New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine
Abstract
1. Introduction
2. Developmental Aspects of Potential Antioxidant Drugs for Clinical Use
2.1. Limitations in the Use of Repurposed Drugs and Nutraceuticals in Medicine
2.2. General Characteristics and Requirements for Antioxidant Drugs in Medicine
3. Iron Chelating Drugs with Antioxidant Effects in Medicine
3.1. The General Iron Chelating Properties of Deferiprone, Deferoxamine and Deferasirox
3.2. Toxicity Limitations in the Use of Deferiprone, Deferoxamine, and Deferasirox
3.3. Repurposing of the Iron Chelating/Antioxidant Drug Deferiprone in Non-Iron-Loaded Diseases
3.4. The Repurposing Prospects of Deferoxamine, Deferasirox, and EDTA as Antioxidant Drugs
4. Iron-Binding Drugs, Pro-Drugs, and Drug Metabolites with Antioxidant Properties
4.1. The Antioxidant Clinical Effects of N-Acetylcysteine and Its Iron-Binding Properties
4.2. Pro-Drugs and Drug Metabolites with Iron-Binding and Antioxidant Properties
5. Future Prospects and Strategies in Antioxidant Therapeutics
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADMET | absorption, distribution, metabolism, elimination, and toxicity |
| DF | deferoxamine |
| DFRA | deferasirox |
| DTPA | diethylenetriaminepenntaacetic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FR | free radicals |
| GSH | reduced glutathione |
| IV | intravenous |
| L1 | deferiprone |
| L1-G | deferiprone glucuronide metabolite |
| LVEF | left- venricular ejection fraction |
| MRI | magnetic resonance imaging |
| NBIA | neurodegeneration with brain iron accumulation |
| NCA | N-acetylcysteine |
| OST | oxidative stress toxicity |
| PKAN | pantothenate kinase 2-associated neurodegeneration |
| ROS | reactive oxygen species |
| TM | thalassaemia major |
| TAOC | total antioxidant capacity |
| SC | subcutaneous |
References
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: Abingdon, UK, 2005. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C.; Cross, C.E. Free radicals, antioxidants and human disease: Where are we now? J. Lab. Clin. Med. 1992, 119, 598–620. [Google Scholar] [PubMed]
- Reddy, V.P. Oxidative Stress in Health and Disease. Biomedicines 2023, 11, 2925. [Google Scholar] [CrossRef] [PubMed]
- Iakovou, E.; Kourti, M. A Comprehensive Overview of the Complex Role of Oxidative Stress in Aging, The Contributing Environmental Stressors and Emerging Antioxidant Therapeutic Interventions. Front. Aging Neurosci. 2022, 14, 827900. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, A.; Reddy, A.B. Regulation of circadian clocks by redox homeostasis. J. Biol. Chem. 2013, 288, 26505–26511. [Google Scholar] [CrossRef]
- Forman, H.J. Redox signaling: An evolution from free radicals to aging. Free Radic. Biol. Med. 2016, 97, 398–407. [Google Scholar] [CrossRef]
- Su, Z.; Hu, Q.; Li, X.; Wang, Z.; Xie, Y. The Influence of Circadian Rhythms on DNA Damage Repair in Skin Photoaging. Int. J. Mol. Sci. 2024, 25, 10926. [Google Scholar] [CrossRef]
- Arevalo, J.A.; Vázquez-Medina, J.P. The Role of Peroxiredoxin 6 in Cell Signaling. Antioxidants 2018, 7, 172. [Google Scholar] [CrossRef]
- Chakraborty, P.; Dewanjee, S. Unrevealing the mechanisms behind the cardioprotective effect of wheat polyphenolics. Arch. Toxicol. 2024, 98, 3543–3567. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules 2015, 20, 20841–20872. [Google Scholar] [CrossRef]
- Black, H.S.; Boehm, F.; Edge, R.; Truscott, T.G. The Benefits and Risks of Certain Dietary Carotenoids that Exhibit both Anti- and Pro-Oxidative Mechanisms—A Comprehensive Review. Antioxidants 2020, 9, 264. [Google Scholar] [CrossRef]
- Pagano, G.; Talamanca, A.A.; Castello, G.; Cordero, M.D.; D’iSchia, M.; Gadaleta, M.N.; Pallardó, F.V.; Petrović, S.; Tiano, L.; Zatterale, A. Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward mitochondria-targeted clinical strategies. Oxidative Med. Cell. Longev. 2014, 2014, 541230. [Google Scholar] [CrossRef]
- Vyas, S.; Zaganjor, E.; Haigis, M.C. Mitochondria and Cancer. Cell 2016, 166, 555–566. [Google Scholar] [CrossRef]
- Kathiresan, D.S.; Balasubramani, R.; Marudhachalam, K.; Jaiswal, P.; Ramesh, N.; Sureshbabu, S.G.; Puthamohan, V.M.; Vijayan, M. Role of Mitochondrial Dysfunctions in Neurodegenerative Disorders: Advances in Mitochondrial Biology. Mol. Neurobiol. 2025, 62, 6827–6855. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S. Reassessment of a free radical theory of cancer with emphasis on ultraviolet carcinogenesis. Integr. Cancer Ther. 2004, 3, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.; Shrivastava, R.; Mittal, M. Chemistry and pharmacological properties of some natural and synthetic antioxidants for heavy metal toxicity. Curr. Med. Chem. 2013, 20, 4540–4574. [Google Scholar] [CrossRef]
- Xiao, C.; Lai, D. Impact of oxidative stress induced by heavy metals on ovarian function. J. Appl. Toxicol. 2025, 45, 107–116. [Google Scholar] [CrossRef]
- Pan, Z.; Gong, T.; Liang, P. Heavy Metal Exposure and Cardiovascular Disease. Circ. Res. 2024, 134, 1160–1178. [Google Scholar] [CrossRef]
- Marant-Micallef, C.; Shield, K.D.; Vignat, J.; Cléro, E.; Kesminiene, A.; Hill, C.; Rogel, A.; Vacquier, B.; Bray, F.; Laurier, D.; et al. The risk of cancer attributable to diagnostic medical radiation: Estimation for France in 2015. Int. J. Cancer 2019, 144, 2954–2963. [Google Scholar] [CrossRef]
- Ambra, R.; Lucchetti, S.; Pastore, G. A Review of the Effects of Olive Oil-Cooking on Phenolic Compounds. Molecules 2022, 27, 661. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.D.; Dizdaroglu, M.; Cooke, M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. 2004, 567, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun-Waterhouse, D.; Yao, W.; Li, X.; Zhao, M.; You, L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chem. 2021, 365, 130524. [Google Scholar] [CrossRef]
- Kern, C.; Bonventre, J.V.; Justin, A.W.; Kashani, K.; Reynolds, E.; Siew, K.; Davis, B.; Karakoy, H.; Grzesiak, N.; Bailey, D.M. Necrosis as a fundamental driver of loss of resilience and biological decline: What if we could intervene? Oncogene 2025, 44, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Pompella, A.; Corti, A. Editorial: The changing faces of glutathione, a cellular protagonist. Front. Pharmacol. 2015, 6, 98. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Topic, A.; Francuski, D.; Markovic, B.; Stankovic, M.; Dobrivojevic, S.; Drca, S.; Radojkovic, D. Gender-related reference intervals of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine determined by liquid chromatography-tandem mass spectrometry in Serbian population. Clin. Biochem. 2013, 46, 321–326. [Google Scholar] [CrossRef]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells 2020, 9, 1456. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Porwal, S.K.; Tartakoff, A.; Devireddy, L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012, 51, 5705–5724. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef]
- Cao, J.Y.; Dixon, S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016, 73, 2195–2209. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Li, M.; Luo, Z. Autophagy-Dependent Ferroptosis as a Therapeutic Target in Cancer. ChemMedChem 2021, 16, 2942–2950. [Google Scholar] [CrossRef]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: Opportunities and challenges in cancer. J. Hematol. Oncol. 2019, 12, 34. [Google Scholar] [CrossRef]
- Catapano, A.; Cimmino, F.; Petrella, L.; Pizzella, A.; D’ANgelo, M.; Ambrosio, K.; Marino, F.; Sabbatini, A.; Petrelli, M.; Paolini, B.; et al. Iron metabolism and ferroptosis in health and diseases: The crucial role of mitochondria in metabolically active tissues. J. Nutr. Biochem. 2025, 140, 109888. [Google Scholar] [CrossRef]
- Teschke, R.; Eickhoff, A. Wilson Disease: Copper-Mediated Cuproptosis, Iron-Related Ferroptosis, and Clinical Highlights, with Comprehensive and Critical Analysis Update. Int. J. Mol. Sci. 2024, 25, 4753. [Google Scholar] [CrossRef]
- Mao, C.; Wang, M.; Zhuang, L.; Gan, B. Metabolic cell death in cancer: Ferroptosis, cuproptosis, disulfidptosis, and beyond. Protein Cell 2024, 15, 642–660. [Google Scholar] [CrossRef]
- Lu, K.; Wijaya, C.S.; Yao, Q.; Jin, H.; Feng, L. Cuproplasia and cuproptosis, two sides of the coin. Cancer Commun. 2025, 45, 505–524. [Google Scholar] [CrossRef]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Potential clinical applications of chelating drugs in diseases targeting transferrin-bound iron and other metals. Expert Opin. Investig. Drugs 2013, 22, 591–618. [Google Scholar] [CrossRef]
- Arosio, P. New Advances in Iron Metabolism, Ferritin and Hepcidin Research. Int. J. Mol. Sci. 2022, 23, 14700. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Importance and Essentiality of Natural and Synthetic Chelators in Medicine: Increased Prospects for the Effective Treatment of Iron Overload and Iron Deficiency. Int. J. Mol. Sci. 2024, 25, 4654. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, L.N.; Kontoghiorghes, G.J. Competition between deferiprone, desferrioxamine and other chelators for iron and the effect of other metals. Arzneimittelforschung 1993, 43, 659–663. [Google Scholar]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Andreou, N.; Constantinou, K.; Kontoghiorghes, G.J. World health dilemmas: Orphan and rare diseases, orphan drugs and orphan patients. World J. Methodol. 2014, 4, 163–188. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Prospects for the introduction of targeted antioxidant drugs for the prevention and treatment of diseases related to free radical pathology. Expert Opin. Investig. Drugs 2019, 28, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Bruzzese, A.; Martino, E.A.; Mendicino, F.; Lucia, E.; Olivito, V.; Bova, C.; Filippelli, G.; Capodanno, I.; Neri, A.; Morabito, F.; et al. Iron chelation therapy. Eur. J. Haematol. 2023, 110, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.E.; Muttar, S.; Badawy, S.M. The challenges of iron chelation therapy in thalassemia: How do we overcome them? Expert Rev. Hematol. 2025, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Inusa, B.P.; Atoyebi, W.; Andemariam, B.; Hourani, J.N.; Omert, L. Global burden of transfusion in sickle cell disease. Transfus. Apher. Sci. 2023, 62, 103764. [Google Scholar] [CrossRef]
