Abstract
To minimize the deleterious effects of oxidative stress and improve oocyte competence, we assessed the impact of melatonin during in vitro pre-maturation (pre-IVM) in bovine cumulus–oocyte complexes (COCs). We compared three groups: control (conventional IVM), pre-IVM control (without melatonin), and pre-IVM + MTn (with melatonin). The analyses included levels of reactive oxygen species (ROS), mitochondrial activity, oocyte lipid content, and the expression of genes related to oxidative stress and lipid metabolism in oocytes and cumulus cells. We also examined embryo quality by evaluating kinetics of development and gene expression. The pre-IVM + MTn group exhibited an increase (p ≤ 0.05) in ROS levels and a decrease (p ≤ 0.05) in lipid content, while maintaining mitochondrial activity similar (p > 0.05) to that of the control group. Regarding gene expression, the effect of pre-IVM, independent of melatonin, was characterized by a decrease in FABP3 transcripts in cumulus cells and reductions in GSS and NFE2L2 transcripts in oocytes (p ≤ 0.05). The pre-IVM + MTn group also displayed a decrease (p ≤ 0.05) in CAT and SOD2 transcript levels. In terms of embryonic development, the pre-IVM + MTn group achieved a higher blastocyst rate on D7 (p ≤ 0.05) compared to the control group (30.8% versus 25.8%), but with similar rates (p > 0.05) to the pre-IVM control group (30.8% versus 35.9%). However, there was a decrease in the levels of the PLAC8 transcript. This study indicates that, under the conditions tested, melatonin did not significantly benefit oocyte competence.
1. Introduction
Oocytes commonly used for in vitro embryo production (IVP) are typically obtained from antral follicles that have not yet completed their natural developmental process. As a result, these oocytes may be less competent when undergoing the complete maturation process [1]. This reduced competence can negatively impact IVP outcomes, as the efficiency of embryo development largely depends on the quality and developmental potential of the oocytes.
When oocytes are removed from the follicular environment, they spontaneously resume meiosis and progress to metaphase II, a stage indicative of nuclear maturation [2]. However, the cytoplasmic maturation process, which includes the redistribution of organelles and protein synthesis, may not occur effectively, potentially compromising the developmental competence of the oocytes [3,4]. Additionally, once retrieved, and placed in vitro conditions, oocytes undergo changes in redox potential, partly due to the loss of antioxidant defense from the follicular fluid and an increased production of reactive oxygen species (ROS). Factors such as exposure to light, oxygen concentrations, and medium composition, which differ significantly from the optimal in vivo environment, contribute to this oxidative imbalance. At the molecular level, ROS such as superoxide anion (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (•OH) are primarily generated in the mitochondria through electron leakage from the electron transport chain during oxidative phosphorylation, a process that becomes exacerbated in in vitro environments due to metabolic stress and reduced antioxidant defenses [5,6]. Another factor that can adversely impact oocyte quality is the lipid accumulation during the in vitro maturation (IVM) phase [7], which can elevate oxidative stress by promoting the lipoperoxidation of free fatty acids [8]. Together, these conditions can impair the oocyte’s ability to support subsequent embryonic development.
To overcome these limitations and enhance the quality of oocytes used for IVP, several strategies have been explored. One such approach involves the use of a pre-maturation methodology, which has been extensively studied in various species [9,10,11,12,13] and recently applied in the in vitro maturation of human oocytes [14,15,16,17]. This technique consists of a culture system—referred to as pre-in vitro maturation (pre-IVM or PIVM)—that precedes the conventional IVM phase. The goal is to provide oocytes with additional time after removal from the follicular environment, by blocking meiosis resumption and prolonging gap junction communication between oocytes and cumulus cells (CCs), thereby continuing the oocyte’s developmental programming [12,18]. Although pre-IVM systems in bovines have not consistently improved embryo development rates [18,19,20,21], few studies have explored the impact of offering optimized conditions during this meiotic arrest period.
In this context, another promising strategy is to optimize the oocyte’s environment immediately after retrieval. Mimicking in vivo conditions in the IVM medium, particularly through antioxidant supplementation, can help protect oocytes from oxidative stress induced by elevated ROS levels in the in vitro culture [6]. Among various antioxidants, melatonin has been extensively studied and shown to have protective effects during IVM across several species, improving embryo outcomes in goats [22,23,24], swine [25,26,27], rodents [28,29,30,31], sheep [32], and bovine [33,34].
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone secreted by the pineal gland in mammals and acts as a broad-spectrum antioxidant [35,36]. During in vivo development, melatonin and other substances are secreted into the follicular fluid by granulosa cells, contributing to the nutrition and quality of oocytes, but these protections are lost during in vitro culture [37]. In bovine IVM systems, melatonin has been reported to reduce ROS levels, increase glutathione (GSH) and ATP content, promote proper organelles distribution, improve mitochondrial function, and regulate the expression of genes involved in antioxidant defense, cumulus expansion, and anti-apoptotic pathways [38,39,40,41,42,43,44,45,46]. In pigs, besides its cytoplasmic benefits, melatonin has also been shown to regulate lipid metabolism via receptor-mediated pathways, maintaining a balance between lipolysis and lipogenesis [47,48,49]. Although numerous studies have explored the role of melatonin during IVM, none have evaluated its effects during the pre-IVM phase in cattle. However, the benefits of melatonin use during pre-IVM have been reported for oocytes obtained from early antral follicles, after undergoing in vitro growth, also supplemented with melatonin [50].
Considering the individual benefits of both pre-IVM and melatonin supplementation, combining these two strategies may represent a promising approach to improve oocyte quality for IVP. Pre-IVM offers oocytes additional time to acquire developmental competence by temporarily maintaining meiotic arrest, while melatonin, with its antioxidant and regulatory properties, may protect and support cellular processes during this sensitive window. The presence of melatonin during pre-IVM could help preserve redox balance and promote a more synchronized progression of cytoplasmic and nuclear maturation, an essential factor for improving oocyte competence. Therefore, we hypothesize that melatonin supplementation during the pre-IVM phase can enhance oocyte quality and developmental potential and consequently improve the rate of bovine embryo development and embryo quality.
2. Materials and Methods
The reagents used were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.1. Experimental Design
To assess whether melatonin supplementation during pre-IVM could improve oocyte quality, several key parameters were evaluated. After retrieval and selection, COCs were randomly allocated to three treatment groups: (1) control: COCs matured in vitro for 24 h; (2) pre-IVM control: COCs underwent pre-IVM for 6 h, followed by 24 h of IVM; and (3) pre-IVM + MTn: COCs pre-matured with melatonin for 6 h, followed by 24 h of IVM.
Initially, we assessed the following morphological and functional parameters: nuclear maturation, ROS quantification, lipid content, mitochondrial activity, and gene expression. For this, COCs from all treatment groups were collected at specific time points: 0 h for the immature control (IC) and 24 h for the mature control (MC); after 6 h of pre-IVM and after 24 h of IVM for groups without melatonin supplementation (PMIC and PMMC) and with melatonin supplementation (PMMI and PMMM), respectively. At each time point, oocytes were denuded, fixed, stained, and analyzed using confocal or epifluorescence microscopy.
For gene-expression analysis, COCs were denuded, and oocytes and cumulus cells were then stored separately at −80 °C until further analysis. The quantification of genes associated with oxidative stress and lipid metabolism was performed in oocytes (CAT, SOD1, SOD2, GSS, NFE2L2, PPARγ, CPT1A, ACSS2, FABP3, and PLIN2) and in cumulus cells (SOD1, SOD2, PPARγ, and FABP3) by qPCR.
Subsequently, to evaluate the effect of treatment on oocyte competence, we monitored embryonic development. COCs underwent the four treatments described above and, post maturation, were co-incubated with sperm for 22 h. Zygotes were then cultured until day 7 (D7) of development. Cleavage rates were assessed on day 2 (D2, two days post fertilization), and blastocyst rates were evaluated on days 6 (D6) and 7 (D7). Embryos classified as expanded blastocysts on D7 were washed and stored in RNAlater at −80 °C for subsequent gene-expression analysis. The quantification of four genes associated with embryo quality (PLAC8, KRT8, PRDX6, and SLC2A3) was carried out by qPCR, using four pools of 15 embryos from the various treatments.
2.2. Recovery of Cumulus–Oocyte Complexes
Ovaries were obtained from a local slaughterhouse and transported to the laboratory at a temperature of 34 °C ± 2. They were then washed with a 0.9% sodium chloride solution supplemented with 100 μg/mL streptomycin sulfate and 100 IU/mL penicillin G. Follicles measuring between 3 and 8 mm were aspirated using 18 G needles attached to 10 mL syringes. COCs classified as grade I or II [51] were divided into three groups: control (conventional IVM), pre-IVM control [100 nM of natriuretic peptide precursor C, NPPC], and pre-IVM with the addition of melatonin [10−9 M]. The COCs from each group were washed and transferred to 150 μL drops of their respective medium, covered with mineral oil.
2.3. Pre-Maturation (Pre-IVM)
The pre-IVM medium was adapted from previously described methods [21]. It consisted of TCM-199 with Earle’s salts (Gibco®, Invitrogen, Carlsbad, CA, USA) supplemented with 0.075 mg/mL amikacin, 10% fetal bovine serum (FBS) (Gibco®), 0.68 mM L-glutamine, 1 mM sodium pyruvate, 0.1 μM cysteamine, and 10−4 IU/mL recombinant follicle-stimulating hormone (Gonal-F®, Merck Serono, Rockland, MA, USA). NPPC was added at a concentration of 100 nM with or without the addition of 10−9 M melatonin [52,53], diluted in DMSO. The COCs were incubated for 6 h at 38.5 °C in an atmosphere of 5% CO2, 5% O2, and 90% N2. After this period, the structures were transferred to the IVM medium.
2.4. In Vitro Maturation
The IVM medium consisted of TCM-199 with Earle’s salts (Gibco®) supplemented with 10% FBS (Gibco®), 0.01 IU/mL recombinant follicle-stimulating hormone (Gonal-F®, Merck Serono, Rockland, MA, USA), 0.1 mg/mL L-glutamine, 0.075 mg/mL amikacin, 0.1 μM cysteamine, and 0.2 mM sodium pyruvate [7]. IVM was conducted for 22–24 h at 38.5 °C under 5% CO2, 5% O2, and 90% N2.
2.5. In Vitro Fertilization and Culture
Post-IVM, COCs were washed and transferred to fertilization medium, Tyrode’s Albumin Lactate and Pyruvate (TALP), supplemented with 2 mM penicillamine, 1 mM hypotaurine, 250 μM epinephrine, and 10 μg/mL heparin. Frozen semen from a Nellore bull (Bos taurus indicus) was used for fertilization at a final concentration of 1 × 106 sperm/mL. The gametes were co-incubated for 18–20 h. Subsequently, the presumptive zygotes were washed and transferred to synthetic oviduct fluid (SOF) culture medium supplemented with essential and non-essential amino acids, 0.35 mM sodium tricitrate, 2.8 mM myo-inositol [54], and 2.5% FBS (Gibco®). They were incubated under the same conditions previously mentioned. The cleavage rate was assessed on day 2 (D2) post-fertilization, and the blastocyst rate on days 6 (D6) and 7 (D7). Embryos classified as expanded blastocysts on D7 were washed with PBS and stored in RNAlater at −80 °C.
