Development of Glycyrrhizic Acid Nanoparticles for Modulating Gastric Ulcer Healing: A Comparative In Vivo Study Targeting Oxidative Stress and Inflammatory Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GLY-NPs
2.2.1. Characterization of GLY-NPs
2.2.2. In Vitro Drug Release
2.2.3. Entrapment Efficiency (EE%)
2.3. Induction of GUs and Experimental Grouping
2.3.1. Assessment of Gastric Mucosal Damage, Ulcer Index (UI%), and Ulcer Inhibition (%)
2.3.2. Biochemical Analysis
Spectrophotometric and Enzyme-Linked Immunosorbent Assay (ELISA)
RNA Extraction and Quantitative RT-PCR Analysis
2.3.3. Histopathological and Immunohistochemical (IHC) Assay
2.4. Statistical Analysis
3. Results
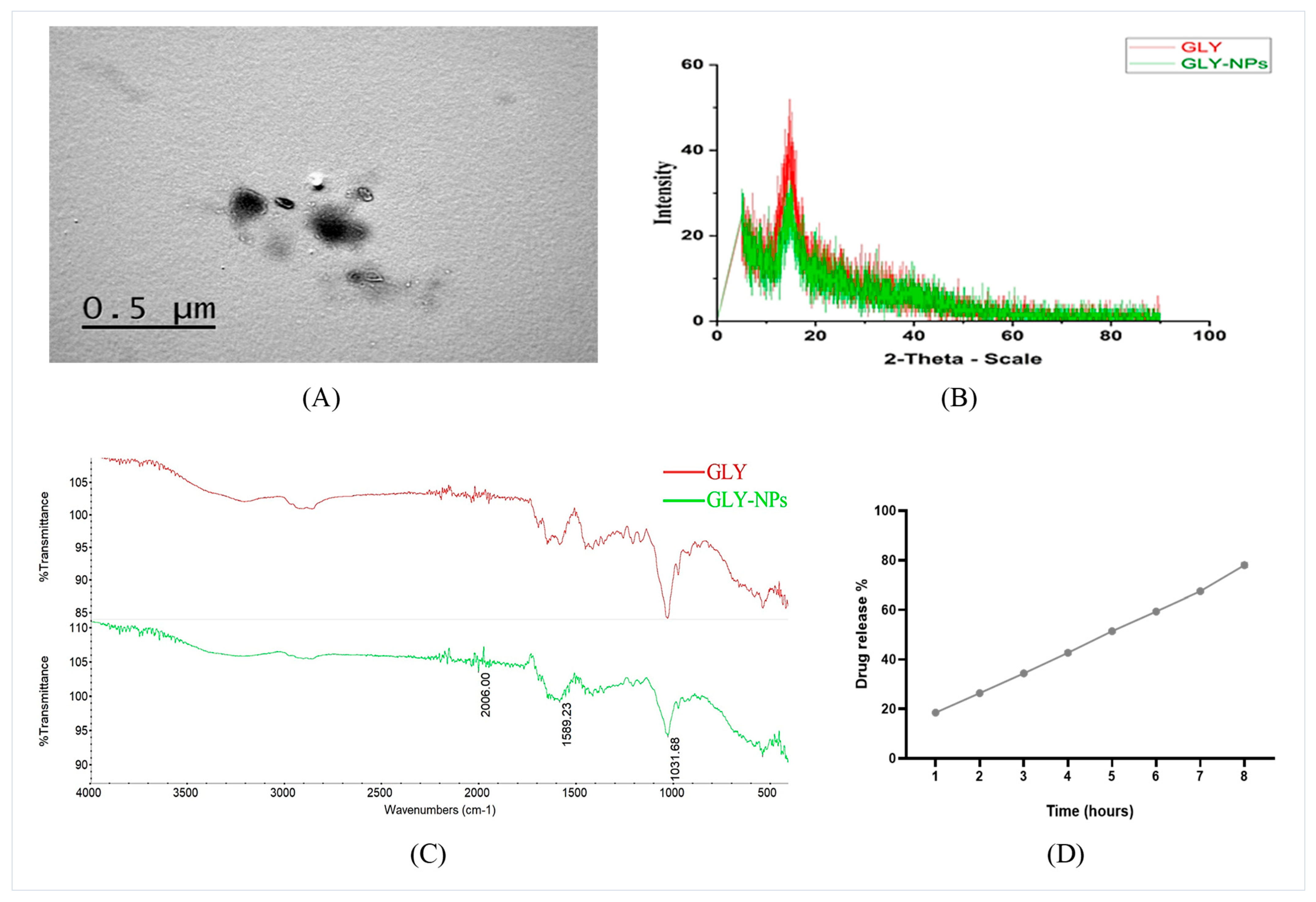
3.1. Zeta Potential, Average Particle Size Evaluation, and TEM Imaging of GLY-NPs
3.2. XRD and FTIR Spectra Analysis of GLY and GLY-NPs
3.3. In Vitro Release Profile and EE% of GLY-NPs
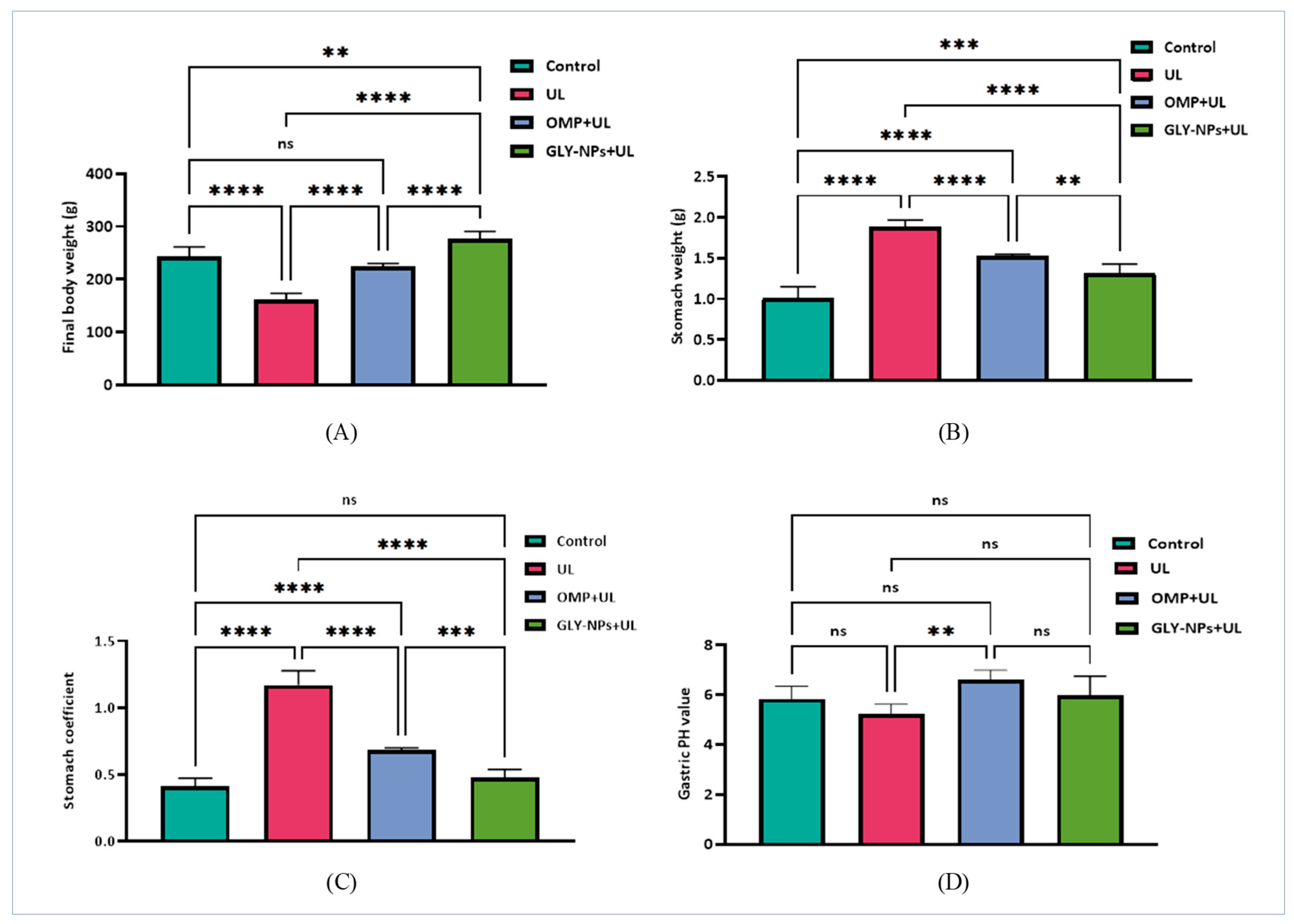
3.4. Effects of GLY-NPs and OMP on Body Weight, Stomach Weight, Stomach Coefficient, and Gastric pH in Ethanol-Induced GUs in Rats
3.5. Macroscopic Evaluation of Gastric Mucosa, UI %, and Ulcer Inhibition (%) in Ethanol-Induced GUs in Rats
3.6. Impact of Ethanol-Induced GUs and Treatments on Renal Biomarkers and Hepatic Enzyme Levels
3.7. Impact of GLY-NPs and OMP on Gastrin and Somatotropin Hormone Levels in Ethanol-Induced GUs in Rats
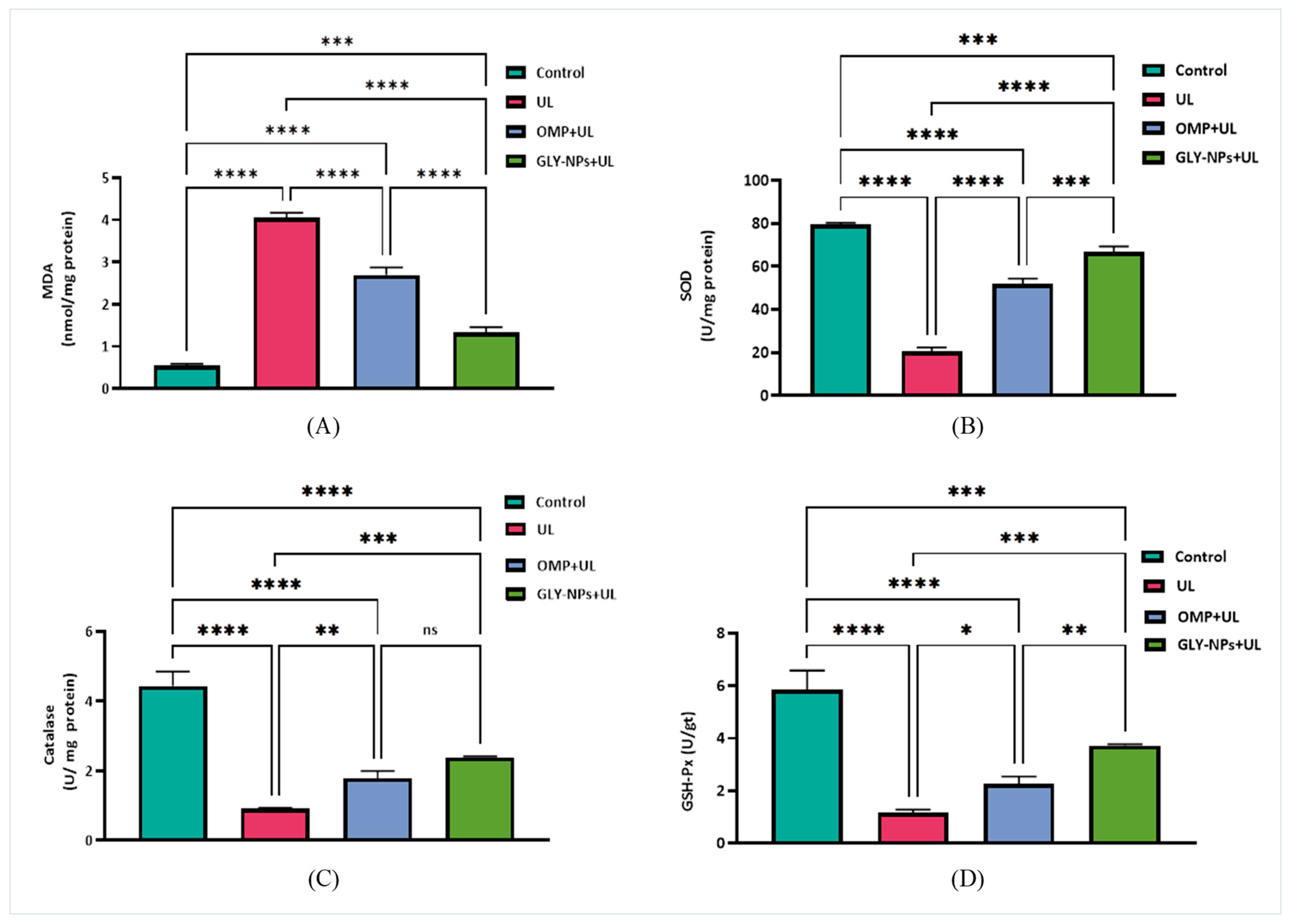
3.8. Protective and Therapeutic Effects of Treatments on Oxidative Stress Biomarkers and Antioxidant Levels
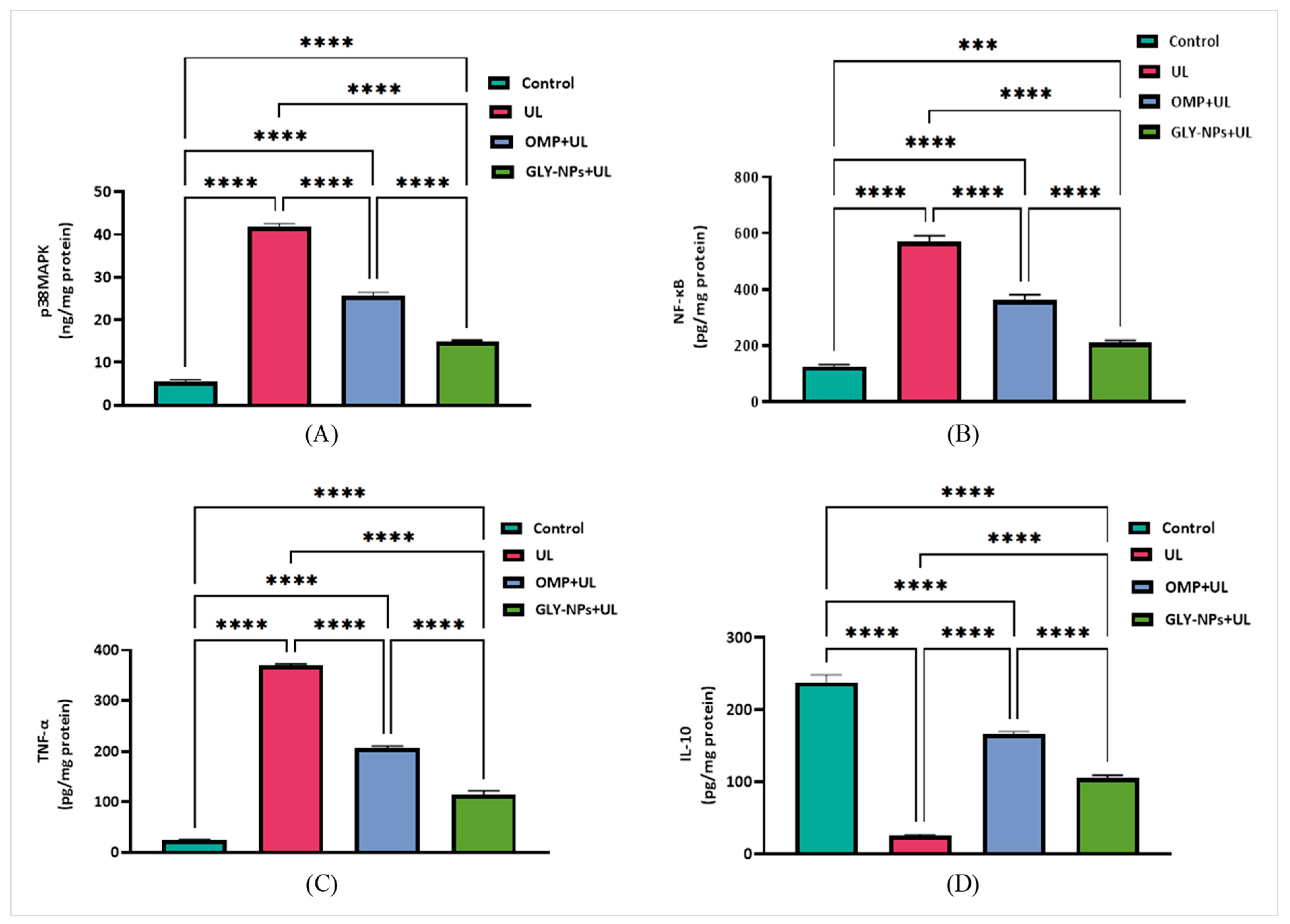
3.9. Modulatory Effects of GLY-NPs and OMP on p38MAPK, NF-κB, and Inflammatory Cytokines in Ethanol-Induced GUs in Rats
3.10. Modulation of TGF-β1/Smad3 and JAK2/STAT3 Signaling Pathway by GLY-NPs and OMP in Ethanol-Induced GUs Model
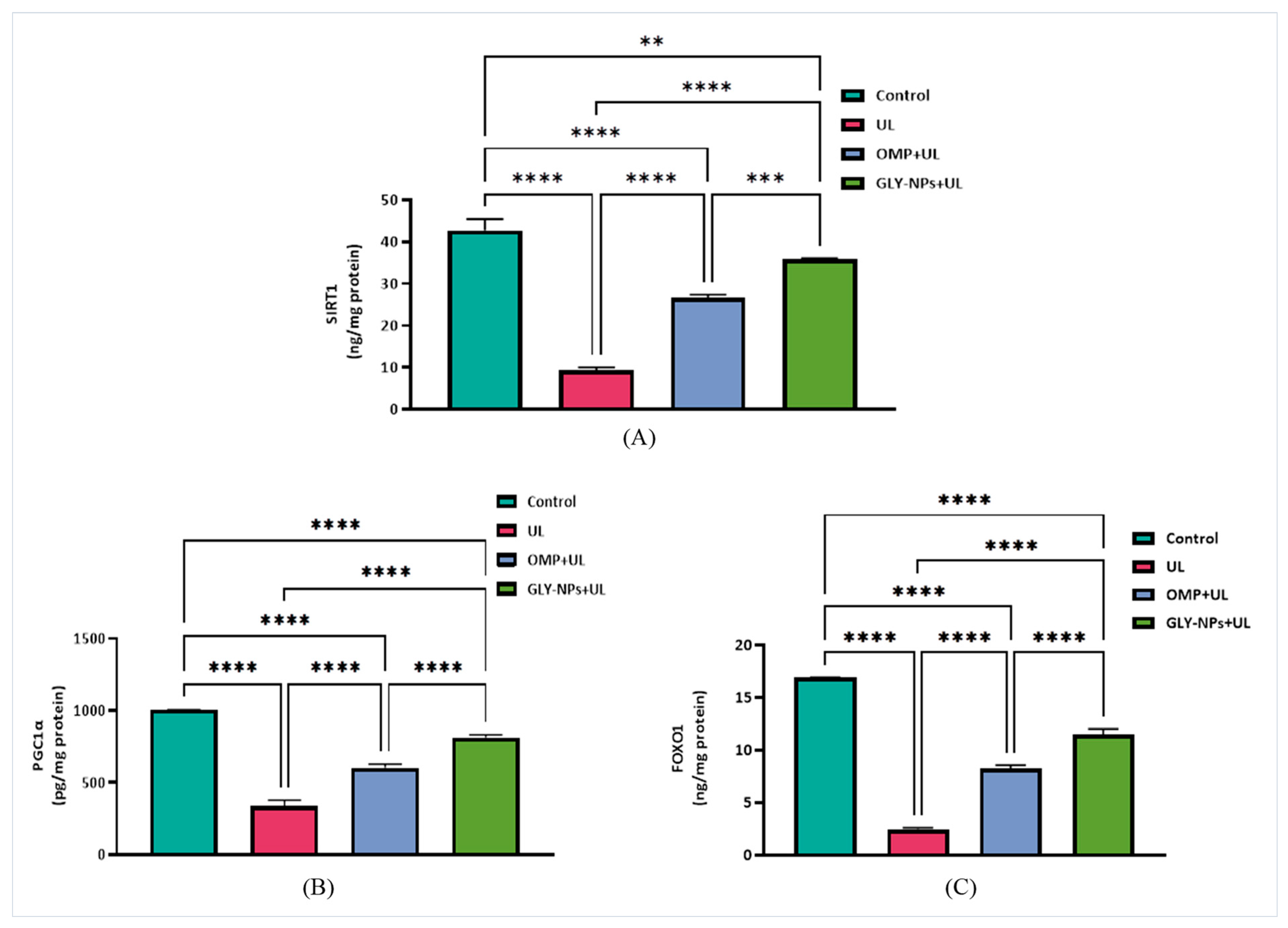
3.11. Restorative Effects of GLY-NPs and OMP on SIRT1, FOXO1, and PGC-1α Levels in Ethanol-Induced GUs
3.12. Histopathological Evaluation
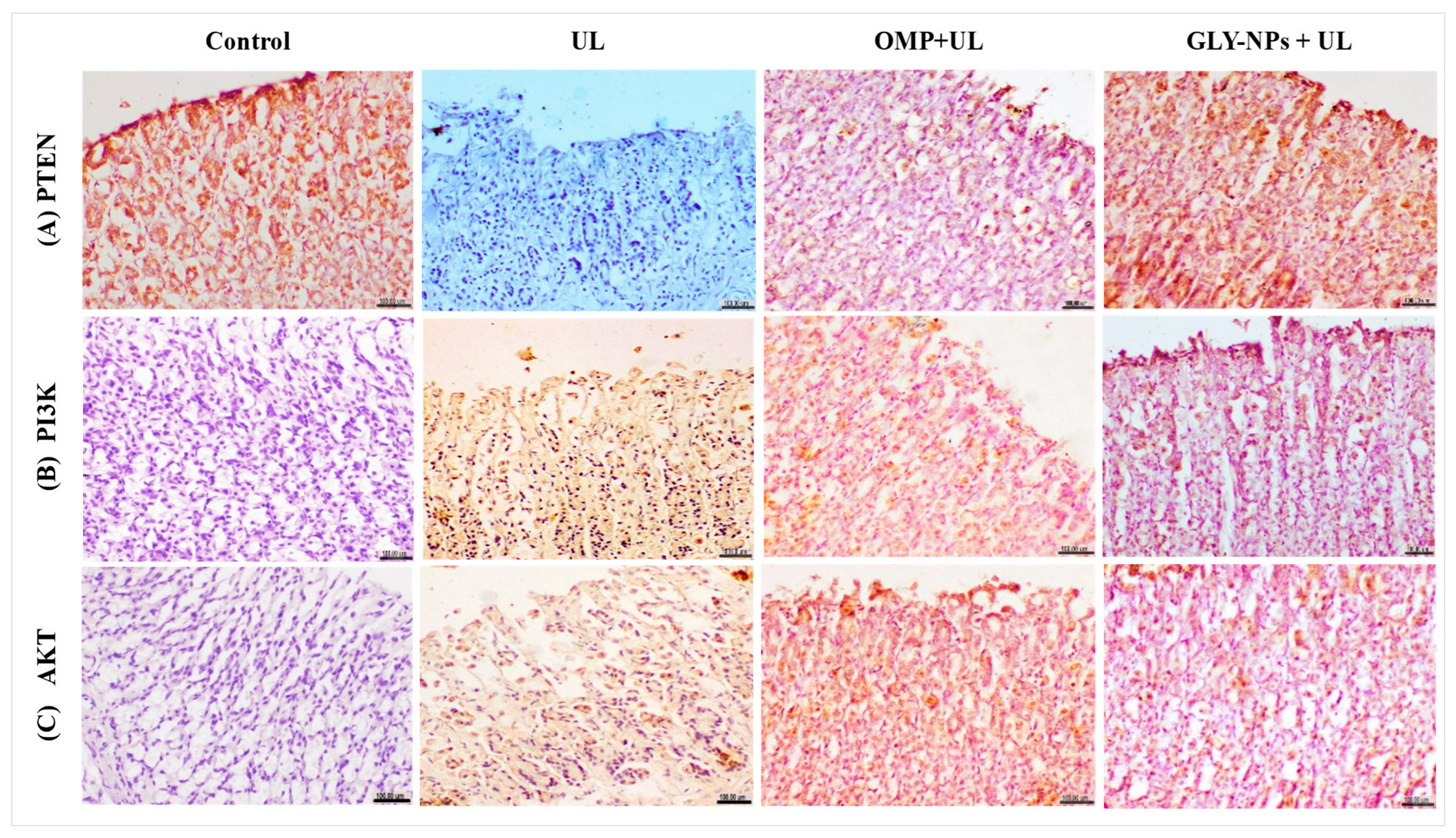
3.13. Immunohistochemical Evaluation of PTEN, PI3K, and AKT
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GUs | Gastric Ulcers |
| OMP | Omeprazole |
| GLY | Glycyrrhizic Acid |
| GLY-NPs | Glycyrrhizic Acid Nanoparticles |
| ROS | Reactive Oxygen Species |
| MDA | Malondialdehyde |
| TNF-α | Tumor Necrosis Factor-Alpha |
| SOD | Superoxide Dismutase |
| MAPKs | Mitogen-Activated Protein Kinases |
| NF-κB | Nuclear Factor Kappa B |
| JAK | Janus Kinase |
| STAT | Signal Transducer and Activator of Transcription |
| IL-10 | Interleukin-10 |
| TGF-β | Transforming Growth Factor-Beta |
| PTEN | Phosphatase and Tensin Homolog |
| PI3K | Phosphatidylinositol 3-Kinase |
| AKT | Protein Kinase B |
| SIRT1 | Sirtuin 1 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator Alpha |
| GSH-Px | Glutathione Peroxidase |
References
- Kamada, T.; Satoh, K.; Itoh, T.; Ito, M.; Iwamoto, J.; Okimoto, T.; Kanno, T.; Sugimoto, M.; Chiba, T.; Nomura, S.; et al. Evidence-Based Clinical Practice Guidelines for Peptic Ulcer Disease 2020. J. Gastroenterol. 2021, 56, 303–322. [Google Scholar] [CrossRef]
- Drini, M. Peptic Ulcer Disease and Non-Steroidal Anti-Inflammatory Drugs. Aust. Prescr. 2017, 40, 91–95. [Google Scholar] [CrossRef]
- Roy, A.J.; Maut, C.; Gogoi, H.K.; Ahmed, S.I.; Kashyap, A. A Review on Herbal Drugs Used in the Treatment of Peptic Ulcer. Curr. Drug Discov. Technol. 2023, 20, 4–15. [Google Scholar] [CrossRef]
