Association Between Oxidative–Inflammation Biomarkers and Incident Chronic Kidney Disease in People with High Cardiovascular Risk: A Nested Case–Control Study
Abstract
1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Evaluation of Kidney Function and Ascertainment of Incident CKD
2.3. Serum Inflammatory and Oxidative Stress Biomarker Assessment (Exposure Variables)
2.4. Assessment of Other Covariables
2.5. Statistical Analysis
3. Results
4. Discussion
5. Strengths and Limitations of the Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Kovesdy, C.; Langham, R.; Rosenberg, M.; Jha, V.; Zoccali, C. A single number for advocacy and communication—Worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019, 96, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Llisterri, J.L.; Micó-Pérez, R.M.; Velilla-Zancada, S.; Rodríguez-Roca, G.C.; Prieto-Díaz, M.Á.; Martín-Sánchez, V.; Barquilla, A.; Polo-García, J.; Segura-Fragoso, A.; Cinza-Sanjurjo, S.; et al. Prevalence of chronic kidney disease and associated factors in the Spanish population attended in primary care: Results of the IBERICAN study. Med. Clin. 2021, 156, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Gorostidi, M.; Sánchez-Martínez, M.; Ruilope, L.M.; Graciani, A.; de la Cruz, J.J.; Santamaría, R.; del Pino, M.D.; Guallar-Castillón, P.; de Álvaro, F.; Rodríguez-Artalejo, F.; et al. Prevalencia de enfermedad renal crónica en España: Impacto de la acumulación de factores de riesgo cardiovascular. Nefrología 2018, 38, 606–615. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Fernández López, P.; Romero Lerma, Á. Consideraciones sobre el consenso español multisociedad de manejo de la enfermedad renal crónica. Med. Fam. Semer. 2023, 49, 102017. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of Oxidative Stress in Metabolic Syndrome and Associated Diseases. Oxid. Med. Cell Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
- Lerman, L.O.; Lerman, A. The metabolic syndrome and early kidney disease: Another link in the chain? Rev. Esp. Cardiol. 2011, 64, 358–360. [Google Scholar] [CrossRef]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes. Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Ryter, S.W.; Choi, M.E. Oxidative stress and autophagy: Crucial modulators of kidney injury. Redox Biol. 2015, 4, 208–214. [Google Scholar] [CrossRef]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Piko, N.; Bevc, S.; Hojs, R.; Ekart, R. The Role of Oxidative Stress in Kidney Injury. Antioxidants 2023, 12, 1772. [Google Scholar] [CrossRef]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem. Res. Int. 2013, 2013, 358985. [Google Scholar] [CrossRef]
