Upcycling of Waste Cherries Produces an Anthocyanin-Rich Powder That Protects Against Amyloid-β Toxicity in C. elegans
Abstract
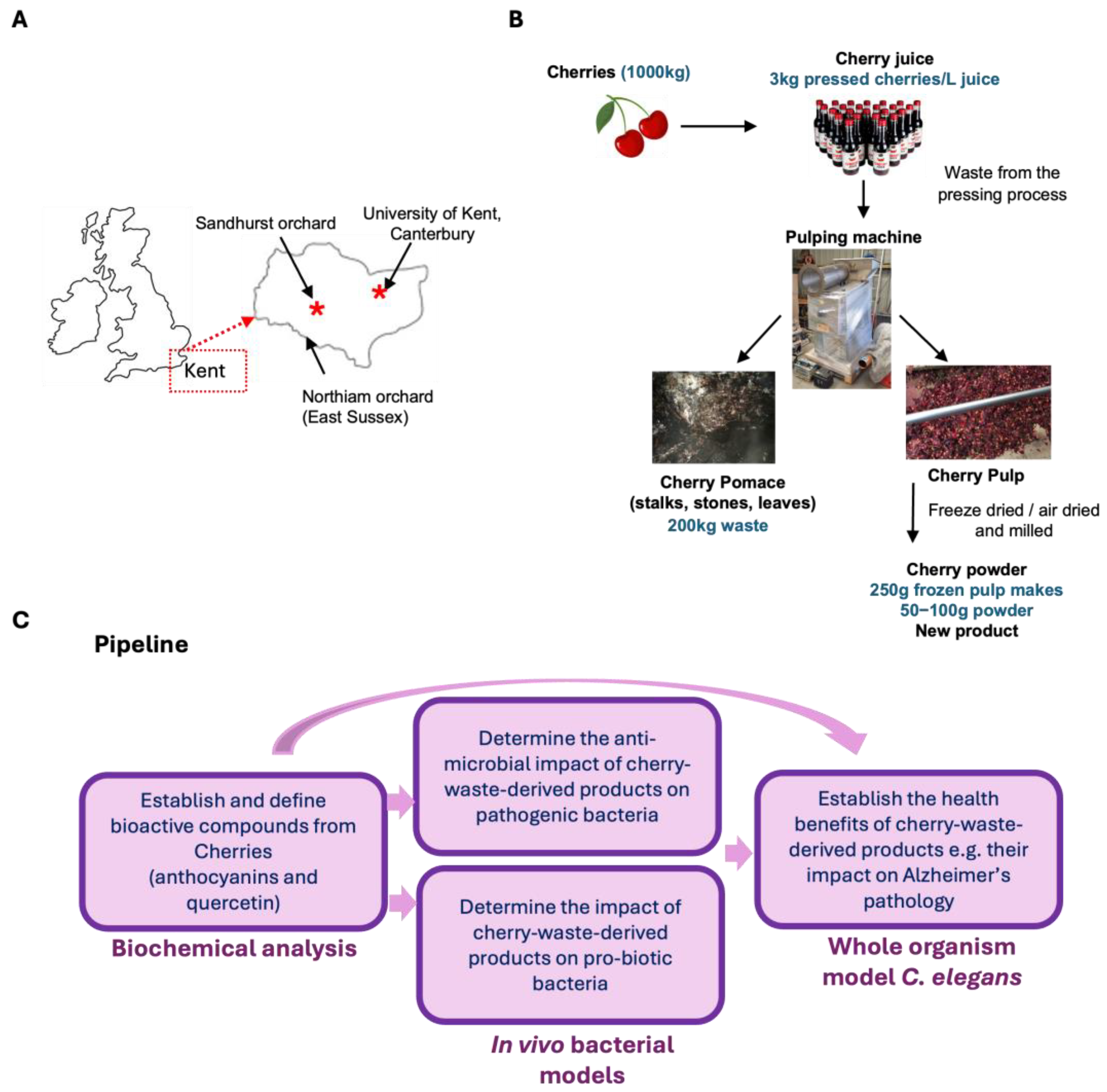
1. Introduction
2. Materials and Methods
2.1. Ultra-Performance Liquid Chromatography (UPLC-MS) Coupled with Quadrupole Time-of-Flight Mass Spectrometry (UPLC-QToF)
2.1.1. Sample Preparation
2.1.2. Ethanol Extraction
2.1.3. Standards
2.1.4. UPLC-QToF
2.1.5. UPLC-MS/MS
2.2. Preparation of Cherry Product Stock Solutions for Bioactivity Assays
2.2.1. Cherry Juice
2.2.2. Cherry Pulp Powder
2.2.3. Blackberry Powder
2.2.4. Kirby–Bauer Analysis
2.2.5. Growth Measurements of E. coli OP50
2.2.6. Growth Measurement of Lactobacillus Strains
2.2.7. C. elegans Culture Methods and Strains
2.2.8. Developmental Assays
2.2.9. Proteotoxicity Assay
2.2.10. Imaging of GST-4::GFP
2.2.11. Statistical Analysis
3. Results
3.1. Quantification and Identification of Anthocyanins in Kent Cherry Products
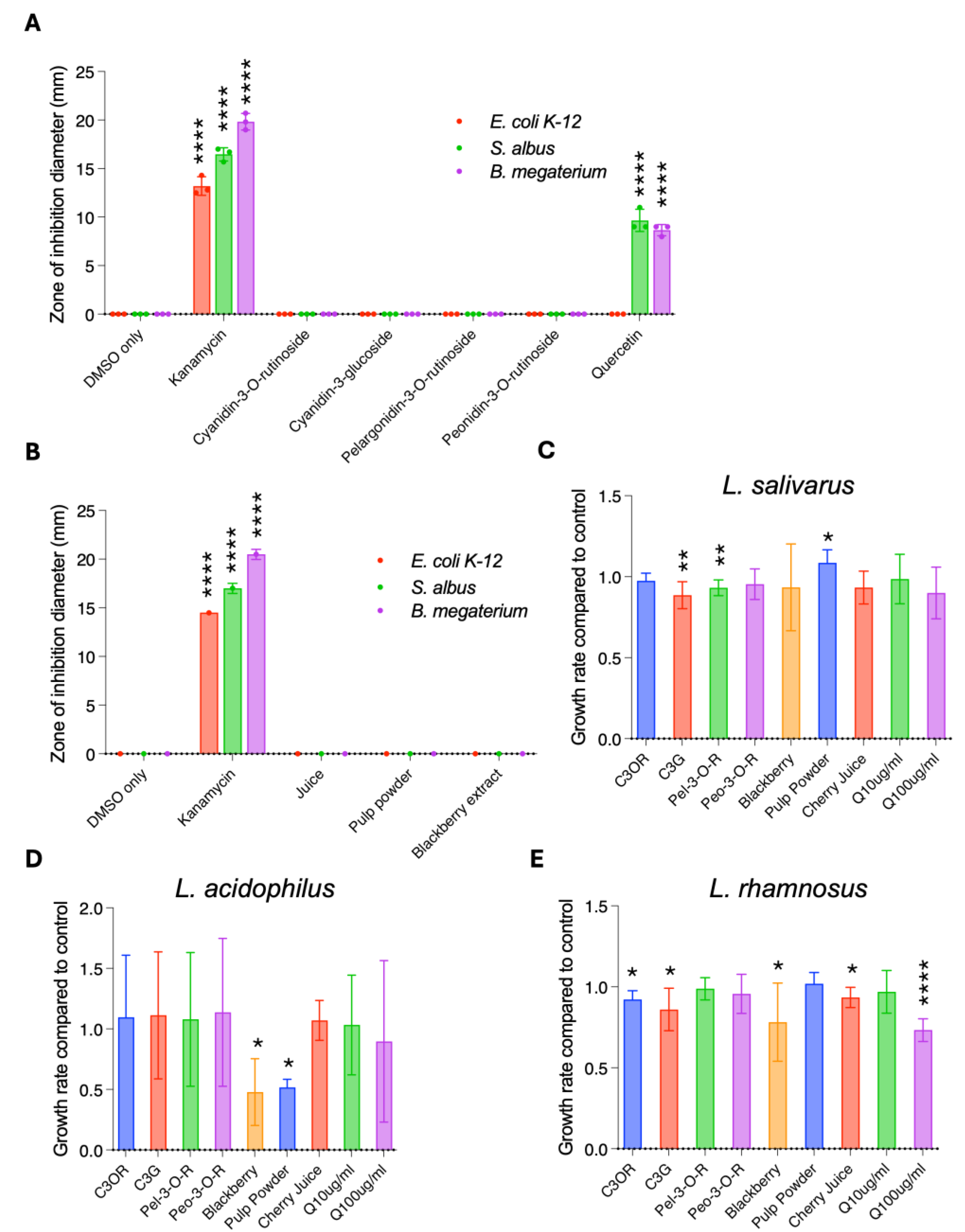