- Woolf, A.; Rose, R. Gastric Ulcer. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, CA, USA, 2025. [Google Scholar] [PubMed]
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-κB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Chen, R.; Chen, R.; Li, T.; Wu, W.; Huang, Z. Esomeprazole Alleviates the Damage to Stress Ulcer in Rats through Not Only Its Antisecretory Effect but Its Antioxidant Effect by Inactivating the p38 MAPK and NF-κB Signaling Pathways. Drug Des. Dev. Ther. 2019, 12, 2969–2984. [Google Scholar] [CrossRef]
- Jia, Y.T.; Ma, B.; Wei, W.; Xu, Y.; Wang, Y.; Tang, H.T.; Xia, Z.F. Sustained Activation of Nuclear Factor-κB by Reactive Oxygen Species Is Involved in the Pathogenesis of Stress-Induced Gastric Damage in Rats. Crit. Care Med. 2007, 35, 1582–1591. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Z.; Mao, N.; Xie, Y. The Protective of Hydrogen on Stress-Induced Gastric Ulceration. Int. Immunopharmacol. 2012, 13, 197–203. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, D.; Gong, Q.; Xu, Q.; Pan, D.; Lu, F.; Tang, Q. Elucidation of SIRT-1/PGC-1α-Associated Mitochondrial Dysfunction and Autophagy in Nonalcoholic Fatty Liver Disease. Lipids Health Dis. 2021, 20, 40. [Google Scholar] [CrossRef]
- Samarakoon, R.; Overstreet, J.M.; Higgins, P.J. TGF-β Signaling in Tissue Fibrosis: Redox Controls, Target Genes and Therapeutic Opportunities. Cell. Signal. 2013, 25, 264–268. [Google Scholar] [CrossRef]
- Stolfi, C.; Troncone, E.; Marafini, I.; Monteleone, G. Role of TGF-β and Smad7 in Gut Inflammation, Fibrosis and Cancer. Biomolecules 2020, 11, 17. [Google Scholar] [CrossRef]
- Zhang, Y.; Dees, C.; Beyer, C.; Lin, N.Y.; Distler, A.; Zerr, P.; Palumbo, K.; Susok, L.; Kreuter, A.; Distler, O.; et al. Inhibition of Casein Kinase II Reduces TGFβ-Induced Fibroblast Activation and Ameliorates Experimental Fibrosis. Ann. Rheum. Dis. 2015, 74, 936–943. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving Cognition of the JAK-STAT Signaling Pathway: Autoimmune Disorders and Cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Ugwu, F.N.; Yu, A.P.; Sin, T.K.; Tam, B.T.; Lai, C.W.; Wong, S.C.; Siu, P.M. Protective Effect of Unacylated Ghrelin on Compression-Induced Skeletal Muscle Injury Mediated by SIRT1-Signaling. Front. Physiol. 2017, 8, 962. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, Q.; Zeng, Z.; Wu, J.; Zhang, Y.; Chen, Z. Sirt1 Inhibits Oxidative Stress in Vascular Endothelial Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7543973. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and Aging Related Signaling Pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef]
- Ren, H.; Shao, Y.; Wu, C.; Ma, X.; Lv, C.; Wang, Q. Metformin Alleviates Oxidative Stress and Enhances Autophagy in Diabetic Kidney Disease via AMPK/SIRT1-FoxO1 Pathway. Mol. Cell. Endocrinol. 2020, 500, 110628. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liao, H.; Liu, Y.; Zheng, Y.; Wu, X.; Su, Z.; Zhang, X.; Lai, Z.; Lai, X.; Lin, Z.X.; et al. Protective Effects of Pogostone from Pogostemonis Herba against Ethanol-Induced Gastric Ulcer in Rats. Fitoterapia 2015, 100, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Tarnawski, A.S.; Ahluwalia, A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells 2021, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Tavirani, M.R.; Jahani-Sherafat, S.; Tavirani, S.R.; Esmaeili, S.; Ansari, M.; Ahmad-zadeh, A. Side Effects of Omeprazole: A System Biology Study. Gastroenterol. Hepatol. Bed Bench 2021, 14, 334. [Google Scholar]
