Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis
Abstract
1. Introduction
2. Gut Microbiota–Pregnancy Axis: A Central Regulator of Pregnancy Homeostasis
3. Autophagy and Ferroptosis: The Basic Molecular Mechanisms
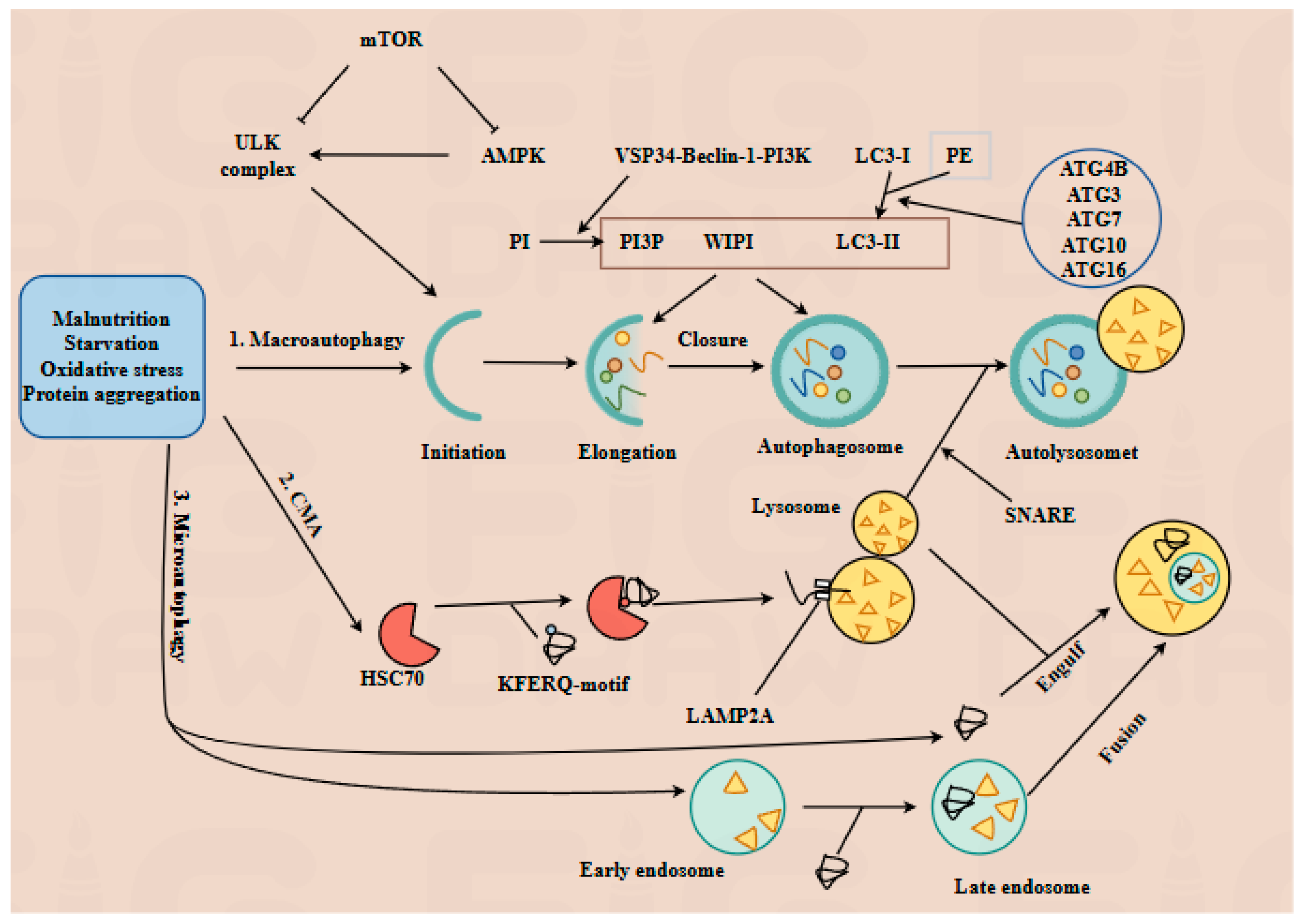
3.1. Core Mechanisms of Autophagy
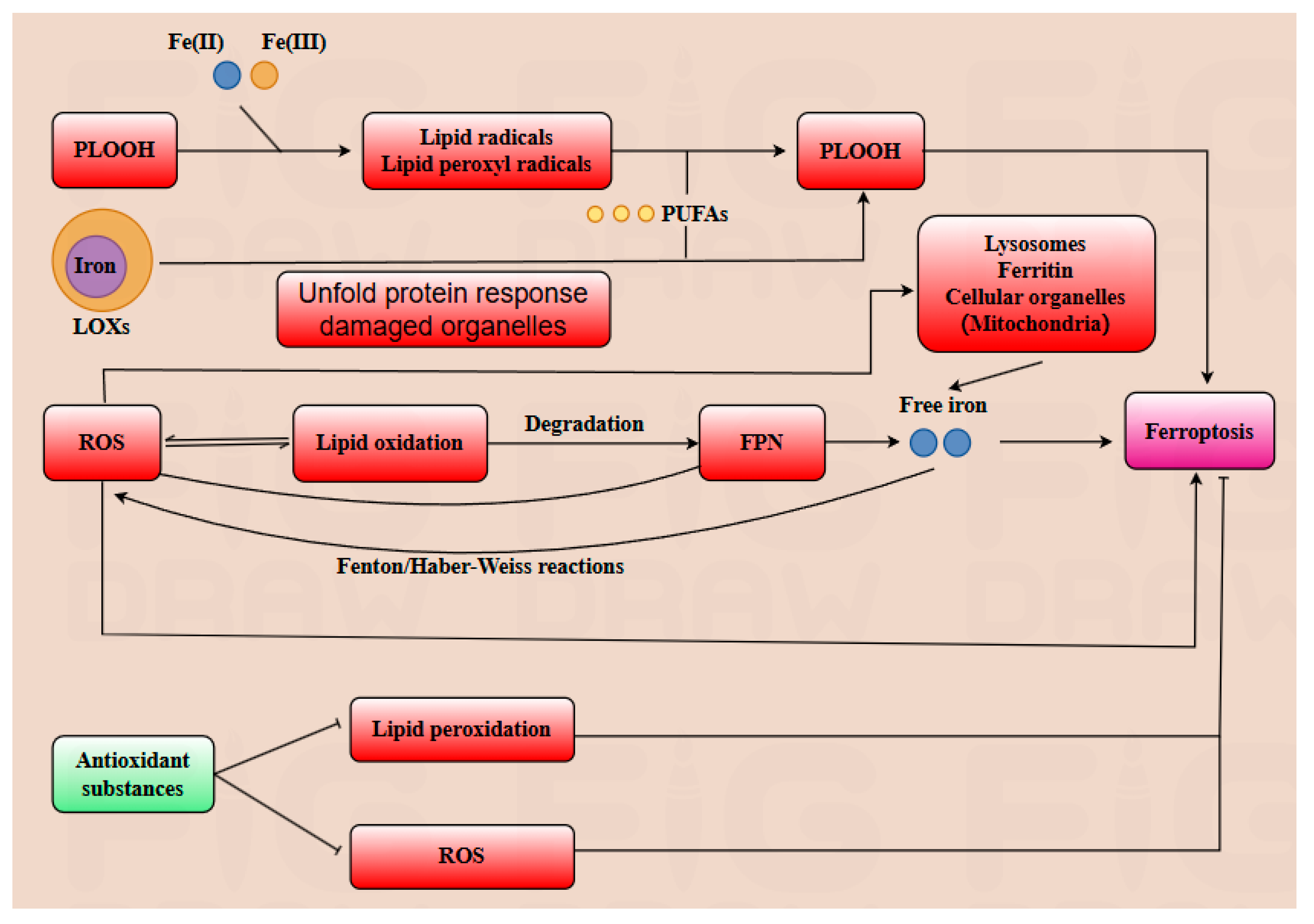
3.2. Core Mechanisms of Ferroptosis
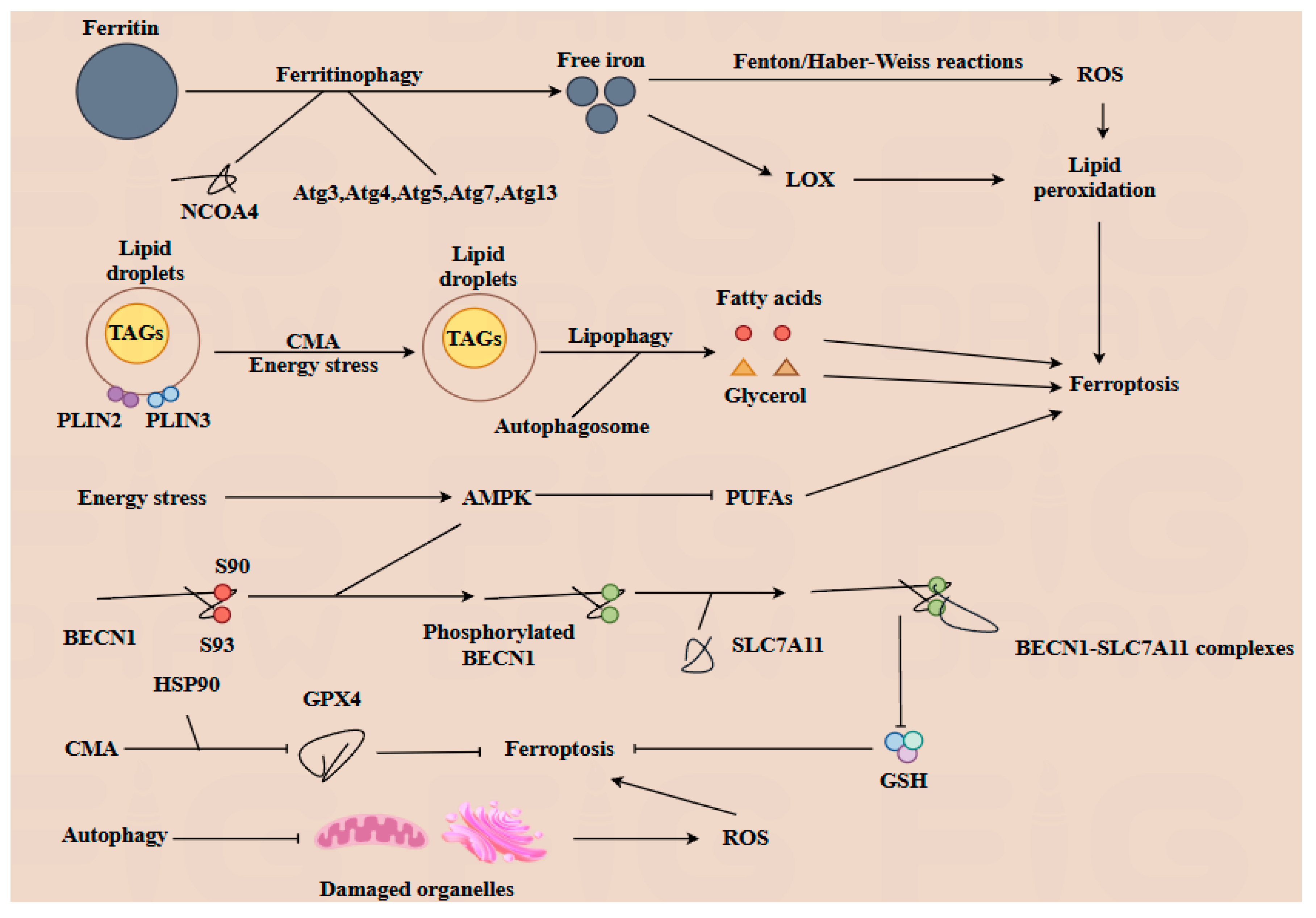
3.3. Autophagy–Ferroptosis Crosstalk in Cellular Homeostasis
4. Dynamic Interplay of Placental Autophagy and Ferroptosis in Pregnancy Physiology and Pathology
4.1. Autophagy in Placental Development
4.2. Ferroptosis in Placental Development and Autophagy
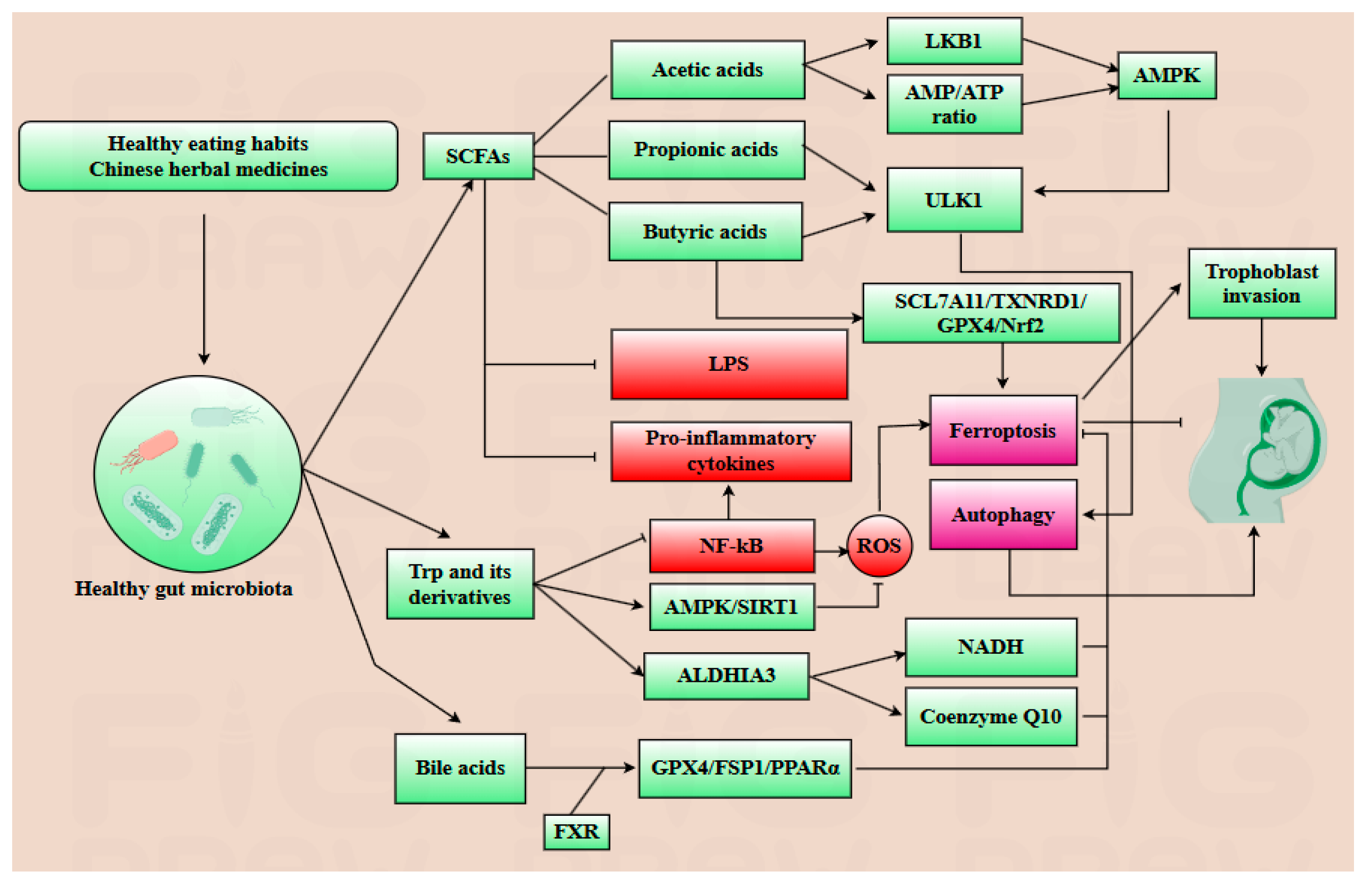
5. Gut Microbiota–Metabolite Axis Orchestrates Placental Autophagy–Ferroptosis Balance in Pregnancy
6. Translational Applications: From Mechanisms to Precision Medicine
7. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACSL4 | Long-chain acyl-coenzyme A synthase 4 |
| AMPK | AMP-activated protein kinase |
| CDCA | Chenodeoxycholic acid |
| CMA | Chaperone-mediated autophagy |
| DCA | Deoxycholic acid |
| ESCs | Embryonic stem cells |
| EVTs | Extravillous trophoblasts |
| FGR | Fetal growth restriction |
| FPN | Ferroportin |
| FSP1 | Ferroptosis suppression protein 1 |
| FXR | Farnesoid X receptor |
| GDM | Gestational diabetes mellitus |
| GPX4 | Glutathione peroxidase 4 |
| GSH | Glutathione |
| HSC70 | Heat-shock cognate protein 70 |
| HSP | Heat shock protein |
| ICM | Inner cell mass |
| LAMP-2A | Lysosome-associated membrane protein type 2A |
| LC3 | Microtubule-associated protein 1 light chain 3 |
| LDs | Lipid droplets |
| LPS | Lipopolysaccharide |
| NCOA4 | Nuclear receptor coactivator 4 |
| PE | Preeclampsia |
| PI | Phosphatidylinositol |
| PI3P | Phosphatidylinositol 3-triphosphate |
| PLIN2 | Perilipin 2 |
| PLIN3 | Perilipin 3 |
| PLOOH | Phospholipid hydroperoxides |
| PUFA | Polyunsaturated fatty acids |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SLC7A11 | Cystine transporter solute carrier family 7 member 11 |
| STBs | Syncytiotrophoblasts |
| TAGs | Triacylglycerols |
| TE | Trophectoderm |
| TMAO | Trimethylamine N-oxide |
| Trp | Tryptophan |
References
- Sharma, A.; Flora, S.J.S. Positive and Negative Regulation of Ferroptosis and Its Role in Maintaining Metabolic and Redox Homeostasis. Oxidative Med. Cell. Longev. 2021, 2021, 9074206. [Google Scholar] [CrossRef]
- Shen, X.; Obore, N.; Wang, Y.; Yu, T.; Yu, H. The Role of Ferroptosis in Placental-Related Diseases. Reprod. Sci. 2023, 30, 2079–2086. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Wang, J.X.; Chen, M.H.; Xu, J.J.; Jiang, M.H.; Feng, Y.L.; Gu, Y.F. miR-30-5p-mediated ferroptosis of trophoblasts is implicated in the pathogenesis of preeclampsia. Redox Biol. 2020, 29, 101402. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cho, M.; Do, Y.; Park, J.K.; Bae, S.J.; Joo, J.; Ha, K.T. Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharmaceuticals 2021, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Tra, T.; Gong, L.; Kao, L.P.; Li, X.L.; Grandela, C.; Devenish, R.J.; Wolvetang, E.; Prescott, M. Autophagy in human embryonic stem cells. PLoS ONE 2011, 6, e27485. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, L.; Gutiérrez, J.; Morselli, E.; Leiva, A. Autophagy Process in Trophoblast Cells Invasion and Differentiation: Similitude and Differences With Cancer Cells. Front. Oncol. 2021, 11, 637594. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, N.; Li, J.; Liang, J.; Zhou, D.; Cao, Q.; Li, X.; Jiang, N. The interplay between autophagy and ferroptosis presents a novel conceptual therapeutic framework for neuroendocrine prostate cancer. Pharmacol. Res. 2024, 203, 107162. [Google Scholar] [CrossRef]