2.6. Gene Expression
Gene expression was analyzed using four pools of twenty oocytes, cumulus cells from twenty oocytes collected before and after IVM, and four pools of 15 expanded blastocysts collected on D7. Cumulus–oocyte complexes were denuded by successive pipetting in PBS without Ca2+ and Mg2+ for immature oocytes and in maintenance medium with 1% hyaluronidase for COCs post-IVM. After separating oocytes and cumulus cells, they were washed three times in PBS without Ca2+ and Mg2+ and subsequently stored in RNAlater at −80 °C until further use.
The extraction of total RNA from oocytes, cumulus cells, and blastocysts was performed separately using the RNeasy Plus Micro kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracted RNA was subsequently used for cDNA synthesis utilizing the GoScript Reverse Transcriptase Kit (Promega, Madison, WI, USA), also following the manufacturer’s guidelines. The first reaction involved an initial incubation at 70 °C for 5 min, followed by a step at 4 °C for 5 min. The second reaction consisted of an annealing step at 25 °C for 5 min, followed by an extension at 42 °C for 60 min and enzyme inactivation at 70 °C for 15 min.
The qPCR reactions were conducted using the GoTaq qPCR Master Mix Kit (Promega). For cumulus cells and blastocyst samples, the reaction conditions were set at 95 °C for 5 min, followed by 50 cycles of denaturation at 95 °C for 15 s, and annealing and extension at 62 °C for 30 s. For oocyte samples, the qPCR protocol was adjusted to 95 °C for 1 min, followed by 50 cycles of denaturation at 95 °C for 15 s, with annealing and extension at 60 °C for 1 min.
Each sample was analyzed in triplicate. The specificity of the PCR reactions was verified by examining the melting curves and the sizes of the amplicons on agarose gels. The nomenclature, primer sequences and concentrations, amplicon sizes, and GenBank accession numbers for each primer pair are detailed in Table 1. GAPDH was selected as the reference gene for normalizing the data.

Table 1.
Information about the specific primers used for the amplification of gene fragments for the quantitative polymerase chain reaction analysis.
2.7. Measurement of Reactive Oxygen Species Levels in Oocytes
Intracellular ROS levels in oocytes were quantified using H2DCFDA (Thermo Fisher, Waltham, MA, USA), dissolved in dimethyl sulfoxide (DMSO) and diluted in PBS with 1% BSA to a final concentration of 50 μM. Denuded oocytes were washed three times in PBS-BSA and then incubated in a 400 μL drop of H2DCFDA solution for 30 min at 38.5 °C in a dark environment. Subsequently, the oocytes were washed three times in PBS-BSA, transferred to a glass slide, and covered with mineral oil for fluorescence microscopy using a Zeiss® Axiophot microscope (Carl Zeiss AG, Oberkochen, Germany). Fluorescence signal intensities (pixels) were quantified at a wavelength of 488 nm, with an excitation power of 9.14%, and emission recorded between 475 and 535 nm. Images were analyzed using ImageJ® imaging software version 1.54 (National Institutes of Health, Bethesda, MD, USA).
2.8. Mitochondria, Lipids, and Chromatin Staining
Denuded oocytes were washed three times in PBS supplemented with 0.3% polyvinylpyrrolidone (PVP) as described previously and incubated for 30 min with MitoTracker Deep Red FM at 400 nM diluted in bench medium at a temperature of 35 to 37 °C. Afterward, the oocytes were washed three times in PBS-PVP and the methodology described previously for lipid staining was followed [7]. The oocytes were fixed for one hour in 4% paraformaldehyde, washed three times in PBS, and stored at 4 °C in 1% paraformaldehyde for up to seven days. Subsequently, they were washed twice in PBS-PVP and incubated for 30 min in PBS supplemented with 0.2% Triton X-100 at 25 to 27 °C. After three washes in PBS with 0.3% PVP, the oocytes were stained with Bodipy 493/503 (Molecular Probes, Eugene, OR, USA) at a concentration of 20 μg/mL (diluted in 50 μL of absolute ethanol and 950 μL of PBS) for one hour. The oocytes were then washed three times in PBS with 0.3% PVP, placed in 35 mm plates in 8 μL drops of anti-fade solution (SlowFade; Molecular Probes, Eugene, OR, USA), and examined under an LSM Leica Sp8 microscope (Leica microsystems, New Orleans, LA, USA). All samples were analyzed and photographed with a 20× objective and a 488 nm argon laser to visualize lipid droplets, with a fluorescence spectrum between 495 and 505 nm. For mitochondrial evaluation, a 638 nm laser was used, with excitation/emission of 644/665 nm. The oocytes underwent up to 20 transverse sections at 4 μm intervals. Z-stacking was employed to create an image of overlapping sections. After constructing the final image of each oocyte, they were adjusted to a grayscale (8-bit image) using the ImageJ program (National Institutes of Health), and the background was corrected. Mitochondrial activity was quantified by the mean number of pixels, and lipid measurements were expressed as a ratio of the area occupied by lipids (number of pixels) to the total area of the oocyte (μm).
After staining with MitoTracker and BODIPY, the oocytes were washed three times in PBS-PVP and then stained with Hoechst 33342 (10 mg/mL) for 15 min in the dark. Following staining, they were washed three times in PBS-PVP and mounted on slides in 8 μL drops of anti-fade solution (SlowFade; Molecular Probes). Chromatin conformation, indicating the meiotic stage, was assessed using fluorescence microscopy (Zeiss® Axioplot). The chromatin was classified into stages: germinative vesicle (GV), germinative vesicle breakdown (GVBD), metaphase I (MI), and metaphase II (MII).
2.9. Statistical Analysis
The Shapiro–Wilk test was employed to assess the normality of the data. Parametric data were analyzed using either the ANOVA test or the unpaired t-test, while non-parametric data were evaluated using the Kruskal–Wallis or Mann–Whitney tests. The chi-squared test was applied to analyze the progression of meiosis and embryonic development. The relative expression of each gene was calculated using the ΔΔCt method with efficiency correction, as described previously [55]. Differences with a p-value of ≤0.05 were considered statistically significant. All analyses were conducted using GraphPad Prism software, version 8.0.1.
3. Results
3.1. Effect of Pre-Maturation on the Meiotic Progression of Oocytes
The meiotic progression of immature oocytes was assessed for the presence of GV or GVBD at 0 h and 6 h pre-IVM. No statistical difference (p > 0.05) was observed among the IC (20/20, 100%), PMIC (18/18, 100%), and PMMI (12/12, 100%) groups. Additionally, after IVM, the oocytes were evaluated for their ability to reach MII, and once again, no statistical difference (p > 0.05) was noted among the MC (17/18, 94.4%), PMMC (9/9, 100%), and PMMM (12/12, 100%) groups. Thus, pre-IVM treatment, whether in the absence or presence of melatonin, did not interfere with the oocytes’ ability to complete nuclear maturation.
3.2. Effect of Melatonin During Pre-Maturation on Reactive Oxygen Species Levels in Oocytes
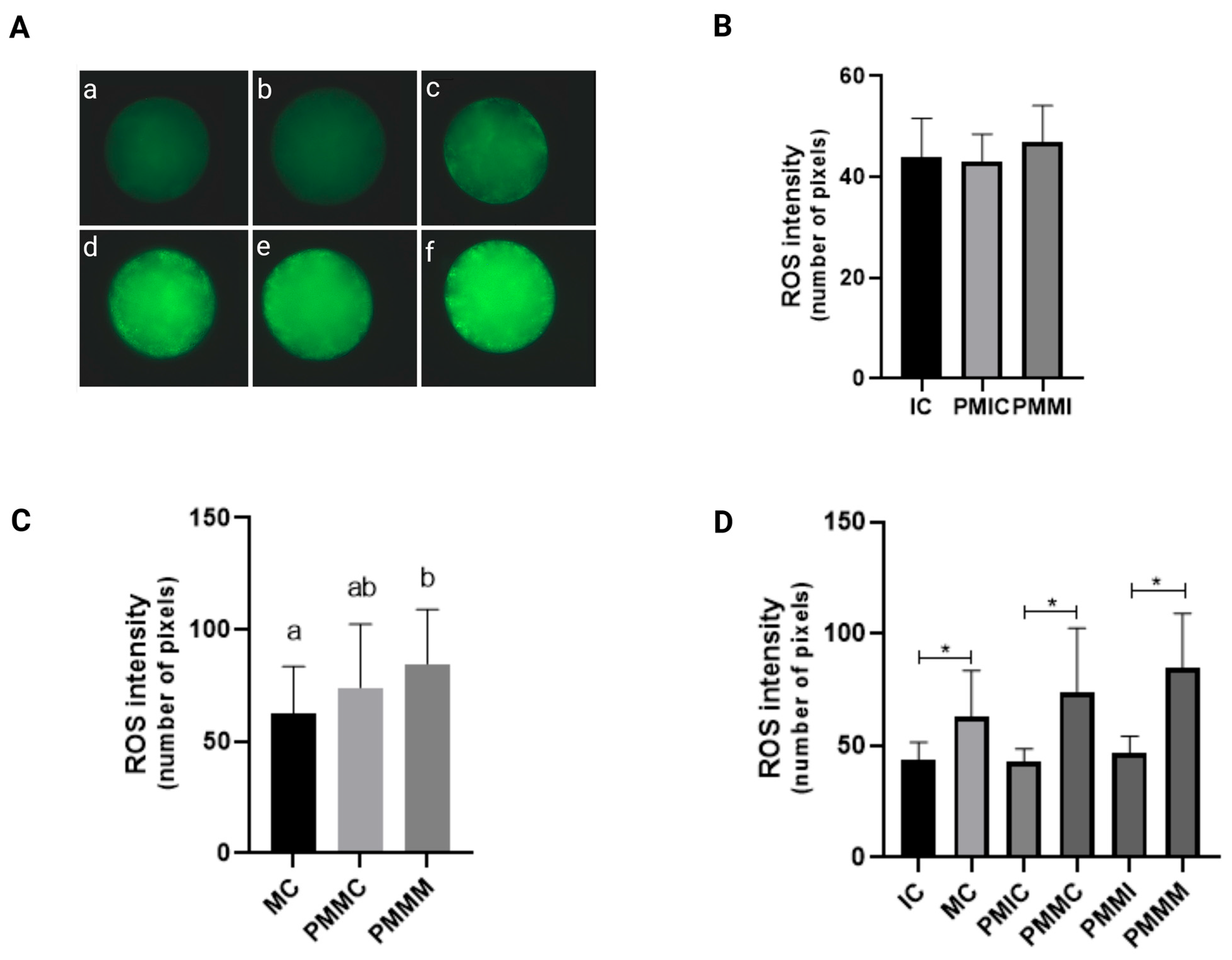
The levels of ROS were measured in oocytes subjected to different treatments and collected at different time points (before and after IVM). Initially, the immature groups—the control (IC) and those retained for 6 h before maturation (PMIC and PMMI)—were compared. The results demonstrated that ROS levels were similar (p > 0.05) across all groups. However, upon evaluation after IVM, the group that underwent pre-IVM with melatonin (PMMI) exhibited higher ROS levels (p ≤ 0.05) compared to the MC group and similar levels to the PMMC group. When all groups were compared at 24 h of maturation, an increase in ROS levels (p ≤ 0.05) during IVM was observed across all groups (Figure 1).