- Navaneethabalakrishnan, S.; An, X.; Vinchi, F. Heme- and iron-activated macrophages in sickle cell disease: An updated perspective. Curr. Opin. Hematol. 2024, 31, 275–284. [Google Scholar] [CrossRef]
- Küpesiz, F.T.; Hazar, V.; Eker, N.; Guler, E.; Yesilipek, M.A.; Tuysuz, G.; Kupesiz, A. Retrospective Evaluation of Relationship Between Iron Overload and Transplantation Complications in Pediatric Patient Who Underwent Allogeneic Stem Cell Transplantation Due to Acute Leukemia and Myelodysplastic Syndrome. J. Pediatr. Hematol. Oncol. 2020, 42, e315–e320. [Google Scholar] [CrossRef]
- Baskin-Miller, J.; Carson, S.; Jaffray, J.; Fletcher, C.; Singer, J.; Freyer, D.R.; Wood, J.; Coates, T.D.; Denton, C.C. Transfusional hemosiderosis in childhood cancer patients and survivors. Pediatr. Blood Cancer 2024, 71, e31220. [Google Scholar] [CrossRef]
- Gao, Q.; Zhou, Y.; Chen, Y.; Hu, W.; Jin, W.; Zhou, C.; Yuan, H.; Li, J.; Lin, Z.; Lin, W. Role of iron in brain development, aging, and neurodegenerative diseases. Ann. Med. 2025, 57, 2472871. [Google Scholar] [CrossRef]
- Klein, H.G.; Spahn, D.R.; Carson, J.L. Red blood cell transfusion in clinical practice. Lancet 2007, 370, 415–426. [Google Scholar] [CrossRef]
- Cazzola, M.; Borgna-Pignatti, C.; Locatelli, F.; Ponchio, L.; Beguin, Y.; De Stefano, P. A moderate transfusion regimen may reduce iron loading in beta-thalassemia major without producing excessive expansion of erythropoiesis. Transfusion 1997, 37, 135–140. [Google Scholar] [CrossRef]
- Iancu, T.C. Iron overload. Mol. Asp. Med. 1983, 6, 1–100. [Google Scholar] [CrossRef]
- Pippard, M.J.; Weatherall, D.J. Iron absorption in non-transfused iron loading anaemias: Prediction of risk for iron loading, and response to iron chelation treatment, in beta thalassaemia intermedia and congenital sideroblastic anaemias. Haematologia 1984, 17, 17–24. [Google Scholar]
- Zurlo, M.; De Stefano, P.; Borgna-Pignatti, C.; Di Palma, A.; Melevendi, C.; Piga, A.; Di Gregorio, F.; Burattini, M.; Terzoli, S. Survival and causes of death in thalassaemia major. Lancet 1989, 2, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Khan, M.; Darlison, M. Survival in beta-thalassaemia major in the UK: Data from the UK Thalassaemia Register. Lancet 2000, 355, 2051–2052. [Google Scholar] [CrossRef] [PubMed]
- Telfer, P.; Coen, P.G.; Christou, S.; Hadjigavriel, M.; Kolnakou, A.; Pangalou, E.; Pavlides, N.; Psiloines, M.; Simamonian, K.; Skordos, G.; et al. Survival of medically treated thalassemia patients in Cyprus. Trends and risk factors over the period 1980–2004. Haematologica 2006, 91, 1187–1192. [Google Scholar] [PubMed]
- Kolnagou, A.; Kontoghiorghes, G.J. Advances in the prevention and treatment are changing thalassemia from a fatal to a chronic disease. experience from a Cyprus model and its use as a paradigm for future applications. Hemoglobin 2009, 33, 287–295. [Google Scholar] [CrossRef]
- Modell, B.; Khan, M.; Darlison, M.; Westwood, M.A.; Ingram, D.; Pennell, D.J. Improved survival of thalassaemia major in the UK and relation to T2* cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2008, 10, 42. [Google Scholar] [CrossRef]
- Au, W.Y.; Lee, V.; Lau, C.W.; Yau, J.; Chan, D.; Chan, E.Y.T.; Cheung, W.W.W.; Ha, S.Y.; Kho, B.; Lee, C.Y.; et al. A synopsis of current care of thalassaemia major patients in Hong Kong. Hong Kong Med. J. 2011, 17, 261–266. [Google Scholar]
- Maggio, A.; Filosa, A.; Vitrano, A.; Aloj, G.; Kattamis, A.; Ceci, A.; Fucharoen, S.; Cianciulli, P.; Grady, R.W.; Prossomariti, L.; et al. Iron chelation therapy in thalassemia major: A systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol. Dis. 2011, 47, 166–175. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Iron Load Toxicity in Medicine: From Molecular and Cellular Aspects to Clinical Implications. Int. J. Mol. Sci. 2023, 24, 12928. [Google Scholar] [CrossRef]
- Adams, P.C. Epidemiology and diagnostic testing for hemochromatosis and iron overload. Int. J. Lab. Hematol. 2015, 37, 25–30. [Google Scholar] [CrossRef]
- Brissot, P.; Troadec, M.-B.; Loréal, O.; Brissot, E. Pathophysiology and classification of iron overload diseases; update 2018. Transfus. Clin. Biol. 2019, 26, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2021, 513, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.V.; Rajapurkar, M.M. The Role of Labile Iron in Kidney Disease and Treatment with Chelation. Hemoglobin 2009, 33, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Kyriacou, K.; Michaelides, Y.; Senkus, R.; Simamonian, K.; Pavlides, N.; Antoniades, L.; Zambartas, C. Ultrastructural pathology of the heart in patients with beta-thalassaemia major. Ultrastruct. Pathol. 2000, 24, 75–81. [Google Scholar] [CrossRef]
- Vassiliou, E.; Farias-Pereira, R. Impact of Lipid Metabolism on Macrophage Polarization: Implications for Inflammation and Tumor Immunity. Int. J. Mol. Sci. 2023, 24, 12032. [Google Scholar] [CrossRef]
- Denz, H.; Huber, P.; Landmann, R.; Orth, B.; Wachter, H.; Fuchs, D. Association between the activation of macrophages, changes of iron metabolism and the degree of anaemia in patients with malignant disorders. Eur. J. Haematol. 1992, 48, 244–248. [Google Scholar] [CrossRef]
- Waldvogel, D.; van Gelderen, P.; Hallett, M. Increased iron in the dentate nucleus of patients with Friedrich’s ataxia. Ann. Neurol. 1999, 46, 123–125. [Google Scholar] [CrossRef]
- Economides, C.P.; Soteriades, E.S.; Hadjigavriel, M.; Seimenis, I.; Karantanas, A. Iron deposits in the knee joints of a thalassemic patient. Acta Radiol. Short Rep. 2013, 2, 2047981613477401. [Google Scholar] [CrossRef]
- Meloni, A.; Pistoia, L.; Gamberini, M.R.; Ricchi, P.; Cecinati, V.; Sorrentino, F.; Cuccia, L.; Allò, M.; Righi, R.; Fina, P.; et al. The Link of Pancreatic Iron with Glucose Metabolism and Cardiac Iron in Thalassemia Intermedia: A Large, Multicenter Observational Study. J. Clin. Med. 2021, 10, 5561. [Google Scholar] [CrossRef]
- Lehéricy, S.; Roze, E.; Goizet, C.; Mochel, F. MRI of neurodegeneration with brain iron accumulation. Curr. Opin. Neurol. 2020, 33, 462–473. [Google Scholar] [CrossRef]
- Drayer, B.P.; Olanow, W.; Burger, P.; Johnson, G.A.; Herfkens, R.; Riederer, S. Parkinson plus syndrome: Diagnosis using high field MR imaging of brain iron. Radiology 1986, 159, 493–498. [Google Scholar] [CrossRef]
- Norfray, J.F.; Chiaradonna, N.L.; Heiser, W.J.; Song, S.H.; Manyam, B.V.; Devleschoward, A.B.; Eastwood, L.M. Brain iron in patients with Parkinson disease: MR visualization using gradient modification. Am. J. Neuroradiol. 1988, 9, 237–240. [Google Scholar]
- Bartzokis, G.; Sultzer, D.; Mintz, J.; Holt, L.E.; Marx, P.; Phelan, C.K.; Marder, S.R. In vivo evaluation of brain iron in Alzheimer’s disease and normal subjects using MRI. Biol. Psychiatry 1994, 35, 480–487. [Google Scholar] [CrossRef]
- Brar, S.; Henderson, D.; Schenck, J.; Zimmerman, E.A. Iron Accumulation in the Substantia Nigra of Patients with Alzheimer Disease and Parkinsonism. Arch. Neurol. 2009, 66, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Suhara, T.; Baba, Y.; Kawasaki, N.K.; Higa, J.K.; Matsui, T. Pathological Roles of Iron in Cardiovascular Disease. Curr. Drug Targets 2018, 19, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B. Influence of desferrioxamine on the renal excretion of iron. Preliminary report. Acta Med. Scand. 1963, 173, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Wöhler, F. The Treatment of Haemochromatosis with Desferrioxamine. Acta Haematol. 1963, 30, 65–87. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kleanthous, M.; Kontoghiorghe, C.N. The History of Deferiprone (L1) and the Paradigm of the Complete Treatment of Iron Overload in Thalassaemia. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020011. [Google Scholar] [CrossRef]
- Nisbet-Brown, E.; Olivieri, N.F.; Giardina, P.J.; Grady, R.W.; Neufeld, E.J.; Séchaud, R.; Krebs-Brown, A.J.; Anderson, J.R.; Alberti, D.; Sizer, K.C.; et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: A randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet 2003, 361, 1597–1602. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kontoghiorghe, C.N. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016, 10, 465–481. [Google Scholar] [CrossRef]
- Dézsi, L.; Vécsei, L. Clinical implications of irregular ADMET properties with levodopa and other antiparkinson’s drugs. Expert Opin. Drug Metab. Toxicol. 2014, 10, 409–424. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Clinical Review: Exjade (Deferasirox, ICL-670). 2005. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021882_s000_Exjade_BioPharmr.pdf (accessed on 6 August 2025).
- Exjade (Deferasirox) Tablets for Oral Suspension [Prescribing Information]; Novartis Pharmaceutical Corporation: East Hanover, NJ, USA, 2011. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021882s010lbl.pdf (accessed on 1 December 2015).
- Kontoghiorghes, G.J.; Kolnagou, A.; Peng, C.T.; Shah, S.V.; Aessopos, A. Safety issues of iron chelation therapy in patients with normal range iron stores including thalassaemia, neurodegenerative, renal and infectious diseases. Expert Opin. Drug Saf. 2010, 9, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. A record number of fatalities in many categories of patients treated with deferasirox: Loopholes in regulatory and marketing procedures undermine patient safety and misguide public funds? Expert Opin. Drug Saf. 2013, 12, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Chuang, G.T.; Tsai, I.J.; Tsau, Y.K.; Lu, M.Y. Transfusion-dependent thalassaemic patients with renal Fanconi syndrome due to deferasirox use. Nephrology 2015, 20, 931–935. [Google Scholar] [CrossRef]
- Al-Khabori, M.; Bhandari, S.; Al-Huneini, M.; Al-Farsi, K.; Panjwani, V.; Daar, S. Side effects of Deferasirox Iron Chelation in Patients with Beta Thalassemia Major or Intermedia. Oman Med. J. 2013, 28, 121–124. [Google Scholar] [CrossRef]
- Naderi, M.; Sadeghi-Bojd, S.; Valeshabad, A.K.; Jahantigh, A.; Alizadeh, S.; Dorgalaleh, A.; Tabibian, S.; Bamedi, T. A prospective study of tubular dysfunction in pediatric patients with Beta thalassemia major receiving deferasirox. Pediatr. Hematol. Oncol. 2013, 30, 748–754. [Google Scholar] [CrossRef]
- Dee, C.M.; Cheuk, D.K.; Ha, S.Y.; Chiang, A.K.; Chan, G.C. Incidence of deferasirox-associated renal tubular dysfunction in children and young adults with beta-thalassaemia. Br. J. Haematol. 2014, 167, 434–436. [Google Scholar] [CrossRef]