- Liu, M.; Wu, K.; Wu, Y. The emerging role of ferroptosis in female reproductive disorders. Biomed. Pharmacother. 2023, 166, 115415. [Google Scholar] [CrossRef]
- Jin, J.; Gao, L.; Zou, X.; Zhang, Y.; Zheng, Z.; Zhang, X.; Li, J.; Tian, Z.; Wang, X.; Gu, J.; et al. Gut Dysbiosis Promotes Preeclampsia by Regulating Macrophages and Trophoblasts. Circ. Res. 2022, 131, 492–506. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Zhang, X.; Yao, G.; Jin, J.; Zhang, T.; Sun, C.; Wang, Z.; Zhang, Q. Intestinal flora and pregnancy complications: Current insights and future prospects. iMeta 2024, 3, e167. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Liu, Y.; Gao, Y.; Yang, R. Regulation of gut microbiota on immune cell ferroptosis: A novel insight for immunotherapy against tumor. Cancer Lett. 2024, 598, 217115. [Google Scholar] [CrossRef]
- Argaw-Denboba, A.; Schmidt, T.S.B.; Di Giacomo, M.; Ranjan, B.; Devendran, S.; Mastrorilli, E.; Lloyd, C.T.; Pugliese, D.; Paribeni, V.; Dabin, J.; et al. Paternal microbiome perturbations impact offspring fitness. Nature 2024, 629, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, Y.; Li, H.; Song, F.; Li, J.; Zhang, Y.; Lin, Y.; Zhang, H.; Fan, J.; Wu, W. Autophagic flux inhibition, apoptosis, and mitochondrial dysfunction in bile acids-induced impairment of human placental trophoblast. J. Cell. Physiol. 2022, 237, 3080–3094. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zheng, X.; Wu, C.; Ma, W.; Wang, Y.; Wang, J.; Wei, Y.; Zeng, X.; Zhang, S.; Guan, W.; et al. Melatonin alleviates high temperature exposure induced fetal growth restriction via the gut-placenta-fetus axis in pregnant mice. J. Adv. Res. 2025, 68, 131–146. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Brummer, R.J.M.; Rastall, R.A.; Weersma, R.K.; Harmsen, H.J.M.; Faas, M.; Eggersdorfer, M. The role of the microbiome for human health: From basic science to clinical applications. Eur. J. Nutr. 2018, 57, 1–14. [Google Scholar] [CrossRef]
- Ktistakis, N.T.; Tooze, S.A. Digesting the Expanding Mechanisms of Autophagy. Trends Cell Biol. 2016, 26, 624–635. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Kuma, A.; Murakami, M.; Kishi, C.; Yamamoto, A.; Mizushima, N. Autophagy is essential for preimplantation development of mouse embryos. Science 2008, 321, 117–120. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Lei, P.; Bai, T.; Sun, Y. Mechanisms of Ferroptosis and Relations With Regulated Cell Death: A Review. Front. Physiol. 2019, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002, 19, 43–55. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.D.; Ma, M.Q.; Liang, Y.; Cai, Y.B.; Zhu, Z.X.; Xu, T.; Zhu, L.; Ren, K. The mechanisms of ferroptosis and its role in atherosclerosis. Biomed. Pharmacother. 2024, 171, 116112. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhang, J.; Zhang, A.; Liu, Y.; Zhou, J.; Wang, X.; Zhang, J. Ferroptosis in Glioma Immune Microenvironment: Opportunity and Challenge. Front. Oncol. 2022, 12, 917634. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Zandkarimi, F.; Zhang, Y.; Meena, J.K.; Kim, J.; Zhuang, L.; Tyagi, S.; Ma, L.; Westbrook, T.F.; Steinberg, G.R.; et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 2020, 22, 225–234. [Google Scholar] [CrossRef]