3.2. Anti-Bacterial and Prebiotic Properties of Cherry Juice and Cherry Pulp Powder
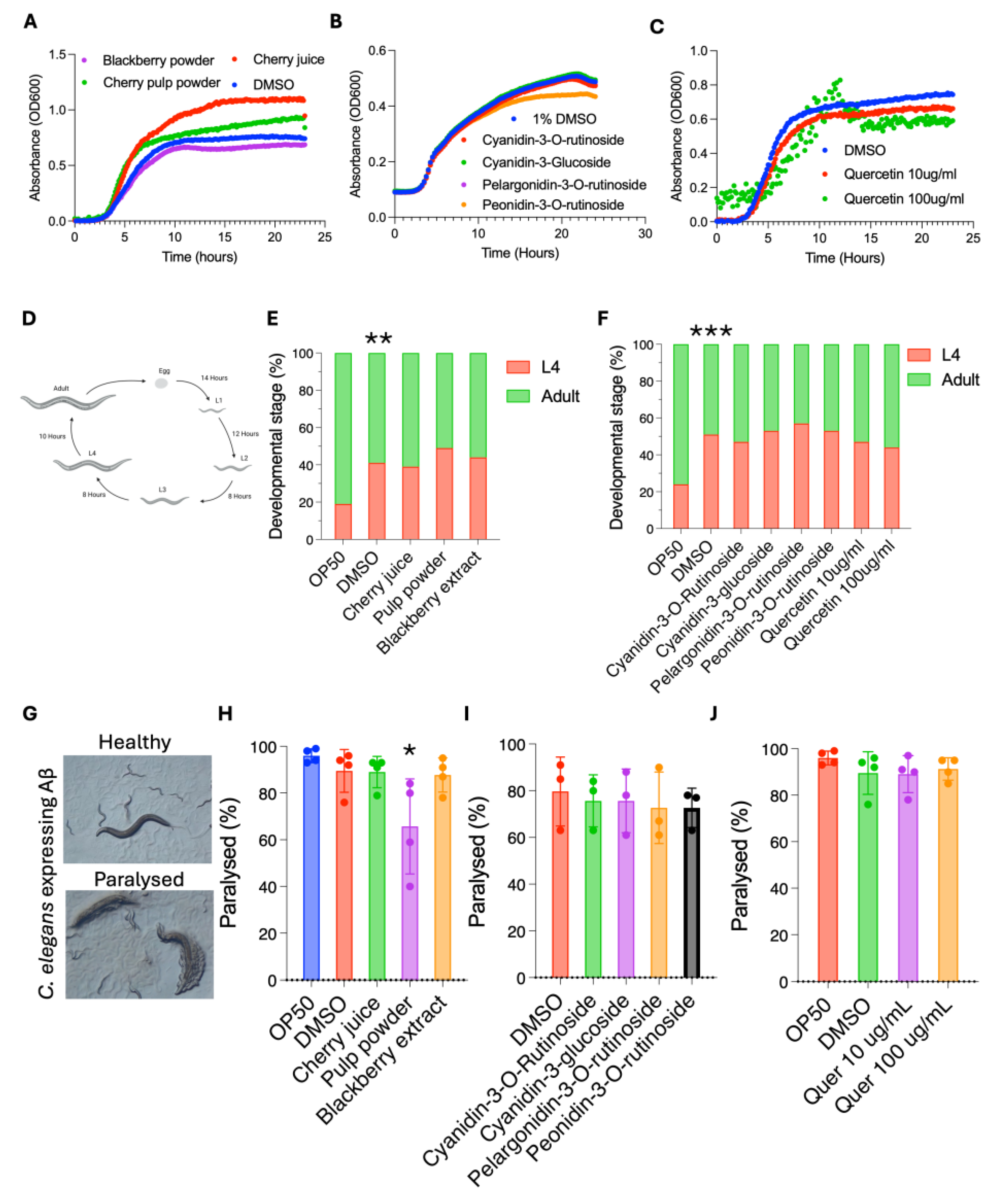
3.3. Cherry Products Do Not Have a Negative Impact on C. elegans Growth or Development
3.4. Cherry Pulp Powder Protects Against Amyloid-β Proteotoxicity
4. Discussion
4.1. Insights from Analysing Waste Cherry Products
4.2. Mechanisms by Which Cherry Powder May Protect Against Aβ Toxicity
4.3. An Opportunity to Use Waste to Produce Inexpensive Healthy Foods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Think Eat Save Tracking Progress to Halve Global Food Waste. Sav. Lives Chang. Lives 2024, 11822, 45230. [Google Scholar]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture Waste Valorisation as a Source of Antioxidant Phenolic Compounds within a Circular and Sustainable Bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Gutiérrez-del-Río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and Polyphenols as Natural Antioxidant Agents in Food Preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Jennings, A.; Thompson, A.S.; Tresserra-Rimbau, A.; O’Neill, J.K.; Hill, C.; Bondonno, N.P.; Kühn, T.; Cassidy, A. Flavonoid-Rich Foods, Dementia Risk, and Interactions with Genetic Risk, Hypertension, and Depression. JAMA Netw. Open 2024, 7, e2434136. [Google Scholar] [CrossRef]
- Hole, K.L.; Williams, R.J. Flavonoids as an Intervention for Alzheimer’s Disease: Progress and Hurdles Towards Defining a Mechanism of Action1. Brain Plast. 2021, 6, 167–192. [Google Scholar] [CrossRef]
- Lakshmikanthan, M.; Muthu, S.; Krishnan, K.; Altemimi, A.B.; Haider, N.N.; Govindan, L.; Selvakumari, J.; Alkanan, Z.T.; Cacciola, F.; Francis, Y.M. A Comprehensive Review on Anthocyanin-Rich Foods: Insights into Extraction, Medicinal Potential, and Sustainable Applications. J. Agric. Food Res. 2024, 17, 101245. [Google Scholar] [CrossRef]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Chemical Characterisation and Bioactive Properties of Prunus Avium L.: The Widely Studied Fruits and the Unexplored Stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Chezanoglou, E.; Mourtzinos, I.; Goula, A.M. Sweet Cherry and Its By-Products as Sources of Valuable Phenolic Compounds. Trends Food Sci. Technol. 2024, 145, 104367. [Google Scholar] [CrossRef]
- Matli, Ö. Economic Analysis Report of Postharvest Losses for Some Fruits; European Commission: Brussels, Belgium; Turkish National Agency: Ankara, Turkish, 2019. [Google Scholar]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Corsi, A.K.; Wightman, B.; Chalfie, M. A Transparent Window into Biology: A Primer on Caenorhabditis Elegans. Genetics 2015, 200, 387–407. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors Affecting Their Stability and Degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef]
- UK Met Office Weather and Climate Summaries. Available online: https://www.metoffice.gov.uk/research/climate/maps-and-data/summaries/index (accessed on 7 November 2024).