- Maximova, N.; Gregori, M.; Simeone, R.; Sonzogni, A.; Zanon, D.; Boz, G.; D’Antiga, L. Total body irradiation and iron chelation treatment are associated with pancreatic injury following pediatric hematopoietic stem cell transplantation. Oncotarget 2018, 9, 19543–19554. [Google Scholar] [CrossRef]
- Fucile, C.; Mattioli, F.; Marini, V.; Gregori, M.; Sonzogni, A.; Martelli, A.; Maximova, N. What is known about deferasirox chelation therapy in pediatric HSCT recipients: Two case reports of metabolic acidosis. Ther. Clin. Risk Manag. 2018, 14, 1649–1655. [Google Scholar] [CrossRef]
- Boelaert, J.R.; Fenves, A.Z.; Coburn, J.W. Deferoxamine therapy and mucormycosis in dialysis patients: Report of an international registry. Am. J. Kidney Dis. 1991, 18, 660–667. [Google Scholar] [CrossRef]
- Orton, R.B.; de Veber, L.L.; Sulh, H.M. Ocular and auditory toxicity of long-term, high-dose subcutaneous deferoxamine therapy. Can. J. Ophthalmol. 1985, 20, 153–156. [Google Scholar] [PubMed]
- Cases, A.; Kelly, J.; Sabater, F.; Torras, A.; Griño, M.C.; Lopez-Pedret, J.; Revert, L. Ocular and auditory toxicity in hemodialyzed patients receiving desferrioxamine. Nephron 1990, 56, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ioannides, A.S.; Panisello, J.M. Acute respiratory distress syndrome in children with acute iron poisoning: The role of intravenous desferrioxamine. Eur. J. Pediatr. 2000, 159, 158–159. [Google Scholar] [CrossRef]
- Cohen, A.R.; Galanello, R.; Piga, A.; De Sanctis, V.; Tricta, F. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood 2003, 102, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Ceci, A.; Baiardi, P.; Felisi, M.; Cappellini, M.D.; Carnelli, V.; De Sanctis, V.; Galanello, R.; Maggio, A.; Masera, G.; Piga, A.; et al. The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br. J. Haematol. 2002, 118, 330–336. [Google Scholar] [CrossRef]
- Galanello, R. Deferiprone in the treatment of transfusion-dependent thalassemia: A review and perspective. Ther. Clin. Risk Manag. 2007, 3, 795–805. [Google Scholar] [PubMed]
- Mazza, P.; Amurri, B.; Lazzari, G.; Masi, C.; Palazzo, G.; Spartera, M.A.; Giua, R.; Sebastio, A.M.; Suma, V.; De Marco, S.; et al. Oral iron chelating therapy. A single center interim report on deferiprone (L1) in thalassemia. Haematologica 1998, 83, 496–501. [Google Scholar] [PubMed]
- Calvaruso, G.; Vitrano, A.; Di Maggio, R.; Lai, E.; Colletta, G.; Quota, A.; Gerardi, C.; Rigoli, L.C.; Sacco, M.; Pitrolo, L.; et al. Deferiprone versus deferoxamine in thalassemia intermedia: Results from a 5-year long-term Italian multicenter randomized clinical trial. Am. J. Hematol. 2015, 90, 634–638. [Google Scholar] [CrossRef]
- Agarwal, M.B. L1 arthropathy syndrome. J. Assoc. Physicians India 1994, 42, 929–930. [Google Scholar] [PubMed]
- Kolnagou, A.; Kleanthous, M.; Kontoghiorghes, G.J. Benefits and Risks in Polypathology and Polypharmacotherapy Challenges in the Era of the Transition of Thalassaemia from a Fatal to a Chronic or Curable Disease. Front. Biosci. (Elite Ed.) 2022, 14, 18. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Vital Role Played by Deferiprone in the Transition of Thalassaemia from a Fatal to a Chronic Disease and Challenges in Its Repurposing for Use in Non-Iron-Loaded Diseases. Pharmaceuticals 2023, 16, 1016. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, G.; Swaak, A.J.; Kontoghiorghes, G.J.; van Eijk, H.G. Efficacy and safety of oral iron chelator L1 in anaemic rheumatoid arthritis patients. Lancet 1989, 2, 1398–1399. [Google Scholar] [CrossRef] [PubMed]
- van der Kraaij, A.M.; van Eijk, H.G.; Koster, J.F. Prevention of postischemic cardiac injury by the orally active iron chelator 1,2-dimethyl-3-hydroxy-4-pyridone (L1) and the antioxidant (+)-cyanidanol-3. Circulation 1989, 80, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, G.; Kontoghiorghes, G.J.; Van Eijk, H.G.; Swaak, A.J. Impaired erythropoietin responsiveness to the anaemia in rheumatoid arthritis. A possible inverse relationship with iron stores and effects of the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Clin. Exp. Rheumatol. 1991, 9, 35–40. [Google Scholar] [PubMed]
- Kontoghiorghes, G.J.; Barr, J.; Baillod, R.A. Studies of aluminium mobilization in renal dialysis patients using the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Arzneimittelforschung 1994, 44, 522–526. [Google Scholar] [PubMed]
- Matthews, A.J.; Vercellotti, G.M.; Menchaca, H.J.; Bloch, P.H.; Michalek, V.N.; Marker, P.H.; Murar, J.; Buchwald, H. Iron and atherosclerosis: Inhibition by the iron chelator deferiprone (L1). J. Surg. Res. 1997, 73, 35–40. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Neocleous, K.; Kolnagou, A. Benefits and risks of deferiprone in iron overload in Thalassaemia and other conditions: Comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003, 26, 553–584. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Barr, J.; Nortey, P.; Sheppard, L. Selection of a new generation of orally active alpha-ketohydroxypyridine iron chelators intended for use in the treatment of iron overload. Am. J. Hematol. 1993, 42, 340–349. [Google Scholar] [CrossRef]
- Olivieri, N.F.; Koren, G.; Matsui, D.; Liu, P.P.; Blendis, L.; Cameron, R.; McClelland, R.A.; Templeton, D.M. Reduction of tissue iron stores and normalization of serum ferritin during treatment with the oral iron chelator L1 in thalassemia intermedia. Blood 1992, 79, 2741–2748. [Google Scholar] [CrossRef]
- Kolnagou, A.; Kleanthous, M.; Kontoghiorghes, G.J. Reduction of body iron stores to normal range levels in thalassaemia by using a deferiprone/deferoxamine combination and their maintenance thereafter by deferiprone monotherapy. Eur. J. Haematol. 2010, 85, 430–438. [Google Scholar] [CrossRef]
- Farmaki, K.; Tzoumari, I.; Pappa, C.; Chouliaras, G.; Berdoukas, V. Normalisation of total body iron load with very intensive combined chelation reverses cardiac and endocrine complications of thalassaemia major. Br. J. Haematol. 2010, 148, 466–475. [Google Scholar] [CrossRef]
- Kolnagou, A.; Kontoghiorghe, C.N.; Kontoghiorghes, G.J. Prevention of Iron Overload and Long Term Maintenance of Normal Iron Stores in Thalassaemia Major Patients using Deferiprone or Deferiprone Deferoxamine Combination. Drug Res. 2017, 67, 404–411. [Google Scholar] [CrossRef]
- Pennell, D.J. T2* magnetic resonance and myocardial iron in thalassemia. Ann. N. Y. Acad. Sci. 2005, 1054, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Papakonstantinou, O.; Alexopoulou, E.; Economopoulos, N.; Benekos, O.; Kattamis, A.; Kostaridou, S.; Ladis, V.; Efstathopoulos, E.; Gouliamos, A.; Kelekis, N.L. Assessment of iron distribution between liver, spleen, pancreas, bone marrow, and myocardium by means of R2 relaxometry with MRI in patients with beta-thalassemia major. J. Magn. Reson. Imaging 2009, 29, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kolnagou, A.; Natsiopoulos, K.; Kleanthous, M.; Ioannou, A.; Kontoghiorghes, G.J. Liver iron and serum ferritin levels are misleading for estimating cardiac, pancreatic, splenic and total body iron load in thalassemia patients: Factors influencing the heterogenic distribution of excess storage iron in organs as identified by MRI T2*. Toxicol. Mech. Methods 2013, 23, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Iankova, V.; Karin, I.; Klopstock, T.; Schneider, S.A. Emerging Disease-Modifying Therapies in Neurodegeneration with Brain Iron Accumulation (NBIA) Disorders. Front. Neurol. 2021, 12, 629414. [Google Scholar] [CrossRef]
- Wallis, L.I.; Paley, M.N.; Graham, J.M.; Grünewald, R.A.; Wignall, E.L.; Joy, H.M.; Griffiths, P.D. MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J. Magn. Reson. Imaging 2008, 28, 1061–1067. [Google Scholar] [CrossRef]
- Rajapurkar, M.M.; Lele, S.S.; Malavade, T.S.; Kansara, M.R.; Hegde, U.N.; Gohel, K.D.; Gang, S.D.; Shah, S.V.; Mukhopadhyay, B.N. Serum catalytic Iron: A novel biomarker for coronary artery disease in patients on maintenance hemodialysis. Indian J. Nephrol. 2013, 23, 332–337. [Google Scholar] [CrossRef]
- Wilson, M.T.; Reeder, B.J. The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions. Mol. Asp. Med. 2022, 84, 101045. [Google Scholar] [CrossRef]
- Li, J.Y.; Liu, S.Q.; Yao, R.Q.; Tian, Y.P.; Yao, Y.M. A Novel Insight Into the Fate of Cardiomyocytes in Ischemia-Reperfusion Injury: From Iron Metabolism to Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 799499. [Google Scholar] [CrossRef] [PubMed]
- Boddaert, N.; Le Quan Sang, K.H.; Rötig, A.; Leroy-Willig, A.; Gallet, S.; Brunelle, F.; Sidi, D.; Thalabard, J.C.; Munnich, A.; Cabantchik, Z.I. Selective iron chelation in Friedreich ataxia: Biologic and clinical implications. Blood 2007, 110, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, G.; Cossu, G.; Balocco, M.; Marchese, R.; Murgia, D.; Melis, M.; Galanello, R.; Barella, S.; Matta, G.; Ruffinengo, U.; et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica 2011, 96, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Abbruzzese, G.; Matta, G.; Murgia, D.; Melis, M.; Ricchi, V.; Galanello, R.; Barella, S.; Origa, R.; Balocco, M.; et al. Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): Results from a four years follow-up. Park. Relat. Disord. 2014, 20, 651–654. [Google Scholar] [CrossRef]
- Zorzi, G.; Zibordi, F.; Chiapparini, L.; Bertini, E.; Russo, L.; Piga, A.; Longo, F.; Garavaglia, B.; Aquino, D.; Savoiardo, M.; et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: Results of a phase II pilot trial. Mov. Disord. 2011, 26, 1756–1759. [Google Scholar] [CrossRef]
- Forni, G.L.; Balocco, M.; Cremonesi, L.; Abbruzzese, G.; Parodi, R.C.; Marchese, R. Regression of symptoms after selective iron chelation therapy in a case of neurodegeneration with brain iron accumulation. Mov. Disord. 2008, 23, 904–907. [Google Scholar] [CrossRef]
- Rohani, M.; Razmeh, S.; Shahidi, G.A.; Alizadeh, E.; Orooji, M. A pilot trial of deferiprone in pantothenate kinase-associated neurodegeneration patients. Neurol. Int. 2018, 9, 7279. [Google Scholar] [CrossRef]
- Devos, D.; Cabantchik, Z.I.; Moreau, C.; Danel, V.; Mahoney-Sanchez, L.; Bouchaoui, H.; Gouel, F.; Rolland, A.S.; Duce, J.A.; Devedjian, J.C.; et al. Conservative iron chelation for neurodegenerative diseases such as Parkinson’s disease and amyotrophic lateral sclerosis. J. Neural Transm. 2020, 127, 189–203. [Google Scholar] [CrossRef]