- Xu, J.; Tian, H.; Ji, Y.; Dong, L.; Liu, Y.; Wang, Y.; Gao, X.; Shi, H.; Li, H.; Yang, L. Urolithin C reveals anti-NAFLD potential via AMPK-ferroptosis axis and modulating gut microbiota. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2687–2699. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Y.; Wu, X.; Chen, J.; Zhou, Q.; Liu, B.; Zhang, L.; Yi, C. Interplay of energy metabolism and autophagy. Autophagy 2024, 20, 4–14. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, C.; Zheng, Y.; Wu, D.; Chen, X.; Lan, H.; Zheng, X.; Wu, H.; Li, S. Glycine regulates lipid peroxidation promoting porcine oocyte maturation and early embryonic development. J. Anim. Sci. 2023, 101, skac425. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Kang, R.; Tang, D. Interplay Between Lipid Metabolism and Autophagy. Front. Cell Dev. Biol. 2020, 8, 431. [Google Scholar] [CrossRef]
- Liu, J.; Kuang, F.; Kroemer, G.; Klionsky, D.J.; Kang, R.; Tang, D. Autophagy-Dependent Ferroptosis: Machinery and Regulation. Cell Chem. Biol. 2020, 27, 420–435. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Xu, W.; Liu, Z.; Huang, K. AMP-activated protein kinase alpha1 phosphorylates PHD2 to maintain systemic iron homeostasis. Clin. Transl. Med. 2022, 12, e854. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Zhu, H.L.; Xu, X.F.; Zhang, J.; Ling, Q.; Zhang, S.; Chang, W.; Xiong, Y.W.; Xu, D.X.; Wang, H. Activation of Atg5-dependent placental lipophagy ameliorates cadmium-induced fetal growth restriction. Environ. Pollut. 2023, 328, 121602. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, L.; Hong, R.; Li, Y.; Wang, Y.; Qi, X.; Ning, W.; Gao, D.; Xu, T.; Ma, Y.; et al. METTL3-mediated m6A methylation negatively modulates autophagy to support porcine blastocyst developmentdouble dagger. Biol. Reprod. 2021, 104, 1008–1021. [Google Scholar] [CrossRef]
- Song, S.; Guo, Q.; Zhu, Y.; Yuan, P.; Yan, Z.; Yan, L.; Qiao, J. Exploring the role of autophagy during early human embryonic development through single-cell transcriptome and methylome analyses. Sci. China Life Sci. 2022, 65, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, M.; Wang, J.; Han, X.; Yang, X.; Zhang, H.; Zhong, D.; Qiu, S.; Yu, S.; Wang, L.; et al. Hypoxia-Inducible Factor 1alpha Affects Yak Oocyte Maturation and Early Embryonic Development by Regulating Autophagy. Antioxidants 2024, 13, 840. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, Y.; Liu, X.; Zha, X.; Elsabagh, M.; Ma, Y.; Jiang, H.; Wang, H.; Wang, M. Autophagy attenuates placental apoptosis, oxidative stress and fetal growth restriction in pregnant ewes. Environ. Int. 2023, 173, 107806. [Google Scholar] [CrossRef]
- Zhou, J.; He, H.; Zhang, J.J.; Liu, X.; Yao, W.; Li, C.; Xu, T.; Yin, S.Y.; Wu, D.Y.; Dou, C.L.; et al. ATG7-mediated autophagy facilitates embryonic stem cell exit from naive pluripotency and marks commitment to differentiation. Autophagy 2022, 18, 2946–2968. [Google Scholar] [CrossRef]
- Yang, N.; Sun, Y.; Han, B.; Deng, N.; Li, G.; Han, Q.; Wang, Y.; Cai, H.; Liu, F.; Cao, B.; et al. Trophoblastic signals facilitate endometrial interferon response and lipid metabolism, ensuring normal decidualization. Cell Rep. 2024, 43, 114246. [Google Scholar] [CrossRef]
