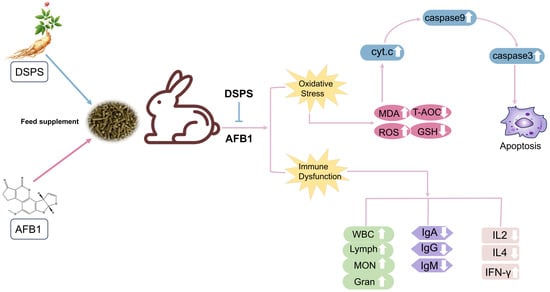
Danshen Polysaccharides Alleviate Aflatoxin B1-Induced Liver Damage and Immune Disorders by Inhibiting the ROS-Mediated Mitochondrial Apoptosis Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Biochemical Indicators
2.3. ELISA
2.4. The Fluorescence Probe Method (DCFH-DA)
2.5. Histopathological Analysis and TUNEL Reaction
2.6. QPCR and Western Blotting
2.7. Statistical Analyses
3. Results
3.1. The Effects of DSPS on Growth Performance
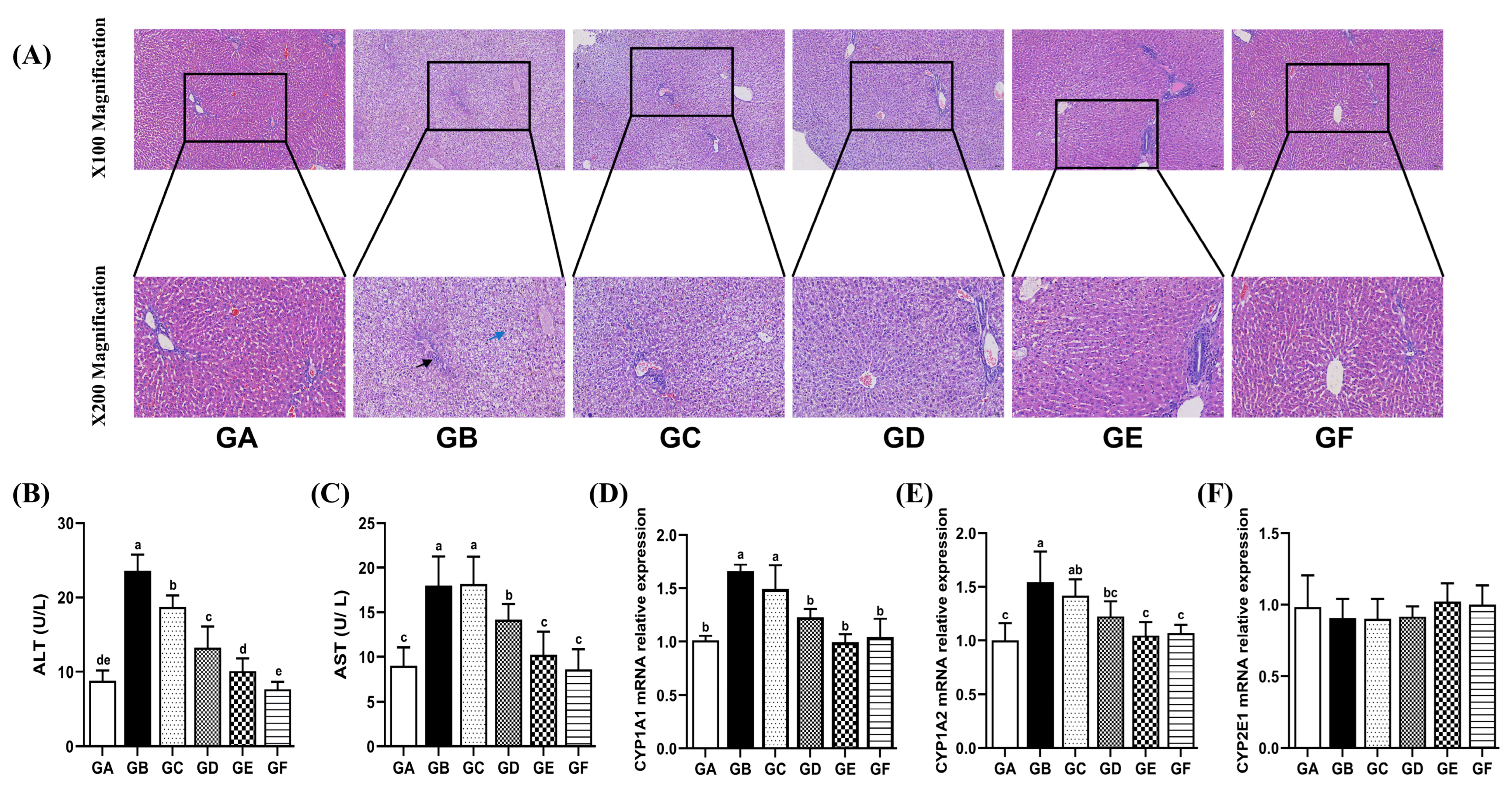
3.2. The Effects of DSPS on Liver Function
3.3. The Effects of DSPS on Drug Metabolizing Enzymes
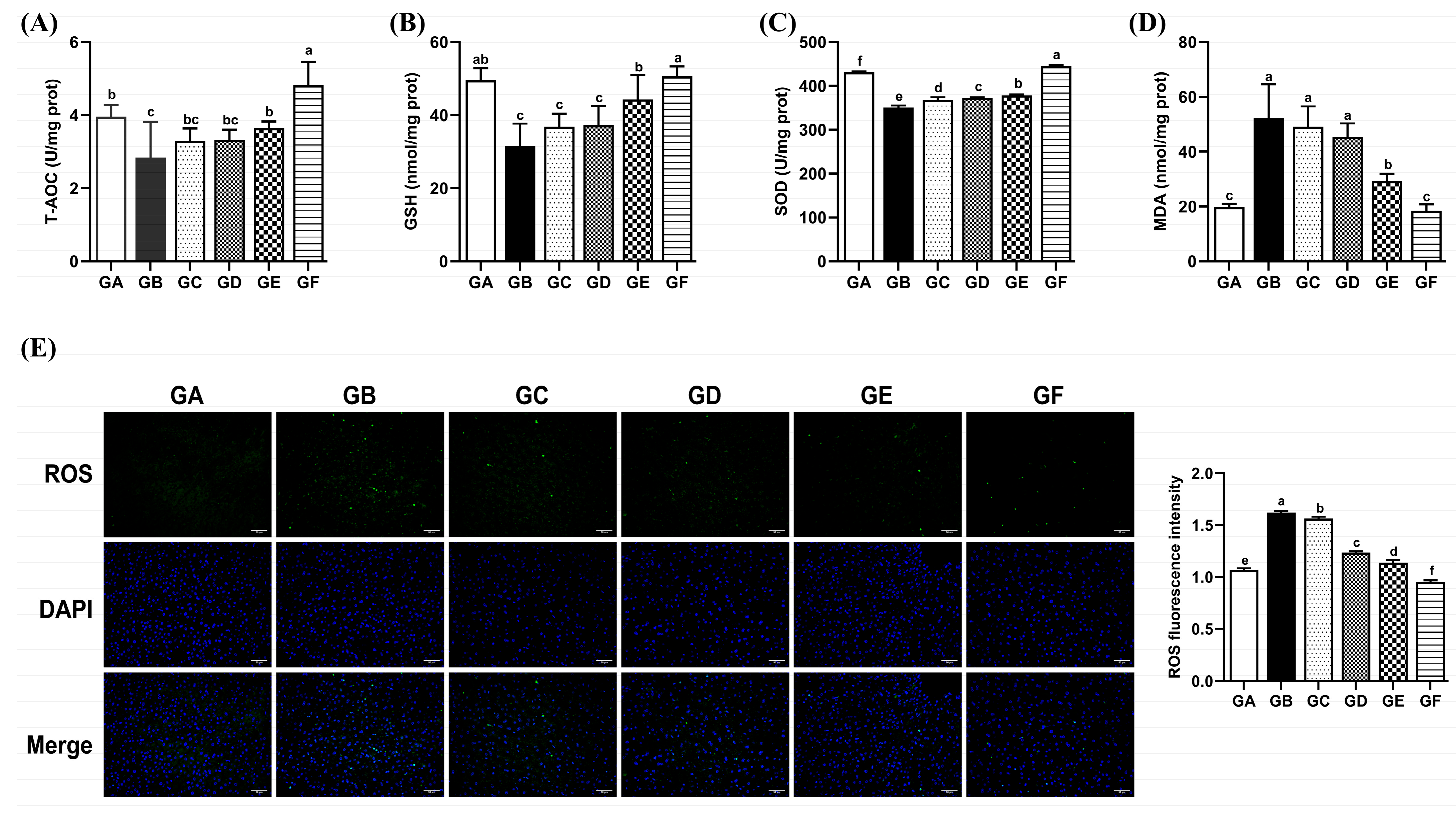
3.4. The Effects of DSPS on Antioxidant Capacity
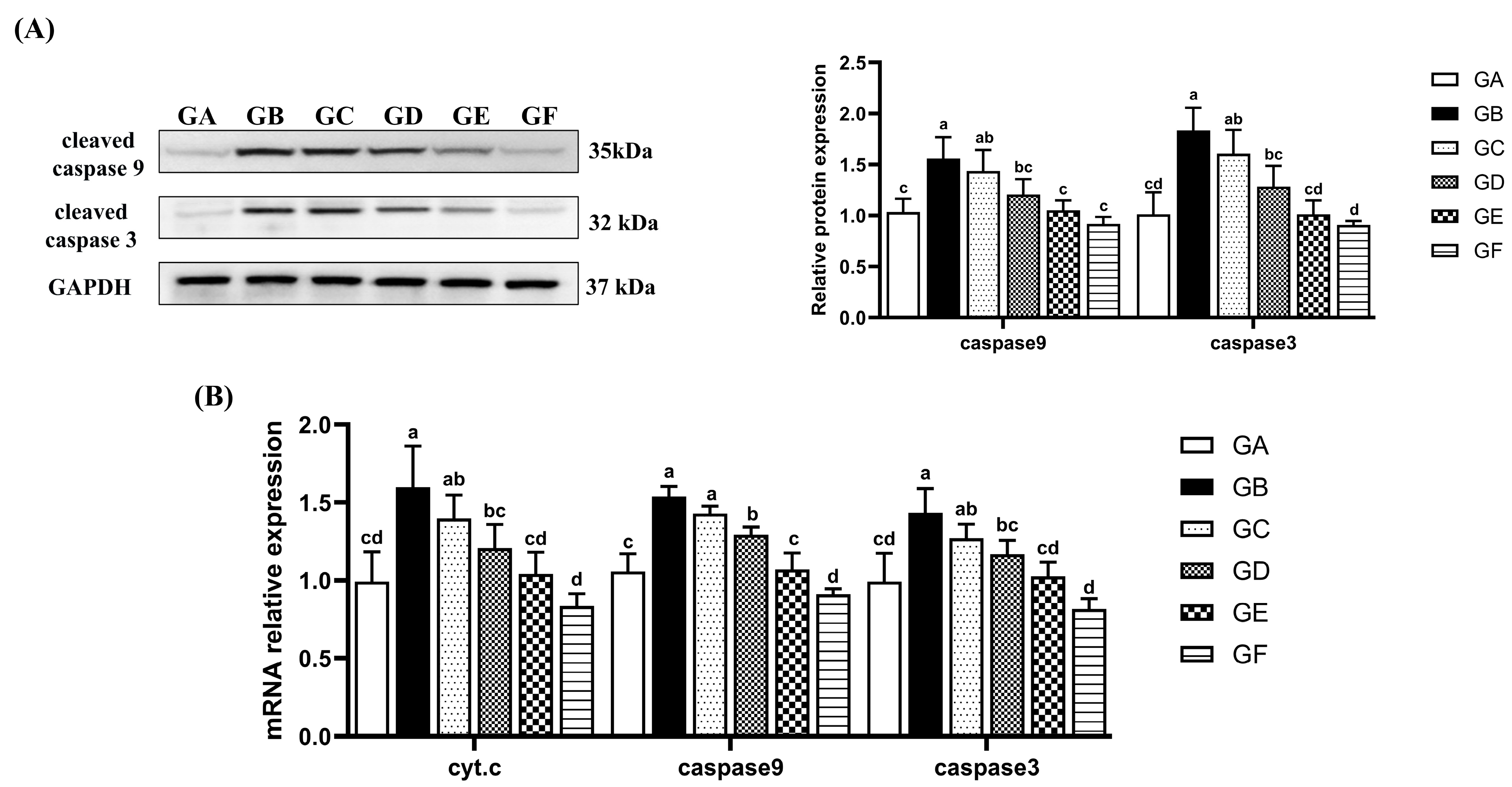
3.5. The Effects of DSPS on Hepatocyte Apoptosis
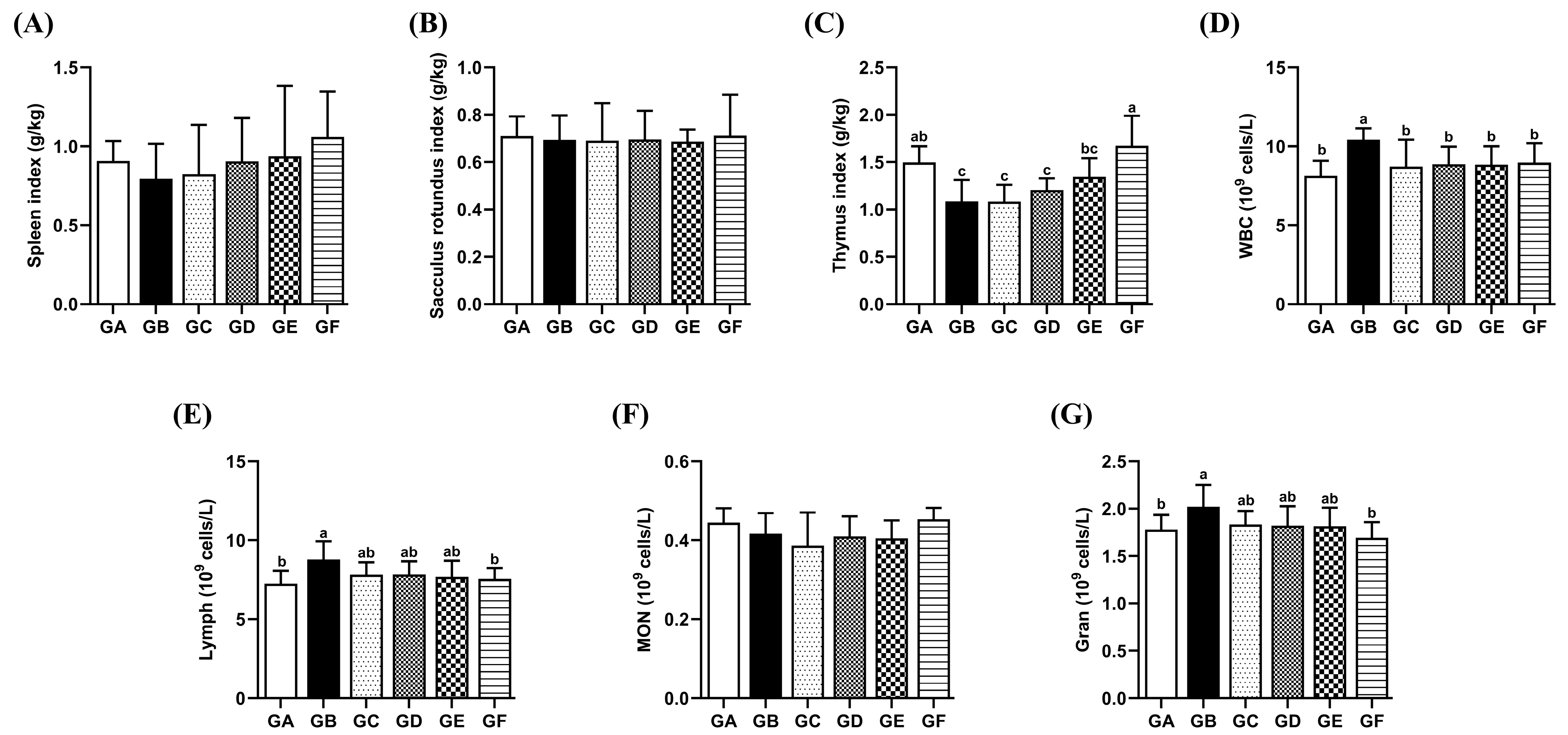
3.6. The Effects of DSPS on the Immune Organ Index
3.7. The Effects of DSPS on the Quantity of Leukocytes and Their Subpopulations
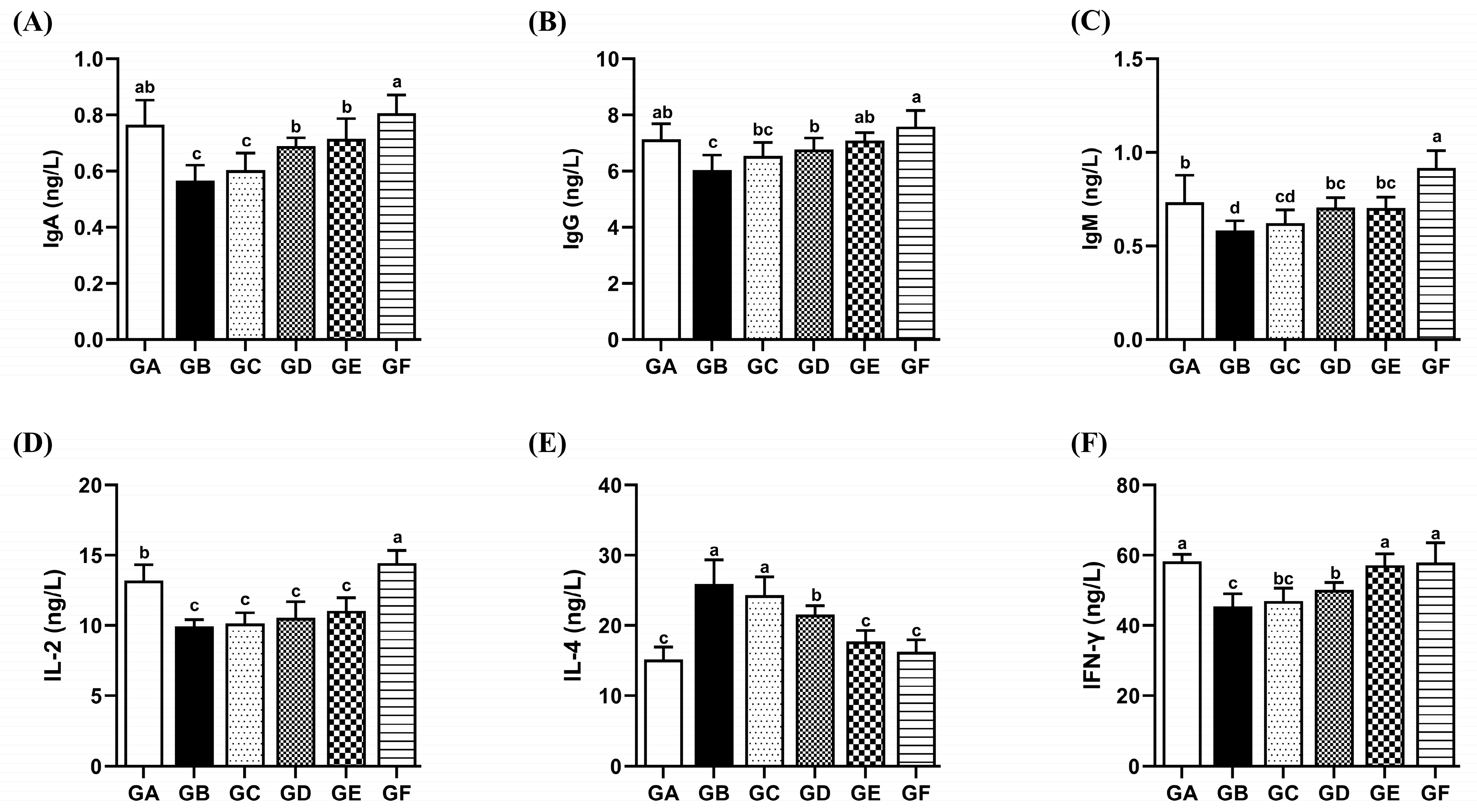
3.8. The Effects of DSPS on Immunoglobulins
3.9. The Effects of DSPS on Immune Cell Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lahouar, A.; Marin, S.; Crespo-Sempere, A.; Saïd, S.; Sanchis, V. Effects of temperature, water activity and incubation time on fungal growth and aflatoxin B1 production by toxinogenic isolates on sorghum seeds. Rev. Argent. Microbiol. 2016, 48, 78–85. [Google Scholar] [CrossRef]
- Sheth, K.; Bankey, P. The liver as an immune organ. Curr. Opin. Crit. Care 2001, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, S.W.; Liu, Y.; Cui, Y.Q.; Zhu, Y.X.; Bao, Y.Z.; Chen, B.J.; Shi, W.Y. Aflatoxin B1 triggers apoptosis in rabbit hepatocytes via mediating oxidative stress and switching on the mitochondrial apoptosis pathway. Ecotox Environ. Saf. 2023, 264, 115478. [Google Scholar] [CrossRef]