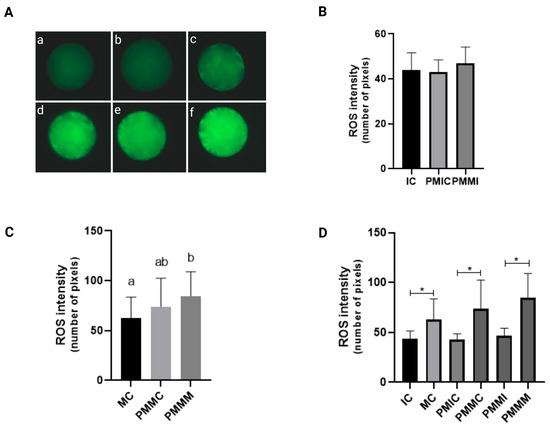
Figure 1.
Representative images of bovine oocytes stained with H2DCFDA (A): Intracellular levels of reactive oxygen species in oocytes in different treatments at different time points. Immature control, 0 h ((a): IC; n = 25); pre-matured for 6 h ((b): PMIC; n = 26); pre-matured in the presence of melatonin for 6 h ((c): PMMI; n = 27); matured control, 24 h ((d): MC; n = 27); pre-matured for 6 h and matured for 24 h ((e): PMMC; n = 23); and pre-matured in the presence of melatonin for 6 h and matured for 24 h ((f): PMMM; n = 16). (B): immature groups; (C): matured groups; (D) before and after in vitro maturation. a,b Different superscripts or * between groups indicate significant differences (p ≤ 0.05).
3.3. Effect of Melatonin During Pre-Maturation on Mitochondrial Activity in Oocytes
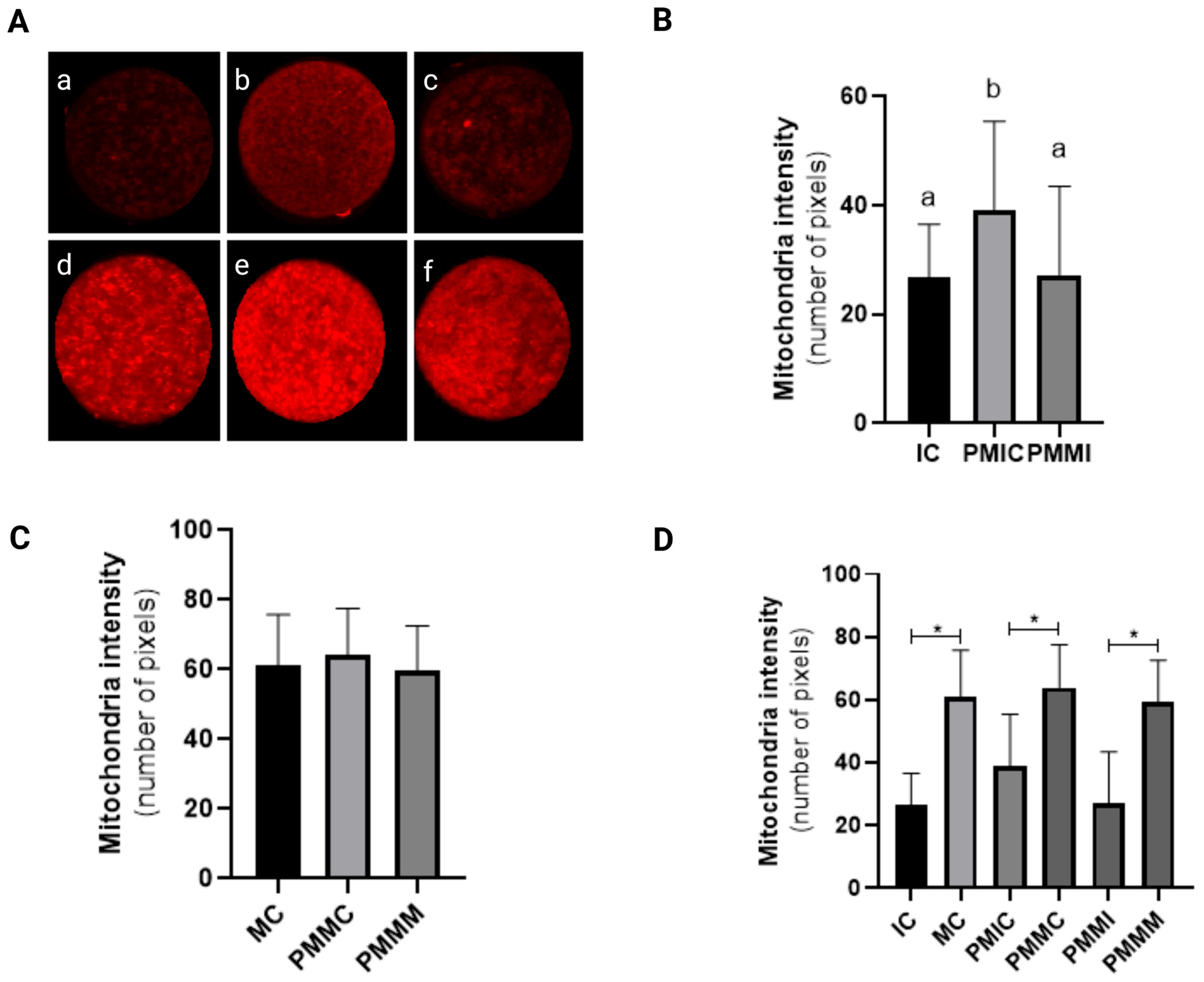
Before IVM, oocytes from the PMIC group displayed higher mitochondrial fluorescence intensity (p ≤ 0.05) compared to those from the IC and PMMI groups. Following IVM, mitochondrial fluorescence levels were similar across all groups (MC, PMMC, and PMMM) (p > 0.05). Additionally, an increase in mitochondrial fluorescence intensity post-IVM was observed in all groups (p ≤ 0.05) when compared to their respective immature stages (Figure 2).
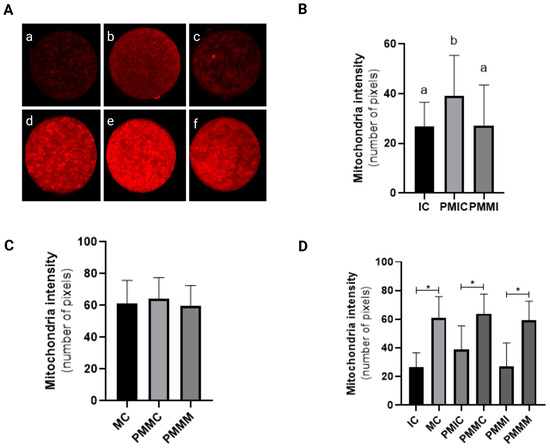
Figure 2.
Representative images of oocytes stained with MitoTracker Deep Red (A). Level of mitochondrial fluorescence intensity in oocytes stained with MitoTracker Deep Red in the different groups. Immature control, 0 h ((a): IC; n = 32); pre-matured for 6 h ((b): PMIC; n = 31); pre-matured in the presence of melatonin for 6 h ((c): PMMI; n = 23); matured control, 24 h ((d): MC; n = 20); pre-matured for 6 h and matured for 24 h ((e): PMMC; n = 17); and pre-matured in the presence of melatonin for 6 h and matured for 24 h ((f): PMMM; n = 20); (B): immature groups; (C): matured groups; (D) before and after in vitro maturation. a,b Different superscripts or * between groups indicate significant differences (p ≤ 0.05).
3.4. Effect of Melatonin During Pre-Maturation on the Lipid Content of Oocytes
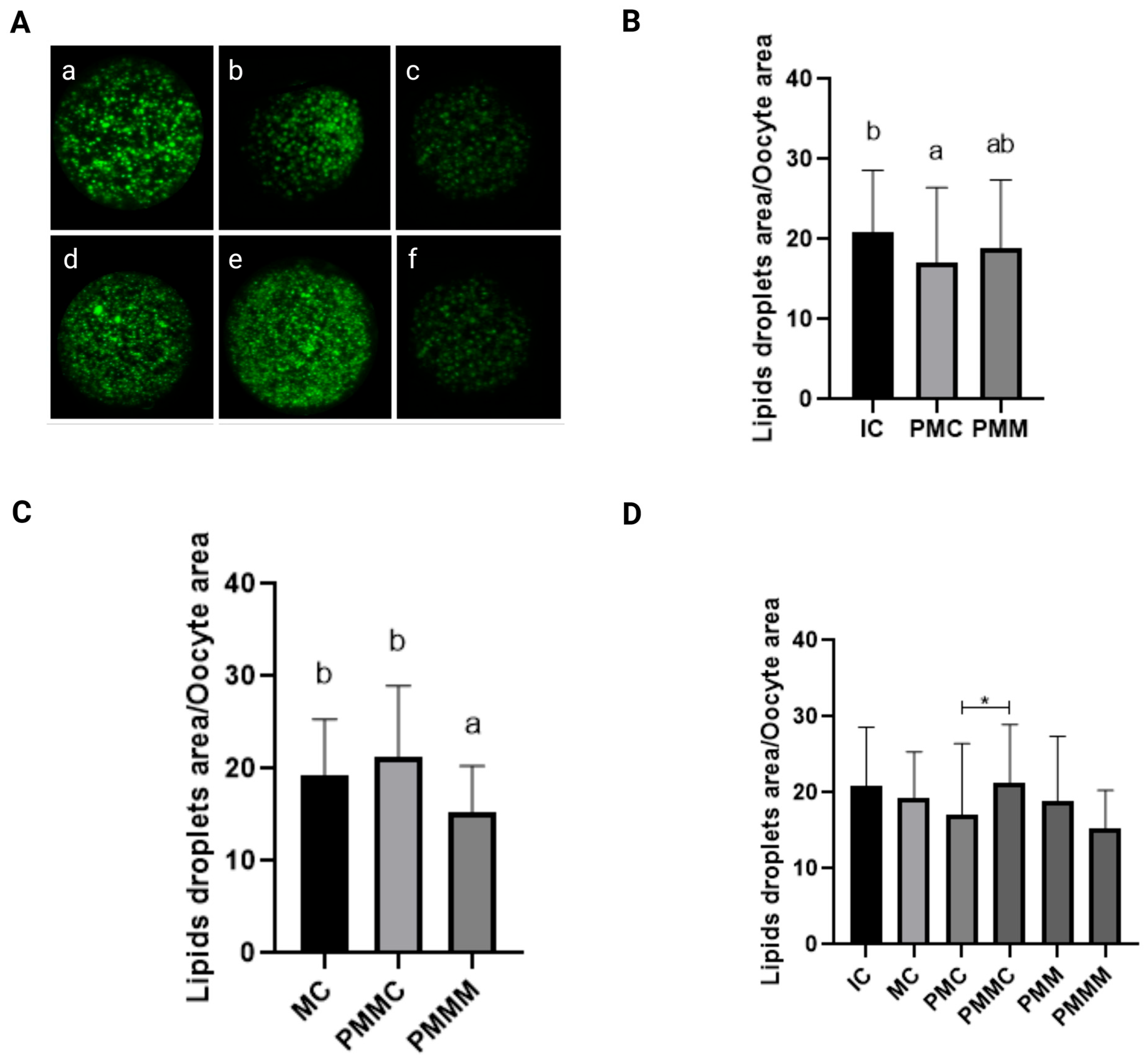
Lipid content in oocytes was quantitatively assessed (Figure 3). Before IVM, oocytes from the pre-IVM control group (PMIC) exhibited a reduced quantity of lipid droplets (p ≤ 0.05) compared to the IC group. Their lipid levels were comparable (p > 0.05) to those in the group pre-matured with melatonin exposure. However, post 24 h of maturation, the group treated with melatonin displayed the lowest amount of lipid droplets (p ≤ 0.05).