- Lu, Y.; Shao, Y.; Cui, W.; Jia, Z.; Zhang, Q.; Zhao, Q.; Chen, Z.J.; Yan, J.; Chu, B.; Yuan, J. Excessive Lipid Peroxidation in Uterine Epithelium Causes Implantation Failure and Pregnancy Loss. Adv. Sci. 2024, 11, e2302887. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, A.K.; Chadchan, S.B.; Popli, P.; Medvedeva, A.; Rowen, M.N.; Stephens, C.S.; Xu, R.; Lydon, J.P.; Demayo, F.J.; Jungheim, E.S.; et al. The Autophagy Gene Atg16L1 is Necessary for Endometrial Decidualization. Endocrinology 2019, 161, bqz039. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, J.J.; He, J.L.; Liu, X.Q.; Chen, X.M.; Ding, Y.B.; Tong, C.; Peng, C.; Geng, Y.Q.; Wang, Y.X.; et al. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. 2020, 98, 555–567. [Google Scholar] [CrossRef]
- Nakashima, A.; Tsuda, S.; Kusabiraki, T.; Aoki, A.; Ushijima, A.; Shima, T.; Cheng, S.B.; Sharma, S.; Saito, S. Current Understanding of Autophagy in Pregnancy. Int. J. Mol. Sci. 2019, 20, 2342. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka-Tatematsu, M.; Nakashima, A.; Fujita, N.; Shima, T.; Yoshimori, T.; Saito, S. Autophagy induced by HIF1alpha overexpression supports trophoblast invasion by supplying cellular energy. PLoS ONE 2013, 8, e76605. [Google Scholar] [CrossRef]
- Lim, H.J.; Song, H. Evolving tales of autophagy in early reproductive events. Int. J. Dev. Biol. 2014, 58, 183–187. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, J.; Wang, J.; Liu, X. When autophagy meets placenta development and pregnancy complications. Front. Cell Dev. Biol. 2024, 12, 1327167. [Google Scholar] [CrossRef]
- Cao, S.; Shen, W.B.; Reece, E.A.; Yang, P. Deficiency of the oxidative stress-responsive kinase p70S6K1 restores autophagy and ameliorates neural tube defects in diabetic embryopathy. Am. J. Obstet. Gynecol. 2020, 223, 753.e1–753.e14. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, L.; Xu, J.; Wang, J.; Yu, Z.; Zhao, K.; Zhang, H.; Cheng, S.; Sharma, S.; Liao, A.; et al. Recent insight into autophagy and immunity at the maternal-fetal interface. J. Reprod. Immunol. 2023, 155, 103781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, Y.; Jin, L. Iron Metabolism and Ferroptosis in Physiological and Pathological Pregnancy. Int. J. Mol. Sci. 2022, 23, 9395. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Adel, N.; Abdulghaffar, S.; Elmahdy, M.; Nabil, M.; Ghareeb, D.; Maghraby, H. Autophagy-related gene and protein expressions during blastocyst development. J. Assist. Reprod. Genet. 2023, 40, 323–331. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamanaka-Tatematsu, M.; Fujita, N.; Koizumi, K.; Shima, T.; Yoshida, T.; Nikaido, T.; Okamoto, A.; Yoshimori, T.; Saito, S. Impaired autophagy by soluble endoglin, under physiological hypoxia in early pregnant period, is involved in poor placentation in preeclampsia. Autophagy 2013, 9, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Alahari, S.; Ausman, J.; Liu, R.; Nguyen, F.; Sallais, J.; Post, M.; Caniggia, I. Placental Hypoxia-Induced Ferroptosis Drives Vascular Damage in Preeclampsia. Circ. Res. 2025, 136, 361–378. [Google Scholar] [CrossRef]
- Gallo, G.; Volpe, M.; Savoia, C. Endothelial Dysfunction in Hypertension: Current Concepts and Clinical Implications. Front. Med. 2021, 8, 798958. [Google Scholar] [CrossRef] [PubMed]
- Beharier, O.; Kajiwara, K.; Sadovsky, Y. Ferroptosis, trophoblast lipotoxic damage, and adverse pregnancy outcome. Placenta 2021, 108, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Yu, L.; Deng, Y. PPARgamma alleviates preeclampsia development by regulating lipid metabolism and ferroptosis. Commun. Biol. 2024, 7, 429. [Google Scholar] [CrossRef]