- Tugume, P.; Nyakoojo, C. Ethno-pharmacological survey of herbal remedies used in the treatment of paediatric diseases in Buhunga parish, Rukungiri District, Uganda. BMC Complement. Altern. Med. 2019, 19, 353. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lai, W.Y.; Sun, M.F.; Lin, C.C.; Chen, B.C.; Lin, H.J.; Chang, C.M.; Yang, C.H.; Huang, K.C.; Yen, H.R. Prescription patterns of traditional Chinese medicine for peptic ulcer disease in Taiwan: A nationwide population-based study. J. Ethnopharmacol. 2015, 176, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Akbar, N.; Kawish, M.; Khan, N.A.; Shah, M.R.; Alharbi, A.M.; Alfahemi, H.; Siddiqui, R. Hesperidin-, curcumin-, and amphotericin B-based nano-formulations as potential antibacterials. Antibiotics 2022, 11, 696. [Google Scholar] [CrossRef]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice To Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Tong, T.; Hu, H.; Zhou, J.; Deng, S.; Zhang, X.; Tang, W.; Fang, L.; Xiao, S.; Liang, J. Glycyrrhizic-Acid-Based Carbon Dots with High Antiviral Activity by Multisite Inhibition Mechanisms. Small 2020, 16, e1906206. [Google Scholar] [CrossRef]
- Ganjhu, R.K.; Mudgal, P.P.; Maity, H.; Dowarha, D.; Devadiga, S.; Nag, S.; Arunkumar, G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease 2015, 26, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Luo, S.; Lv, X.; Deng, Y.; Huang, H.; Zhao, B.; Zhang, Q.; Li, G. Formulation of injectable glycyrrhizic acid-hydroxycamptothecin micelles as new generation of DNA topoisomerase I inhibitor for enhanced antitumor activity. Int. J. Pharm. 2019, 571, 118693. [Google Scholar] [CrossRef]
- Lei, Y.; Kong, Y.; Sui, H.; Feng, J.; Zhu, R.; Wang, W. Enhanced oral bioavailability of glycyrrhetinic acid via nanocrystal formulation. Drug Deliv. Transl. Res. 2016, 6, 519–525. [Google Scholar] [CrossRef]
- Hassan, A.; Al-Salmi, F.A.; Abuamara, T.M.M.; Matar, E.R.; Amer, M.E.; Fayed, E.M.M.; Hablas, M.G.A.; Mohammed, T.S.; Ali, H.E.; Abd El-Fattah, F.M.; et al. Ultrastructural analysis of zinc oxide nanospheres enhances anti-tumor efficacy against Hepatoma. Front. Oncol. 2022, 12, 933750. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Elebeedy, D.; Matar, E.R.; Fahmy Mohamed Elsayed, A.; Abd El Maksoud, A.I. Investigation of angiogenesis and wound healing potential mechanisms of zinc oxide nanorods. Front. Pharmacol. 2021, 12, 661217. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nano-particles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.D.; Penon, O.; Monferrer, D.; Rivera-Gil, P. Classification system for nanotechnology-enabled health products with both scientific and regulatory application. Front. Med. 2023, 10, 1212949. [Google Scholar] [CrossRef]
- Singh, S.; Dobhal, A.K.; Jain, A.; Pandit, J.K.; Chakraborty, S. Formulation and evaluation of solid lipid nanoparticles of a water-soluble drug: Zidovudine. Chem. Pharm. Bull. 2010, 58, 650–655. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, L.; Zheng, H.; Xu, S.; Hong, X.; Cai, T.; Xu, J.; Zhang, W.; Mai, Y.; Li, J.; et al. Protective effect of Amauroderma rugosum ethanol extract and its primary bioactive compound, ergosterol, against acute gastric ulcers based on LXR-mediated gastric mucus secretions. Phytomedicine 2024, 123, 155236. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.N.; Abdel Aziz, I.; Nur Azlina, M.F. Piper sarmentosum Roxb. methanolic extract prevents stress-induced gastric ulcer by modulating oxidative stress and inflammation. Front. Pharmacol. 2023, 13, 971443. [Google Scholar] [CrossRef]