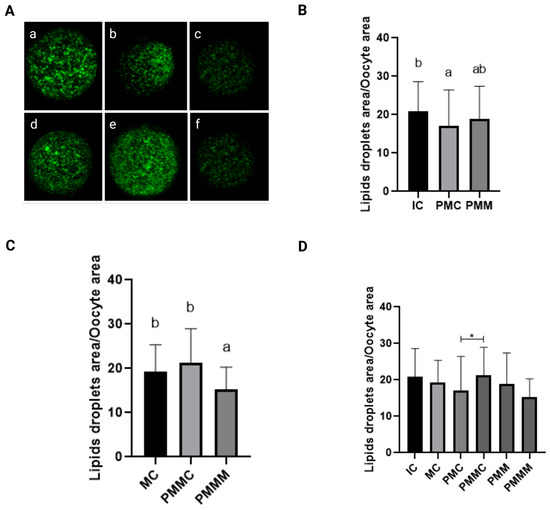
Figure 3.
Representative images of oocytes stained with Bodipy 493/503 (A). Immature control, 0 h ((a): IC; n = 32); pre-matured for 6 h ((b): PMIC; n = 31); pre-matured in the presence of melatonin for 6 h ((c): PMMI; n = 23); matured control, 24 h ((d): MC; n = 20); pre-matured for 6 h and matured for 24 h ((e): PMMC; n = 17); and pre-matured in the presence of melatonin for 6 h and matured for 24 h ((f): PMMM; n = 20); Area of lipid droplets relative to the total area of the oocytes to evaluate the lipid droplets in the different groups. (B): immature groups; (C): matured groups; (D) before and after in vitro maturation. a,b Different superscripts or * between groups indicate significant differences (p ≤ 0.05).
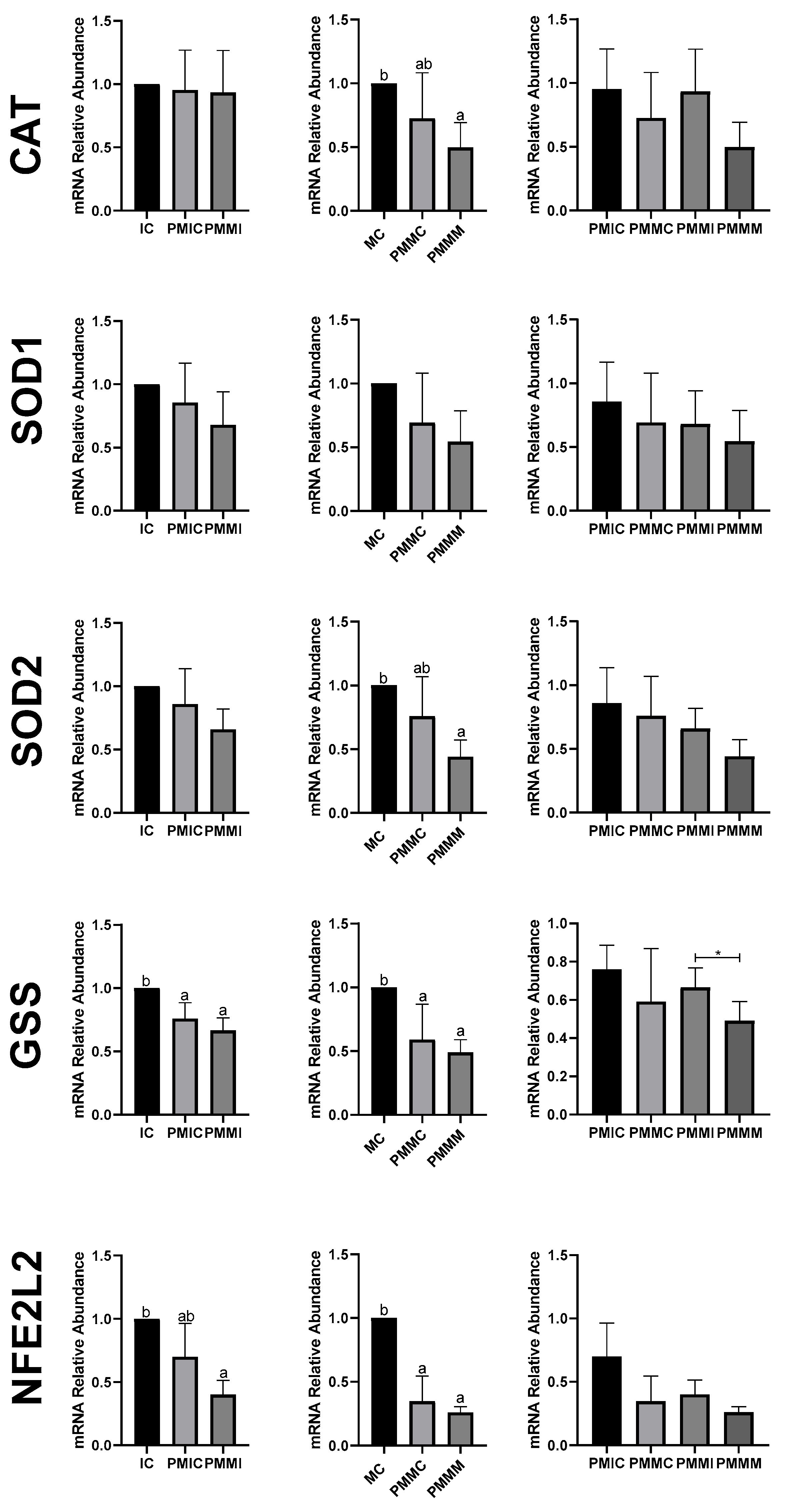
3.5. Effect of Melatonin During Pre-Maturation on Gene Expression in Cumulus Cells and Oocytes
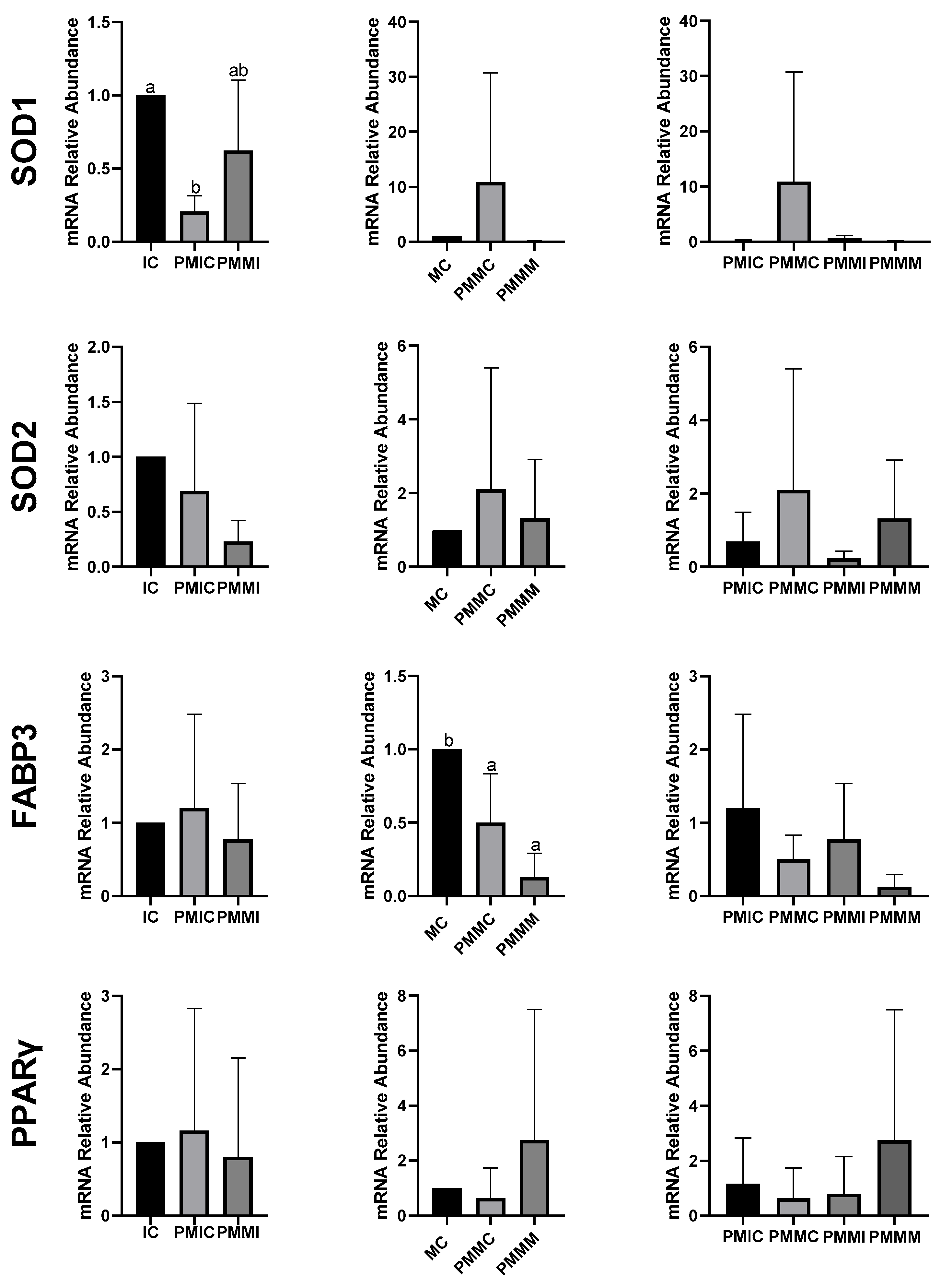
The transcript levels of genes involved in antioxidant defense (SOD1 and SOD2) and lipid metabolism (FABP3 and PPARγ) were quantified in cumulus cells from all groups, before and after IVM. The results are depicted in Figure 4. The PMIC group showed lower transcript levels of the SOD1 gene (p ≤ 0.05) compared to the IC group. However, transcript levels for the PMMI group were similar to those of the IC and PMIC groups. Post-IVM, similar (p > 0.05) transcript levels of SOD1 were observed across the MC, PMMC, and PMMM groups. Regarding the FABP3 gene, after maturation, transcript levels decreased in both groups subjected to pre-IVM, regardless of melatonin exposure (PMMC and PMMM), compared to the MC group (Figure 4).
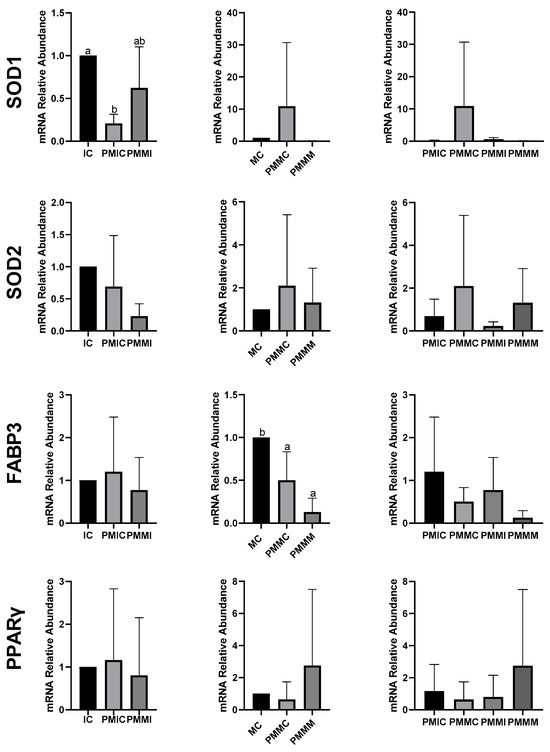
Figure 4.