- Co, H.K.C.; Wu, C.C.; Lee, Y.C.; Chen, S.H. Emergence of large-scale cell death through ferroptotic trigger waves. Nature 2024, 631, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Zaugg, J.; Solenthaler, F.; Albrecht, C. Materno-fetal iron transfer and the emerging role of ferroptosis pathways. Biochem. Pharmacol. 2022, 202, 115141. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Hu, H.; Zhang, M.; Long, W.; Liu, J.; Jiang, J.; Yu, B. Iron deficiency in late pregnancy and its associations with birth outcomes in Chinese pregnant women: A retrospective cohort study. Nutr. Metab. 2019, 16, 30. [Google Scholar] [CrossRef]
- Sangkhae, V.; Fisher, A.L.; Ganz, T.; Nemeth, E. Iron Homeostasis During Pregnancy: Maternal, Placental, and Fetal Regulatory Mechanisms. Annu. Rev. Nutr. 2023, 43, 279–300. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin and Iron in Health and Disease. Annu. Rev. Med. 2023, 74, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Xu, Z.; Guo, J.; Zhao, J.; Chen, W.; Huang, W.; Wang, M.; Mi, C.; Zhang, Y.; Yang, Y.; et al. Hypoxia causes trophoblast cell ferroptosis to induce miscarriage through lnc-HZ06/HIF1alpha-SUMO/NCOA4 axis. Redox Biol. 2024, 70, 103073. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, D.; Hu, H.; Zhang, P.; Xie, R.; Cui, W. HIF-1alpha/BNIP3 signaling pathway-induced-autophagy plays protective role during myocardial ischemia-reperfusion injury. Biomed. Pharmacother. 2019, 120, 109464. [Google Scholar] [CrossRef]
- Mulder, J.; Kusters, D.M.; Roeters van Lennep, J.E.; Hutten, B.A. Lipid metabolism during pregnancy: Consequences for mother and child. Curr. Opin. Lipidol. 2024, 35, 133–140. [Google Scholar] [CrossRef]
- Li, Y.Y.; Peng, Y.Q.; Yang, Y.X.; Shi, T.J.; Liu, R.X.; Luan, Y.Y.; Yin, C.H. Baicalein improves the symptoms of polycystic ovary syndrome by mitigating oxidative stress and ferroptosis in the ovary and gravid placenta. Phytomedicine 2024, 128, 155423. [Google Scholar] [CrossRef]
- Ortega, M.A.; Garcia-Puente, L.M.; Fraile-Martinez, O.; Pekarek, T.; Garcia-Montero, C.; Bujan, J.; Pekarek, L.; Barrena-Blazquez, S.; Gragera, R.; Rodriguez-Rojo, I.C.; et al. Oxidative Stress, Lipid Peroxidation and Ferroptosis Are Major Pathophysiological Signatures in the Placental Tissue of Women with Late-Onset Preeclampsia. Antioxidants 2024, 13, 591. [Google Scholar] [CrossRef]
- Nakashima, A.; Shima, T.; Aoki, A.; Kawaguchi, M.; Yasuda, I.; Tsuda, S.; Yoneda, S.; Yamaki-Ushijima, A.; Cheng, S.; Sharma, S.; et al. Placental autophagy failure: A risk factor for preeclampsia. J. Obstet. Gynaecol. Res. 2020, 46, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, F.; Wang, X.; Mo, C. Role of ferroptosis in pregnancy related diseases and its therapeutic potential. Front. Cell Dev. Biol. 2023, 11, 1083838. [Google Scholar] [CrossRef]
- Hu, C.; Yan, Y.; Ji, F.; Zhou, H. Maternal Obesity Increases Oxidative Stress in Placenta and It Is Associated With Intestinal Microbiota. Front. Cell. Infect. Microbiol. 2021, 11, 671347. [Google Scholar] [CrossRef]
- Hung, T.H.; Huang, S.Y.; Chen, S.F.; Wu, C.P.; Hsieh, T.T. Decreased placental apoptosis and autophagy in pregnancies complicated by gestational diabetes with large-for-gestational age fetuses. Placenta 2020, 90, 27–36. [Google Scholar] [CrossRef]
- Cao, B.; Macones, C.; Mysorekar, I.U. ATG16L1 governs placental infection risk and preterm birth in mice and women. JCI Insight 2016, 1, e86654. [Google Scholar] [CrossRef]