- Stanoeva, J.P.; Balshikevska, E.; Stefova, M.; Tusevski, O.; Simic, S.G. Comparison of the Effect of Acids in Solvent Mixtures for Extraction of Phenolic Compounds From Aronia Melanocarpa. Nat. Prod. Commun. 2020, 15, 1934578X20934675. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, S.; Fei, Y.; Liu, G.; Jang, H.; Fang, J. Antimicrobial Activity of Anthocyanins and Catechins against Foodborne Pathogens Escherichia Coli and Salmonella. Food Control 2019, 106, 106712. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Morón-Ortiz, Á.; Karamalegkos, A.A.; Mapelli-Brahm, P.; Ezcurra, M.; Meléndez-Martínez, A.J. Phytoene and Phytoene-Rich Microalgae Extracts Extend Lifespan in C. Elegans and Protect against Amyloid-β Toxicity in an Alzheimer’s Disease Model. Antioxidants 2024, 13, 931. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Khan, M.; Shah, S.A.; Saeed, K.; Kim, M.O. Natural Antioxidant Anthocyanins—A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients 2019, 11, 1195. [Google Scholar] [CrossRef]
- McColl, G.; Roberts, B.R.; Pukala, T.L.; Kenche, V.B.; Roberts, C.M.; Link, C.D.; Ryan, T.M.; Masters, C.L.; Barnham, K.J.; Bush, A.I.; et al. Utility of an Improved Model of Amyloid-Beta (Aβ1-42) Toxicity in Caenorhabditis Elegansfor Drug Screening for Alzheimer’s Disease. Mol. Neurodegener. 2012, 7, 57. [Google Scholar] [CrossRef]
- Leung, C.K.; Empinado, H.; Choe, K.P. Depletion of a Nucleolar Protein Activates Xenobiotic Detoxification Genes in Caenorhabditis Elegans via Nrf/SKN-1 and P53/CEP-1. Free Radic. Biol. Med. 2012, 52, 937–950. [Google Scholar] [CrossRef]
- Whyte, A.R.; Cheng, N.; Fromentin, E.; Williams, C.M. A Randomized, Double-Blinded, Placebo-Controlled Study to Compare the Safety and Efficacy of Low Dose Enhanced Wild Blueberry Powder and Wild Blueberry Extract (ThinkBlueTM) in Maintenance of Episodic and Working Memory in Older Adults. Nutrients 2018, 10, 660. [Google Scholar] [CrossRef]
- Boespflug, E.L.; Eliassen, J.C.; Dudley, J.A.; Shidler, M.D.; Kalt, W.; Summer, S.S.; Stein, A.L.; Stover, A.N.; Krikorian, R. Enhanced Neural Activation with Blueberry Supplementation in Mild Cognitive Impairment. Nutr. Neurosci. 2018, 21, 297–305. [Google Scholar] [CrossRef]
- Krikorian, R.; Skelton, M.R.; Summer, S.S.; Shidler, M.D.; Sullivan, P.G. Blueberry Supplementation in Midlife for Dementia Risk Reduction. Nutrients 2022, 14, 1619. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a Polyphenol-Rich Grape and Blueberry Extract (MemophenolTM) on Cognitive Function in Older Adults with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Psychol. 2023, 14, 1144231. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Mesnage, R.; Fernandes, F.; Abhayaratne, N.; Xu, Y.; Zhang, Z.; Bell, L.; Williams, C.; Rodriguez-Mateos, A. Wild Blueberry (Poly)Phenols Can Improve Vascular Function and Cognitive Performance in Healthy Older Individuals: A Double-Blind Randomized Controlled Trial. Am. J. Clin. Nutr. 2023, 117, 1306–1319. [Google Scholar] [CrossRef]