- Lin, L.X.; Fu, P.F.; Chen, N.M.; Gao, N.Y.; Cao, Q.Q.; Yue, K.; Xu, T.T.; Zhang, C.D.; Zhang, C.; Liu, F.; et al. Total flavonoids of Rhizoma Drynariae protect hepatocytes against aflatoxin B1-induced oxidative stress and apoptosis in broiler chickens. Ecotox Environ. Saf. 2022, 230, 113148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zhang, Q.; Yao, W.; Zhao, Z.; Wang, X.; Bao, Y.; Shi, W. Salvia miltiorrhiza polysaccharide mitigates AFB1-induced liver injury in rabbits. Ecotoxicol. Environ. Saf. 2024, 276, 116344. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful Effects and Control Strategies of Aflatoxin B1 Produced by Aspergillus flavus and Aspergillus parasiticus Strains on Poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.T.; An, W. Roles of liver innate immune cells in nonalcoholic fatty liver disease. World J. Gastroenterol. 2010, 16, 4652–4660. [Google Scholar] [CrossRef]
- Heymann, F.; Tacke, F. Immunology in the liver--from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhan, D.L.; Chen, Y.Y.; Wang, W.H.; He, C.Y.; Lin, Y.; Lin, Y.C.; Lin, Z.N. Aflatoxin B1 enhances pyroptosis of hepatocytes and activation of Kupffer cells to promote liver inflammatory injury via dephosphorylation of cyclooxygenase-2: An in vitro, ex vivo and in vivo study. Arch. Toxicol. 2019, 93, 3305–3320. [Google Scholar] [CrossRef]
- Zhang, L.W.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.H.; Peng, Y.; Duan, X.L.; Meng, E.; Zhang, C.L.; Zeng, T.; et al. Role of macrophage AHR/TLR4/STAT3 signaling axis in the colitis induced by non-canonical AHR ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef]