- Zietek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Liu, X.J.; Wang, B.W.; Zhao, M.; Zhang, C.; Chen, Y.H.; Hu, C.Q.; Zhao, H.; Wang, H.; Chen, X.; Tao, F.B.; et al. Effects of maternal LPS exposure during pregnancy on metabolic phenotypes in female offspring. PLoS ONE 2014, 9, e114780. [Google Scholar] [CrossRef][Green Version]
- Wang, J.; Gu, X.; Yang, J.; Wei, Y.; Zhao, Y. Gut Microbiota Dysbiosis and Increased Plasma LPS and TMAO Levels in Patients With Preeclampsia. Front. Cell. Infect. Microbiol. 2019, 9, 409. [Google Scholar] [CrossRef] [PubMed]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef]
- Wang, G.; Qin, S.; Chen, L.; Geng, H.; Zheng, Y.; Xia, C.; Yao, J.; Deng, L. Butyrate dictates ferroptosis sensitivity through FFAR2-mTOR signaling. Cell Death Dis. 2023, 14, 292. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Liu, Z.; Wang, J.; Wang, J.; Gao, M.; Zhang, Y.; Yang, C.; Zhang, A.; Li, G.; Li, X.; et al. Immunoregulatory role of the gut microbiota in inflammatory depression. Nat. Commun. 2024, 15, 3003. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Q. Short-Chain Fatty Acids Attenuate Renal Fibrosis and Enhance Autophagy of Renal Tubular Cells in Diabetic Mice Through the HDAC2/ULK1 Axis. Endocrinol. Metab. 2022, 37, 432–443. [Google Scholar] [CrossRef]
- Li, P.; Wang, H.; Guo, L.; Gou, X.; Chen, G.; Lin, D.; Fan, D.; Guo, X.; Liu, Z. Association between gut microbiota and preeclampsia-eclampsia: A two-sample Mendelian randomization study. BMC Med. 2022, 20, 443. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Tang, L.; Liu, H.; Chen, W.; Chen, Q.; Zhong, M.; Yin, A. Potential roles of the interactions between gut microbiota and metabolites in LPS-induced intrauterine inflammation (IUI) and associated preterm birth (PTB). J. Transl. Med. 2024, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Geng, H.; Liang, C.; Xiong, X.; Du, X.; Zhuan, Q.; Liu, Z.; Meng, L.; Zhou, D.; Zhang, L.; et al. Leonurine restrains granulosa cell ferroptosis through SLC7A11/GPX4 axis to promote the treatment of polycystic ovary syndrome. Free Radic. Biol. Med. 2025, 226, 330–347. [Google Scholar] [CrossRef]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Huo, X.; Li, J.; Cao, Y.F.; Li, S.N.; Shao, P.; Leng, J.; Li, W.; Liu, J.; Yang, K.; Ma, R.C.W.; et al. Trimethylamine N-Oxide Metabolites in Early Pregnancy and Risk of Gestational Diabetes: A Nested Case-Control Study. J. Clin. Endocrinol. Metab. 2019, 104, 5529–5539. [Google Scholar] [CrossRef]
- Gao, N.; Yang, Y.; Liu, S.; Fang, C.; Dou, X.; Zhang, L.; Shan, A. Gut-Derived Metabolites from Dietary Tryptophan Supplementation Quench Intestinal Inflammation through the AMPK-SIRT1-Autophagy Pathway. J. Agric. Food Chem. 2022, 70, 16080–16095. [Google Scholar] [CrossRef]
- Yue, Y.; Ke, Y.; Zheng, J.; Wang, Z.; Liu, H.; Liu, S. Microbiota-derived tryptophan metabolism and AMPK/mTOR pathway mediate antidepressant-like effect of Shugan Hewei Decoction. Front. Pharmacol. 2024, 15, 1466336. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Tang, R.; Wang, J.; Wan, D.; Yin, Y.; Xie, L. Gut microbiota bridges the iron homeostasis and host health. Sci. China Life Sci. 2023, 66, 1952–1975. [Google Scholar] [CrossRef]
- Xie, Y.; Kang, R.; Klionsky, D.J.; Tang, D. GPX4 in cell death, autophagy, and disease. Autophagy 2023, 19, 2621–2638. [Google Scholar] [CrossRef]