Relative mRNA levels of genes related to antioxidant defense (SOD1 and SOD2) and lipid metabolism (FABP3 and PPARγ) in cumulus cells of the cumulus–oocyte complex from different treatments and time points: immature control, 0 h (IC); pre-matured for 6 h (PMIC); pre-matured in the presence of melatonin for 6 h (PMMI); matured control, 24 h (MC); pre-matured for 6 h and matured for 24 h (PMMC); and pre-matured in the presence of melatonin for 6 h and matured for 24 h (PMMM). a,b Different superscripts indicate significant differences between groups (p ≤ 0.05).
In oocytes (Figure 5), transcript levels of the GSS gene were reduced (p ≤ 0.05) after 6 h of pre-IVM in both the PMIC and PMMI groups compared to the IC group. The level of NFE2L2 transcripts decreased only in the PMMI group compared to the IC group. Post-IVM, transcript levels of CAT and SOD2 were lower in the PMMM group compared to the MC group, while GSS and NFE2L2 transcripts were reduced in both pre-IVM groups (PMMC and PMMM) relative to the control group (MC).
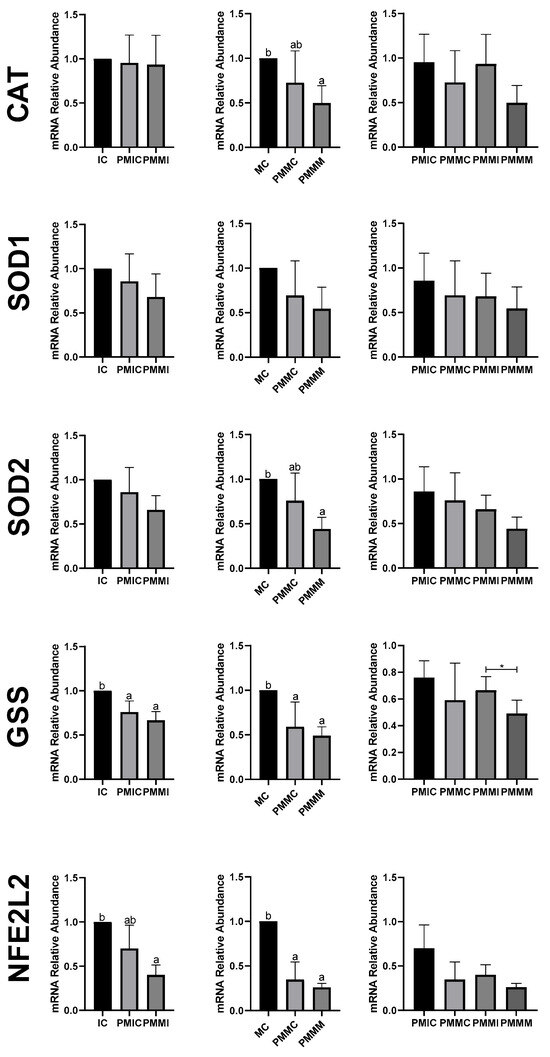
Figure 5.
Relative mRNA levels of genes related to antioxidant defense (CAT, SOD1, SOD2, GSS, and NFE2L2) in oocytes in different treatments at different time points: immature control, 0 h (IC); pre-matured for 6 h (PMIC); pre-matured in the presence of melatonin for 6 h (PMMI); matured control, 24 h (MC); pre-matured for 6 h and matured for 24 h (PMMC); and pre-matured in the presence of melatonin for 6 h and matured for 24 h (PMMM). a,b Different superscripts or * between groups indicate significant differences (p ≤ 0.05).
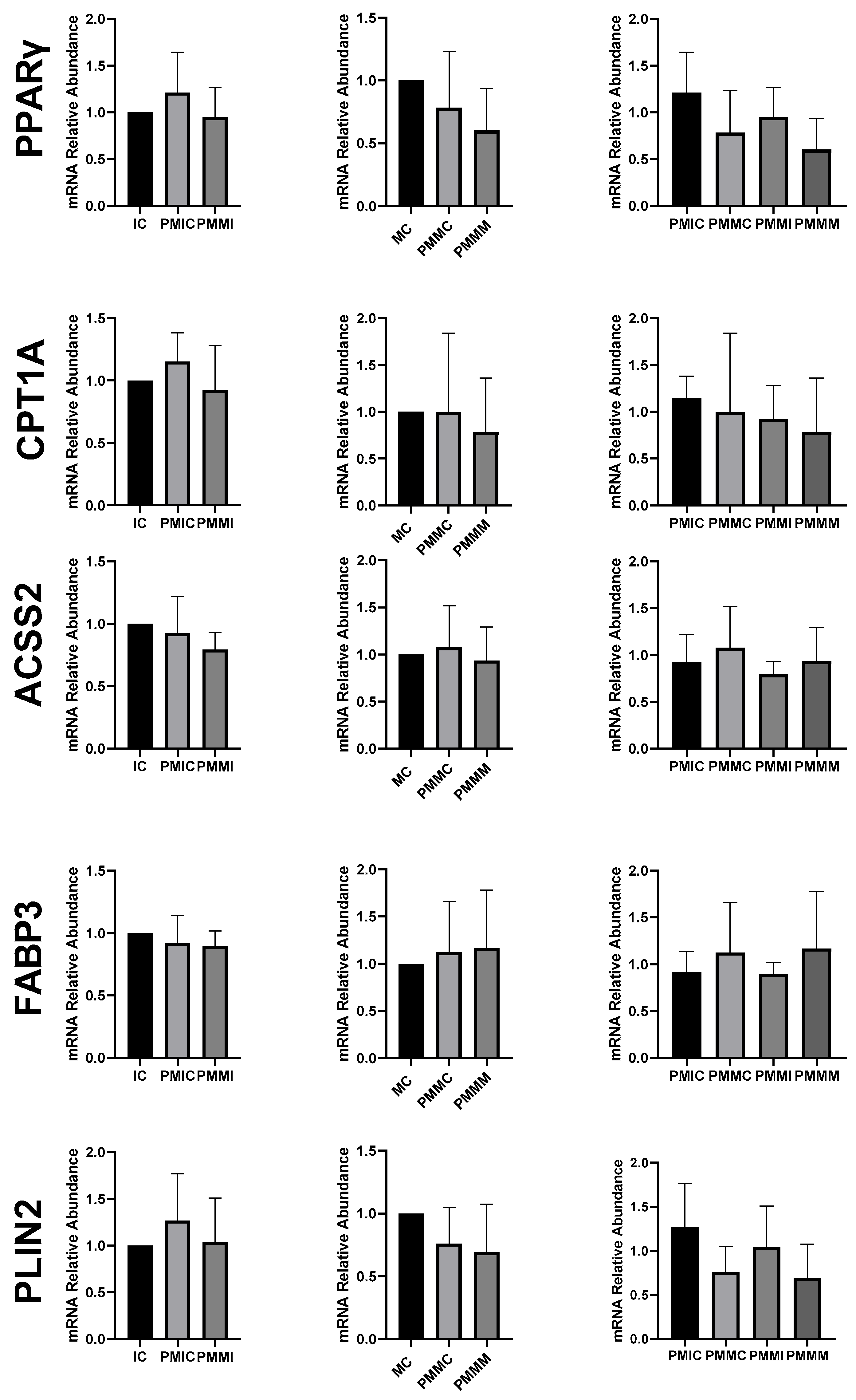
The expression of genes associated with lipid metabolism (PPARγ, CPT1A, ACSS2, FABP3, and PLIN2) did not differ (p > 0.05) among the oocytes of the evaluated groups (Figure 6).
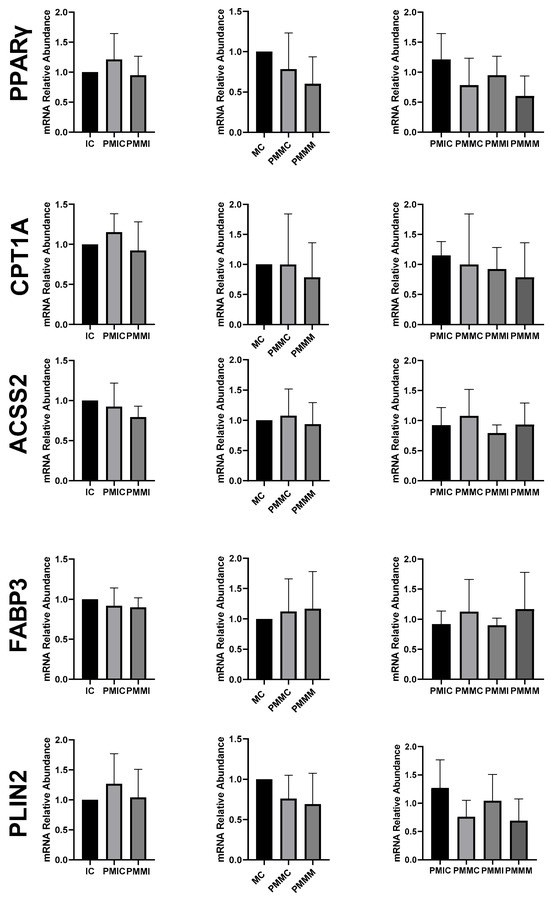
Figure 6.
Relative mRNA levels of genes associated with lipid metabolism (PPARγ, CPT1A, ACSS2, FABP3, and PLIN2) in oocytes in different treatments at different time points: immature control, 0 h (IC); pre-matured for 6 h (PMIC); pre-matured in the presence of melatonin for 6 h (PMMI); matured control, 24 h (MC); pre-matured for 6 h and matured for 24 h (PMMC); and pre-matured in the presence of melatonin for 6 h and matured for 24 h (PMMM).
3.6. Effect of Melatonin During Pre-Maturation on Embryonic Development
Embryonic development (Table 2) was evaluated by assessing 1752 COCs randomly assigned to different treatment groups. The COCs from the pre-IVM control and pre-IVM + MTn groups demonstrated superior (p ≤ 0.05) embryonic development compared to the control group in terms of cleavage rates at D2 (79.4% and 66.0%, respectively) and blastocyst formation at D7 (35.9% and 25.8%, respectively). However, at D6, the pre-IVM + MTn group exhibited a lower (p ≤ 0.05) blastocyst rate (14.1%) compared to the control (18.5%) and pre-IVM control (20.3%) groups. No differences were observed among groups in the different embryonic developmental stages at D6 or D7 (p > 0.05).

Table 2.
Embryonic development of pre-matured cumulus–oocyte complex in the presence of melatonin.
