased PPARα expression. | NS | NS |
| Mogale et al., 2011 [22] | South Africa | Alloxan monohydrate-induced DM in albino Wistar rats. 300 mg/kg of S. birrea bark aqueous, methanolic, acetone, and hexane extracts were administered orally. | Inhibited α-amylase and α-glucosidase. Reduced postprandial blood glucose levels. | NS | NS | NS |
| Ndifossap et al., 2010 [29] | Cameroon | Nicotinamide and STZ-induced DM in Wistar rats. 150 or 300 mg/kg of S. birrea stem bark extract was administered orally for 14 days. | Decreased FBG, restored insulin levels. | NS | NS | NS |
| Gondwe et al., 2008 [33] | South Africa | STZ-induced DM in Wistar rats. 60, 120, and 240 mg/kg of S. birrea bark ethanol extract administered orally for 5 weeks. | Reduced blood glucose and increased hepatic glycogen. No effect on insulin. | NS | NS | Reduced mean arterial pressure. |
| Dimo et al., 2006 [34] | Cameroon | STZ-induced DM in Wistar rats, with 150 and 300 mg/kg methanolic/methylene chloride extract of S. birrea stem bark administered orally for 21 days. | Decreased blood glucose levels, polyphagia, and polydipsia. Increased plasma insulin. | TC and TG levels returned to normal at a dose of 300 mg/kg. | NS | NS |
| Ojewole et al., 2003 [35] | South Africa | STZ-induced DM in Balb C mice and Wistar rats. 100–800 mg/kg methanol/methylene chloride extract of S. birrea stem bark administered orally for 8 h. | Decreased FBG levels. | NS | NS | NS |
| Reference and Country | Experimental Design | S. birrea Dose and Duration of Intervention | Antidiabetic Activities | Hypolipidemic Activities | Inflammatory and Antioxidant Activities | Antihypertensive Activities |
|---|---|---|---|---|---|---|
| Coulidiaty et al., 2025 [45] Burkina Faso | Phase I open-label clinical trial 10 healthy males, aged 18 to 40 years | Oral administration of 1800 mg of S. birrea leaf powder for 14 days | No significant changes in glycemia | Decreased TC | NS | Reduced BP |
| Victoria-Montesinos et al., 2021 [43] Spain | Randomized, double-blind, placebo-controlled trial 67 patients with prediabetes (33 on S. birrea and 34 on placebo), aged 18 to 65 years | Daily ingestion of 100 mg S. birrea stem bark aqueous powder per day for 90 days | No change in FBG or HbA1c Increased FI, HOMA-IR, and QUICKI index Decreased 2 h post-OGTT | No significant changes in serum TC, LDL, HDL, or TG | Decreased E-selectin No effect on IL-6 levels | Reduced SBP No change in DBP Improved FMD |
| Borochov-Neori et al., 2008 [44] Israel | Phase I open-label, non-controlled clinical trial 10 healthy males aged 18 to 40 years | 200 mL of S. birrea juice per day for 3 weeks | No effect on serum glucose levels | Decreased TC, LDL, and TG, increased HDL | Inhibited ox-LDL Reduced lipid peroxides High in vitamin C, phenols, and increased FRAP Strong free radical scavenging ability | No effect on SBP or DBP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashaba, G.R.; Mokgalaboni, K.; Lebelo, S.L. Potential Impact of Sclerocarya birrea on Cardiovascular Health and Related Risk Factors: Review of Existing Evidence. Antioxidants 2025, 14, 997. https://doi.org/10.3390/antiox14080997
Mashaba GR, Mokgalaboni K, Lebelo SL. Potential Impact of Sclerocarya birrea on Cardiovascular Health and Related Risk Factors: Review of Existing Evidence. Antioxidants. 2025; 14(8):997. https://doi.org/10.3390/antiox14080997
Chicago/Turabian StyleMashaba, Given R., Kabelo Mokgalaboni, and Sogolo L. Lebelo. 2025. "Potential Impact of Sclerocarya birrea on Cardiovascular Health and Related Risk Factors: Review of Existing Evidence" Antioxidants 14, no. 8: 997. https://doi.org/10.3390/antiox14080997
APA StyleMashaba, G. R., Mokgalaboni, K., & Lebelo, S. L. (2025). Potential Impact of Sclerocarya birrea on Cardiovascular Health and Related Risk Factors: Review of Existing Evidence. Antioxidants, 14(8), 997. https://doi.org/10.3390/antiox14080997