- Fujii, J.; Yamada, K.I. Defense systems to avoid ferroptosis caused by lipid peroxidation-mediated membrane damage. Free Radic. Res. 2023, 57, 353–372. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Cao, J.; Wang, F.; Han, J.R.; Wu, T.W.; Li, L.; Yu, J.; Fan, Y.; Xie, G.; et al. ACSL4 and polyunsaturated lipids support metastatic extravasation and colonization. Cell 2025, 188, 412–429 e427. [Google Scholar] [CrossRef]
- Park, M.; Park, S.; Choi, Y.; Cho, Y.-L.; Kim, M.J.; Park, Y.-J.; Chung, S.W.; Lee, H.; Lee, S.-J. The mechanism underlying correlation of particulate matter-induced ferroptosis with inflammasome activation and iron accumulation in macrophages. Cell Death Discov. 2024, 10, 144. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-dependent autophagic degradation of GPX4 drives ferroptosis. Autophagy 2023, 19, 1982–1996. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Straub, A.C.; Tyurina, Y.Y.; Kapralov, A.A.; Hall, R.; Wenzel, S.E.; Mallampalli, R.K.; Bayir, H. Vitamin E/Coenzyme Q-Dependent "Free Radical Reductases": Redox Regulators in Ferroptosis. Antioxid. Redox Signal. 2024, 40, 317–328. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Duan, Y.; Zheng, X.; Lin, Z.; Zhou, J. Nrf2/FSP1/CoQ10 axis-mediated ferroptosis is involved in sodium aescinate-induced nephrotoxicity. Arch. Biochem. Biophys. 2024, 759, 110100. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Chen, H.; Yang, Z.; Chen, S.; Wang, J.; Zhou, Y.; Xuan, R. The Diagnostic Potential of Gut Microbiota-Derived Short-Chain Fatty Acids in Preeclampsia. Front. Pediatr. 2022, 10, 878924. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Yan, C.; Xie, R.; Guo, Z.; Wang, S.; Zhang, Y.; Li, Z.; Wang, B.; Cao, H. Diammonium Glycyrrhizinate Protects against Nonalcoholic Fatty Liver Disease in Mice through Modulation of Gut Microbiota and Restoration of Intestinal Barrier. Mol. Pharm. 2018, 15, 3860–3870. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, N.; Wang, H.; Tran, H.; Ivanovska, J.; Pan, J.; Miraglia, E.; Leung, S.; Posiewko, M.; Li, D.; Mohammadi, A.; et al. L-citrulline attenuates lipopolysaccharide-induced inflammatory lung injury in neonatal rats. Pediatr. Res. 2023, 94, 1684–1695. [Google Scholar] [CrossRef]
- Catassi, G.; Aloi, M.; Giorgio, V.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. The Role of Diet and Nutritional Interventions for the Infant Gut Microbiome. Nutrients 2024, 16, 400. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.E.; O’Brien, E.C.; Moore, R.L.; Byrne, D.F.; Geraghty, A.A.; Saldova, R.; Murphy, E.F.; Van Sinderen, D.; Cotter, P.D.; McAuliffe, F.M. The association between the maternal diet and the maternal and infant gut microbiome: A systematic review. Br. J. Nutr. 2023, 129, 1491–1499. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Gohir, W.; Kennedy, K.M.; Wallace, J.G.; Saoi, M.; Bellissimo, C.J.; Britz-McKibbin, P.; Petrik, J.J.; Surette, M.G.; Sloboda, D.M. High-fat diet intake modulates maternal intestinal adaptations to pregnancy and results in placental hypoxia, as well as altered fetal gut barrier proteins and immune markers. J. Physiol. 2019, 597, 3029–3051. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, Y.; Ren, S.; Li, J.; Chen, S.; Feng, J.; He, B.; Zhou, Y.; Xuan, R. Gut microbiota-derived trimethylamine N-Oxide: A novel target for the treatment of preeclampsia. Gut Microbes 2024, 16, 2311888. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Sun, J.H.; Wang, W.J. Gut microbiota in gastrointestinal diseases during pregnancy. World J. Clin. Cases 2022, 10, 2976–2989. [Google Scholar] [CrossRef]