3.7. Effect of Melatonin During Pre-Maturation on Gene Expression in Blastocysts
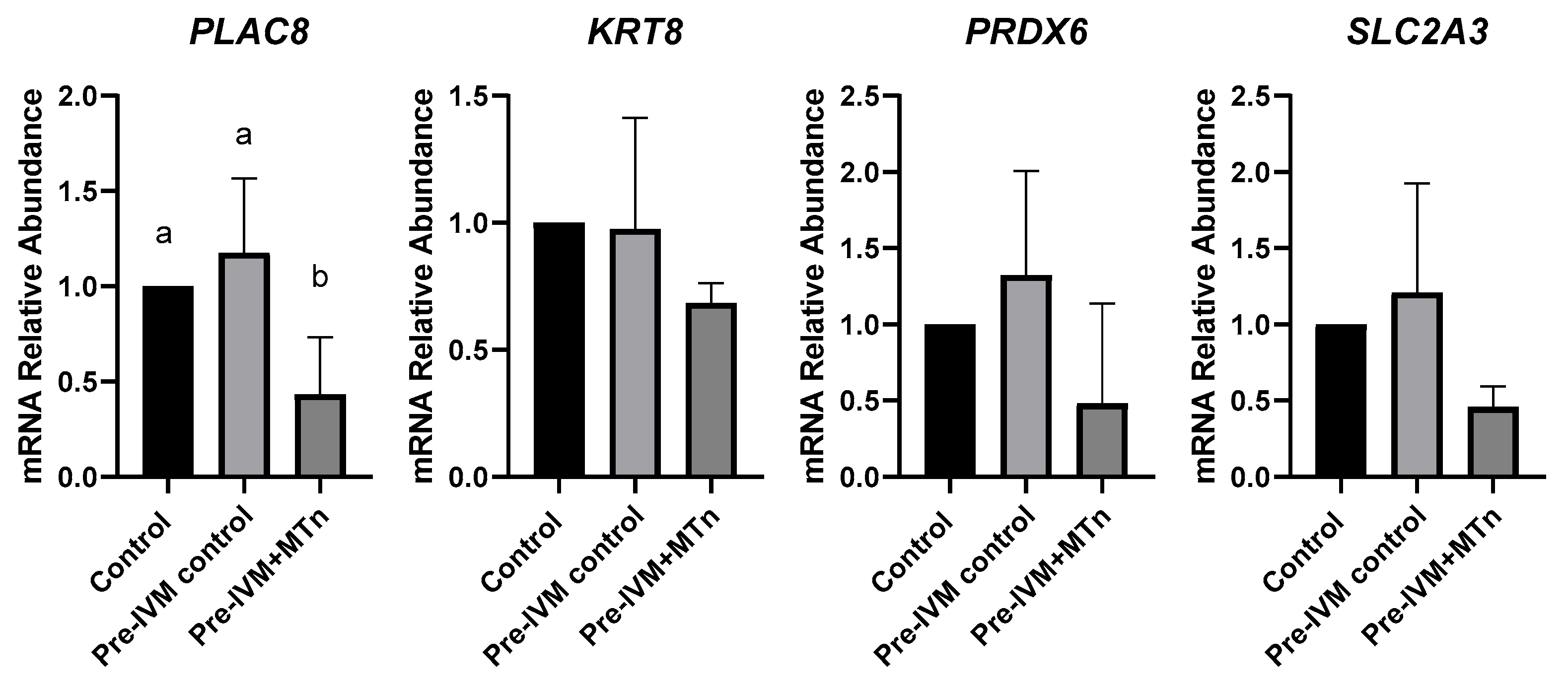
To evaluate embryonic quality, the transcript levels of the PLAC8, KRT8, PRDX6, and SLC2A3 genes were quantified in blastocysts from the three treatments (control, pre-IVM control, and pre-IVM + MTn). Only the PLAC8 gene showed a significant difference between the investigated genes, with its transcription level reduced (p ≤ 0.05) in the pre-IVM + MTn group (Figure 7).
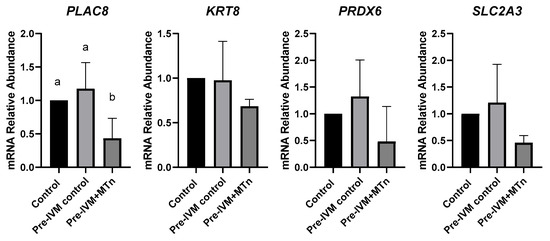
Figure 7.
Relative mRNA levels of genes related to embryo quality (PLAC8, KRT8, PRDX6, and SLC2A3) in expanded blastocysts from three groups: control (in vitro maturation, 24 h); pre-IVM control (pre-IVM for 6 h and MIV for 24 h); and pre-IVM + MTn (pre-IVM in the presence of melatonin for 6 h, and MIV for 24 h). a,b Different superscripts indicate significant differences between groups (p ≤ 0.05).
4. Discussion
Since oocytes spontaneously resume meiosis upon removal from the follicular environment, regardless of their developmental competence, many oocytes subjected to IVM are not fully competent to support embryonic development. To address this, a pre-IVM period can provide additional time for essential processes such as cytoplasmic reorganization and better synchronization with nuclear events. We hypothesized that enhancing the pre-IVM environment with melatonin, known for its potent antioxidant properties and potential regulation of lipid metabolism, could improve developmental competence. This hypothesis is supported by employing the physiological agent NPPC to delay meiosis for 6 h [21], alongside with melatonin at a concentration of 10−9 M established for bovine oocytes and embryos [46,52,53].
To investigate the effects of melatonin supplementation during meiotic arrest on oocyte quality, we initially evaluated intracellular ROS levels across experimental groups. Melatonin supplementation during the 6 h pre-IVM did not reduce ROS levels, as expected. Previous studies suggest that melatonin can improve oocyte quality and embryo development rate, mainly by reducing oxidative damage during IVM [41]. However, the beneficial effects of melatonin can be potentiated in conditions that cause high oxidative stress [35,44,56,57]. The use of a system with low oxygen tension, which reduces ROS production [58,59], may have constrained the additional antioxidant benefits of melatonin. Another contributing factor is that the 6 h period might not have been adequate to induce a reduction in ROS. In certain contexts, antioxidants like melatonin can have pro-oxidant effects, depending on the dosage and cellular environment, potentially increasing ROS levels instead of reducing them [60,61]. Moreover, an increase in ROS levels after IVM was observed in all groups. This increase can be attributed to various intrinsic and extrinsic factors related to the in vitro environment, which is more stressful than the physiological environment [62]. The exposure of oocytes to artificial conditions such as temperature, pH, medium composition, and atmospheric oxygen can induce ROS production [6].
The increase in ROS levels coincided with an increase in mitochondrial activity observed during IVM in all evaluated groups. During maturation, the oocyte’s energy demand increases, and the production of ATP through the mitochondrial electron transport chain results in the formation of ROS [63]. The efficiency of the repair mechanisms determines the amount of ROS [64]. The absence of significant differences between the groups after maturation indicates that melatonin did not interfere with mitochondrial activity.
Considering that melatonin, in addition to its antioxidant potential, is also associated with lipid metabolism [47,48], we evaluated whether its presence had any effect on oocytes. Melatonin supplementation did not affect lipid storage when immature oocytes were evaluated after the retention period. However, after IVM, oocytes exposed to melatonin exhibited the lowest concentration of lipids. This effect of melatonin was particularly evident when comparing the pre-IVM groups, which, despite having similar lipid content after retention, displayed a difference in content at 24 h of maturation, suggesting that these oocytes used lipids more efficiently to support the high energy metabolism necessary during maturation. Another possible explanation for the reduction in lipids could be that melatonin reduced the lipogenic capacity of the oocytes to store fatty acids in lipid droplets.
Lipids are essential for cytoplasmic maturation and provide the necessary energy during the early stages of embryonic development [65]. The storage of lipids in oocytes is a natural process, crucial for ensuring energy availability. However, the quantity and balance of these lipids are critical. Excess lipids in the oocyte cytoplasm can lead to problems that affect oocyte quality and embryonic development, such as oxidative stress, mitochondrial dysfunction, and abnormalities in cytoplasmic maturation [66]. In fact, oocytes matured in vitro have been shown to have a higher lipid content than those matured in vivo, indicating that the in vitro environment is more challenging and detrimental to oocyte quality [7].
To investigate the effect of melatonin, we analyzed the expression of genes involved in oxidative stress and lipid metabolism in cumulus cells and oocytes, both after retention and after IVM. Melatonin supplementation in the pre-IVM phase did not increase the transcript levels of genes associated with antioxidant defense (CAT, SOD1, SOD2, GSS, and NFE2L2) in oocytes at any of the time points evaluated. On the contrary, a reduction in CAT and SOD2 levels was observed after IVM, as well as in GSS and NFE2L2 both before and after IVM, contrasting with studies that supplemented melatonin directly during IVM [43,46,52,67,68]. Although antioxidant transcript levels were already altered between the groups after pre-IVM, no phenotypic change was observed until the MIV phase, when energy demand increases, and modulation of gene expression adjusts ROS levels. These results suggest that, in the long term, transcript levels may play a determining role. However, this effect does not seem to be exclusive to melatonin supplementation, as the pre-IVM control group also showed similar results in terms of the abundance of mRNA transcripts and the level of ROS. It has been reported that C-type natriuretic peptide (CNP) treatment in pre-IVM increases mitochondrial activity and antioxidant defense by up-regulating genes [69], but this effect was not observed in the present study.
Regarding lipid metabolism, melatonin appears to have had little influence through gene regulation. The reduction in FABP3 transcript levels in cumulus cells after IVM occurred in the pre-IVM groups, independently of the presence of melatonin. The FABP gene is responsible for encoding proteins that bind long-chain fatty acids and is associated with lipid accumulation during IVM, as well as with increased transcript levels in cumulus cells [70]. Although the group treated with melatonin at pre-IVM showed a slight reduction in lipid droplets, supplementation did not alter the expression of genes related to lipid metabolism (PPARγ, CPT1A, FABP3, PLIN2, and ACSS2). This result differs from those observed in porcine oocytes matured with melatonin, where the transcription of genes such as PPARγ, PLIN2, and CPT1A was increased [47,71].
Despite the apparent reduction in FABP3 transcripts in cumulus cells, the lipid content of the oocytes in the pre-IVM control group remained similar to that of the post-IVM control group. This suggests that the decrease in lipid droplets observed in pre-matured oocytes treated with melatonin is not due to a lower transport of fatty acids mediated by FABP3, but rather to a reduced capacity to synthesize new droplets for energy storage. The increase in free fatty acids in the cytoplasm may contribute to the rise in ROS in oocytes pre-matured with melatonin due to lipoperoxidation, and not necessarily mitochondrial activity, as no statistical differences were present between the groups assessed by MitoTracker staining or in the levels of CPT1A gene transcripts.
The most critical assessment of the oocyte’s competence is its ability to cleave and develop into an embryo after IVF and IVC. Although the implementation of the pre-IVM phase generates controversial results regarding the improvement of embryonic development, sometimes showing no influence [18] or providing benefits [72,73], we observed that the pre-IVM groups had higher cleavage (D2) and blastocyst (D7) rates than the control group. This suggests that pre-IVM improved the quality of oocyte development and could be an attractive alternative to the low-oxygen tension system, given the inconsistent results on embryonic development in published works [74,75,76,77] and the low rates obtained in this study. Melatonin supplementation, on the other hand, did not enhance the beneficial effect observed with pre-IVM treatment.
Finally, embryonic quality was assessed by the expression of genes associated with placentation (PLAC8 and KRT8), antioxidant defense (PDRX6), and cell metabolism (SLC2A3). Only the PLAC8 gene showed a reduction in transcript levels. This gene is related to placentation, playing a role in placental development and maternal–fetal communication [78]. Its expression has been positively associated with D7 embryos that progress to a live birth [79] but contrasting results have been observed in high- and low-quality D14 embryos [80]. Thus, it is possible that melatonin during pre-IVM may have influenced later embryonic development.