- Barfoot, K.L.; May, G.; Lamport, D.J.; Ricketts, J.; Riddell, P.M.; Williams, C.M. The Effects of Acute Wild Blueberry Supplementation on the Cognition of 7–10-Year-Old Schoolchildren. Eur. J. Nutr. 2019, 58, 2911–2920. [Google Scholar] [CrossRef]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of Anthocyanin-Rich Cherry Juice for 12 Weeks Improves Memory and Cognition in Older Adults with Mild-to-Moderate Dementia. Eur. J. Nutr. 2017, 56, 333–341. [Google Scholar] [CrossRef]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-Loaded PEG-Gold Nanoparticles Enhanced the Neuroprotection of Anthocyanins in an Aβ1–42 Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-Enriched Bilberry and Blackcurrant Extracts Modulate Amyloid Precursor Protein Processing and Alleviate Behavioral Abnormalities in the APP/PS1 Mouse Model of Alzheimer’s Disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin Suppresses the Toxicity of Aβ Deposits through Diversion of Molecular Forms in in Vitro and in Vivo Models of Alzheimer’s Disease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef]
- Tarozzi, A.; Morroni, F.; Merlicco, A.; Bolondi, C.; Teti, G.; Falconi, M.; Cantelli-Forti, G.; Hrelia, P. Neuroprotective Effects of Cyanidin 3-O-Glucopyranoside on Amyloid Beta (25–35) Oligomer-Induced Toxicity. Neurosci. Lett. 2010, 473, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.M. The Food Matrix: Implications in Processing, Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2019, 59, 3612–3629. [Google Scholar] [CrossRef] [PubMed]
- Kumkum, R.; Aston-Mourney, K.; McNeill, B.A.; Hernández, D.; Rivera, L.R. Bioavailability of Anthocyanins: Whole Foods versus Extracts. Nutrients 2024, 16, 1403. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on Healthy Diets from Sustainable Food Systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and Relative Stabilities of Acylated and Nonacylated Anthocyanins in Beverage Systems. J. Food Sci. Technol. 2022, 59, 831–845. [Google Scholar] [CrossRef]
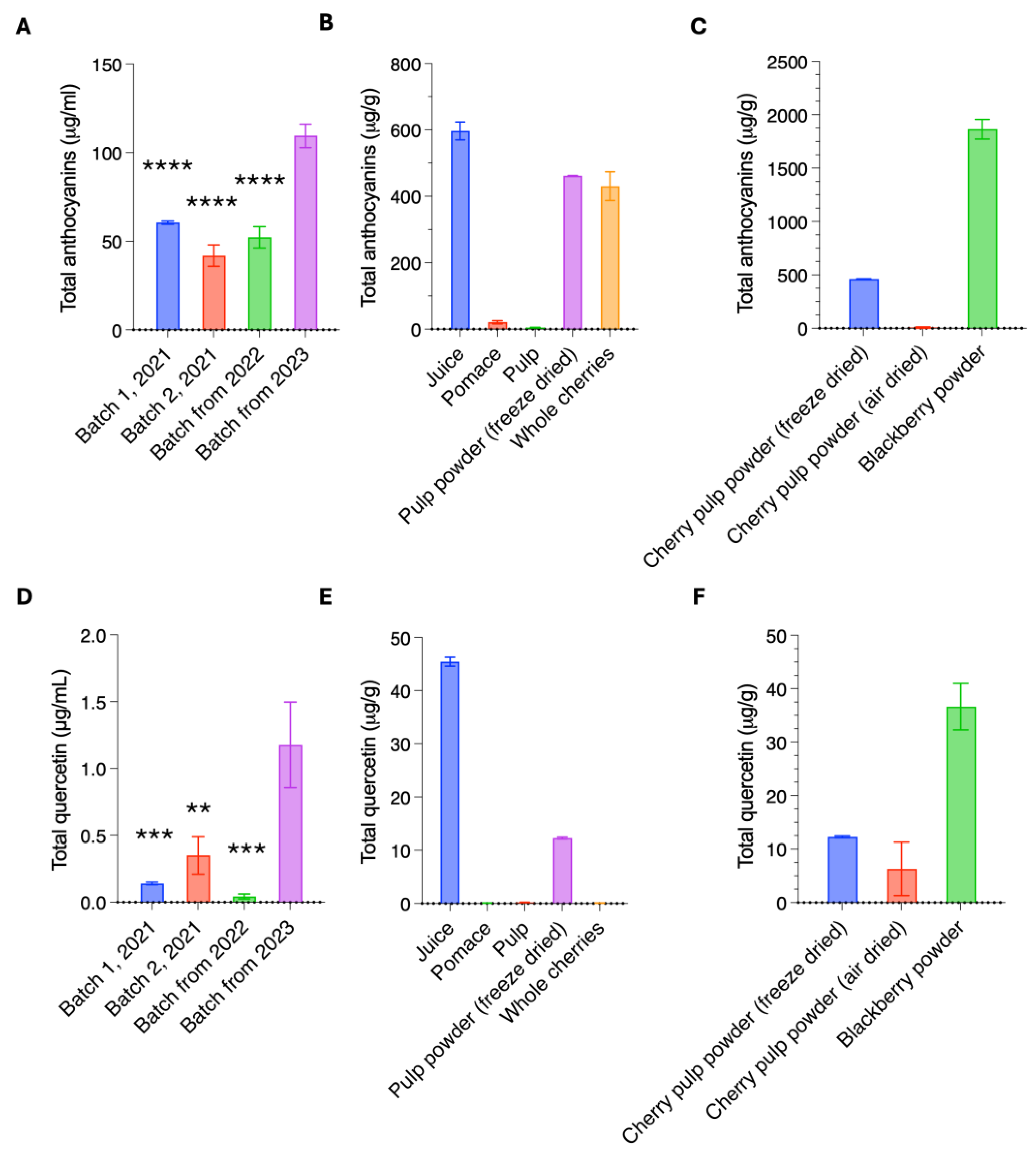
| Sample Name | Biological Replicate | Cyanidin-3-glucoside | Cyanidin-3-O-rutinoside | Pelargonidin-3-glucoside | Pelargonidin-3-O-rutinoside | Delphinidinin-3-glucoside | Delphinidinin-3-rutinoside | Petunidin-3-glucoside | Petunidin-3-rutinoside | Peonidin-3-rutinoside | Peonidin-3-glucoside | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dallaways cherry juice—Batch 1, 2021 | 1 | 5.9 | 46.6 | 0.1 | 0.9 | 0.0 | 0.1 | 0.0 | 0.0 | 5.6 | 0.0 | 59.2 |