- Liu, F.; Nong, X.; Qu, W.; Li, X. Weikangling capsules combined with omeprazole ameliorates ethanol-induced chronic gastritis by regulating gut microbiota and EGF-EGFR-ERK pathway. Life Sci. 2023, 315, 121368. [Google Scholar] [CrossRef]
- Zeeshan, M.; Ali, H.; Khan, S.; Mukhtar, M.; Khan, M.I.; Arshad, M. Glycyrrhizic acid-loaded pH-sensitive poly-(lactic-co-glycolic acid) nanoparticles for the amelioration of inflammatory bowel disease. Nanomedicine 2019, 14, 1945–1969. [Google Scholar] [CrossRef]
- Shareef, S.H.; Al-Medhtiy, M.H.; Ibrahim, I.A.; Alzahrani, A.R.; Jabbar, A.A.; Galali, Y.; Shakir Agha, N.F.; Aziz, P.Y.; Thabit, M.A.; Agha, D.N.F.; et al. Gastroprophylactic effects of p-cymene in ethanol-induced gastric ulcer in rats. Processes 2022, 10, 1314. [Google Scholar] [CrossRef]
- Szabo, S.; Hollander, D. Pathways of gastrointestinal protection and repair: Mechanisms of action of sucralfate. Am. J. Med. 1989, 86, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Q.; Xu, N.; Cai, J.; Luo, D.; Zhang, Q.; Su, Z.; Gao, C.; Liu, Y. Antioxidative and anti-inflammatory effects of water extract of Acrostichum aureum Linn. against ethanol-induced gastric ulcer in rats. Evid.-Based Complement. Alternat. Med. 2018, 2018, 3585394. [Google Scholar] [CrossRef]
- Al Asmari, A.; Al Shahrani, H.; Al Masri, N.; Al Faraidi, A.; Elfaki, I.; Arshaduddin, M. Vanillin abrogates ethanol induced gastric injury in rats via modulation of gastric secretion, oxidative stress and inflammation. Toxicol. Rep. 2016, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, R.R.; Mansour, D.F.; Salama, A.A.; Abdel-Rahman, R.F.; Hassan, A.M. Regulation of PKB/Akt-pathway in the chemopreventive effect of lactoferrin against diethylnitrosamine-induced hepatocarcinogenesis in rats. Pharmacol. Rep. 2019, 71, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sistani Karampour, N.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef]
- Beiranvand, M. A review of the most common in vivo models of stomach ulcers and natural and synthetic anti-ulcer compounds: A comparative systematic study. Phytomedicine Plus 2022, 2, 100264. [Google Scholar] [CrossRef]
- Maestrini, M.; Molento, M.B.; Forzan, M.; Perrucci, S. In vitro anthelmintic activity of an aqueous extract of Glycyrrhiza glabra and of glycyrrhetinic acid against gastrointestinal nematodes of small ruminants. Parasite 2021, 28, 64. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, Y.; Wang, H.; Chun, C.; Chen, L.; Wang, K.; Lu, Z.; Zhao, Y.; Li, X. Protective Effects of Chitosan-Bilirubin Nanoparticles Against Ethanol-Induced Gastric Ulcers. Int. J. Nanomed. 2021, 16, 8235–8250. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, Y.; Zhu, L.-J.; Liu, Z.-Q. Nanocarriers: A general strategy for enhancement of oral bioavailability of poorly absorbed or pre-systemically metabolized drugs. Curr. Drug Metab. 2010, 11, 197–207. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Singh, A.; Neupane, Y.R.; Panda, B.P.; Kohli, K. Lipid Based Nanoformulation of Lycopene Improves Oral Delivery: Formulation Optimization, Ex Vivo Assessment and Its Efficacy against Breast Cancer. J. Microencapsul. 2017, 34, 416–429. [Google Scholar] [CrossRef]