- Ishizaka, Y.; Yamakado, M.; Toda, A.; Tani, M.; Ishizaka, N. Relationship between estimated glomerular filtration rate, albuminuria, and oxidant status in the Japanese population. BMC Nephrol. 2013, 14, 191. [Google Scholar] [CrossRef]
- Cottone, S.; Mule, G.; Guarneri, M.; Palermo, A.; Lorito, M.C.; Riccobene, R.; Arsena, R.; Vaccaro, F.; Vadala, A.; Nardi, E.; et al. Endothelin-1 and F2-isoprostane relate to and predict renal dysfunction in hypertensive patients. Nephrol. Dial. Transplant. 2008, 24, 497–503. [Google Scholar] [CrossRef]
- Xu, G.; Luo, K.; Liu, H.; Huang, T.; Fang, X.; Tu, W. The progress of inflammation and oxidative stress in patients with chronic kidney disease. Ren. Fail. 2015, 37, 45–49. [Google Scholar] [CrossRef]
- Neelofar, K.; Arif, Z.; Arafat, M.Y.; Alam, K.; Ahmad, J. A study on correlation between oxidative stress parameters and inflammatory markers in type 2 diabetic patients with kidney dysfunction in north Indian population. J. Cell Biochem. 2019, 120, 4892–4902. [Google Scholar] [CrossRef] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef]
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between Albuminuria, Kidney Function, and Inflammatory Biomarker Profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Larson, M.G.; Guo, C.-Y.; Vasan, R.S.; Lipinska, I.; O’Donnell, C.J.; Kathiresan, S.; Meigs, J.B.; Keaney, J.F.; Rong, J.; et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol. Dial. Transplant. 2011, 26, 920–926. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Lee, H.; Fessler, M.B.; Qu, P.; Heymann, J.; Kopp, J.B. Macrophage polarization in innate immune responses contributing to pathogenesis of chronic kidney disease. BMC Nephrol. 2020, 21, 270. [Google Scholar] [CrossRef]
- Dousdampanis, P.; Aggeletopoulou, I.; Mouzaki, A. The role of M1/M2 macrophage polarization in the pathogenesis of obesity-related kidney disease and related pathologies. Front. Immunol. 2025, 15, 1534823. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yang, H.; Zhang, D.; Zhang, Y.; Liu, B.; Wang, Y.; Zhou, H.; Xu, Z.-X.; Wang, Y. The role of macrophages in fibrosis of chronic kidney disease. Biomed. Pharmacother. 2024, 177, 117079. [Google Scholar] [CrossRef]
- Brennan, E.; Kantharidis, P.; Cooper, M.E.; Godson, C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism and kidney function. Nat. Rev. Nephrol. 2021, 17, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.; Green, M.; Fainaru, M.; Smetana, S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999, 56, 1078–1083. [Google Scholar] [CrossRef]
- Shao, B.; Pennathur, S.; Pagani, I.; Oda, M.N.; Witztum, J.L.; Oram, J.F.; Heinecke, J.W. Modifying apolipoprotein A-I by malondialdehyde, but not by an array of other reactive carbonyls, blocks cholesterol efflux by the ABCA1 pathway. J. Biol. Chem. 2010, 285, 18473–18484. [Google Scholar] [CrossRef]