5. Conclusions
We conclude that melatonin supplementation during pre-IVM had no beneficial effects on oocyte competence and bovine embryonic development. This result can be attributed to the brief exposure period of 6 h, considering that previous studies have shown positive effects of melatonin, such as the reduction in ROS and improvement in mitochondrial activity, when used throughout the 24 h IVM period in cattle. In addition, the role of melatonin as a regulator of lipid metabolism has been investigated predominantly in porcine oocytes, in IVM protocols with 41 h of exposure. This suggests that the duration and context of supplementation may be crucial factors in observing the potential benefits of melatonin. Although the mechanism by which pre-IVM affects gene regulation, especially in relation to antioxidant defense factors, is still unclear, pre-IVM treatment, independently of melatonin supplementation, showed promising results for embryonic development. This pre-IVM protocol could therefore be an interesting option for low-oxygen tension systems.
Author Contributions
L.K.L.P. was involved in the preparation of the manuscript; substantial contributions to conception and design, data acquisition, data analysis, and interpretation; N.R.K.: Data acquisition, data analysis and interpretation; J.E.V.C.: Data acquisition, data analysis and interpretation; H.B.S.A.: Data acquisition, data analysis and interpretation; M.M.F.: Data acquisition, data analysis and interpretation; J.F.W.S.: Accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; M.A.N.D.: Accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council for Scientific and Technological Development (CNPq), Brazilian Agricultural Research Corporation (EMBRAPA) and the Federal District Research Support Foundation (FAPDF, Grant No.: 00193.10832025/2025-64).
Institutional Review Board Statement
The use of biological material from animals registered under protocol no. MB65/23 is in accordance with the precepts of Law No. 11794 of 8 October 2008, Decree No. 6899 of 15 July 2009, and with the standards issued by the National Council for the Control of Animal Experimentation and was approved by the Ethics Committee on the Use of Animals of the University Federal of Goiás.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors Daniel Graziani for assistance with confocal microscopy, and Frigoias and Bom Corte (Anápolis and Formosa-GO, Brazil) for providing the biological materials required for this experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Pre-IVM | pre-in vitro maturation |
| COCs | cumulus-oocyte complexes |
| IVP | in vitro embryo production |
| ROS | reactive oxygen species |
| IVM | in vitro maturation |
| CCs | cumulus cells |
| Pre-IVM Control | COCs underwent pre-IVM for 6 h, followed by 24 h of IVM |
| Pre-IVM + MTn | COCs pre-matured with melatonin for 6 h, followed by 24 h of IVM |
| IC | immature control |
| MC | mature control |
| PMIC | COCs after 6 h of pre-IVM for groups without melatonin supplementation |
| PMMC | after 6 h of pre-IVM and after 24 h of IVM for groups without melatonin supplementation |
| PMMI | after 6 h of pre-IVM for groups with melatonin supplementation |
| PMMM | after 6 h of pre-IVM and after 24 h of IVM for groups with melatonin supplementation |
| D | days post-fertilization |
| NPPC | Natriuretic peptide precursor C |
| CNP | C-type natriuretic peptide |
References
- Caixeta, E.S.; Ripamonte, P.; Franco, M.M.; Junior, J.B.; Dode, M.A.N. Effect of follicle size on mRNA expression in cumulus cells and oocytes of Bos indicus: An approach to identify marker genes for developmental competence. Reprod. Fertil. Dev. 2009, 21, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Strączyńska, P.; Papis, K.; Morawiec, E.; Czerwiński, M.; Gajewski, Z.; Olejek, A.; Bednarska-Czerwińska, A. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod. Biol. Endocrinol. 2022, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Roelen, B.A.J. Bovine oocyte maturation: Acquisition of developmental competence. Reprod. Fertil. Dev. 2019, 32, 98–103. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Pan, X.; Wang, P.; Yuan, X.; Ma, B. Advances in Oocyte Maturation In Vivo and In Vitro in Mammals. Int. J. Mol. Sci. 2023, 24, 9059. [Google Scholar] [CrossRef]
- Takahashi, M. Oxidative Stress and Redox Regulation on In Vitro Development of Mammalian Embryos. J. Reprod. Dev. 2012, 58, 1–9. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Paramio, M.-T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef]
- Faria, O.A.C.; Kawamoto, T.S.; Dias, L.R.O.; Fidelis, A.A.G.; Leme, L.O.; Caixeta, F.M.C.; Gomes, A.C.M.M.; Sprícigo, J.F.W.; Dode, M.A.N. Maturation system affects lipid accumulation in bovine oocytes. Reprod. Fertil. Dev. 2021, 33, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Franciosi, F.; Coticchio, G.; Lodde, V.; Tessaro, I.; Modina, S.C.; Fadini, R.; Dal Canto, M.; Renzini, M.M.; Albertini, D.F.; Luciano, A.M. Natriuretic peptide precursor C delays meiotic resumption and sustains gap junction-mediated communication in bovine cumulus-enclosed oocytes. Biol. Reprod. 2014, 91, 61. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, Q.; Cai, J.; Zhao, X.; Ma, B. Effect of C-Type Natriuretic Peptide on Maturation and Developmental Competence of Goat Oocytes Matured In Vitro. PLoS ONE 2015, 10, e0132318. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.-Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Fan, X.; Li, R.; Zhang, J. Effect of C-type natriuretic peptide pretreatment on in vitro bovine oocyte maturation. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 199–206. [Google Scholar] [CrossRef]
- Zhong, Y.; Lin, J.; Liu, X.; Hou, J.; Zhang, Y.; Zhao, X. C-Type natriuretic peptide maintains domestic cat oocytes in meiotic arrest. Reprod. Fertil. Dev. 2016, 28, 1553–1559. [Google Scholar] [CrossRef]
- Gong, X.; Li, H.; Zhao, Y. The Improvement and Clinical Application of Human Oocyte In Vitro Maturation (IVM). Reprod. Sci. 2022, 29, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.H.; Tran, V.Q.; Le, A.H.; Nguyen, D.L.; Pham, T.D.; Vu, A.L.; Le, T.K.; Le, H.L.; Huynh, B.G.; Ho, T.M.; et al. Impact of low versus high oxygen tension on human oocyte maturation during biphasic capacitation IVM (CAPA-IVM). J. Assist. Reprod. Genet. 2025, 1–8. [Google Scholar] [CrossRef]
- Sanchez, F.; Le, A.H.; Ho, V.N.A.; Romero, S.; Van Ranst, H.; De Vos, M.; Gilchrist, R.B.; Ho, T.M.; Vuong, L.N.; Smitz, J. Biphasic in vitro maturation (CAPA-IVM) specifically improves the developmental capacity of oocytes from small antral follicles. J. Assist. Reprod. Genet. 2019, 36, 2135–2144. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Ho, T.M.; De Vos, M.; Sanchez, F.; Romero, S.; Ledger, W.L.; Anckaert, E.; Vuong, L.N.; Smitz, J. A fresh start for IVM: Capacitating the oocyte for development using pre-IVM. Hum. Reprod. Update 2024, 30, 3–25. [Google Scholar] [CrossRef]
- Zhenwei, J.; Xianhua, Z. Pre-IVM treatment with C-type natriuretic peptide in the presence of cysteamine enhances bovine oocytes antioxidant defense ability and developmental competence in vitro. Iran. J. Vet. Res. 2019, 20, 173–179. [Google Scholar] [PubMed]
- Diógenes, M.N.; Guimarães, A.L.S.; Leme, L.O.; Maurício, M.F.; Dode, M.A.N. Effect of prematuration and maturation with fibroblast growth factor 10 (FGF10) on in vitro development of bovine oocytes. Theriogenology 2017, 102, 190–198. [Google Scholar] [CrossRef]
- Guimarães, A.L.S.; Pereira, S.A.; Leme, L.O.; Dode, M.A.N. Evaluation of the simulated physiological oocyte maturation system for improving bovine in vitro embryo production. Theriogenology 2015, 83, 52–57. [Google Scholar] [CrossRef]
- Caixeta, F.M.C.; Sousa, R.V.; Guimarães, A.L.; Leme, L.O.; Sprícigo, J.F.W.; Netto, S.B.S.; Pivato, I.; Dode, M.A.N. Meiotic arrest as an alternative to increase the production of bovine embryos by somatic cell nuclear transfer. Zygote 2017, 25, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Saeedabadi, S.; Abazari-Kia, A.H.; Rajabi, H.; Parivar, K.; Salehi, M. Melatonin Improves The Developmental Competence of Goat Oocytes. Int. J. Fertil. Steril. 2018, 12, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Roura, M.; Catalá, M.G.; Menéndez-Blanco, I.; Izquierdo, D.; Fouladi-Nashta, A.A.; Paramio, M.T. Beneficial effects of melatonin on in vitro embryo production from juvenile goat oocytes. Reprod. Fertil. Dev. 2018, 30, 253–261. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Catalá, M.-G.; Roura, M.; Menéndez-Blanco, I.; Piras, A.-R.; Izquierdo, D.; Paramio, M.-T. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 2019, 54, 381–390. [Google Scholar] [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Il Jin, D. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol. Reprod. Dev. 2018, 85, 665–681. [Google Scholar] [CrossRef]
- Park, H.-J.; Park, J.-Y.; Kim, J.-W.; Yang, S.-G.; Jung, J.-M.; Kim, M.-J.; Kang, M.-J.; Cho, Y.H.; Wee, G.; Yang, H.-Y.; et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 2018, 64, e12458. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, Z.; Cui, M.; Zhang, L.; Li, Q. Melatonin Restores the Developmental Competence of Heat Stressed Porcine Oocytes, and Alters the Expression of Genes Related to Oocyte Maturation. Animals 2021, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Du, M.; Zhuan, Q.; Luo, Y.; Li, J.; Hou, Y.; Zeng, S.; Zhu, S.; Fu, X. Melatonin rescues the aneuploidy in mice vitrified oocytes by regulating mitochondrial heat product. Cryobiology 2019, 89, 68–75. [Google Scholar] [CrossRef]
- Keshavarzi, S.; Salehi, M.; Farifteh-Nobijari, F.; Hosseini, T.; Hosseini, S.; Ghazifard, A.; Ghaffari Novin, M.; Fallah-Omrani, V.; Nourozian, M.; Hosseini, A. Melatonin Modifies Histone Acetylation During In Vitro Maturation of Mouse Oocytes. Cell J. 2018, 20, 244–249. [Google Scholar] [CrossRef]
- Nasheed Hamad Almohammed, Z.; Moghani-Ghoroghi, F.; Ragerdi-Kashani, I.; Fathi, R.; Tahaei, L.S.; Naji, M.; Pasbakhsh, P. The Effect of Melatonin on Mitochondrial Function and Autophagy in In Vitro Matured Oocytes of Aged Mice. Cell J. 2020, 22, 9–16. [Google Scholar] [CrossRef]
- Nikmard, F.; Hosseini, E.; Bakhtiyari, M.; Ashrafi, M.; Amidi, F.; Aflatoonian, R. The boosting effects of melatonin on the expression of related genes to oocyte maturation and antioxidant pathways: A polycystic ovary syndrome- mouse model. J. Ovarian Res. 2022, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Nandi, S.; Gupta, P.S.P.; Mondal, S. Antioxidants supplementation improves the quality of in vitro produced ovine embryos with amendments in key development gene expressions. Theriogenology 2023, 201, 41–52. [Google Scholar] [CrossRef]