- Chen, X.P.; Ishfaq, M.; Wang, J. Effects of Lactobacillus salivarius supplementation on the growth performance, liver function, meat quality, immune responses and Salmonella Pullorum infection resistance of broilers challenged with Aflatoxin B1. Poult. Sci. 2022, 101, 101651. [Google Scholar] [CrossRef]
- Jia, Q.; Zhu, R.; Tian, Y.; Chen, B.; Li, R.; Li, L.; Wang, L.; Che, Y.; Zhao, D.; Mo, F.; et al. Salvia miltiorrhiza in diabetes: A review of its pharmacology, phytochemistry, and safety. Phytomedicine 2019, 58, 152871. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yang, L.; Yang, F.; Pei, Q.; Lu, L.; Huang, X.; Ouyang, P.; Geng, Y.; Li, Z.; Zhang, X.; et al. Salvia miltiorrhiza polysaccharide activated macrophages and improved the disease resistance of sturgeon against Aeromonas hydrophila. Fish Shellfish Immunol. 2022, 127, 594–603. [Google Scholar] [CrossRef]
- Shi, M.; Huang, F.; Deng, C.; Wang, Y.; Kai, G. Bioactivities, biosynthesis and biotechnological production of phenolic acids in Salvia miltiorrhiza. Crit. Rev. Food Sci. Nutr. 2019, 59, 953–964. [Google Scholar] [CrossRef]
- Fu, Y.P.; Peng, X.; Zhang, C.W.; Jiang, Q.X.; Li, C.Y.; Paulsen, B.S.; Rise, F.; Huang, C.; Feng, B.; Li, L.X.; et al. Salvia miltiorrhiza polysaccharide and its related metabolite 5-methoxyindole-3-carboxaldehyde ameliorate experimental colitis by regulating Nrf2/Keap1 signaling pathway. Carbohydr. Polym. 2023, 306, 120626. [Google Scholar] [CrossRef]
- Meng, H.; Wu, J.; Shen, L.; Chen, G.; Jin, L.; Yan, M.; Wan, H.; He, Y. Microwave assisted extraction, characterization of a polysaccharide from Salvia miltiorrhiza Bunge and its antioxidant effects via ferroptosis-mediated activation of the Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2022, 215, 398–412. [Google Scholar] [CrossRef]