- Tucker, P.S.; Dalbo, V.J.; Han, T.; Kingsley, M.I. Clinical and research markers of oxidative stress in chronic kidney disease. Biomarkers 2013, 18, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Buil-Cosiales, P.; Corella, D.; Bulló, M.; Fitó, M.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Wärnberg, J.; López-Miranda, J.; et al. Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial. Int. J. Epidemiol. 2019, 48, 387–388o. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Díaz-López, A.; Ruiz-Canela, M.; Basora, J.; Fitó, M.; Corella, D.; Serra-Majem, L.; Wärnberg, J.; Romaguera, D.; Estruch, R.; et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: One-year results of the PREDIMED-Plus trial. Diabetes Care 2019, 42, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.L.P.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef]
- Rossi, M.; Campbell, K.L.; Johnson, D.W.; Stanton, T.; Vesey, D.A.; Coombes, J.S.; Weston, K.S.; Hawley, C.M.; McWhinney, B.C.; Ungerer, J.P.J.; et al. Protein-bound Uremic Toxins, Inflammation and Oxidative Stress: A Cross-sectional Study in Stage 3–4 Chronic Kidney Disease. Arch. Med. Res. 2014, 45, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Stevens, P.E.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- MM, B. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vart, P.; Gansevoort, R.T.; Coresh, J.; Reijneveld, S.A.; Bültmann, U. Socioeconomic Measures and CKD in the United States and The Netherlands. Clin. J. Am. Soc. Nephrol. 2013, 8, 1685–1693. [Google Scholar] [CrossRef]
- Schröder, H.; Zomeño, M.D.; Martínez-González, M.A.; Salas-Salvadó, J.; Corella, D.; Vioque, J.; Romaguera, D.; Martínez, J.A.; Tinahones, F.J.; Miranda, J.L.; et al. Validity of the energy-restricted Mediterranean Diet Adherence Screener. Clin. Nutr. 2021, 40, 4971–4979. [Google Scholar] [CrossRef]
- Schröder, H.; Covas, M.I.; Marrugat, J.; Vila, J.; Pena, A.; Alcántara, M.; Masiá, R. Use of a three-day estimated food record, a 72-h recall and a food-frequency questionnaire for dietary assessment in a Mediterranean Spanish population. Clin. Nutr. 2001, 20, 429–437. [Google Scholar] [CrossRef]
- Molina, L.; Sarmiento, M.; Peñafiel, J.; Donaire, D.; Garcia-Aymerich, J.; Gomez, M.; Ble, M.; Ruiz, S.; Frances, A.; Schröder, H.; et al. Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population. PLoS ONE 2017, 12, e0168148. [Google Scholar] [CrossRef]
- International Expert Committee Report on the Role of the A1C Assay in the Diagnosis of Diabetes. Diabetes Care 2009, 32, 1327–1334. [CrossRef]