- Shimojo, A.A.; Fernandes, A.R.V.; Ferreira, N.R.; Sanchez-Lopez, E.; Santana, M.H.; Souto, E.B. Evaluation of the influence of process parameters on the properties of resveratrol-loaded NLC using 22 full factorial design. Antioxidants 2019, 8, 272. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as particulate drug formulations in therapy: Rationale for development and what we can expect for the future. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef]
- Hajipour, H.; Ghorbani, M.; Kahroba, H.; Mahmoodzadeh, F.; Emameh, R.Z.; Taheri, R.A. Arginyl-glycyl-aspartic acid (RGD) containing nanostructured lipid carrier co-loaded with doxorubicin and sildenafil citrate enhanced anti-cancer effects and overcomes drug resistance. Process Biochem. 2019, 84, 172–179. [Google Scholar] [CrossRef]
- Csicsák, D.; Szolláth, R.; Kádár, S.; Ambrus, R.; Bartos, C.; Balogh, E.; Antal, I.; Köteles, I.; Tőzsér, P.; Bárdos, V.; et al. The effect of the particle size reduction on the biorelevant solubility and dissolution of poorly soluble drugs with different acid-base character. Pharmaceutics 2023, 15, 278. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.U.; Singh, S.; Sharma, P.; Prajapati, B.G. Amorphization of low soluble drug with amino acids to improve its therapeutic efficacy: A state-of-art review. AAPS PharmSciTech 2023, 24, 253. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Kulkarni, G.T. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: Where do we stand? Drug Deliv. 2016, 23, 378–394. [Google Scholar] [CrossRef]
- Marques, S.S.; Segundo, M.A. Nanometrics goes beyond the size: Assessment of nanoparticle concentration and encapsulation efficiency in nanocarriers. TrAC Trends Anal. Chem. 2024, 174, 117672. [Google Scholar] [CrossRef]
- Tong, R.; Cheng, J. Ring-opening polymerization-mediated controlled formulation of polylactide-drug nanoparticles. J. Am. Chem. Soc. 2009, 131, 4744–4754. [Google Scholar] [CrossRef]
- Rahman, Z.; Dwivedi, D.K.; Jena, G.B. Ethanol-induced gastric ulcer in rats and intervention of tert-butylhydroquinone: Involvement of Nrf2/HO-1 signalling pathway. Hum. Exp. Toxicol. 2020, 39, 547–562. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Mohammed, H.S.; Aboushousha, T.; Lotfy, D.M.; El-Shazly, M.A.; Sobeh, M.; Taha, E.F. Ipomoea carnea mitigates ethanol-induced ulcers in irradiated rats via Nrf2/HO-1 pathway: An in vivo and in silico study. Sci. Rep. 2024, 14, 3469. [Google Scholar] [CrossRef]
- El-Din, M.I.G.; Youssef, F.S.; Said, R.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Chemical constituents and gastro-protective potential of Pachira glabra leaves against ethanol-induced gastric ulcer in experimental rat model. Inflammopharmacology 2021, 29, 317–332. [Google Scholar] [CrossRef]
- Albalawi, M.; Khateeb, S. Enhanced therapeutic efficacy of omeprazole nanosuspension in ethanol-induced gastric ulcer: A focus on oxidative stress and inflammatory pathways. Biomolecules 2025, 15, 902. [Google Scholar] [CrossRef]
- Ju, S.M.; Kim, M.S.; Jo, Y.S.; Jeon, Y.M.; Bae, J.S.; Pae, H.O.; Jeon, B.H. Licorice and its active compound glycyrrhizic acid ameliorates cisplatin-induced nephrotoxicity through inactivation of p53 by scavenging ROS and overexpression of p21 in human renal proximal tubular epithelial cells. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 890–899. [Google Scholar]
- Sun, F.P.; Song, Y.G.; Cheng, W.; Zhao, T.; Yao, Y.L. Gastrin, somatostatin, G and D cells of gastric ulcer in rats. World J. Gastroenterol. 2002, 8, 375. [Google Scholar] [CrossRef]