- Song, Y.H.; Liu, Q.; Lv, Z.P.; Chen, Y.Y.; Zhou, Y.C.; Sun, X.G. Protection of a polysaccharide from Salvia miltiorrhiza, a Chinese medicinal herb, against immunological liver injury in mice. Int. J. Biol. Macromol. 2008, 43, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wei, Y.Y.; Wang, X.; Ba, C.J.; Shi, W.Y. Protective effect of Salvia miltiorrhiza polysaccharides on liver injury in chickens. Poult. Sci. 2019, 98, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gong, X.; Zhang, S.; Cui, C.; Zhang, Q.; Wang, X.; Shi, W.; Bao, Y. Danshen polysaccharides alleviate AFB1 induced Jejunal injury. Ecotoxicol. Environ. Saf. 2024, 285, 117115. [Google Scholar] [CrossRef]
- Li, S.H.; Liu, R.M.; Xia, S.; Wei, G.Q.; Ishfaq, M.; Zhang, Y.X.; Zhang, X.Y. Protective role of curcumin on aflatoxin B1-induced TLR4/RIPK pathway mediated-necroptosis and inflammation in chicken liver. Ecotox Environ. Saf. 2022, 233, 113319. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, W.; Yin, P.; Gao, J.; Na, L.; Sun, Y.; Wang, Z.; Zhang, Z.B.; Zhao, C.H. Ruxolitinib Alleviates Renal Interstitial Fibrosis in UUO Mice. Int. J. Biol. Sci. 2020, 16, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Chen, H.D.; Tsim, K.W.K.; Shen, X.; Li, X.M.; Li, X.L.; Lei, H.T.; Liu, Y.L. Aflatoxin B1 Induces Inflammatory Liver Injury via Gut Microbiota in Mice. J. Agr. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef]
- Potz, B.A.; Lawandy, I.J.; Clements, R.T.; Sellke, F.W. Alcohol modulates autophagy and apoptosis in pig liver tissue. J. Surg. Res. 2016, 203, 154–162. [Google Scholar] [CrossRef]
- Lu, Q.; Hu, Y.; Nabi, F.; Li, Z.; Janyaro, H.; Zhu, W.; Liu, J. Effect of Penthorum Chinense Pursh Compound on AFB1-Induced Immune Imbalance via JAK/STAT Signaling Pathway in Spleen of Broiler Chicken. Vet. Sci. 2023, 10, 521. [Google Scholar] [CrossRef]