- Khalid, U.B.; Haroon, Z.H.; Aamir, M.; Ain, Q.U.; Mansoor, K.; Jaffar, S.R. Comparison of Estimated Glomerular Filtration Rate with Both Serum Creatinine and Cystatin C (eGFRcr-cys) versus Single Analyte (eGFRcr or eGFRcys) Using CKD-EPI and MDRD Equations in Tertiary Care Hospital Settings. J. Coll. Physicians Surg. Pak. 2020, 30, 701–706. [Google Scholar] [PubMed]
- Monserrat-Mesquida, M.; Quetglas-Llabrés, M.; Capó, X.; Bouzas, C.; Mateos, D.; Pons, A.; Tur, J.A.; Sureda, A. Metabolic Syndrome Is Associated with Oxidative Stress and Proinflammatory State. Antioxidants 2020, 9, 236. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2014, 18, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Jialal, I. Chemerin levels in metabolic syndrome: A promising biomarker. Arch. Physiol. Biochem. 2023, 129, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. Message Transmission Between Adipocyte and Macrophage in Obesity. In Obesity and Lipotoxicity; Springer: Berlin/Heidelberg, Germany, 2024; pp. 273–295. [Google Scholar]
- Lin, L.; Tan, W.; Pan, X.; Tian, E.; Wu, Z.; Yang, J. Metabolic Syndrome-Related Kidney Injury: A Review and Update. Front. Endocrinol. 2022, 13, 904001. [Google Scholar] [CrossRef]
- Islamuddin, M.; Qin, X. Renal macrophages and NLRP3 inflammasomes in kidney diseases and therapeutics. Cell Death Discov. 2024, 10, 229. [Google Scholar] [CrossRef]
- Balakrishnan, V.S.; Schmid, C.H.; Jaber, B.L.; Natov, S.N.; King, A.J.; Pereira, B.J.G. Interleukin-1 Receptor Antagonist Synthesis by Peripheral Blood Mononuclear Cells. J. Am. Soc. Nephrol. 2000, 11, 2114–2121. [Google Scholar] [CrossRef]
- Batal, I.; De Serres, S.A.; Mfarrej, B.G.; Grafals, M.; Pinkus, G.S.; Kalra, A.; Weins, A.; Bijol, V.; Rennke, H.G.; Guleria, I.; et al. Glomerular Inflammation Correlates With Endothelial Injury and With IL-6 and IL-1β Secretion in the Peripheral Blood. Transplantation 2014, 97, 1034–1042. [Google Scholar] [CrossRef]
- Shankar, A.; Sun, L.; Klein, B.E.K.; Lee, K.E.; Muntner, P.; Nieto Javier, F.; Tsai, M.Y.; Cruickshanks, K.J.; Schubert, C.R.; Brazy, P.C.; et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: A population-based cohort study. Kidney Int. 2011, 80, 1231–1238. [Google Scholar] [CrossRef]
- Kawamoto, R.; Akase, T.; Ninomiya, D.; Kumagi, T.; Kikuchi, A. Metabolic syndrome is a predictor of decreased renal function among community-dwelling middle-aged and elderly Japanese. Int. Urol. Nephrol. 2019, 51, 2285–2294. [Google Scholar] [CrossRef]
- Foster, M.C.; Hwang, S.-J.; Larson, M.G.; Lichtman, J.H.; Parikh, N.I.; Vasan, R.S.; Levy, D.; Fox, C.S. Overweight, Obesity, and the Development of Stage 3 CKD: The Framingham Heart Study. Am. J. Kidney Dis. 2008, 52, 39–48. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Hwang, J.Y.; Kim, E.H.; Park, J.-Y.; Kim, H.-K.; Lee, W.J. The risk of chronic kidney disease in a metabolically healthy obese population. Kidney Int. 2015, 88, 843–850. [Google Scholar] [CrossRef]