| 2 | 5.9 | 46.7 | 0.1 | 1.0 | 0.0 | 0.1 | 0.0 | 0.0 | 5.9 | 0.0 | 59.7 | |
| 3 | 6.2 | 48.8 | 0.1 | 1.0 | 0.0 | 0.1 | 0.0 | 0.0 | 6.0 | 0.0 | 62.2 | |
| Dallaways cherry juice—Batch 2, 2021 | 1 | 3.2 | 44.1 | 0.1 | 1.0 | 0.0 | 0.1 | 0.0 | 0.0 | 5.4 | 0.0 | 53.9 |
| 2 | 1.9 | 29.7 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 3.5 | 0.0 | 35.8 | |
| 3 | 1.9 | 30.0 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 3.5 | 0.0 | 36.1 | |
| Dallaways cherry juice—Batch from 2022 | 1 | 3.3 | 50.9 | 0.1 | 1.1 | 0.0 | 0.1 | 0.0 | 0.0 | 3.7 | 0.0 | 59.2 |
| 2 | 2.0 | 35.2 | 0.0 | 0.7 | 0.0 | 0.0 | 0.0 | 0.0 | 2.4 | 0.0 | 40.3 | |
| 3 | 3.3 | 49.1 | 0.1 | 1.1 | 0.0 | 0.1 | 0.0 | 0.0 | 3.5 | 0.0 | 57.2 | |
| Dallaways cherry juice—Batch from 2023 | 1 | 10.7 | 71.8 | 0.2 | 1.8 | 0.0 | 0.1 | 0.0 | 0.0 | 12.3 | 0.0 | 96.9 |
| 2 | 16.2 | 82.8 | 0.3 | 2.5 | 0.0 | 0.1 | 0.0 | 0.0 | 17.0 | 0.0 | 118.9 | |
| 3 | 15.1 | 79.5 | 0.2 | 2.3 | 0.0 | 0.1 | 0.0 | 0.0 | 15.6 | 0.0 | 112.8 |
| Sample Name | Biological Replicate Number | Cyanidin-3-glucoside | Cyanidin-3-O-rutinoside | Pelargonidin-3-glucoside | Pelargonidin-3-O-rutinoside | Delphinidinin-3-glucoside | Delphinidinin-3-rutinoside | Petunidin-3-glucoside | Petunidin-3-rutinoside | Peonidin-3-rutinoside | Peonidin-3-glucoside | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Juice | 1 | 91.5 | 429.0 | 1.5 | 14.5 | 0.0 | 0.0 | 0.0 | 0.0 | 99.5 | 0.0 | 636.0 |
| 2 | 64.5 | 392.5 | 1.0 | 11.0 | 0.0 | 0.0 | 0.0 | 0.0 | 75.5 | 0.0 | 544.5 | |
| 3 | 86.0 | 420.5 | 1.5 | 13.0 | 0.0 | 0.0 | 0.0 | 0.0 | 90.0 | 0.0 | 611.0 | |
| Pomace | 1 | 2.0 | 20.0 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 0.0 | 26.0 |
| 2 | 2.0 | 19.5 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.0 | 0.0 | 25.5 | |
| 3 | 1.0 | 8.5 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.0 | 11.5 | |
| Pulp | 1 | 0.5 | 3.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 4.5 |
| 2 | 0.5 | 4.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 5.0 | |
| 3 | 0.5 | 5.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.5 | 0.0 | 6.0 | |
| Pulp powder(freeze dried) | 1 | 28.0 | 399.5 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.5 | 0.0 | 460.5 |
| 2 | 28.0 | 401.5 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.0 | 0.0 | 463.0 | |
| 3 | 28.5 | 401.0 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 462.0 | |
| Whole cherries | 1 | 17.0 | 325.5 | 0.0 | 6.0 | 0.0 | 0.5 | 0.0 | 0.0 | 29.0 | 0.0 | 378.0 |
| 2 | 33.5 | 434.5 | 0.5 | 8.5 | 0.0 | 0.5 | 0.0 | 0.0 | 39.5 | 0.0 | 517.0 | |