- Taranu, I.; Hermenean, A.; Bulgaru, C.; Pistol, G.C.; Ciceu, A.; Grosu, I.A.; Marin, D.E. Diet containing grape seed meal by-product counteracts AFB1 toxicity in liver of pig after weaning. Ecotoxicol. Environ. Saf. 2020, 203, 110899. [Google Scholar] [CrossRef]
- Liu, S.P.; Kang, W.L.; Mao, X.R.; Ge, L.; Du, H.; Li, J.Y.; Hou, L.L.; Liu, D.D.; Yin, Y.L.; Liu, Y.H.; et al. Melatonin mitigates aflatoxin B1-induced liver injury via modulation of gut microbiota/intestinal FXR/liver TLR4 signaling axis in mice. J. Pineal Res. 2022, 73, 12812. [Google Scholar] [CrossRef]
- Lori, S.; Lahtela-Kakkonen, M.; D’Onofrio, C.; Maietti, F.; Mucignat, G.; Bardhi, A.; Barbarossa, A.; Zaghini, A.; Pauletto, M.; Dacasto, M.; et al. New insights into aflatoxin B1 mechanistic toxicology in cattle liver: An integrated approach using molecular docking and biological evaluation in CYP1A1 and CYP3A74 knockout BFH12 cell lines. Arch. Toxicol. 2024, 98, 3097–3108. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Zeng, H.; Fu, W.; Wang, J.; Tan, Y.; Zheng, C.; Qiu, Z.; Luo, J.; Lv, C.; et al. Low-dose microcystin-LR antagonizes aflatoxin B1 induced hepatocarcinogenesis through decreasing cytochrome P450 1A2 expression and aflatoxin B1-DNA adduct generation. Chemosphere 2020, 248, 126036. [Google Scholar] [CrossRef]
- Liu, R.M.; Ding, Y.X.; Li, W.N.; Li, S.H.; Li, X.T.; Zhao, D.M.; Zhang, Y.X.; Wei, G.Q.; Zhang, X.Y. Protective role of curcumin on broiler liver by modulating aflatoxin B1-induced DNA methylation and CYPs expression. Ecotoxicol. Environ. Saf. 2023, 260, 115086. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Mendoza, K.M.; Reed, K.M.; Coulombe, R.A., Jr. Structure, genetic mapping, and function of the cytochrome P450 3A37 gene in the turkey (Meleagris gallopavo). Cytogenet. Genome Res. 2009, 125, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, Q.; Liang, Q.; Wu, Y.; Ye, Z.; Wu, H.; Sun, Q.; Tang, H.; Liu, Y.; Liu, Q.; et al. Human CYP1A1-activated aneugenicity of aflatoxin B1 in mammalian cells and its combined effect with benzo(a)pyrene. Chem. Biol. Interact. 2024, 392, 110923. [Google Scholar] [CrossRef]