- Moeinzadeh, F.; Rouhani, M.H.; Seirafian, S.; Vahdat, S.; Mortazavi, M.; Clark, C.C.T.; Shahdadian, F. Metabolic health status and renal disorders: A cross-sectional study. Sci. Rep. 2023, 13, 20794. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, F.-H.; Chang, C.-J.; Wang, R.-S.; Yang, Y.-C.; Chang, Y.-F.; Wu, J.-S. Metabolic abnormalities, but not obesity per se, associated with chronic kidney disease in a Taiwanese population. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cai, R.; Sun, J.; Dong, X.; Huang, R.; Tian, S.; Wang, S. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: A systematic review and meta-analysis. Endocrine 2017, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Schold, J.D.; Kirwan, J.P.; Arrigain, S.; Jolly, S.E.; Poggio, E.D.; Beddhu, S.; Nally, J.V. Metabolic Syndrome, ESRD, and Death in CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Hsiung, J.-T.; Arif, Y.; Hashemi, L.; Sumida, K.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Streja, E. Triglycerides and Renal Outcomes According to Albuminuria and in Consideration of Other Metabolic Syndrome Components in Diabetic US Veterans. Am. J. Nephrol. 2023, 54, 14–24. [Google Scholar] [CrossRef]
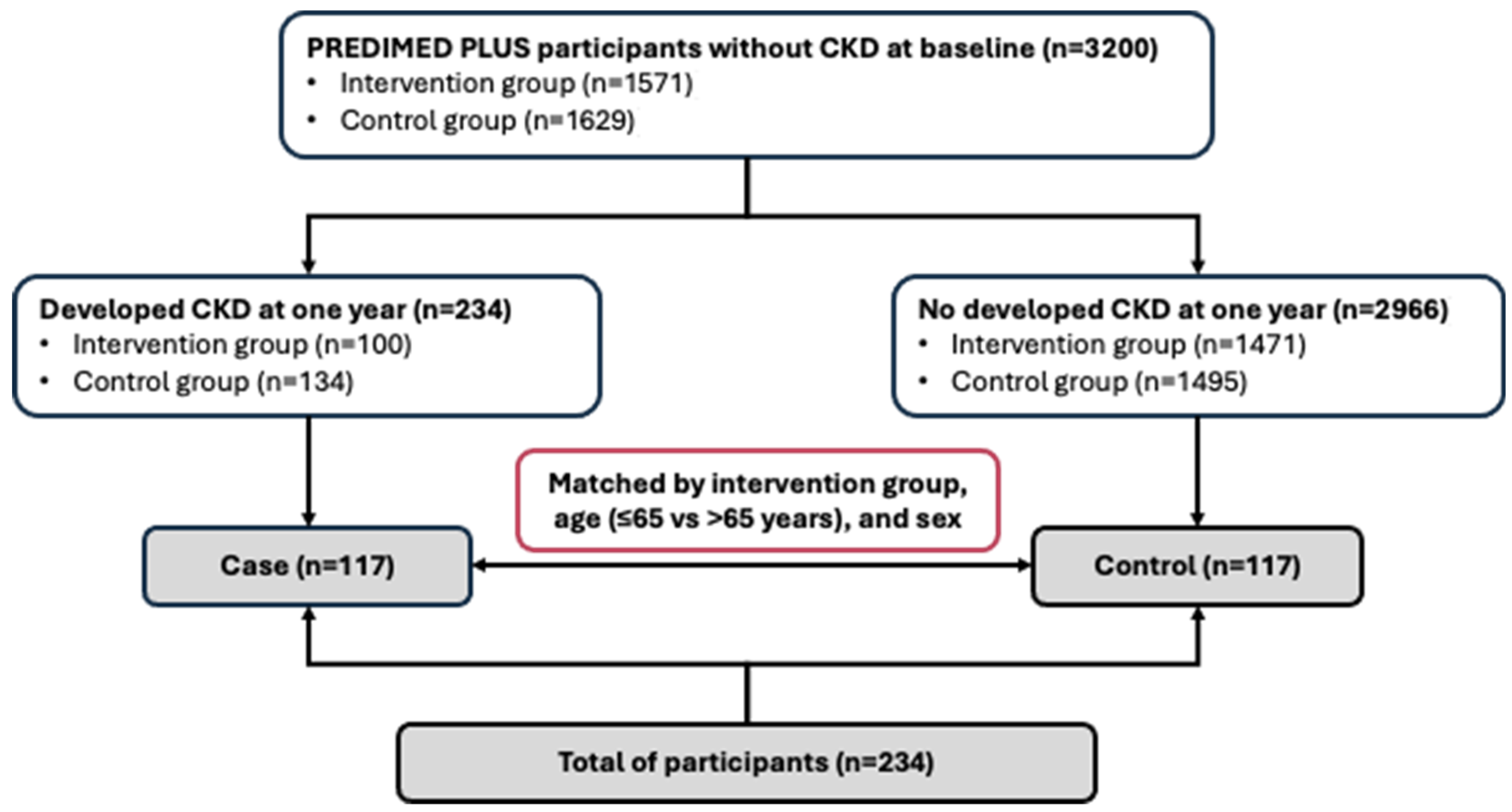

| General Characteristics | Matched Control Subjects (n = 117) | Incident CKD (Case Subjects) (n = 117) | p-Value * | |
|---|---|---|---|---|
| Age, mean (SD), years | 65.7 (4.85) | 66.1 (4.57) | 0.570 | |
| Women, no. (%) | 58 (49.6) | 58 (49.6) | 1.000 | |
| Intervention group, no. (%) | 1.000 | |||
| Lifestyle intervention | 53 (45.3) | 53 (45.3) | ||
| Control | 64 (54.7) | 64 (54.7) | ||
| BMI, mean (SD), kg/m2 | 32.0 (3.51) | 33.2 (3.32) | 0.006 | |
| Obesity (BMI ≥ 30 kg/m2), no. (%) | 79 (67.5) | 91 (77.8) | 0.078 | |
| Education level, no. (%) | 0.043 | |||
| Primary education | 48 (41.0) | 67 (57.3) | ||
| Secondary/academic or graduate | 69 (59.0) | 50 (42.7) | ||
| Smoking status, no. (%) | 0.913 | |||
| Never smoked | 53 (45.3) | 53 (45.3) | ||
| Former smoker | 49 (41.9) | 51 (43.6) | ||
| Current smoker | 15 (12.8) | 13 (11.1) | ||
| Physical activity, mean (SD), METs/min/day | 376.5 (281.1) | 372.2 (355.6) | 0.274 | |
| erMedDiet score, mean (SD), points | 8.77 (2.81) | 8.52 (2.86) | 0.411 | |
| Energy intake, mean (SD), kcal/day | 2462 (634) | 2326 (609) | 0.107 | |
| Systolic blood pressure, mean (SD), mmHg | 138.9 (15.7) | 141.7 (17.3) | 0.159 | |
| Diastolic blood pressure, mean (SD), mmHg | 82.1 (10.7) | 80.7 (9.09) | 0.278 | |