- Devos, D.; Labreuche, J.; Rascol, O.; Corvol, J.C.; Duhamel, A.; Guyon Delannoy, P.; Poewe, W.; Compta, Y.; Pavese, N.; Růžička, E.; et al. Trial of Deferiprone in Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 2045–2055. [Google Scholar] [CrossRef]
- Ayton, S.; Barton, D.; Brew, B.; Brodtmann, A.; Clarnette, R.; Desmond, P.; Devos, D.; Ellis, K.A.; Fazlollahi, A.; Fradette, C.; et al. Deferiprone in Alzheimer Disease: A Randomized Clinical Trial. JAMA Neurol. 2025, 82, 11–18. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Drug Selection and Posology, Optimal Therapies and Risk/Benefit Assessment in Medicine: The Paradigm of Iron-Chelating Drugs. Int. J. Mol. Sci. 2023, 24, 16749. [Google Scholar] [CrossRef]
- Müller, T.; Möhr, J.D. Negative findings from trials with NLY01 or deferiprone for Parkinson’s disease. Lancet Neurol. 2024, 23, 558–559. [Google Scholar] [CrossRef]
- Aronson, J.K.; Heneghan, C.; Ferner, R.E. Drug shortages. Part 1. Definitions and harms. Br. J. Clin. Pharmacol. 2023, 89, 2950–2956. [Google Scholar] [CrossRef]
- Aronson, J.K.; Heneghan, C.; Ferner, R.E. Drug shortages. Part 2: Trends, causes and solutions. Br. J. Clin. Pharmacol. 2023, 89, 2957–2963. [Google Scholar] [CrossRef]
- Djordjevic, D.; McFadyen, A.; AAnderson, J. Ethical challenges and opportunities in the development and approval of novel therapeutics for rare diseases. J. Med. Access 2023, 7, 27550834231177507. [Google Scholar] [CrossRef] [PubMed]
- Rajapurkar, M.M.; Hegde, U.; Bhattacharya, A.; Alam, M.G.; Shah, S.V. Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol. Mech. Methods 2013, 23, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Saxena, D.; Spino, M.; Tricta, F.; Connelly, J.; Cracchiolo, B.M.; Hanauske, A.R.; D’Alliessi Gandolfi, D.; Mathews, M.B.; Karn, J.; Holland, B.; et al. Drug-Based Lead Discovery: The Novel Ablative Antiretroviral Profile of Deferiprone in HIV-1-Infected Cells and in HIV-Infected Treatment-Naive Subjects of a Double-Blind, Placebo-Controlled, Randomized Exploratory Trial. PLoS ONE 2016, 11, e0154842. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Ghosh, K.; Pathare, A.V.; Karnad, D. Deferiprone (L1) as an adjuvant therapy for Plasmodium falciparum malaria. Indian J. Med. Res. 2002, 115, 17–21. [Google Scholar] [PubMed]
- Filosa, A.; Vitrano, A.; Rigano, P.; Calvaruso, G.; Barone, R.; Capra, M.; Cuccia, L.; Gagliardotto, F.; Pitrolo, L.; Prossomariti, L.; et al. Long-term treatment with deferiprone enhances left ventricular ejection function when compared to deferoxamine in patients with thalassemia major. Blood Cells Mol. Dis. 2013, 51, 85–88. [Google Scholar] [CrossRef][Green Version]
- Pepe, A.; Meloni, A.; Capra, M.; Cianciulli, P.; Prossomariti, L.; Malaventura, C.; Putti, M.C.; Lippi, A.; Romeo, M.A.; Bisconte, M.G.; et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: Cardiac iron and function comparison determined by quantitative magnetic resonance imaging. Haematologica 2011, 96, 41–47. [Google Scholar] [CrossRef]
- Pepe, A.; Meloni, A.; Rossi, G.; Cuccia, L.; D’Ascola, G.D.; Santodirocco, M.; Cianciulli, P.; Caruso, V.; Romeo, M.A.; Filosa, A.; et al. Cardiac and hepatic iron and ejection fraction in thalassemia major: Multicentre prospective comparison of combined deferiprone and deferoxamine therapy against deferiprone or deferoxamine monotherapy. J. Cardiovasc. Magn. Reson. 2013, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Vitrano, A.; Lucania, G.; Capra, M.; Cuccia, L.; Gagliardotto, F.; Pitrolo, L.; Prossomariti, L.; Filosa, A.; Caruso, V.; et al. Long-term use of deferiprone significantly enhances left-ventricular ejection function in thalassemia major patients. Am. J. Hematol. 2012, 87, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Morales, N.P.; Rodrat, S.; Piromkraipak, P.; Yamanont, P.; Paiboonsukwong, K.; Fucharoen, S. Iron chelation therapy with deferiprone improves oxidative status and red blood cell quality and reduces redox-active iron in β-thalassemia/hemoglobin E patients. Biomed. Pharmacother. 2022, 145, 112381. [Google Scholar] [CrossRef] [PubMed]
- Tauchenová, L.; Křížová, B.; Kubánek, M.; Fraňková, S.; Melenovský, V.; Tintěra, J.; Kautznerová, D.; Malušková, J.; Jirsa, M.; Kautzner, J. Successful Treatment of Iron-Overload Cardiomyopathy in Hereditary Hemochromatosis with Deferoxamine and Deferiprone. Can. J. Cardiol. 2016, 32, 1574.e1–1574.e3. [Google Scholar] [CrossRef]
- Badat, M.; Kaya, B.; Telfer, P. Combination-therapy with concurrent deferoxamine and deferiprone is effective in treating resistant cardiac iron-loading in aceruloplasminaemia. Br. J. Haematol. 2015, 171, 430–432. [Google Scholar] [CrossRef]
- Elalfy, M.S.; Hamdy, M.; El-Beshlawy, A.; Ebeid, F.S.E.; Badr, M.; Kanter, J.; Inusa, B.; Adly, A.A.M.; Williams, S.; Kilinc, Y.; et al. Deferiprone for transfusional iron overload in sickle cell disease and other anemias: Open-label study of up to 3 years. Blood Adv. 2023, 7, 611–619. [Google Scholar] [CrossRef]
- Sudmantaitė, V.; Čelutkienė, J.; Glaveckaite, S.; Katkus, R. Difficult diagnosis of cardiac haemochromatosis: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–6. [Google Scholar] [CrossRef]
- Tanner, M.A.; Galanello, R.; Dessi, C.; Smith, G.C.; Westwood, M.A.; Agus, A.; Roughton, M.; Assomull, R.; Nair, S.V.; Walker, J.M.; et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation 2007, 115, 1876–1884. [Google Scholar] [CrossRef]
- Chan, S.; Lian, Q.; Chen, M.P.; Jiang, D.; Ho, J.T.K.; Cheung, Y.F.; Chan, G.C. Deferiprone inhibits iron overload-induced tissue factor bearing endothelial microparticle generation by inhibition oxidative stress induced mitochondrial injury, and apoptosis. Toxicol. Appl. Pharmacol. 2018, 338, 148–158. [Google Scholar] [CrossRef]
- Eybl, V.; Caisová, D.; Koutenský, J.; Kontoghiorghes, G.J. Influence of iron chelators, 1,2-dialkyl-3-hydroxypyridin-4-ones, on the lipid peroxidation and glutathione level in the liver of mice. Arch. Toxicol. Suppl. 1991, 14, 185–187. [Google Scholar] [CrossRef]
- Sadrzadeh, S.M.; Nanji, A.A.; Price, P.L. The oral iron chelator, 1,2-dimethyl-3-hydroxypyrid-4-one reduces hepatic-free iron, lipid peroxidation and fat accumulation in chronically ethanol-fed rats. J. Pharmacol. Exp. Ther. 1994, 269, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Jackson, M.J.; Lunec, J. In vitro screening of iron chelators using models of free radical damage. Free Radic. Res. Commun. 1986, 2, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghe, C.N.; Kolnagou, A.; Kontoghiorghes, G.J. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front. Biosci. (Elite Ed.) 2014, 19, 862–885. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Prospects for introducing deferiprone as potent pharmaceutical antioxidant. Front. Biosci. (Elite Ed.) 2009, 1, 161–178. [Google Scholar] [CrossRef]
- Yuan, Y.; Yang, X.; Zhao, Y.; Flores, J.J.; Huang, L.; Gu, L.; Li, R.; Zhang, X.; Zhu, S.; Dong, S.; et al. Mitochondrial ferritin upregulation by deferiprone reduced neuronal ferroptosis and improved neurological deficits via NDRG1/Yap pathway in a neonatal rat model of germinal matrix hemorrhage. J. Cereb. Blood Flow Metab. 2025, 45, 510–527. [Google Scholar] [CrossRef]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Maheshwari, S. Ferroptosis Signaling Pathways: Alzheimer’s Disease. Horm. Metab. Res. 2023, 55, 819–826. [Google Scholar] [CrossRef]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef]
- Yang, B.; Yang, K.; Chen, Y.; Li, Q.; Chen, J.; Li, S.; Wu, Y. Exposure of A2E to blue light promotes ferroptosis in the retinal pigment epithelium. Cell. Mol. Biol. Lett. 2025, 30, 22. [Google Scholar] [CrossRef]
- Yamada, K.; Tazaki, A.; Ushio-Watanabe, N.; Usui, Y.; Takeda, A.; Matsunaga, M.; Suzumura, A.; Shimizu, H.; Zheng, H.; Ariefta, N.R.; et al. Retinal ferroptosis as a critical mechanism for the induction of retinochoroiditis during ocular toxoplasmosis. Redox Biol. 2023, 67, 102890. [Google Scholar] [CrossRef]
- Ye, Z.; Yan, Y.; Jin, F.; Jiang, J.; Deng, C.; Wang, L.; Dong, K. Deferiprone protects photoreceptors by inhibiting ferroptosis after experimental retinal detachment. Exp. Eye Res. 2025, 250, 110156. [Google Scholar] [CrossRef]
- Rayatpour, A.; Foolad, F.; Heibatollahi, M.; Khajeh, K.; Javan, M. Ferroptosis inhibition by deferiprone, attenuates myelin damage and promotes neuroprotection in demyelinated optic nerve. Sci. Rep. 2022, 12, 19630. [Google Scholar] [CrossRef]
- Bao, L.; Zhao, Y.; Duan, S.; Wu, K.; Shan, R.; Liu, Y.; Yang, Y.; Chen, Q.; Song, C.; Li, W. Ferroptosis is involved in Staphylococcus aureus-induced mastitis through autophagy activation by endoplasmic reticulum stress. Int. Immunopharmacol. 2024, 140, 112818. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xie, L.; Xing, Y.; Liu, M.; Yang, J.; Gao, N.; Cai, Y. Iron-overload-induced ferroptosis in mouse cerebral toxoplasmosis promotes brain injury and could be inhibited by Deferiprone. PLoS Neglected Trop. Dis. 2023, 17, e0011607. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, N.; Aboulhoda, B.E.; Khalifa, M.M.; Morcos, G.N.; Morsy, S.A.A.G.; Alghamdi, M.A.; Khalifa, I.M.; Abd Algaleel, W.A. Amelioration of nephrotoxicity by targeting ferroptosis: Role of NCOA4, IREB2, and SLC7a11 signaling. Braz. J. Med. Biol. Res. 2024, 57, e13116. [Google Scholar] [CrossRef] [PubMed]
- Linjacki, S.; Wang, Y.; Baath, N.; Mantle, D.; Yang, G. H2S Protects from Rotenone-Induced Ferroptosis by Stabilizing Fe-S Clusters in Rat Cardiac Cells. Cells 2024, 13, 371. [Google Scholar] [CrossRef]
- El Hajj, S.; Canabady-Rochelle, L.; Fries-Raeth, I.; Gaucher, C. A Smooth Muscle Cell-Based Ferroptosis Model to Evaluate Iron-Chelating Molecules for Cardiovascular Disease Treatment. Curr. Issues Mol. Biol. 2024, 46, 1348–1359. [Google Scholar] [CrossRef]
- Tong, J.; Lan, X.T.; Zhang, Z.; Liu, Y.; Sun, D.Y.; Wang, X.J.; Ou-Yang, S.X.; Zhuang, C.L.; Shen, F.M.; Wang, P.; et al. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: Potential involvement of PANoptosis. Acta Pharmacol. Sin. 2023, 44, 1014–1028. [Google Scholar] [CrossRef]