| 3 | 20.5 | 343.5 | 0.0 | 5.5 | 0.0 | 0.5 | 0.0 | 0.0 | 27.0 | 0.0 | 397.0 |
| Sample Name | Biological Replicate Number | Cyanidin-3-glucoside | Cyanidin-3-O-rutinoside | Pelargonidin-3-glucoside | Pelargonidin-3-O-rutinoside | Delphinidinin-3-glucoside | Delphinidinin-3-rutinoside | Petunidin-3-glucoside | Petunidin-3-rutinoside | Peonidin-3-rutinoside | Peonidin-3-glucoside | Total Anthocyanins |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cherry pulp powder (freeze dried) | 1 | 28.0 | 399.5 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.5 | 0.0 | 460.5 |
| 2 | 28.0 | 401.5 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 26.0 | 0.0 | 463.0 | |
| 3 | 28.5 | 401.0 | 0.5 | 7.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 462.0 | |
| Cherry pulp powder (air dried) | 1 | 2.0 | 15.5 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 0.0 | 19.5 |
| 2 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | |
| 3 | 0.5 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | |
| Blackberry powder | 1 | 1518.5 | 307.5 | 50.5 | 1.5 | 82.5 | 38.5 | 5.5 | 1.0 | 39.5 | 0.0 | 2045.0 |
| 2 | 1414 | 236.5 | 33.0 | 1.0 | 59.5 | 29.5 | 4.0 | 1.0 | 26.5 | 0.0 | 1807.0 | |
| 3 | 1388 | 214.5 | 29.0 | 1.0 | 54.0 | 27.0 | 3.5 | 0.5 | 23.5 | 0.0 | 1741.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blackburn, S.A.; Sullivan, W.G.; Freeman, L.M.; Howland, K.; Karamalegos, A.A.; Dallaway, M.; Philo, M.; Tullet, J.M.A.; Ezcurra, M. Upcycling of Waste Cherries Produces an Anthocyanin-Rich Powder That Protects Against Amyloid-β Toxicity in C. elegans. Antioxidants 2025, 14, 995. https://doi.org/10.3390/antiox14080995
Blackburn SA, Sullivan WG, Freeman LM, Howland K, Karamalegos AA, Dallaway M, Philo M, Tullet JMA, Ezcurra M. Upcycling of Waste Cherries Produces an Anthocyanin-Rich Powder That Protects Against Amyloid-β Toxicity in C. elegans. Antioxidants. 2025; 14(8):995. https://doi.org/10.3390/antiox14080995
Chicago/Turabian StyleBlackburn, Sarah A., William G. Sullivan, Laura M. Freeman, Kevin Howland, Antonis A. Karamalegos, Michael Dallaway, Mark Philo, Jennifer M. A. Tullet, and Marina Ezcurra. 2025. "Upcycling of Waste Cherries Produces an Anthocyanin-Rich Powder That Protects Against Amyloid-β Toxicity in C. elegans" Antioxidants 14, no. 8: 995. https://doi.org/10.3390/antiox14080995
APA StyleBlackburn, S. A., Sullivan, W. G., Freeman, L. M., Howland, K., Karamalegos, A. A., Dallaway, M., Philo, M., Tullet, J. M. A., & Ezcurra, M. (2025). Upcycling of Waste Cherries Produces an Anthocyanin-Rich Powder That Protects Against Amyloid-β Toxicity in C. elegans. Antioxidants, 14(8), 995. https://doi.org/10.3390/antiox14080995