- Chen, X.; Li, P.; Liu, M.; Zheng, H.; He, Y.; Chen, M.X.; Tang, W.; Yue, X.; Huang, Y.; Zhuang, L.; et al. Gut dysbiosis induces the development of pre-eclampsia through bacterial translocation. Gut 2020, 69, 513–522. [Google Scholar] [CrossRef]
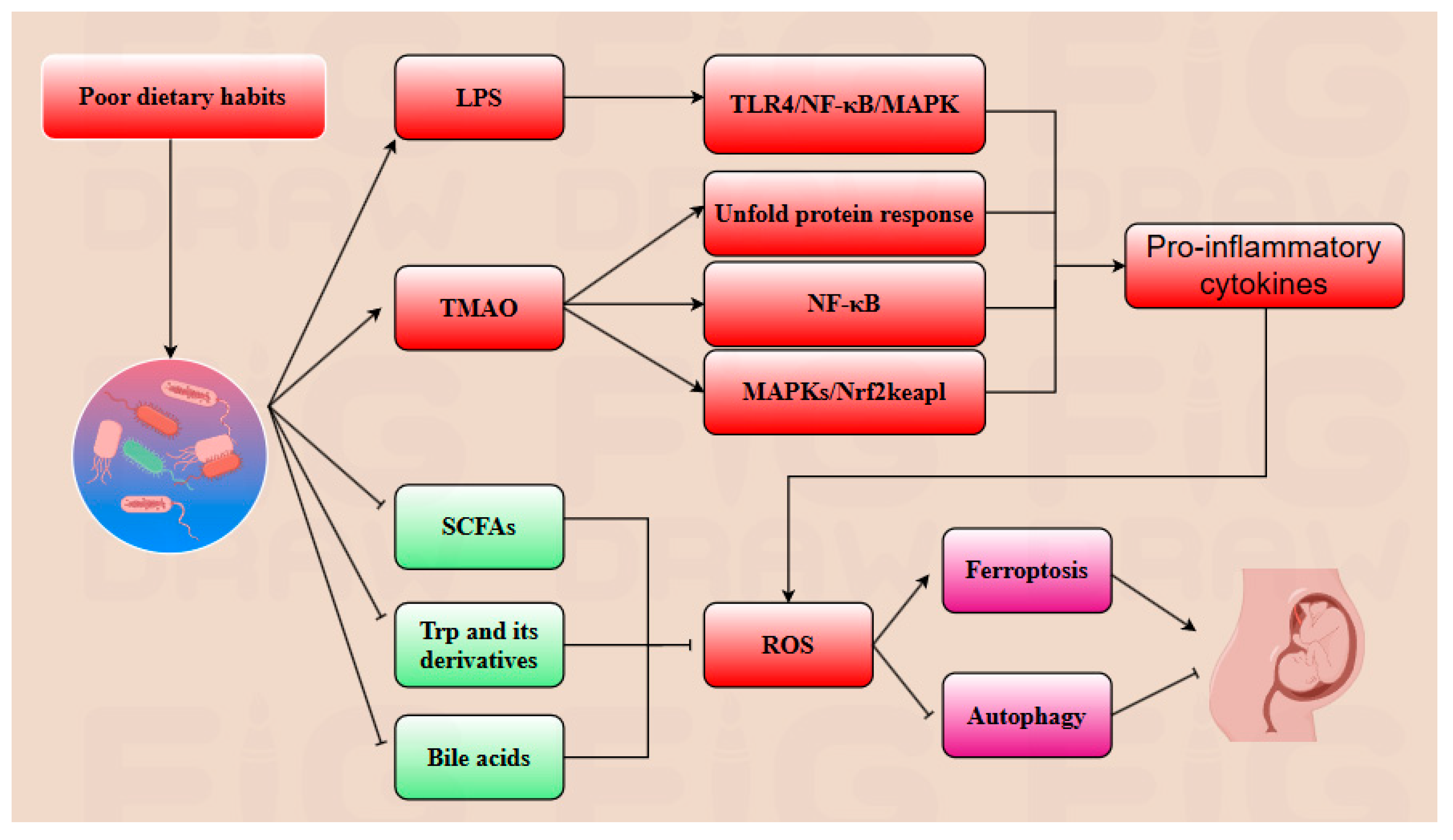
| Metabolites | Influence | Key Reaction | Reference |
|---|---|---|---|
| PLOOH | Promotion | Reacts with iron(II) or iron(III) to generate lipid alkyl radicals and lipid peroxyl radicals, which further react with PUFA to generate more PLOOH. | [90,91] |
| ROS | Promotion | Promotes lipid oxidation, thereby further enhancing the degradation of FPN. | [23] |
| ACSL4 | Promotion | Catalyzes the conversion of PUFA to acyl-CoA, which is further incorporated into phospholipids. | [92] |
| Free iron | Promotion | Facilitates the conversion of PUFA to PLOOH in the cell membrane and promotes ROS production via the Fenton and Haber–Weiss reactions. | [93] |
| LPS | Promotion | LPS promotion promotes secretion of pro-inflammatory cytokines and ROS by activating TLR4/NF-κB/MAPK pathways. | [77] |
| TMAO | Promotion | Promotes the unfolded protein response and upregulates NF-κB/MAPKs/Nrf2-Keap1 pathways. | [86] |
| GPX4 | Inhibition | Utilizes reduced GSH to detoxify harmful lipid peroxides into non-toxic lipid alcohols and scavenges ROS. | [94] |
| Vitamin E | Inhibition | Neutralizes free radicals on the plasma membrane, thereby preventing peroxidation of PUFAs in the membrane. | [95] |
| CoQ10H2 | Inhibition | Neutralizes free radicals and scavenges accumulated lipid peroxides, thereby alleviating oxidative damage. | [95] |
| NADPH | Inhibition | Supplies a large amount of reducing equivalents in the form of hydrogen in cells, and plays a key role in reducing oxidized antioxidants back to their reduced state. | [96] |
| FSP1 | Inhibition | Upregulates the NADP/NADPH ratio and utilizes reducing equivalents to reduce CoQ10 back to CoQ10H2. | [97] |
| SCFAs | Inhibition | Promote ULK1 synthesis, thereby further upregulating the autophagy pathway to inhibit ferroptosis. | [81] |
| Trp and its derivatives | Inhibition | Upregulate AMPK/SIRT1 and ALDH1A3 pathways to inhibit ROS production, thereby suppressing ferroptosis. | [87] |
| Bile acids | Inhibition | Upregulate the GPX4/FSP1/PPARα axis, inhibit ROS generation, thereby further suppressing ferroptosis. | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Elsabagh, M.; He, F.; Wu, H.; Zhang, B.; Fan, K.; Wang, M.; Zhang, H. Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis. Antioxidants 2025, 14, 970. https://doi.org/10.3390/antiox14080970
Du X, Elsabagh M, He F, Wu H, Zhang B, Fan K, Wang M, Zhang H. Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis. Antioxidants. 2025; 14(8):970. https://doi.org/10.3390/antiox14080970
Chicago/Turabian StyleDu, Xingyu, Mabrouk Elsabagh, Feiyang He, Huisi Wu, Bei Zhang, Kewei Fan, Mengzhi Wang, and Hao Zhang. 2025. "Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis" Antioxidants 14, no. 8: 970. https://doi.org/10.3390/antiox14080970
APA StyleDu, X., Elsabagh, M., He, F., Wu, H., Zhang, B., Fan, K., Wang, M., & Zhang, H. (2025). Gut Microbiota and Its Metabolites Modulate Pregnancy Outcomes by Regulating Placental Autophagy and Ferroptosis. Antioxidants, 14(8), 970. https://doi.org/10.3390/antiox14080970