- Peña-Montes, D.J.; Huerta-Cervantes, M.; Riveros-Rosas, H.; Manzo-Avalos, S.; Aguilera-Méndez, A.; Huerta, M.; Trujillo, X.; Cortés-Rojo, C.; Montoya-Pérez, R.; Salgado-Garciglia, R.; et al. Iron chelation mitigates mitochondrial dysfunction and oxidative stress by enhancing nrf2-mediated antioxidant responses in the renal cortex of a murine model of type 2 diabetes. Mitochondrion 2024, 78, 101937. [Google Scholar] [CrossRef]
- Seddiek, H.; Hanna, M.; Hamoud, A.E.M.; Elbaset, M.A.; Akabawy, A.M.A.; Kotb, M.Z.; Khalifa, M.M. Deferiprone ameliorates cisplatin induced peripheral neurotoxicity via ferritinophagy adjustment. Sci. Rep. 2025, 15, 4485. [Google Scholar] [CrossRef]
- Lian, G.; Huang, X.X.; Zeng, Y. Puerarin Induces Ferroptosis in Colorectal Cancer Cells via Triggering NCOA4 Upregulation. Nutr. Cancer 2023, 75, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, Y.; Gao, C.; Li, D.; Chen, L.; Dai, B.; Yang, H.; Han, L.; Deng, Q.; Bian, X. MDH2 Promotes Hepatocellular Carcinoma Growth Through Ferroptosis Evasion via Stabilizing GPX4. Int. J. Mol. Sci. 2024, 25, 11604. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Cho, Y.L.; Choi, Y.; Min, J.K.; Park, Y.J.; Yoon, S.J.; Kim, D.S.; Son, M.Y.; Chung, S.W.; Lee, H.; et al. Particulate matter induces ferroptosis by accumulating iron and dysregulating the antioxidant system. BMB Rep. 2023, 56, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kinsela, A.S.; Waite, T.D. Elucidation of alveolar macrophage cell response to coal dusts: Role of ferroptosis in pathogenesis of coal workers’ pneumoconiosis. Sci. Total Environ. 2022, 823, 153727. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, Y.; Zhang, S.; Chen, G. Mitochondria-Related Ferroptosis Drives Cognitive Deficits in Neonatal Mice Following Sevoflurane Administration. Front. Med. 2022, 9, 887062. [Google Scholar] [CrossRef]
- Nobuta, H.; Yang, N.; Ng, Y.H.; Marro, S.G.; Sabeur, K.; Chavali, M.; Stockley, J.H.; Killilea, D.W.; Walter, P.B.; Zhao, C.; et al. Oligodendrocyte Death in Pelizaeus-Merzbacher Disease Is Rescued by Iron Chelation. Cell Stem Cell 2019, 25, 531–541.e6. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Q.; Chen, K.; Ma, H.; Wang, X.; Tong, J.; Shen, Y.; Cui, H. Uncovering the role of ferroptosis in Bietti crystalline dystrophy and potential therapeutic strategies. Cell Commun. Signal. 2024, 22, 359. [Google Scholar] [CrossRef]
- Paraskevaidis, I.A.; Iliodromitis, E.K.; Vlahakos, D.; Tsiapras, D.P.; Nikolaidis, A.; Marathias, A.; Michalis, A.; Kremastinos, D.T. Deferoxamine infusion during coronary artery bypass grafting ameliorates lipid peroxidation and protects the myocardium against reperfusion injury: Immediate and long-term significance. Eur. Heart J. 2005, 26, 263–270. [Google Scholar] [CrossRef]
- Gajardo Cortez, A.I.J.; Lillo-Moya, J.; San-Martín-Martinez, D.; Pozo-Martinez, J.; Morales, P.; Prieto, J.C.; Aguayo, R.; Puentes, Á.; Ramos, C.; Silva, S.; et al. Safety and Pharmacokinetics of a Combined Antioxidant Therapy against Myocardial Reperfusion Injury: A Phase 1 Randomized Clinical Trial in Healthy Humans. Clin. Pharmacol. Drug Dev. 2024, 13, 1051–1060. [Google Scholar] [CrossRef]
- Lamichhane, A.; Sharma, S.; Bastola, B.; Chhusyabaga, B.; Shrestha, N.; Poudel, P. Unlocking the potential of deferoxamine: A systematic review on its efficacy and safety in alleviating myocardial ischemia-reperfusion injury in adult patients following cardiopulmonary bypass compared to standard care. Ther. Adv. Cardiovasc. Dis. 2024, 18, 17539447241277382. [Google Scholar] [CrossRef]
- Daglas, M.; Adlard, P.A. The Involvement of Iron in Traumatic Brain Injury and Neurodegenerative Disease. Front. Neurosci. 2018, 12, 981. [Google Scholar] [CrossRef]
- Selim, M.; Foster, L.D.; Moy, C.S.; Xi, G.; Hill, M.D.; Morgenstern, L.B.; Greenberg, S.M.; James, M.L.; Singh, V.; Clark, W.M.; et al. Deferoxamine mesylate in patients with intracerebral haemorrhage (i-DEF): A multicentre, randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2019, 18, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.; Robinson, L.; Yeatts, S.D.; Conwit, R.A.; Shehadah, A.; Lioutas, V.; Selim, M.; i-DEF Investigators. Effect of Deferoxamine on Trajectory of Recovery After Intracerebral Hemorrhage: A Post Hoc Analysis of the i-DEF Trial. Stroke 2022, 53, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Millán, M.; DeGregorio-Rocasolano, N.; Pérez de la Ossa, N.; Reverté, S.; Costa, J.; Giner, P.; Silva, Y.; Sobrino, T.; Rodríguez-Yáñez, M.; Nombela, F.; et al. Targeting Pro-Oxidant Iron with Deferoxamine as a Treatment for Ischemic Stroke: Safety and Optimal Dose Selection in a Randomized Clinical Trial. Antioxidants 2021, 10, 1270. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Effenberger, M.; Zoller, H. Iron metabolism in transplantation. Transpl. Int. 2014, 27, 1109–1117. [Google Scholar] [CrossRef]
- Yamada, N.; Karasawa, T.; Wakiya, T.; Sadatomo, A.; Ito, H.; Kamata, R.; Watanabe, S.; Komada, T.; Kimura, H.; Sanada, Y.; et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: Potential role of ferroptosis. Am. J. Transplant. 2020, 20, 1606–1618. [Google Scholar] [CrossRef]
- Arkadopoulos, N.; Nastos, C.; Kalimeris, K.; Economou, E.; Theodoraki, K.; Kouskouni, E.; Pafiti, A.; Kostopanagiotou, G.; Smyrniotis, V. Iron chelation for amelioration of liver ischemia-reperfusion injury. Hemoglobin 2010, 34, 265–277. [Google Scholar] [CrossRef]
- Shen, H.; Ma, Y.; Qiao, Y.; Zhang, C.; Chen, J.; Zhang, R. Application of Deferoxamine in Tissue Regeneration Attributed to Promoted Angiogenesis. Molecules 2024, 29, 2050. [Google Scholar] [CrossRef]
- Parker, J.B.; Griffin, M.F.; Downer, M.A.; Akras, D.; Berry, C.E.; Cotterell, A.C.; Gurtner, G.C.; Longaker, M.T.; Wan, D.C. Chelating the valley of death: Deferoxamine’s path from bench to wound clinic. Front. Med. 2023, 10, 1015711. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Wang, S.; Miao, R.; Zhong, J. Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic Approaches in Cardiovascular Diseases. Nutrients 2023, 15, 591. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.; Marcus, R.E.; Huehns, E.R. Desferrioxamine suppositories. Lancet 1983, 2, 454. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.S.; Ambruso, D.R.; Robinson, W.A.; Githens, J.H. Intranasal administration of deferoxamine to iron overloaded patients. Am. J. Med. Sci. 1989, 297, 280–284, Erratum in: Am. J. Med. Sci. 1990, 300, 32. [Google Scholar] [CrossRef] [PubMed]
- Farr, A.C.; Xiong, M.P. Challenges and Opportunities of Deferoxamine Delivery for Treatment of Alzheimer’s Disease, Parkinson’s Disease, and Intracerebral Hemorrhage. Mol. Pharm. 2021, 18, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef]
- Hanson, L.R.; Frey, W.H., 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef]
- Rao, I.Y.; Hanson, L.R.; Johnson, J.C.; Rosenbloom, M.H.; Frey, W.H., 2nd. Brain Glucose Hypometabolism and Iron Accumulation in Different Brain Regions in Alzheimer’s and Parkinson’s Diseases. Pharmaceuticals 2022, 15, 551. [Google Scholar] [CrossRef]
- McLachlan, D.R.C.; Dalton, A.J.; Kruck, T.P.; Bell, M.Y.; Smith, W.L.; Kalow, W.; Andrews, D.F. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 1991, 337, 1304–1308. [Google Scholar] [CrossRef]
- Donfrancesco, A.; Deb, G.; Dominici, C.; Angioni, A.; Caniglia, M.; De Sio, L.; Fidani, P.; Amici, A.; Helson, L. Deferoxamine, cyclophosphamide, etoposide, carboplatin, and thiotepa (D-CECaT): A new cytoreductive chelation-chemotherapy regimen in patients with advanced neuroblastoma. Am. J. Clin. Oncol. 1992, 15, 319–322. [Google Scholar] [CrossRef]
- Yamasaki, T.; Terai, S.; Sakaida, I. Deferoxamine for advanced hepatocellular carcinoma. N. Engl. J. Med. 2011, 365, 576–578. [Google Scholar] [CrossRef]
- Dreicer, R.; Kemp, J.D.; Stegink, L.D.; Cardillo, T.; Davis, C.S.; Forest, P.K.; See, W.A. A phase II trial of deferoxamine in patients with hormone-refractory metastatic prostate cancer. Cancer Investig. 1997, 15, 311–317. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.; Blake, D. Metal ions and oxygen radical reactions in human inflammatory joint disease. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 311, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Pambianchi, E.; Ferrara, F.; Pecorelli, A.; Benedusi, M.; Choudhary, H.; Therrien, J.P.; Valacchi, G. Deferoxamine Treatment Improves Antioxidant Cosmeceutical Formulation Protection against Cutaneous Diesel Engine Exhaust Exposure. Antioxidants 2021, 10, 1928. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Wang, R.; Lai, T.S.; Wu, K.D. Deferoxamine-related fatal nasal-orbital-cerebral mucormycosis. Kidney Int. 2006, 70, 1888. [Google Scholar] [CrossRef] [PubMed]
- Niihara, Y.; Ge, J.; Shalev, O.; Wu, H.; Tu, A.; Tanaka, K.R. Desferrioxamine decreases NAD redox potential of intact red blood cells: Evidence for desferrioxamine as an inducer of oxidant stress in red blood cells. BMC Clin. Pharmacol. 2002, 2, 8. [Google Scholar] [CrossRef]
- Jia, H.; Liu, X.; Cao, Y.; Niu, H.; Zhang, L.; Li, R.; Li, F.; Sun, D.; Shi, M.; Wa, L.; et al. Deferoxamine ameliorates neurological dysfunction by inhibiting ferroptosis and neuroinflammation after traumatic brain injury. Brain Res. 2023, 1812, 148383. [Google Scholar] [CrossRef]
- Wang, H.; Wu, S.; Li, Q.; Sun, H.; Wang, Y. Targeting Ferroptosis: Acteoside as a Neuroprotective Agent in Salsolinol-Induced Parkinson’s Disease Models. Front. Biosci. 2025, 30, 26679. [Google Scholar] [CrossRef]
- Zhu, D.; Liang, R.; Liu, Y.; Li, Z.; Cheng, L.; Ren, J.; Guo, Y.; Wang, M.; Chai, H.; Niu, Q.; et al. Deferoxamine ameliorated Al(mal)3-induced neuronal ferroptosis in adult rats by chelating brain iron to attenuate oxidative damage. Toxicol. Mech. Methods 2022, 32, 530–541. [Google Scholar] [CrossRef]
- Thorwald, M.A.; Godoy-Lugo, J.A.; Garcia, G.; Silva, J.; Kim, M.; Christensen, A.; Mack, W.J.; Head, E.; O’Day, P.A.; Benayoun, B.A.; et al. Iron-associated lipid peroxidation in Alzheimer’s disease is increased in lipid rafts with decreased ferroptosis suppressors, tested by chelation in mice. Alzheimer’s Dement. 2025, 21, e14541. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, W.; Kong, T.; Hua, T.; Ma, H.; Hu, Y.; Pan, S.; Ling, B.; Yang, M.; Cheng, C. Targeted temperature management alleviates post-resuscitation myocardial dysfunction by inhibiting ferroptosis. Cell Death Discov. 2025, 11, 71. [Google Scholar] [CrossRef]