- Yeo, D.; Hwang, S.J.; Kim, W.J.; Youn, H.J.; Lee, H.J. The aqueous extract from Artemisia capillaris inhibits acute gastric mucosal injury by inhibition of ROS and NF-kB. Biomed. Pharmacother. 2018, 99, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Kim, J.E.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Lee, Y.H.; Song, H.; Hwang, D. Anti-ulcer effect of Gallarhois extract with anti-oxidant activity in an ICR model of ethanol/hydrochloride acid-induced gastric injury. J. Tradit. Complement. Med. 2018, 9, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Ercan, G.; Tartar, R.I.; Solmaz, A.; Gulcicek, O.B.; Karagulle, O.O.; Meric, S.; Cayoren, H.; Kusaslan, R.; Kemik, A.; Kayali, D.G.; et al. Potent therapeutic effects of ruscogenin on gastric ulcer established by acetic acid. Asian J. Surg. 2020, 43, 405–416. [Google Scholar] [CrossRef]
- Wetchakul, P.; Net-Anong, S.; Goon, J.A.; Sanpinit, S. Anti-oxidative stress and gastroprotective effect of Tri-Tharn-Thip tea against ethanol-induced gastric ulcer in rats. S. Afr. J. Bot. 2024, 170, 130–136. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, H.; Jin, Y.; Zhang, M.; Zhao, Q.; Li, H.; Wang, S.; Lu, Y.; Chen, S.; Du, H.; et al. The active components and potential mechanisms of Wuji Wan in the treatment of ethanol-induced gastric ulcer: An integrated metabolomics, network pharmacology and experimental validation. J. Ethnopharmacol. 2024, 326, 117901. [Google Scholar] [CrossRef] [PubMed]
- Konovalova, G.G.; Tikhaze, A.K.; Lankin, V.Z. Antioxidant activity of parapharmaceutics containing natural inhibitors of free radical processes. Bull. Exp. Biol. Med. 2000, 130, 658–660. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, C.; Li, J.; Xiong, L.; Wang, Z.; Liu, J.; Li, P. Evaluation of the gastroprotective effects of 20(S)-ginsenoside Rg3 on gastric ulcer models in mice. J. Ginseng Res. 2019, 43, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-Dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, anti-inflammatory, and antiulcer potential of manuka honey against gastric ulcer in rats. Oxid. Med. Cell. Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef]
- Badr, A.M.; El-Orabi, N.F.; Ali, R.A. The implication of the crosstalk of Nrf2 with NOXs, and HMGB1 in ethanol-induced gastric ulcer: Potential protective effect is afforded by raspberry ketone. PLoS ONE 2019, 14, e0220548. [Google Scholar] [CrossRef]
- Philpott, H.L.; Nandurkar, S.; Lubel, J.; Gibson, P.R. Drug-induced gastrointestinal disorders. Frontline Gastroenterol. 2014, 5, 49–57. [Google Scholar] [CrossRef]
- Kim, S.H.; Choo, G.S.; Yoo, E.S.; Woo, J.S.; Han, S.H.; Lee, J.H.; Jung, J.Y. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol. Rep. 2019, 42, 1904–1914. [Google Scholar] [CrossRef]
- Seo, J.H.; Lim, J.W.; Kim, H.; Kim, K.H. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-κB and induces chemokine expression in gastric epithelial AGS cells. Lab. Investig. 2004, 84, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and antioxidants: Physiological and pathological role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Wang, W.; Luo, M.; Fu, Y.; Wang, S.; Efferth, T.; Zu, Y. Glycyrrhizic acid nanoparticles inhibit LPS-induced inflammatory mediators in 264.7 mouse macrophages compared with unprocessed glycyrrhizic acid. Int. J. Nanomed. 2013, 8, 1377–1383. [Google Scholar] [CrossRef]