| Hypertension, no. (%) | 96 (82.1) | 97 (82.9) | 0.863 | |
| Type 2 diabetes, no. (%) a | 23 (19.7) | 59 (50.4) | <0.001 | |
| Medication use, no. (%) | ||||
| Lipid-lowering drugs | 62 (53.0) | 67 (57.3) | 0.511 | |
| Oral blood glucose-lowering drugs | 22 (18.8) | 48 (41.0) | <0.001 | |
| Insulin treatment | 3 (2.6) | 8 (6.8) | 0.123 | |
| Antihypertensive drugs | 84 (71.8) | 94 (80.3) | 0.125 | |
| ARBs | 35 (29.9) | 45 (38.5) | 0.168 | |
| ACEis | 36 (30.8) | 33 (28.2) | 0.677 | |
| Blood parameters | ||||
| Glucose, mean (SD), mg/dL | 106.3 (18.4) | 120.6 (34.8) | 0.002 | |
| HbA1c, mean (SD), % | 5.91 (0.54) | 6.44 (1.05) | <0.001 | |
| Triglycerides, mean (SD), mg/dL | 137.0 (64.2) | 162.8 (82.5) | 0.008 | |
| LDL-cholesterol, mean (SD), mg/dL | 119.2 (31.2) | 118.0 (32.3) | 0.765 | |
| Kidney function parameters | ||||
| Uric acid, mean (SD), mg/dL | 5.76 (1.31) | 5.86 (1.17) | 0.535 | |
| UACR, mean (SD), mg/g | 5.19 (3.37) | 12.2 (7.81) | <0.001 | |
| Serum Creatinine, mean (SD), mg/dL | 0.76 (0.12) | 0.81 (0.14) | 0.005 | |
| CyC, mean (SD), mg/L | 1.01 (0.13) | 1.09 (0.21) | 0.002 | |
| eGFR-SCr, mean (SD), mL/min/1.73 m2 | 89.9 (6.46) | 85.3 (10.2) | 0.003 | |
| eGFR-cyC, mean (SD), mL/min/1.73 m2 | 74.2 (12.9) | 68.5 (17.0) | 0.005 | |
| eGFR-SCr cyC, mean (SD), mL/min/1.73 m2 | 81.3 (9.8) | 76.4 (13.6) | 0.002 | |
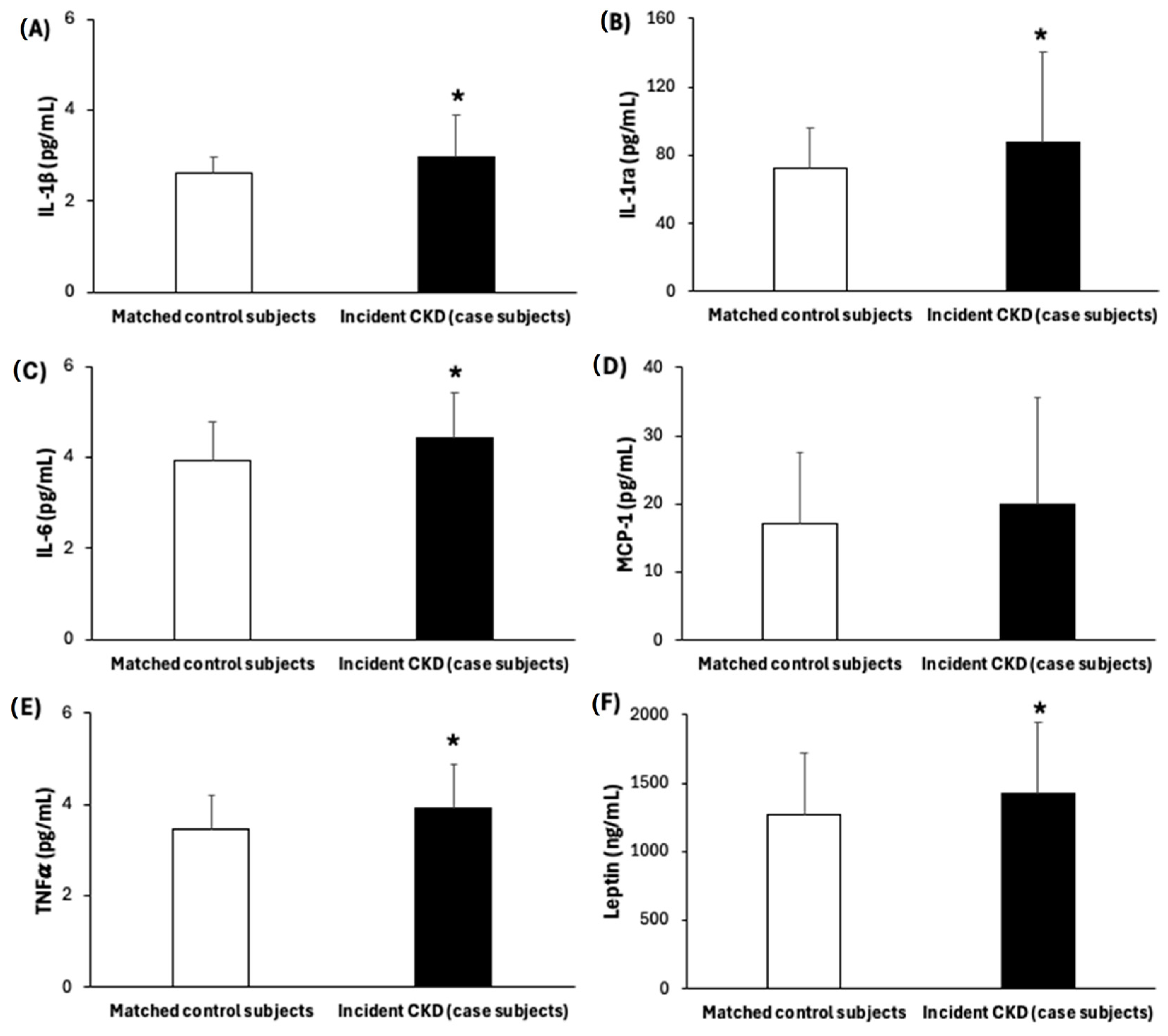
| Inflammatory Biomarkers | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDA, nM | Carbonyls, % | IL-1β, pg/mL | IL-1ra, pg/mL | IL-6, pg/mL | MCP1, pg/mL | TNFα, pg/mL | Leptin, ng/mL | |
| Kidney Function Markers | r | r | r | r | r | r | r | r |
| Bivariable analysis | ||||||||
| UACR, mg/g | 0.14 * | 0.04 | 0.21 ** | 0.03 | 0.10 | 0.10 | 0.20 ** | 0.10 |
| eGFR-SCr, mL/min/1.73 m2 | 0.00 | 0.02 | −0.09 | −0.06 | −0.11 | −0.12 | −0.03 | −0.18 ** |
| eGFR-cyC, mL/min/1.73 m2 | −0.01 | −0.08 | −0.14 * | −0.18 ** | −0.11 | −0.05 | −0.05 | −0.11 |
| eGFR-SCr cyC, mL/min/1.73 m2 | 0.00 | −0.03 | −0.11 | −0.17 * | −0.10 | −0.09 | −0.06 | −0.15 * |
| Multivariate-adjusted models * | ||||||||
| UACR, mg/g | 0.16 * | 0.01 | 0.20 * | 0.03 | 0.11 | 0.09 | 0.19 * | 0.09 |
| eGFR-SCr, mL/min/1.73 m2 | 0.02 | −0.01 | −0.07 | −0.06 | −0.07 | −0.09 | −0.06 | −0.17 * |
| eGFR-cyC, mL/min/1.73 m2 | 0.02 | −0.07 | −0.14 * | −0.14 * | −0.09 | −0.01 | −0.08 | −0.08 |
| eGFR-SCr cyC, mL/min/1.73 m2 | 0.04 | −0.03 | −0.12 | −0.12 | −0.07 | −0.06 | −0.11 | −0.12 |
| Tertile of Oxidative Stress and Inflammatory Biomarkers | ||||||
|---|---|---|---|---|---|---|
| Continuous (per 1-SD Increase) | 1st Tertile | 2nd Tertile OR (95% CI) | 3rd Tertile OR (95% CI) | p for Trend | ||