- Ishteyaque, S.; Yadav, K.S.; Verma, S.; Washimkar, K.R.; Mugale, M.N. CYP2E1 triggered GRP78/ATF6/CHOP signaling axis inhibit apoptosis and promotes progression of hepatocellular carcinoma. Arch. Biochem. Biophys. 2023, 745, 109701. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.Y.; Yu, P.; Yang, K.P.; Cao, D.L. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Method. 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Hesperetin protects hippocampal neurons from the neurotoxicity of Aflatoxin B1 in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115782. [Google Scholar] [CrossRef]
- Deng, J.; Zhao, L.; Zhang, N.Y.; Karrow, N.A.; Krumm, C.S.; Qi, D.S.; Sun, L.H. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat. Res.-Rev. Mutat. 2018, 778, 79–89. [Google Scholar] [CrossRef]
- Dey, D.K.; Kang, S.C. Aflatoxin B1 induces reactive oxygen species-dependent caspase-mediated apoptosis in normal human cells, inhibits Allium cepa root cell division, and triggers inflammatory response in zebrafish larvae. Sci. Total Environ. 2020, 737, 139704. [Google Scholar] [CrossRef]
- Ma, Z.J.; Lu, L.; Yang, J.J.; Wang, X.X.; Su, G.; Wang, Z.L.; Chen, G.H.; Sun, H.M.; Wang, M.Y.; Yang, Y. Lariciresinol induces apoptosis in HepG2 cells via mitochondrial-mediated apoptosis pathway. Eur. J. Pharmacol. 2018, 821, 1–10. [Google Scholar] [CrossRef]