- Hassan, H.M.; El-Kannishy, S.M.; Alattar, A.; Alshaman, R.; Hamdan, A.M.; Al-Gayyar, M.M. Therapeutic effects of blocking β-catenin against hepatocellular carcinoma-induced activation of inflammation, fibrosis and tumor invasion. Biomed. Pharmacother. 2021, 135, 111216. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, A.M.; Al-Gayyar, M.M.; Shams, M.E.E.; Alshaman, U.S.; Prabahar, K.; Bagalagel, A.; Diri, R.; Noor, A.O.; Almasri, D. Thymoquinone therapy remediates elevated brain tissue inflammatory mediators induced by chronic administration of food preservatives. Sci. Rep. 2019, 9, 7026. [Google Scholar] [CrossRef]
- Elsherbiny, N.M.; Al-Gayyar, M.M. Anti-tumor activity of arjunolic acid against Ehrlich Ascites carcinoma cells in vivo and in vitro through blocking TGF-β type 1 receptor. Biomed. Pharmacother. 2016, 82, 28–34. [Google Scholar] [CrossRef]
- de Oliveira Formiga, R.; Júnior, E.B.A.; Vasconcelos, R.C.; Araújo, A.A.; de Carvalho, T.G.; de Araújo Junior, R.F.; Batista, L.M. Effect of p-cymene and rosmarinic acid on gastric ulcer healing—Involvement of multiple endogenous curative mechanisms. Phytomedicine 2021, 86, 153497. [Google Scholar] [CrossRef]
- Stewart, A.G.; Thomas, B.; Koff, J. TGF-β: Master regulator of inflammation and fibrosis. Respirology 2018, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Mao, Y.; Wu, J.; Feng, J.; Li, J.; Wu, L.; Yu, Q.; Zhou, Y.; Zhang, J.; Chen, J.; et al. TGF-β/Smad and JAK/STAT pathways are involved in the anti-fibrotic effects of propylene glycol alginate sodium sulfate on hepatic fibrosis. J. Cell. Mol. Med. 2020, 24, 5224–5237. [Google Scholar] [CrossRef]
- Dong, Z.; Zhao, X.; Tai, W.; Lei, W.; Wang, Y.; Li, Z.; Zhang, T. IL-27 attenuates the TGF-β1-induced proliferation, differentiation and collagen synthesis in lung fibroblasts. Life Sci. 2016, 146, 24–33. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming growth factor-β (TGF-β) directly activates the JAK1-STAT3 axis to induce hepatic fibrosis in coordination with the SMAD pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef] [PubMed]
- Cordes, F.; Lenker, E.; Weinhage, T.; Spille, L.J.; Bettenworth, D.; Varga, G.; Schmidt, H.H.; Foell, D. Impaired IFN-γ-dependent STAT3 activation is associated with dysregulation of regulatory and inflammatory signaling in monocytes of ulcerative colitis patients. Inflamm. Bowel Dis. 2021, 27, 887–901. [Google Scholar] [CrossRef]
- van Gennep, S.; Fung, I.C.N.; Jong, D.C.; Ramkisoen, R.K.; Clasquin, E.; de Jong, J.; de Vries, L.C.S.; de Jonge, W.J.; Gecse, K.B.; Löwenberg, M.; et al. Histological outcomes and JAK-STAT signalling in ulcerative colitis patients treated with tofacitinib. J. Crohns Colitis 2024, 18, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Milovanova, T.N. Mucosal immunity and the FOXO1 transcription factors. Front. Immunol. 2019, 10, 2530. [Google Scholar] [CrossRef]
- Kim, M.E.; Kim, D.H.; Lee, J.S. FoxO transcription factors: Applicability as novel immune cell regulators and therapeutic targets in oxidative stress-related diseases. Int. J. Mol. Sci. 2022, 23, 11877. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Song, M.Y.; Song, E.K.; Kim, E.K.; Moon, W.S.; Han, M.K.; Park, J.W.; Kwon, K.B.; Park, B.H. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor-κB signaling pathway. Diabetes 2009, 58, 344–351. [Google Scholar] [CrossRef]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef]
- Panes, J.D.; Godoy, P.A.; Silva-Grecchi, T.; Celis, M.T.; Ramirez-Molina, O.; Gavilan, J.; Muñoz-Montecino, C.; Castro, P.A.; Moraga-Cid, G.; Yévenes, G.E.; et al. Changes in PGC-1α/SIRT1 signaling impact on mitochondrial homeostasis in amyloid-beta peptide toxicity model. Front. Pharmacol. 2020, 11, 709. [Google Scholar] [CrossRef]