| MDA, nM | ≤2.77 | 2.77–3.73 | >3.73 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 31 (13.2)/41 (17.5) | 37 (15.8)/38 (16.2) | 49 (20.9)/38 (16.2) | ||
| Crude (matched a) model | 1.10 (0.85–1.41) | 1.00 ref | 1.29 (0.67–2.46) | 1.64 (0.89–3.02) | 0.121 | |
| Multivariable model † | 1.03 (0.53–2.02) | 1.00 ref | 1.70 (0.49–5.86) | 0.90 (0.24–3.44) | 0.646 | |
| Carbonyls, % | ≤70.68 | 70.68–107.17 | ≥107.17 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 20 (8.5)/39 (16.7) | 56 (23.9)/39 (16.7) | 41 (17.5)/39 (16.7) | ||
| Crude (matched a) model | 1.12 (0.86–1.45) | 1.00 ref | 2.70 (1.36–5.35) ** | 2.04 (1.03–4.06) * | 0.253 | |
| Multivariable model † | 0.87 (0.45–1.66) | 1.00 ref | 3.99 (0.89–17.9) | 2.95 (0.64–13.7) | 0.655 | |
| IL-1β, pg/mL | ≤2.42 | 2.42–2.57 | >2.57 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 29 (12.4)/38 (16.2) | 32 (13.7)/39 (16.7) | 56 (23.9)/40 (17.1) | ||
| Crude (matched a) model | 2.03 (1.33–3.08) ** | 1.00 ref | 1.06 (0.54–2.08) | 1.85 (0.97–3.53) | 0.038 | |
| Multivariable model † | 2.84 (1.04–7.74) * | 1.00 ref | 1.28 (0.35–4.75) | 2.94 (0.70–12.31) | 0.111 | |
| IL-1ra, pg/mL | ≤59.23 | 59.23–69.77 | >69.77 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 29 (12.4)/49 (20.9) | 31 (13.2)/28 (12.0) | 57 (24.4)/40 (17.1) | ||
| Crude (matched a) model | 1.57 (1.10–2.24) * | 1.00 ref | 1.88 (0.92–3.85) | 2.22 (1.22–4.04) ** | 0.022 | |
| Multivariable model † | 2.15 (1.13–4.11) * | 1.00 ref | 4.27 (0.93–19.3) | 4.27 (0.93–19.7) | 0.089 | |
| IL-6, pg/mL | ≤3.57 | 3.57–3.81 | >3.81 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 8 (3.4)/39 (16.7) | 42 (17.9)/41 (17.5) | 67 (28.6)/37 (15.8) | ||
| Crude (matched a) model | 1.68 (1.23–2.29) ** | 1.00 ref | 4.86 (1.92–12.33) ** | 7.03 (2.88–17.14) ** | <0.001 | |
| Multivariable model † | 2.09 (1.11–3.95) * | 1.00 ref | 7.32 (1.03–52.09) * | 7.94 (1.45–43.6) * | 0.065 | |
| MCP1, pg/mL | ≤10.3 | 10.3–19.9 | >19.9 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 36 (15.4)/40 (17.1) | 38 (16.2)/38 (16.2) | 43 (18.4)/39 (16.7) | ||
| Crude (matched a) model | 1.26 (0.96–1.67) | 1.00 ref | 1.10 (0.60–2.00) | 1.22 (0.66–2.28) | 0.530 | |
| Multivariable model † | 1.46 (0.75–2.85) | 1.00 ref | 0.90 (0.24–3.42) | 0.90 (0.23–3.54) | 0.899 | |
| TNFα, pg/mL | ≤3.17 | 3.17–3.52 | >3.52 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 19 (8.1)/39 (16.7) | 30 (12.8)/38 (16.2) | 68 (29.1)/40 (17.1) | ||
| Crude (matched a) model | 1.80 (1.27–2.56) ** | 1.00 ref | 1.87 (0.87–4.05) | 3.79 (1.79–8.02) ** | <0.001 | |
| Multivariable model † | 2.27 (1.01–5.09) * | 1.00 ref | 2.05 (0.37–11.3) | 4.30 (0.86–21.55) | 0.069 | |