- Sang, R.; Ge, B.J.; Li, H.F.; Zhou, H.Y.; Yan, K.X.; Wang, W.; Cui, Q.C.; Zhang, X.M. Taraxasterol alleviates aflatoxin B1-induced liver damage in broiler chickens via regulation of oxidative stress, apoptosis and autophagy. Ecotoxicol. Environ. Saf. 2023, 251, 114546. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Tian, J.; Muhammad, I.; Liu, M.; Wu, C.; Xu, C.; Zhang, X. Ferulic acid prevents aflatoxin B1-induced liver injury in rats via inhibiting cytochrome P450 enzyme, activating Nrf2/GST pathway and regulating mitochondrial pathway. Ecotoxicol. Environ. Saf. 2021, 224, 112624. [Google Scholar] [CrossRef]
- Manoharan, R.R.; Prasad, A.; Pospisil, P.; Kzhyshkowska, J. ROS signaling in innate immunity via oxidative protein modifications. Front. Immunol. 2024, 15, 1359600. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Yuan, S.; Chen, J.; Peng, X.; Wang, F.; Cui, H.; Fang, J. Effects of sodium selenite on the decreased percentage of T cell subsets, contents of serum IL-2 and IFN-γ induced by aflatoxin B1 in broilers. Res. Vet. Sci. 2013, 95, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Cemerski, S.; van Meerwijk, J.P.M.; Romagnoli, P. Oxidative-stress-induced T lymphocyte hyporesponsiveness is caused by structural modification rather than proteasomal degradation of crucial TCR signaling molecules. Eur. J. Immunol. 2003, 33, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Wang, X.; Zhang, D.; Wei, Y.Y.; Cui, Y.Q.; Shi, W.Y.; Bao, Y.Z. Synergistic use of florfenicol and Salvia miltiorrhiza polysaccharide can enhance immune responses in broilers. Ecotoxicol. Environ. Saf. 2021, 210, 111825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Li, M.; Niu, S.; Wang, J.; Shao, H.; Li, T.; Wang, H. Salvia miltiorrhiza polysaccharide activates T Lymphocytes of cancer patients through activation of TLRs mediated-MAPK and-NF-κB signaling pathways. J. Ethnopharmacol. 2017, 200, 165–173. [Google Scholar] [CrossRef]

| Items | GA | GB | GC | GD | GE | GF |
|---|---|---|---|---|---|---|