- Kumfu, S.; Sripetchwandee, J.; Thonusin, C.; Sumneang, N.; Maneechote, C.; Arunsak, B.; Chunchai, T.; Oo, T.T.; Kongkaew, A.; Chattipakorn, S.C.; et al. Ferroptosis inhibitor improves cardiac function more effectively than inhibitors of apoptosis and necroptosis through cardiac mitochondrial protection in rats with iron-overloaded cardiomyopathy. Toxicol. Appl. Pharmacol. 2023, 479, 116727. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, Y.; Cai, X.; Huang, X.; Fu, W.; Wang, L.; Qiu, L.; Li, J.; Sun, L. Ferroptosis, a new target for treatment of renal injury and fibrosis in a 5/6 nephrectomy-induced CKD rat model. Cell Death Discov. 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, H.; Huang, Y.; Lu, J.; Du, H.; Wang, B. Mesenchymal stem cell-derived exosomal miR-26a induces ferroptosis, suppresses hepatic stellate cell activation, and ameliorates liver fibrosis by modulating SLC7A11. Open Med. 2024, 19, 20240945. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, Y.; Yan, B.; Zhang, J.; Wang, T.; Fang, Y.; Ye, Z.; Zhang, N.; Zhang, N.; Wu, Z.; et al. Intracellular Magnetic Hyperthermia Sensitizes Sorafenib to Orthotopic Hepatocellular Carcinoma Via Amplified Ferroptosis. ACS Nano 2024, 18, 29804–29819. [Google Scholar] [CrossRef]
- Zhang, S.; Chang, W.; Wu, H.; Wang, Y.H.; Gong, Y.W.; Zhao, Y.L.; Liu, S.H.; Wang, H.Z.; Svatek, R.S.; Rodriguez, R.; et al. Pan-cancer analysis of iron metabolic landscape across the Cancer Genome Atlas. J. Cell. Physiol. 2020, 235, 1013–1024. [Google Scholar] [CrossRef]
- Kumada, H.; Itoh, M.; Tohda, S. Effect of Ferroptosis Inducers and Inhibitors on Cell Proliferation in Acute Leukemia. Anticancer Res. 2024, 44, 1003–1010. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Xu, M.; Zheng, G.; Chen, J.; Li, S.; Cui, J.; Zhang, S. Implication of ferroptosis in hepatic toxicity upon single or combined exposure to polystyrene microplastics and cadmium. Environ. Pollut. 2023, 334, 122250. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Zhang, M.; OuYang, H.; Li, M.; Jia, D.; Wang, R.; Zhou, W.; Liu, H.; Hu, Y.; et al. Cadmium exposure during puberty damages testicular development and spermatogenesis via ferroptosis caused by intracellular iron overload and oxidative stress in mice. Environ. Pollut. 2023, 325, 121434. [Google Scholar] [CrossRef]
- Pan, L.; Yu, Z.; Xiang, W.X.; Sun, S.R.; Li, J.X.; Deng, Y.K.; Wang, M.C.; Zhong, J.X.; Huang, K.; Gao, P.S.; et al. Cigarette smoke-induced epithelial cell ferroptosis promotes neutrophilic inflammation in patients with nasal polyps. J. Allergy Clin. Immunol. 2025, 15, 1882–1897. [Google Scholar] [CrossRef]
- Walter, P.B.; Macklin, E.A.; Porter, J.; Evans, P.; Kwiatkowski, J.L.; Neufeld, E.J.; Coates, T.; Giardina, P.J.; Vichinsky, E.; Olivieri, N.; et al. Inflammation and oxidant-stress in beta-thalassemia patients treated with iron chelators deferasirox (ICL670) or deferoxamine: An ancillary study of the Novartis CICL670A0107 trial. Haematologica 2008, 93, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Murillo Ortiz, B.O.; Ramírez Emiliano, J.; Romero Vázquez, M.J.; Amador Medina, L.F.; Martínez Garza, S.; Ramos Rodríguez, E.M. Impact of iron chelation with deferasirox on telomere length and oxidative stress in hemodialysis patients: A randomized study. Nefrologia 2025, 45, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Ghoti, H.; Fibach, E.; Merkel, D.; Perez-Avraham, G.; Grisariu, S.; Rachmilewitz, E.A. Changes in parameters of oxidative stress and free iron biomarkers during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndromes. Haematologica 2010, 95, 1433–1434. [Google Scholar] [CrossRef] [PubMed]
- Neaimy, K.; Alsarraf, O.; Alkhyatt, M. Comparative Study of Oxidative Stress in Patients with Β-Thalassemia Major on Deferasirox Versus Deferoxamine Therapy. Georgian Med. News 2024, 348, 99–102. [Google Scholar] [PubMed]
- Belini Junior, E.; da Silva, D.G.; Torres Lde, S.; de Almeida, E.A.; Cancado, R.D.; Chiattone, C.; Bonini-Domingos, C.R. Oxidative stress and antioxidant capacity in sickle cell anaemia patients receiving different treatments and medications for different periods of time. Ann. Hematol. 2012, 91, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Menaker, N.; Halligan, K.; Shur, N.; Paige, J.; Hickling, M.; Nepo, A.; Weintraub, L. Acute Liver Failure During Deferasirox Chelation: A Toxicity Worth Considering. J. Pediatr. Hematol. Oncol. 2017, 39, 217–222. [Google Scholar] [CrossRef]
- Caranfa, J.; Carrera, W.; Marmalidou, A.; Desai, S.; Baumal, C. Multimodal imaging in deferasirox-mediated retinopathy. Eur. J. Ophthalmol. 2024, 34, NP33–NP37. [Google Scholar] [CrossRef]
- Scoglio, M.; Cappellini, M.D.; D’Angelo, E.; Bianchetti, M.G.; Lava, S.A.G.; Agostoni, C.; Milani, G.P. Kidney Tubular Damage Secondary to Deferasirox: Systematic Literature Review. Children 2021, 8, 1104. [Google Scholar] [CrossRef]
- Badeli, H.; Baghersalimi, A.; Eslami, S.; Saadat, F.; Rad, A.H.; Basavand, R.; Papkiadeh, S.R.; Darbandi, B.; Kooti, W.; Peluso, I. Early Kidney Damage Markers after Deferasirox Treatment in Patients with Thalassemia Major: A Case-Control Study. Oxidative Med. Cell. Longev. 2019, 2019, 5461617. [Google Scholar] [CrossRef]
- García-Fariña, B.; Rink, L.; Santarini, V.; Westkemper, M.; Dohna-Schwake, C.; Möhlendick, B. Case report: Acute liver failure during deferasirox therapy and the potential role of pharmacogenetics. Front. Pharmacol. 2024, 15, 1477755. [Google Scholar] [CrossRef]
- Delgado, Y.; Torres-Sanchez, A.; Perez, D.; Torres, G.; Estrada, S.; Ortiz Alvelo, N.; Vega, J.; Santos, L.; Torres, A.; Madera, B.A.; et al. Deferasirox’s Anti-Chemoresistance and Anti-Metastatic Effect on Non-Small Cell Lung Carcinoma. Biomedicines 2024, 12, 2272. [Google Scholar] [CrossRef]
- Mo, M.; Pan, L.; Deng, L.; Liang, M.; Xia, N.; Liang, Y. Iron Overload Induces Hepatic Ferroptosis and Insulin Resistance by Inhibiting the Jak2/stat3/slc7a11 Signaling Pathway. Cell Biochem. Biophys. 2024, 82, 2079–2094. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Wu, M.; Li, Z. Electroacupuncture improves cognitive function in APP/PS1 mice by inhibiting oxidative stress related hippocampal neuronal ferroptosis. Brain Res. 2024, 1831, 148744. [Google Scholar] [CrossRef]
- Hsu, W.Y.; Wang, L.T.; Lin, P.C.; Liao, Y.M.; Hsu, S.H.; Chiou, S.S. Deferasirox Causes Leukaemia Cell Death through Nrf2-Induced Ferroptosis. Antioxidants 2024, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, K.; Ikeda, M.; Miyamoto, H.D.; Furusawa, S.; Abe, K.; Watanabe, M.; Kanamura, T.; Fujita, S.; Nishimura, R.; Toyohara, T.; et al. Deferasirox Targeting Ferroptosis Synergistically Ameliorates Myocardial Ischemia Reperfusion Injury in Conjunction with Cyclosporine A. J. Am. Heart Assoc. 2024, 13, e031219. [Google Scholar] [CrossRef] [PubMed]
- Thapa, K.; Singh, T.G.; Kaur, A. Targeting ferroptosis in ischemia/reperfusion renal injury. Naunyn Schmiedeberg’s Arch. Pharmacol. 2022, 395, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ran, L.; Yang, Y.; Gao, X.; Peng, M.; Liu, S.; Sun, L.; Wan, J.; Wang, Y.; Yang, K.; et al. Deferasirox alleviates DSS-induced ulcerative colitis in mice by inhibiting ferroptosis and improving intestinal microbiota. Life Sci. 2023, 314, 121312. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Kichigina, L.A.; Selyutina, O.Y.; Polyakov, N.E.; Kontoghiorghes, G.J. Antioxidant Activity of Deferasirox and Its Metal Complexes in Model Systems of Oxidative Damage: Comparison with Deferiprone. Molecules 2021, 26, 5064. [Google Scholar] [CrossRef]
- Pippard, M.J.; Jackson, M.J.; Hoffman, K.; Petrou, M.; Modell, C.B. Iron chelation using subcutaneous infusions of diethylene triamine penta-acetic acid (DTPA). Scand. J. Haematol. 1986, 36, 466–472. [Google Scholar] [CrossRef]
- Born, T.; Kontoghiorghe, C.N.; Spyrou, A.; Kolnagou, A.; Kontoghiorghes, G.J. EDTA chelation reappraisal following new clinical trials and regular use in millions of patients: Review of preliminary findings and risk/benefit assessment. Toxicol. Mech. Methods 2013, 23, 11–17. [Google Scholar] [CrossRef]
- Fan, W.; Guo, M. Research progress on nanotoxicity and detoxification of cobalt in metal-based implants. Ann Med. 2025, 57, 2532120. [Google Scholar] [CrossRef]
- Dumala, N.; Chintala, S.; Mangalampalli, B.; Venkata, R.P. Elucidation of 28 day repeated oral dose induced genotoxicity potential of nickel (II) oxide nanoparticles in wistar albino rats. Regul Toxicol Pharmacol. 2025, 161, 105858. [Google Scholar] [CrossRef]
- Mantle, D.; Golomb, B.A. Coenzyme Q10 and Xenobiotic Metabolism: An Overview. Int J Mol Sci. 2025, 26, 5788. [Google Scholar] [CrossRef] [PubMed]
- Arruebarrena, M.A.; Hawe, C.T.; Lee, Y.M.; Branco, R.C. Mechanisms of Cadmium Neurotoxicity. Int. J. Mol. Sci. 2023, 24, 16558. [Google Scholar] [CrossRef] [PubMed]
- Wax, P.M. Current use of chelation in American health care. J. Med. Toxicol. 2013, 9, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Lamas, G.A.; Goertz, C.; Boineau, R.; Mark, D.B.; Rozema, T.; Nahin, R.L.; Lindblad, L.; Lewis, E.F.; Drisko, J.; Lee, K.L.; et al. Effect of disodium EDTAchelation regimen on cardiovascular events in patients with previous myocardial infarction: The TACTrandomized trial. JAMA 2013, 309, 1241–1250. [Google Scholar] [CrossRef]
- Ravalli, F.; Vela Parada, X.; Ujueta, F.; Pinotti, R.; Anstrom, K.J.; Lamas, G.A.; Navas-Acien, A. Chelation Therapy in Patients with Cardiovascular Disease: A Systematic Review. J. Am. Heart Assoc. 2022, 11, e024648. [Google Scholar] [CrossRef]
- Ujueta, F.; Lamas, G.A.; Anstrom, K.J.; Navas-Acien, A.; Boineau, R.; Rosenberg, Y.; Stylianou, M.; Jones, T.L.Z.; Joubert, B.R.; Yu, Q.; et al. Multivitamins After Myocardial Infarction in Patients with Diabetes: A Randomized Clinical Trial. JAMA Intern. Med. 2025, 185, 540–548. [Google Scholar] [CrossRef]
- Vezzoli, A.; Mrakic-Sposta, S.; Dellanoce, C.; Montorsi, M.; Vietti, D.; Ferrero, M.E. Chelation Therapy Associated with Antioxidant Supplementation Can Decrease Oxidative Stress and Inflammation in Multiple Sclerosis: Preliminary Results. Antioxidants 2023, 12, 1338. [Google Scholar] [CrossRef]
- Fulgenzi, A.; Vietti, D.; Ferrero, M.E. EDTA Chelation Therapy in the Treatment of Neurodegenerative Diseases: An Update. Biomedicines 2020, 8, 269. [Google Scholar] [CrossRef]