| Leptin, ng/mL | ≤1101.4 | 1101.4–1518.11 | >1518.11 ng/mL | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 50 (21.4)/39 (16.7) | 36 (15.4)/40 (17.1) | 31 (13.2)/38 (16.2) | ||
| Crude (matched a) model | 1.38 (1.05–1.82) * | 1.00 ref | 0.72 (0.40–1.29) | 0.65 (0.35–1.21) | 0.163 | |
| Multivariable model † | 1.97 (0.91–4.28) | 1.00 ref | 2.09 (0.52–8.49) | 3.39 (0.59–19.52) | 0.170 | |
| Inflammatory–oxidative score, mean (SD), point | 13.8 (1.30), ≤15 | 16.5 (0.51), 15–17 | 19.7 (1.58), >17 | |||
| No. Case (%)/control (%) | 117(50)/117(50) | 16 (6.8)/60 (25.6) | 40 (17.1)/29 (12.4) | 61 (26.1)/28 (12.0) | ||
| Crude (matched a) model | 2.06 (1.49–2.83) ** | 1.00 ref | 7.89 (2.88–21.63) ** | 11.36 (4.28–30.16) ** | <0.001 | |
| Multivariable model † | 2.14 (1.13–4.07) * | 1.00 ref | 41.66 (2.47–703.91) ** | 22.39 (2.16–232.51) ** | 0.011 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quetglas-Llabrés, M.M.; Díaz-López, A.; Bouzas, C.; Monserrat-Mesquida, M.; Salas-Salvadó, J.; Ruiz-Canela, M.; Martínez, J.A.; Santos-Lozano, J.M.; García, S.; Estruch, R.; et al. Association Between Oxidative–Inflammation Biomarkers and Incident Chronic Kidney Disease in People with High Cardiovascular Risk: A Nested Case–Control Study. Antioxidants 2025, 14, 975. https://doi.org/10.3390/antiox14080975
Quetglas-Llabrés MM, Díaz-López A, Bouzas C, Monserrat-Mesquida M, Salas-Salvadó J, Ruiz-Canela M, Martínez JA, Santos-Lozano JM, García S, Estruch R, et al. Association Between Oxidative–Inflammation Biomarkers and Incident Chronic Kidney Disease in People with High Cardiovascular Risk: A Nested Case–Control Study. Antioxidants. 2025; 14(8):975. https://doi.org/10.3390/antiox14080975
Chicago/Turabian StyleQuetglas-Llabrés, Maria Magdalena, Andrés Díaz-López, Cristina Bouzas, Margalida Monserrat-Mesquida, Jordi Salas-Salvadó, Miguel Ruiz-Canela, J. Alfredo Martínez, José Manuel Santos-Lozano, Silvia García, Ramon Estruch, and et al. 2025. "Association Between Oxidative–Inflammation Biomarkers and Incident Chronic Kidney Disease in People with High Cardiovascular Risk: A Nested Case–Control Study" Antioxidants 14, no. 8: 975. https://doi.org/10.3390/antiox14080975
APA StyleQuetglas-Llabrés, M. M., Díaz-López, A., Bouzas, C., Monserrat-Mesquida, M., Salas-Salvadó, J., Ruiz-Canela, M., Martínez, J. A., Santos-Lozano, J. M., García, S., Estruch, R., López-Miranda, J., Romaguera, D., Tinahones, F. J., García-Fernández, M., Mas-Fontao, S., Matía-Martín, P., Vioque, J., Bueno, A., Babio, N., ... Sureda, A. (2025). Association Between Oxidative–Inflammation Biomarkers and Incident Chronic Kidney Disease in People with High Cardiovascular Risk: A Nested Case–Control Study. Antioxidants, 14(8), 975. https://doi.org/10.3390/antiox14080975
