| AFB1 (μg/kg) | ND | 25 | 25 | 25 | 25 | ND |
| DON/ZEA | ND | ND | ND | ND | ND | ND |
| ZEA | ND | ND | ND | ND | ND | ND |
| DSPS (mg/kg) | ND | ND | 300 | 600 | 900 | 900 |
| Items | GA | GB | GC | GD | GE | GF |
|---|---|---|---|---|---|---|
| Mortality (%) | 5 | 15 | 15 | 10 | 5 | 0 |
| ADG (g/d) | 47.46 ± 2.03 b | 25.87 ± 1.40 e | 29.37 ± 2.46 e | 33.33 ± 0.85 d | 35.40 ± 1.40 c | 52.06 ± 1.87 a |
| ADFI (g/d) | 114.60 ± 6.72 a | 79.92 ± 9.09 c | 81.27 ± 4.84 c | 92.86 ± 6.75 b | 98.25 ± 3.38 b | 115.24 ± 5.04 a |
| FCR | 2.41 ± 0.06 c | 3.09 ± 0.28 a | 2.78 ± 0.33 a | 2.78 ± 0.14 b | 2.78 ± 0.10 b | 2.21 ± 0.06 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Bao, Y.; Gong, X.; Ma, S.; Wang, X.; Shi, W. Danshen Polysaccharides Alleviate Aflatoxin B1-Induced Liver Damage and Immune Disorders by Inhibiting the ROS-Mediated Mitochondrial Apoptosis Pathway. Antioxidants 2025, 14, 991. https://doi.org/10.3390/antiox14080991
Zhang L, Bao Y, Gong X, Ma S, Wang X, Shi W. Danshen Polysaccharides Alleviate Aflatoxin B1-Induced Liver Damage and Immune Disorders by Inhibiting the ROS-Mediated Mitochondrial Apoptosis Pathway. Antioxidants. 2025; 14(8):991. https://doi.org/10.3390/antiox14080991
Chicago/Turabian StyleZhang, Lu, Yongzhan Bao, Xincheng Gong, Shuang Ma, Xiao Wang, and Wanyu Shi. 2025. "Danshen Polysaccharides Alleviate Aflatoxin B1-Induced Liver Damage and Immune Disorders by Inhibiting the ROS-Mediated Mitochondrial Apoptosis Pathway" Antioxidants 14, no. 8: 991. https://doi.org/10.3390/antiox14080991
APA StyleZhang, L., Bao, Y., Gong, X., Ma, S., Wang, X., & Shi, W. (2025). Danshen Polysaccharides Alleviate Aflatoxin B1-Induced Liver Damage and Immune Disorders by Inhibiting the ROS-Mediated Mitochondrial Apoptosis Pathway. Antioxidants, 14(8), 991. https://doi.org/10.3390/antiox14080991