- Baxter, A.J.; Krenzelok, E.P. Pediatric fatality secondary to EDTA chelation. Clin. Toxicol. 2008, 46, 1083–1084. [Google Scholar] [CrossRef]
- Discalzi, G.; Pira, E.; Herrero Hernandez, E.; Valentini, C.; Turbiglio, M.; Meliga, F. Occupational Mn parkinsonism: Magneticresonance imaging and clinical patterns following CaNa2-EDTA chelation. Neurotoxicology 2000, 21, 863–866. [Google Scholar]
- Brown, M.J.; Willis, T.; Omalu, B.; Leiker, R. Deaths resulting from hypocalcemia after administration of edetate disodium: 2003-2005. Pediatrics 2006, 118, e534–e536. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.W.; Kori, S.; Thomas, J.D. Adverse effects in 5 patients receiving EDTA at an outpatient chelation clinic. Vet. Hum. Toxicol. 2002, 44, 274–276. [Google Scholar] [PubMed]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Roussel, A.M.; Hininger-Favier, I.; Waters, R.S.; Osman, M.; Fernholz, K.; Anderson, R.A. EDTA chelation therapy, withoutadded vitamin C, decreases oxidative DNA damage and lipid peroxidation. Altern. Med. Rev. 2009, 14, 56–61. [Google Scholar] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef]
- Bailey, G.P.; Wood, D.M.; Archer, J.R.; Rab, E.; Flanagan, R.J.; Dargan, P.I. An assessment of the variation in the concentration of acetylcysteine in infusions for the treatment of paracetamol overdose. Br. J. Clin. Pharmacol. 2017, 83, 393–399. [Google Scholar] [CrossRef]
- Clark, R.S.B.; Empey, P.E.; Bayır, H.; Rosario, B.L.; Poloyac, S.M.; Kochanek, P.M.; Nolin, T.D.; Au, A.K.; Horvat, C.M.; Wisniewski, S.R.; et al. Phase I randomized clinical trial of N-acetylcysteine in combination with an adjuvant probenecid for treatment of severe traumatic brain injury in children. PLoS ONE 2017, 12, e0180280. [Google Scholar] [CrossRef]
- Conus, P.; Seidman, L.J.; Fournier, M.; Xin, L.; Cleusix, M.; Baumann, P.S.; Ferrari, C.; Cousins, A.; Alameda, L.; Gholam-Rezaee, M.; et al. N-acetylcysteine in a Double-Blind Randomized Placebo-Controlled Trial: Toward Biomarker-Guided Treatment in Early Psychosis. Schizophr. Bull. 2018, 44, 317–327. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, S.; Li, C. Effects of N-acetylcysteine treatment in acute respiratory distress syndrome: A meta-analysis. Exp. Ther. Med. 2017, 14, 2863–2868. [Google Scholar] [CrossRef]
- Tenório, M.B.; Ferreira, R.C.; Moura, F.A.; Bueno, N.; Goulart, M.; Oliveira, A. Oral antioxidant therapy for prevention and treatment of preeclampsia: Meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Modarresi, A.; Ziaie, S.; Salamzadeh, J.; Sahraei, Z.; Nafar, M.; Panahi, Y.; Parvin, M.; Einollahi, B. Study of The Effects of N-Acetylcysteine on Oxidative Stress Status of Patients on Maintenance-Hemodialysis Undergoing Cadaveric Kidney Transplantation. Iran. J. Pharm. Res. 2017, 16, 1631–1638. [Google Scholar] [PubMed]
- Wallis, R.S.; Sabi, I.; Lalashowi, J.; Bakuli, A.; Mapamba, D.; Olomi, W.; Siyame, E.; Ngaraguza, B.; Chimbe, O.; Charalambous, S.; et al. Adjunctive N-Acetylcysteine and Lung Function in Pulmonary Tuberculosis. NEJM Evid. 2024, 3, EVIDoa2300332. [Google Scholar] [CrossRef] [PubMed]
- Komakula, S.; Bhatia, R.; Sahib, A.; Upadhyay, A.; S, L.J.; Garg, A.; Y, V.V.; Pandit, A.K.; Vibha, D.; Singh, M.B.; et al. Safety and efficacy of N-acetylcysteine (NAC) as an adjunct to standard treatment in patients with acute ischemic stroke: A randomized controlled pilot trial (NACTLYS). Sci. Rep. 2024, 14, 1103. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Z.; Wang, T.; Yu, M.; Li, X. Efficacy and acceptability of adjunctive n-acetylcysteine for psychotic disorders: Systematic review and meta-analysis. Hum. Psychopharmacol. 2024, 39, e2880. [Google Scholar] [CrossRef]
- Babu Balagopal, P.; Kohli, R.; Uppal, V.; Averill, L.; Shah, C.; McGoogan, K.; Di Guglielmo, M.; Goran, M.; Hossain, M.J. Effect of N-acetyl cysteine in children with metabolic dysfunction-associated steatotic liver disease-A pilot study. J. Pediatr. Gastroenterol. Nutr. 2024, 79, 652–660. [Google Scholar] [CrossRef]
- Soleimani, A.; Habibi, M.R.; Hasanzadeh Kiabi, F.; Alipour, A.; Habibi, V.; Azizi, S.; Zeydi, A.E.; Sohrabi, F.B. The effect of intravenous N-acetylcysteine on prevention of atrial fibrillation after coronary artery bypass graft surgery: A double-blind, randomised, placebo-controlled trial. Kardiol. Pol. 2018, 76, 99–106. [Google Scholar] [CrossRef]
- Sharafkhah, M.; Abdolrazaghnejad, A.; Zarinfar, N.; Mohammadbeigi, A.; Massoudifar, A.; Abaszadeh, S. Safety and efficacy of N-acetyl-cysteine for prophylaxis of ventilator-associated pneumonia: A randomized, double blind, placebo-controlled clinical trial. Med. Gas Res. 2018, 8, 19–23. [Google Scholar]
- Mantzarlis, K.; Tsolaki, V.; Zakynthinos, E. Role of Oxidative Stress and Mitochondrial Dysfunction in Sepsis and Potential Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 5985209. [Google Scholar] [CrossRef]
- Bavarsad Shahripour, R.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Wong, G.; Wu, S.Y.; Chen, W.M.; Hsu, P.J.; Chou, T.C.; Chiang, M.F.; Wu, M.S.; Lee, M.C.; Soong, R.S. Effects of N-acetylcysteine on hepatocellular carcinoma in chronic hepatitis C. Am. J. Cancer Res. 2024, 14, 3533–3544. [Google Scholar] [CrossRef] [PubMed]
- van Zandwijk, N.; Dalesio, O.; Pastorino, U.; de Vries, N.; van Tinteren, H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the EUropean Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J. Natl. Cancer Inst. 2000, 92, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, P.B.; Liu, T.; Florell, S.R.; Honeggar, M.; Leachman, S.A.; Boucher, K.M.; Grossman, D. A Phase II Randomized Placebo-Controlled Trial of Oral N-acetylcysteine for Protection of Melanocytic Nevi against UV-Induced Oxidative Stress In Vivo. Cancer Prev. Res. 2017, 10, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Posadzki, P.; Lee, M.S.; Onakpoya, I.; Lee, H.W.; Ko, B.S.; Ernst, E. Dietary supplements and prostate cancer: A systematic review of double-blind, placebo-controlled randomised clinical trials. Maturitas 2013, 75, 125–130. [Google Scholar] [CrossRef]
- Monti, D.; Sotgia, F.; Whitaker-Menezes, D.; Tuluc, M.; Birbe, R.; Berger, A.; Lazar, M.; Cotzia, P.; Draganova-Tacheva, R.; Lin, Z.; et al. Pilot study demonstrating metabolic and anti-proliferative effects of in vivo anti-oxidant supplementation with N-Acetylcysteine in Breast Cancer. Semin. Oncol. 2017, 44, 226–232. [Google Scholar] [CrossRef]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.J. Cisplatin overdose: Toxicities and management. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef]
- Cloos, J.; Bongers, V.; Lubsen, H.; Tobi, H.; Braakhuis, B.J.; Snow, G.B. Lack of effect of daily N-acetylcysteine supplementation on mutagen sensitivity. Cancer Epidemiol. Biomarkers Prev. 1996, 5, 941–944. [Google Scholar]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef]
- Orgel, E.; Knight, K.R.; Chi, Y.Y.; Malvar, J.; Rushing, T.; Mena, V.; Eisenberg, L.S.; Rassekh, S.R.; Ross, C.J.D.; Scott, E.N.; et al. Intravenous N-Acetylcysteine to Prevent Cisplatin-Induced Hearing Loss in Children: A Nonrandomized Controlled Phase I Trial. Clin. Cancer Res. 2023, 29, 2410–2418. [Google Scholar] [CrossRef]
- Zavala-Valencia, A.C.; Velasco-Hidalgo, L.; Martínez-Avalos, A.; Castillejos-López, M.; Torres-Espíndola, L.M. Effect of N-Acetylcysteine on Cisplatin Toxicity: A Review of the Literature. Biologics 2024, 18, 7–19. [Google Scholar] [CrossRef]
- Sins, J.W.R.; Fijnvandraat, K.; Rijneveld, A.W.; Boom, M.B.; Kerkhoffs, J.H.; van Meurs, A.H.; de Groot, M.R.; Heijboer, H.; Dresse, M.F.; Lê, P.Q.; et al. Effect of N-acetylcysteine on pain in daily life in patients with sickle cell disease: A randomised clinical trial. Br. J. Haematol. 2018, 182, 444–448. [Google Scholar] [CrossRef]
- Ozdemir, Z.C.; Koc, A.; Aycicek, A.; Kocyigit, A. N-Acetylcysteine supplementation reduces oxidative stress and DNA damage in children with β-thalassemia. Hemoglobin 2014, 38, 359–364. [Google Scholar] [CrossRef]
- Amen, F.; Machin, A.; Touriño, C.; Rodríguez, I.; Denicola, A.; Thomson, L. N-acetylcysteine improves the quality of red blood cells stored for transfusion. Arch. Biochem. Biophys. 2017, 621, 31–37. [Google Scholar] [CrossRef]
- Ghazaiean, M.; Aliasgharian, A.; Karami, H.; Ghasemi, M.M.; Darvishi-Khezri, H. Antioxidative effects of N-acetylcysteine in patients with β-thalassemia: A quick review on clinical trials. Health Sci. Rep. 2024, 7, e70096. [Google Scholar] [CrossRef]
- Bolarinwa, A.B.; Oduwole, O.; Okebe, J.; Ogbenna, A.A.; Otokiti, O.E.; Olatinwo, A.T. Antioxidant supplementation for sickle cell disease. Cochrane Database Syst. Rev. 2024, 5, CD013590. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, W.; Ito, J.; Henkelmann, B.; Xu, C.; Mishima, E.; Conrad, M. N-acetyl-l-cysteine averts ferroptosis by fostering glutathione peroxidase 4. Cell Chem. Biol. 2025, 32, 767–775.e5. [Google Scholar] [CrossRef]
- Hu, M.; Zhang, Y.; Ma, S.; Li, J.; Wang, X.; Liang, M.; Sferruzzi-Perri, A.N.; Wu, X.; Ma, H.; Brännström, M.; et al. Suppression of uterine and placental ferroptosis by N-acetylcysteine in a rat model of polycystic ovary syndrome. Mol. Hum. Reprod. 2021, 27, gaab067. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhu, Y.; Liu, W.; Balasubramanian, B.; Xu, X.; Yao, J.; Lei, X. Ferroptosis in Liver Disease: Natural Active Compounds and Therapeutic Implications. Antioxidants 2024, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Alin, L.; Chen, Y.; Brand, D.; Bourassa, M.W.; Dietrich, K.; Wilkinson, C.M.; Nadeau, C.A.; Kumar, A.; Perry, S.; et al. N-acetylcysteine targets 5 lipoxygenase-derived, toxic lipids and can synergize with prostaglandin E2 to inhibit ferroptosis and improve outcomes following hemorrhagic stroke in mice. Ann. Neurol. 2018, 84, 854–872. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wang, L.; Fu, K.; Cheng, S.; Wang, S.; Feng, Z.; Yu, S.; Yang, Z. N-Acetylcysteine Alleviates Necrotizing Enterocolitis by Depressing SESN2 Expression to Inhibit Ferroptosis in Intestinal Epithelial Cells. Inflammation 2025, 48, 464–482. [Google Scholar] [CrossRef]