- Alamoudi, J.A.; El-Masry, T.A.; El-Nagar, M.M.F.; El Zahaby, E.I.; Elmorshedy, K.E.; Gaballa, M.M.S.; Alshawwa, S.Z.; Alsunbul, M.; Alharthi, S.; Ibrahim, H.A. Chitosan/hesperidin nanoparticles formulation: A promising approach against ethanol-induced gastric ulcers via Sirt1/FOXO1/PGC-1α/HO-1 pathway. Front. Pharmacol. 2024, 15, 1433793. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Madni, A.; Balasubramanian, V.; Rehman, M.; Correia, A.; Kashif, P.M.; Mäkilä, E.; Salonen, J.; Santos, H.A. Development and optimization of methotrexate-loaded lipid-polymer hybrid nanoparticles for controlled drug delivery applications. Int. J. Pharm. 2017, 533, 156–168. [Google Scholar] [CrossRef]
- Tu, K.; Liu, Z.; Yao, B.; Han, S.; Yang, W. MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT signaling in hepatocellular carcinoma. Int. J. Oncol. 2016, 48, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Yao, Y.; Xia, W.; Setijono, S.R.; Kim, J.H.; Vila, I.K.; Chiu, H.H.; Wu, Y.; Billalabeitia, E.G.; Lee, M.G.; et al. PTEN self-regulates through USP11 via the PI3K-FOXO pathway to stabilize tumor suppression. Nat. Commun. 2019, 10, 636. [Google Scholar] [CrossRef]
- Stambolic, V.; Suzuki, A.; de la Pompa, J.L.; Brothers, G.M.; Mirtsos, C.; Sasaki, T.; Ruland, J.; Penninger, J.M.; Siderovski, D.P.; Mak, T.W. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 1998, 95, 29–39. [Google Scholar] [CrossRef]
- Qiu, W.; Leibowitz, B.; Zhang, L.; Yu, J. Growth factors protect intestinal stem cells from radiation-induced apoptosis by suppressing PUMA through the PI3K/AKT/p53 axis. Oncogene 2010, 29, 1622–1632. [Google Scholar] [CrossRef] [PubMed]





| Forward Sequence | Reverse Sequence | Genes Accession Numbers | |
|---|---|---|---|
| STAT3 | AGAGGCGGCAGCAGATAGC | TTGTTGGCGGGTCTGAAGTT | NM_012747.2 |
| JAK2 | CAATGATAAACAAGGGCAAATGAT | CTTGGCAATCTTCCGTTGCT | NM_031514.1 |
| GAPDH | TGGATTTGGACGCATTGGTC | TTTGCACTGGTACGTGTTGAT | NM_017008.4 |
| Control | UL | OMP + UL | GLY-NPs + UL | |
|---|---|---|---|---|
| Gastrin (pg/mL) | 99.69 ± 2.68 | 420.5 ± 5.92 a | 254.0 ±2.86 ab | 170.8 ± 5.57 abc |
| Somatotropin (pg/mL) | 26.63 ± 2.75 | 215.3 ± 5.23 a | 151.6 ± 2.67 ab | 88.62 ± 2.07 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albalawi, M.; Khateeb, S. Development of Glycyrrhizic Acid Nanoparticles for Modulating Gastric Ulcer Healing: A Comparative In Vivo Study Targeting Oxidative Stress and Inflammatory Pathways. Antioxidants 2025, 14, 990. https://doi.org/10.3390/antiox14080990
Albalawi M, Khateeb S. Development of Glycyrrhizic Acid Nanoparticles for Modulating Gastric Ulcer Healing: A Comparative In Vivo Study Targeting Oxidative Stress and Inflammatory Pathways. Antioxidants. 2025; 14(8):990. https://doi.org/10.3390/antiox14080990
Chicago/Turabian StyleAlbalawi, Mody, and Sahar Khateeb. 2025. "Development of Glycyrrhizic Acid Nanoparticles for Modulating Gastric Ulcer Healing: A Comparative In Vivo Study Targeting Oxidative Stress and Inflammatory Pathways" Antioxidants 14, no. 8: 990. https://doi.org/10.3390/antiox14080990
APA StyleAlbalawi, M., & Khateeb, S. (2025). Development of Glycyrrhizic Acid Nanoparticles for Modulating Gastric Ulcer Healing: A Comparative In Vivo Study Targeting Oxidative Stress and Inflammatory Pathways. Antioxidants, 14(8), 990. https://doi.org/10.3390/antiox14080990