- Zhou, D.; Yang, Y.; Chen, J.; Zhou, J.; He, J.; Liu, D.; Zhang, A.; Yuan, B.; Jiang, Y.; Xia, W.; et al. N-acetylcysteine Protects Against Myocardial Ischemia-Reperfusion Injury Through Anti-ferroptosis in Type 1 Diabetic Mice. Cardiovasc. Toxicol. 2024, 24, 481–498. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B. NAC, NAC, Knockin’ on Heaven’s door: Interpreting the mechanism of action of N-acetylcysteine in tumor and immune cells. Redox Biol. 2022, 57, 102497. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Z.; Sun, K.; Liu, Y.; Fang, Z.; Sun, S.; Li, C.; Wang, Z. Mitochondrial Regulation of Ferroptosis in Cancer Cells. Int. J. Biol. Sci. 2025, 21, 2179–2200. [Google Scholar] [CrossRef] [PubMed]
- Bebber, C.M.; Müller, F.; Prieto Clemente, L.; Weber, J.; von Karstedt, S. Ferroptosis in Cancer Cell Biology. Cancers 2020, 12, 164. [Google Scholar] [CrossRef]
- Jiang, Y.; Glandorff, C.; Sun, M. GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors. Antioxidants 2024, 13, 697. [Google Scholar] [CrossRef]
- Kruck, T.P.; Kalow, W.; McLachlan, D.R. Determination of desferoxamine and a major metabolite by high-performance liquid chromatography. Application to the treatment of aluminium-related disorders. J. Chromatogr. 1985, 341, 123–130. [Google Scholar] [CrossRef]
- Singh, S.; Hider, R.C.; Porter, J.B. Separation and identification of desferrioxamine and its iron chelating metabolites by high-performance liquid chromatography and fast atom bombardment mass spectrometry: Choice of complexing agent and application to biological fluids. Anal. Biochem. 1990, 187, 212–219. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Kontoghiorghe, C.N.; Mourouzidis, L.; Timoshnikov, V.A.; Polyakov, N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines 2020, 7, 45. [Google Scholar] [CrossRef]
- Levy, G. Clinical pharmacokinetics of salicylates: A re-assessment. Br. J. Clin. Pharmacol. 1980, 10 (Suppl. S2), 285S–290S. [Google Scholar] [CrossRef]
- Navarro, S.L.; Saracino, M.R.; Makar, K.W.; Thomas, S.S.; Li, L.; Zheng, Y.; Levy, L.; Schwarz, Y.; Bigler, J.; Potter, J.D.; et al. Determinants of aspirin metabolism in healthy men and women: Effects of dietary inducers of UDP-glucuronosyltransferases. Lifestyle Genom. 2011, 4, 110–118. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. The Puzzle of Aspirin and Iron Deficiency: The Vital Missing Link of the Iron-Chelating Metabolites. Int. J. Mol. Sci. 2024, 25, 5150. [Google Scholar] [CrossRef] [PubMed]
- Malisza, K.L.; Hasinoff, B.B. Doxorubicin reduces the iron(III) complexes of the hydrolysis products of the antioxidant cardioprotective agent dexrazoxane (ICRF-187) and produces hydroxyl radicals. Arch. Biochem. Biophys. 1995, 316, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Hasinoff, B.B. Chemistry of dexrazoxane and analogues. Semin. Oncol. 1998, 25 (4 Suppl. S10), 3–9. [Google Scholar] [PubMed]
- Jirkovský, E.; Jirkovská, A.; Bureš, J.; Chládek, J.; Lenčová, O.; Stariat, J.; Pokorná, Z.; Karabanovich, G.; Roh, J.; Brázdová, P.; et al. Pharmacokinetics of the Cardioprotective Drug Dexrazoxane and Its Active Metabolite ADR-925 with Focus on Cardiomyocytes and the Heart. J. Pharmacol. Exp. Ther. 2018, 364, 433–446. [Google Scholar] [CrossRef]
- Tolani, D.; Wilcox, J.; Shyam, S.; Bansal, N. Cardio-oncology for Pediatric and Adolescent/Young Adult Patients. Curr. Treat. Options Oncol. 2023, 24, 1052–1070. [Google Scholar] [CrossRef]
- Spalato Ceruso, M.; Napolitano, A.; Silletta, M.; Mazzocca, A.; Valeri, S.; Improta, L.; Santini, D.; Tonini, G.; Badalamenti, G.; Vincenzi, B. Use of Cardioprotective Dexrazoxane Is Associated with Increased Myelotoxicity in Anthracycline-Treated Soft-Tissue Sarcoma Patients. Chemotherapy 2019, 64, 105–109. [Google Scholar] [CrossRef]
- Jones, R.L. Utility of dexrazoxane for the reduction of anthracycline-induced cardiotoxicity. Expert Rev. Cardiovasc. Ther. 2008, 6, 1311–1317. [Google Scholar] [CrossRef]
- Barnabé, N.; Zastre, J.A.; Venkataram, S.; Hasinoff, B.B. Deferiprone protects against doxorubicin-induced myocyte cytotoxicity. Free Radic. Biol. Med. 2002, 33, 266–275. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. New Insights into Aspirin’s Anticancer Activity: The Predominant Role of Its Iron-Chelating Antioxidant Metabolites. Antioxidants 2025, 14, 29. [Google Scholar] [CrossRef]
- Guirguis-Blake, J.M.; Evans, C.V.; Perdue, L.A.; Bean, S.I.; Senger, C.A. Aspirin Use to Prevent Cardiovascular Disease and Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 327, 1585–1597. [Google Scholar] [CrossRef]
- Nafisi, S.; Støer, N.C.; Veierød, M.B.; Randel, K.R.; Hoff, G.; Löfling, L.; Bosetti, C.; Botteri, E. Low-Dose Aspirin and Prevention of Colorectal Cancer: Evidence From a Nationwide Registry-Based Cohort in Norway. Am. J. Gastroenterol. 2024, 119, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Sikavi, D.R.; Wang, K.; Ma, W.; Drew, D.A.; Ogino, S.; Giovannucci, E.L.; Cao, Y.; Song, M.; Nguyen, L.H.; Chan, A.T. Aspirin Use and Incidence of Colorectal Cancer According to Lifestyle Risk. JAMA Oncol. 2024, 10, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, K.E.; Hall, L.H.; Ziegler, L.; Foy, R.; Green, S.M.C.; MacKenzie, M.; Taylor, D.G.; Smith, S.G.; Aspirin for Cancer Prevention AsCaP Steering Committee. Acceptability of aspirin for cancer preventive therapy: A survey and qualitative study exploring the views of the UK general population. BMJ Open 2023, 13, e078703. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.R.; Han, D.D.; Wei, W.; Zeng, Q.; Rong, Z.X.; Bai, X.; Zhang, Y.P.; Wang, J.; Cai, X.T.; Rao, X.G.; et al. Regular use of aspirin and statins reduces the risk of cancer in individuals with systemic inflammatory diseases. Cancer Res. 2024, 84, 1889–1897. [Google Scholar] [CrossRef]
- Zheng, G.; Faber, M.T.; Wang, J.; Baandrup, L.; Hertzum-Larsen, R.; Sundström, K.; Kjær, S.K. Low-dose aspirin use and risk of ovarian cancer: A combined analysis from two nationwide studies in Denmark and Sweden. Br. J. Cancer 2024, 130, 1279–1285. [Google Scholar] [CrossRef]
- Sassano, M.; Taborelli, M.; Boccia, S.; Cadoni, G.; La Vecchia, C.; Garavello, W.; Lazarus, P.; Lee, Y.A.; Hashibe, M.; Boffetta, P. Aspirin intake and head and neck cancer: A pooled analysis within the INHANCE consortium. Head Neck 2024, 46, 926–935. [Google Scholar] [CrossRef]
- Yang, J.; Yamashita-Kanemaru, Y.; Morris, B.I.; Contursi, A.; Trajkovski, D.; Xu, J.; Patrascan, I.; Benson, J.; Evans, A.C.; Conti, A.G.; et al. Aspirin prevents metastasis by limiting platelet TXA2 suppression of T cell immunity. Nature 2025, 640, 1052–1061. [Google Scholar] [CrossRef]
- Chen, H.; Qi, Q.; Wu, N.; Wang, Y.; Feng, Q.; Jin, R.; Jiang, L. Aspirin promotes RSL3-induced ferroptosis by suppressing mTOR/SREBP-1/SCD1-mediated lipogenesis in PIK3CA-mutant colorectal cancer. Redox Biol. 2022, 55, 102426. [Google Scholar] [CrossRef]
- Wu, Z.; Li, D.; Tian, D.; Liu, X.; Wu, Z. Aspirin mediates protection from diabetic kidney disease by inducing ferroptosis inhibition. PLoS ONE 2022, 17, e0279010. [Google Scholar] [CrossRef]
- Wang, Y.F.; Feng, J.Y.; Zhao, L.N.; Zhao, M.; Wei, X.F.; Geng, Y.; Yuan, H.F.; Hou, C.Y.; Zhang, H.H.; Wang, G.W.; et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-κB p65-activated SLC7A11 transcription. Acta Pharmacol. Sin. 2023, 44, 1712–1724. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.; Xie, F.; Zhang, L.; Yan, H.; Huang, J.; Zhang, C.; Zhou, F.; Chen, J.; Zhang, L. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022, 42, 88–116. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, J. Targeting ferroptosis regulators by natural products in colorectal cancer. Front. Pharmacol. 2024, 15, 1374722. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Deferiprone and Iron-Maltol: Forty Years since Their Discovery and Insights into Their Drug Design, Development, Clinical Use and Future Prospects. Int. J. Mol. Sci. 2023, 24, 4970. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J.; Kolnagou, A.; Demetriou, T.; Neocleous, M.; Kontoghiorghe, C.N. New Era in the Treatment of Iron Deficiency Anaemia Using Trimaltol Iron and Other Lipophilic Iron Chelator Complexes: Historical Perspectives of Discovery and Future Applications. Int. J. Mol. Sci. 2021, 22, 5546. [Google Scholar] [CrossRef]
- Jung, M.; Mertens, C.; Tomat, E.; Brüne, B. Iron as a Central Player and Promising Target in Cancer Progression. Int. J. Mol. Sci. 2019, 20, 273. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J.; Agarwal, M.B.; Tondury, P.; Kersten, M.J.; Jaeger, M.; Vreugdenhil, G.; Vania, A.; Rahman, Y.E. Future of oral iron chelator deferiprone. (L1) ICOC committee. Lancet 1993, 341, 1479–1480. [Google Scholar] [CrossRef] [PubMed]
- Kolnagou, A.; Kontoghiorghes, G.J. Effective combination therapy of deferiprone and deferoxamine for the rapid clearance of excess cardiac IRON and the prevention of heart disease in thalassemia. The Protocol of the International Committee on Oral Chelators. Hemoglobin 2006, 30, 239–249. [Google Scholar] [CrossRef] [PubMed]
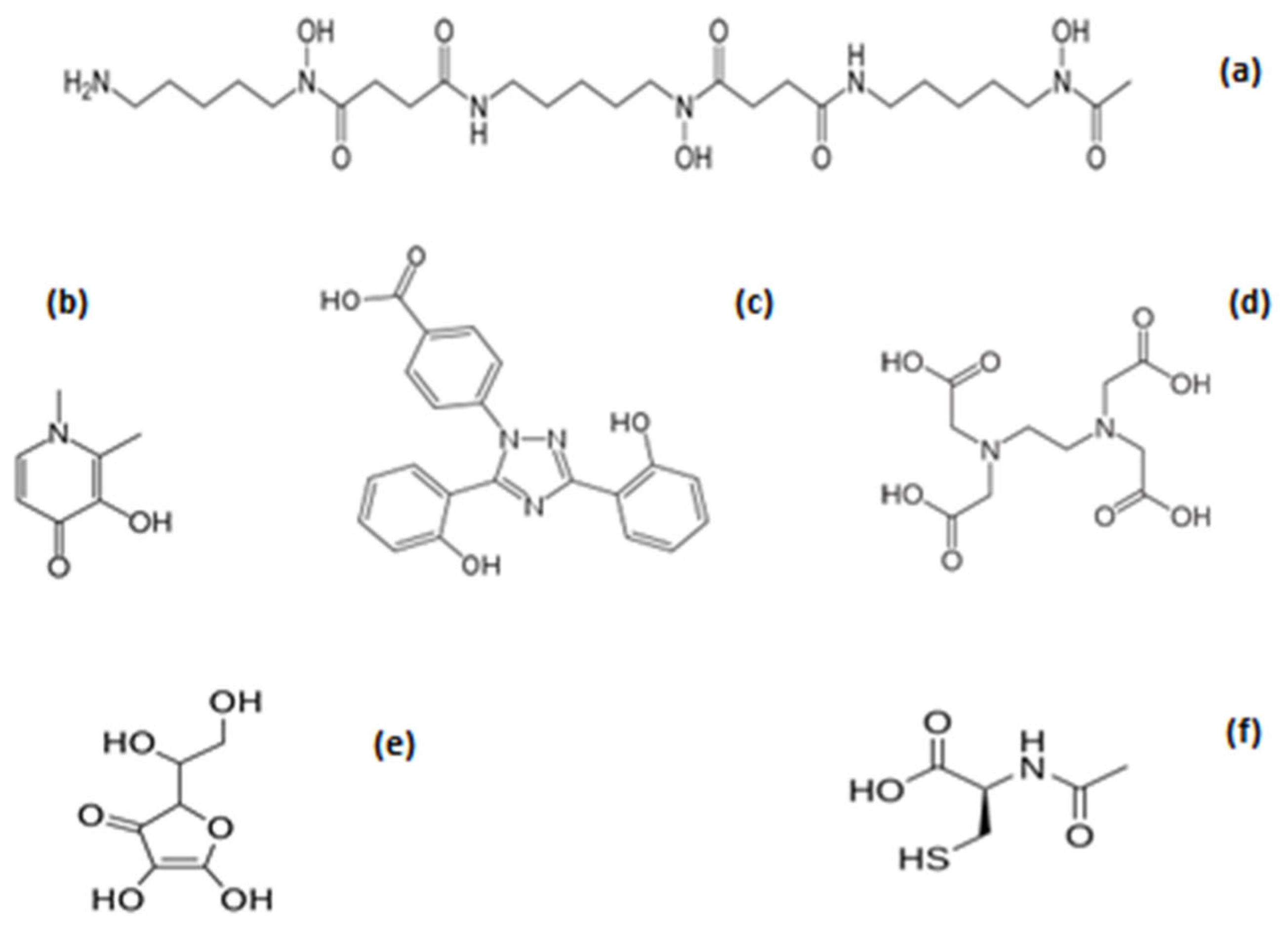
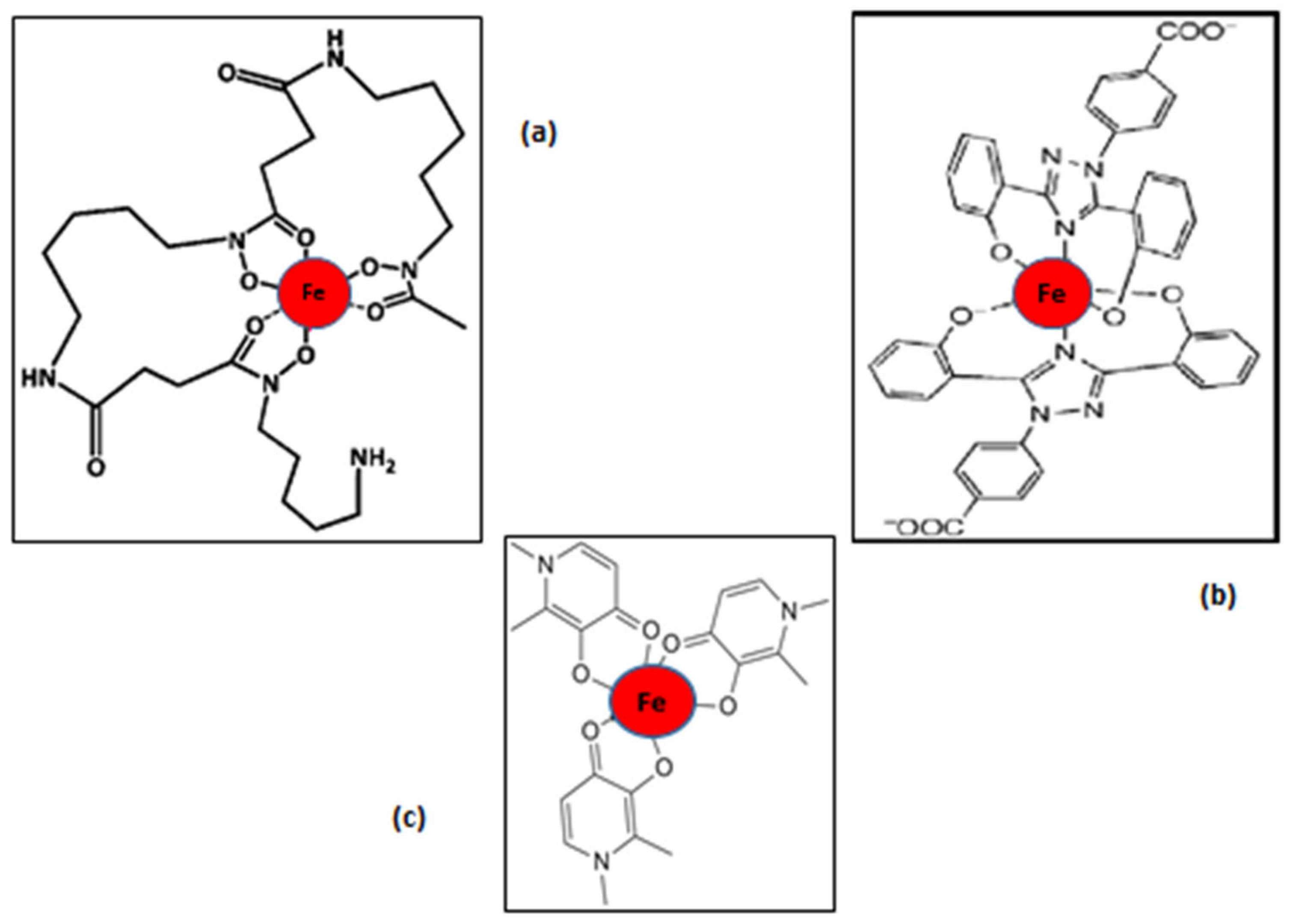

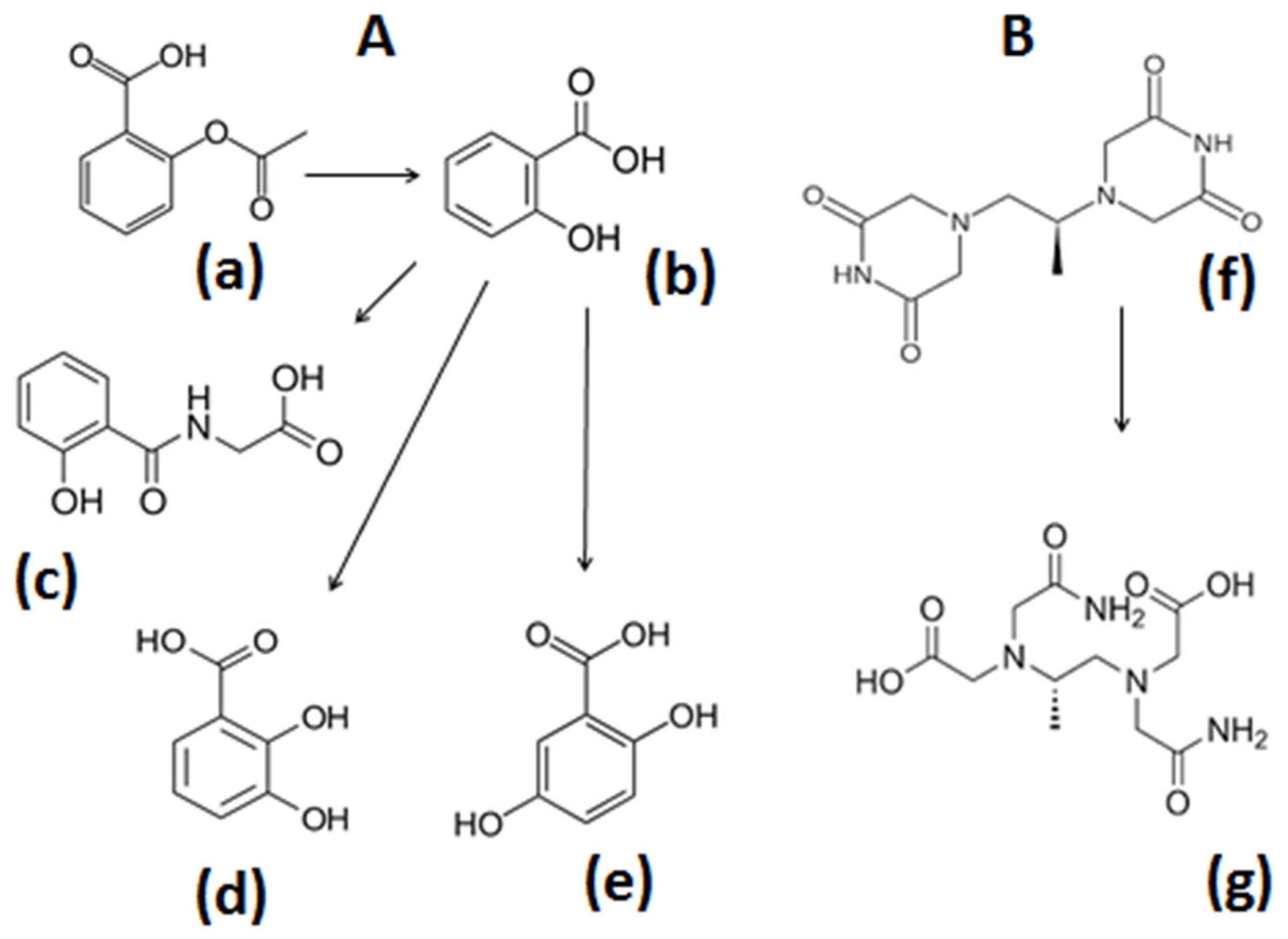
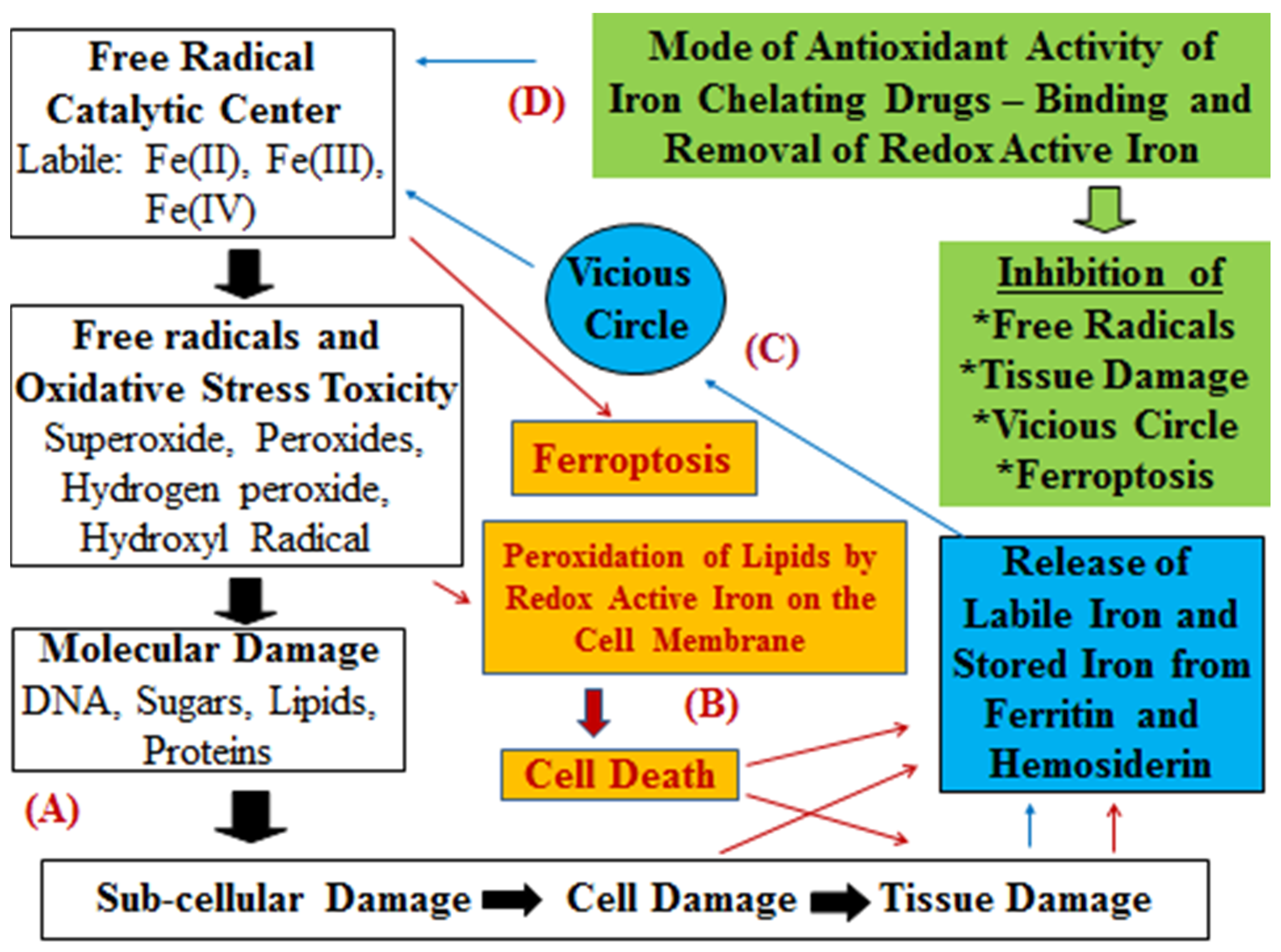
| CLINICAL IRON-RELATED EFFECTS OF THE IRON CHELATING DRUGS |
|
|
|
|
|
|
|
|
|
|
|
| PHARMACOKINETIC, METABOLIC, AND ANTIOXIDANT PROPERTIES OF THE CHELATING DRUGS |
|
|
|
|
|
|
|
|
|
|
| CHEMICAL AND PHYSICOCHEMICAL PROPERTIES OF THE CHELATING DRUGS |
|
|
|
|
|
|
|
|
| DRUG PROPERTIES, USES, AND AVAILABILITY OF DEFERIPRONE |
|
|
|
|
|
|
|
|
|
|
|
|
| SAFETY AND CLINICAL EFFECTS OF DEFERIPRONE IN IRON-LOADED THALASSAEMIA PATIENTS |
|
|
|
|
|
|
| CLINICAL EFFECTS AND POSOLOGY OF DEFERIPRONE IN NON-IRON-LOADED PATIENT CATEGORIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontoghiorghes, G.J. New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine. Antioxidants 2025, 14, 982. https://doi.org/10.3390/antiox14080982
Kontoghiorghes GJ. New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine. Antioxidants. 2025; 14(8):982. https://doi.org/10.3390/antiox14080982
Chicago/Turabian StyleKontoghiorghes, George J. 2025. "New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine" Antioxidants 14, no. 8: 982. https://doi.org/10.3390/antiox14080982
APA StyleKontoghiorghes, G. J. (2025). New Approaches and Strategies for the Repurposing of Iron Chelating/Antioxidant Drugs for Diseases of Free Radical Pathology in Medicine. Antioxidants, 14(8), 982. https://doi.org/10.3390/antiox14080982