- Silva, B.R.; Barrozo, L.G.; Nascimento, D.R.; Costa, F.C.; Azevedo, V.A.N.; Paulino, L.R.F.M.; Lopes, E.P.F.; Batista, A.L.P.S.; Aguiar, F.L.N.; Peixoto, C.A.; et al. Effects of cyclic adenosine monophosphate modulating agents during oocyte pre-maturation and the role of melatonin on in vitro maturation of bovine cumulus-oocyte complexes. Anim. Reprod. Sci. 2023, 257, 107327. [Google Scholar] [CrossRef]
- Tutt, D.A.R.; Guven-Ates, G.; Kwong, W.Y.; Simmons, R.; Sang, F.; Silvestri, G.; Canedo-Ribeiro, C.; Handyside, A.H.; Labrecque, R.; Sirard, M.-A.; et al. Developmental, cytogenetic and epigenetic consequences of removing complex proteins and adding melatonin during in vitro maturation of bovine oocytes. Front. Endocrinol. 2023, 14, 1280847. [Google Scholar] [CrossRef]
- El Sheikh, M.; Mesalam, A.; Mesalam, A.A.; Idrees, M.; Lee, K.-L.; Kong, I.-K. Melatonin Abrogates the Anti-Developmental Effect of the AKT Inhibitor SH6 in Bovine Oocytes and Embryos. Int. J. Mol. Sci. 2019, 20, 2956. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Sananmuang, T.; Puthier, D.; Nguyen, C.; Chokeshaiusaha, K. Novel classifier orthologs of bovine and human oocytes matured in different melatonin environments. Theriogenology 2020, 156, 82–89. [Google Scholar] [CrossRef]
- An, Q.; Peng, W.; Cheng, Y.; Lu, Z.; Zhou, C.; Zhang, Y.; Su, J. Melatonin supplementation during in vitro maturation of oocyte enhances subsequent development of bovine cloned embryos. J. Cell. Physiol. 2019, 234, 17370–17381. [Google Scholar] [CrossRef]
- Cordova, A.; Miranda, M.S.; King, W.A.; Mastromonaco, G.F. Effects of EGF and melatonin on gene expression of cumulus cells and further in vitro embryo development in bovines. Zygote 2022, 30, 600–610. [Google Scholar] [CrossRef]
- Gutiérrez-Añez, J.C.; Henning, H.; Lucas-Hahn, A.; Baulain, U.; Aldag, P.; Sieg, B.; Hensel, V.; Herrmann, D.; Niemann, H. Melatonin improves rate of monospermic fertilization and early embryo development in a bovine IVF system. PLoS ONE 2021, 16, e0256701. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, S.; Sun, Y.; Jiang, X.; Hao, H.; Du, W.; Zhu, H. Protective effects of melatonin on the in vitro developmental competence of bovine oocytes. Anim. Sci. J. 2018, 89, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Cunha, M.C.; Mesquita, L.G.; Bressan, F.; Collado, M.D.; Balieiro, J.C.C.; Schwarz, K.R.L.; de Castro, F.C.; Watanabe, O.Y.; Watanabe, Y.F.; de Alencar Coelho, L.; et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology 2016, 86, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tao, J.; Chai, M.; Wu, H.; Wang, J.; Li, G.; He, C.; Xie, L.; Ji, P.; Dai, Y.; et al. Melatonin Improves the Quality of Inferior Bovine Oocytes and Promoted Their Subsequent IVF Embryo Development: Mechanisms and Results. Molecules 2017, 22, 2059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-M.; Hao, H.-S.; Du, W.-H.; Zhao, S.-J.; Wang, H.-Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.-B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Min, J.-T.; Du, W.-H.; Hao, H.-S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H.-B. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef]
- Zhao, X.-M.; Wang, N.; Hao, H.-S.; Li, C.-Y.; Zhao, Y.-H.; Yan, C.-L.; Wang, H.-Y.; Du, W.-H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Jin, J.-X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Melatonin regulates lipid metabolism in porcine oocytes. J. Pineal Res. 2017, 62, e12388. [Google Scholar] [CrossRef]
- Jin, J.-X.; Sun, J.-T.; Jiang, C.-Q.; Cui, H.-D.; Bian, Y.; Lee, S.; Zhang, L.; Lee, B.C.; Liu, Z.-H. Melatonin Regulates Lipid Metabolism in Porcine Cumulus-Oocyte Complexes via the Melatonin Receptor 2. Antioxidants 2022, 11, 687. [Google Scholar] [CrossRef]
- Zhu, T.; Guan, S.; Lv, D.; Zhao, M.; Yan, L.; Shi, L.; Ji, P.; Zhang, L.; Liu, G. Melatonin Modulates Lipid Metabolism in Porcine Cumulus-Oocyte Complex via Its Receptors. Front. Cell Dev. Biol. 2021, 9, 648209. [Google Scholar] [CrossRef]
- Phuong, L.D.T.; Thien, L.C.; Su Pham, C.D.; Minh, N.U.; Huy Bao, N.T.; Thien Truc, L.N.; Huyen, T.T.N.; Minh, D.T.; Nguyen, N.-T.; Van Thuan, N.; et al. Melatonin and cyclic adenosine monophosphate enhance the meiotic and developmental competence of porcine oocytes from early antral follicles during in vitro growth and pre-maturation culture. Theriogenology 2025, 237, 129–142. [Google Scholar] [CrossRef]
- Stojkovic, M.; Machado, S.A.; Stojkovic, P.; Zakhartchenko, V.; Hutzler, P.; Gonçalves, P.B.; Wolf, E. Mitochondrial Distribution and Adenosine Triphosphate Content of Bovine Oocytes Before and After In Vitro Maturation: Correlation with Morphological Criteria and Developmental Capacity After In Vitro Fertilization and Culture1. Biol. Reprod. 2001, 64, 904–909. [Google Scholar] [CrossRef]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Leme, L.O.; Martins, C.F.; Dode, M.; Alves, B.G.; Costa, F.; de Oliveira, E.B.; Gambarini, M.L. Melatonin reduces apoptotic cells, SOD2 and HSPB1 and improves the in vitro production and quality of bovine blastocysts. Reprod. Domest. Anim. 2018, 53, 226–236. [Google Scholar] [CrossRef]
- Marques, T.C.; da Silva Santos, E.C.; Diesel, T.O.; Martins, C.F.; Cumpa, H.C.B.; de Oliveira Leme, L.; Dode, M.A.N.; Alves, B.G.; Costa, F.P.H.; de Oliveira, E.B.; et al. Blastocoel fluid removal and melatonin supplementation in the culture medium improve the viability of vitrified bovine embryos. Theriogenology 2021, 160, 134–141. [Google Scholar] [CrossRef]
- Holm, P.; Booth, P.J.; Schmidt, M.H.; Greve, T.; Callesen, H. High bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteins. Theriogenology 1999, 52, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- de Castro Cavallari, F.; Leal, C.L.V.; Zvi, R.; Hansen, P.J. Effects of melatonin on production of reactive oxygen species and developmental competence of bovine oocytes exposed to heat shock and oxidative stress during in vitro maturation. Zygote 2019, 27, 180–186. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, M.; Mesalam, A.A.; Song, S.-H.; Ko, J.; Kong, I.-K. Melatonin Alleviates the Toxicity of High Nicotinamide Concentrations in Oocytes: Potential Interaction with Nicotinamide Methylation Signaling. Oxid. Med. Cell Longev. 2021, 2021, 5573357. [Google Scholar] [CrossRef]
- Favetta, L.A.; St John, E.J.; King, W.A.; Betts, D.H. High levels of p66shc and intracellular ROS in permanently arrested early embryos. Free Radic. Biol. Med. 2007, 42, 1201–1210. [Google Scholar] [CrossRef]
- Harvey, A.J. The role of oxygen in ruminant preimplantation embryo development and metabolism. Anim. Reprod. Sci. 2007, 98, 113–128. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; He, Y.; Jia, L.; Yang, C.S.; Reiter, R.J.; Zhang, J. Antioxidant and Pro-Oxidant Activities of Melatonin in the Presence of Copper and Polyphenols In Vitro and In Vivo. Cells 2019, 8, 903. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Guérin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Update 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Sutton, M.L.; Cetica, P.D.; Beconi, M.T.; Kind, K.L.; Gilchrist, R.B.; Thompson, J.G. Influence of oocyte-secreted factors and culture duration on the metabolic activity of bovine cumulus cell complexes. Reproduction 2003, 126, 27–34. [Google Scholar] [CrossRef][Green Version]
- Adhikari, D.; Lee, I.-W.; Yuen, W.S.; Carroll, J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol. Reprod. 2022, 106, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Lipinska, P. Energy metabolism of follicular environment during oocyte growth and maturation. J. Reprod. Dev. 2020, 66, 1–7. [Google Scholar] [CrossRef]
- de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. [Google Scholar] [CrossRef]
- El-Sheikh, M.; Mesalam, A.A.; Kang, S.-M.; Joo, M.-D.; Soliman, S.S.; Khalil, A.A.K.; Ahn, M.-J.; Kong, I.-K. Modulation of Apoptosis and Autophagy by Melatonin in Juglone-Exposed Bovine Oocytes. Animals 2023, 13, 1475. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.R.; Silva, J.R.V. Mechanisms of action of non-enzymatic antioxidants to control oxidative stress during in vitro follicle growth, oocyte maturation, and embryo development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yang, X.; Liu, K. Treatment of cattle oocytes with C-type natriuretic peptide before in vitro maturation enhances oocyte mitochondrial function. Anim. Reprod. Sci. 2021, 225, 106685. [Google Scholar] [CrossRef]
- Del Collado, M.; da Silveira, J.C.; Sangalli, J.R.; Andrade, G.M.; Sousa, L.R.D.S.; Silva, L.A.; Meirelles, F.V.; Perecin, F. Fatty Acid Binding Protein 3 and Transzonal Projections Are Involved in Lipid Accumulation During In Vitro Maturation of Bovine Oocytes. Sci. Rep. 2017, 7, 2645. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.-J.; Zhang, X.; Liu, X.; Zhao, S.; Huo, L.-J.; Zhou, J.; Miao, Y.-L. Melatonin protects against defects induced by malathion during porcine oocyte maturation. J. Cell. Physiol. 2020, 235, 2836–2846. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, X. Effects of C-type natriuretic peptide on meiotic arrest and developmental competence of bovine oocyte derived from small and medium follicles. Sci. Rep. 2020, 10, 18213. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, B.; Xu, X.; Zhang, H.; Feng, X.; Hao, H.; Du, W.; Zhu, H.; Li, S.; Yu, W.; et al. Combination of CNP, MT and FLI during IVM Significantly Improved the Quality and Development Abilities of Bovine Oocytes and IVF-Derived Embryos. Antioxidants 2023, 12, 897. [Google Scholar] [CrossRef]
- Báez, F.; de Brun, V.; Rodríguez-Osorio, N.; Viñoles, C. Low oxygen tension during in vitro embryo production improves the yield, quality, and cryotolerance of bovine blastocysts. Anim. Sci. J. 2024, 95, e13941. [Google Scholar] [CrossRef]
- Gaspar, R.C.; Arnold, D.R.; Corrêa, C.A.P.; da Rocha, C.V.; Penteado, J.C.T.; Del Collado, M.; Vantini, R.; Garcia, J.M.; Lopes, F.L. Oxygen tension affects histone remodeling of in vitro-produced embryos in a bovine model. Theriogenology 2015, 83, 1408–1415. [Google Scholar] [CrossRef]
- Leivas, F.G.; Brum, D.S.; Saliba, W.P.; Alvim, M.T.T.; Bernardi, M.L.; Rubin, M.I.B.; Silva, C.A.M. Oxygen tension in IVM and IVF of bovine oocytes: Effect on embryonic development and pregnancy rate. Anim. Reprod. (AR) 2018, 3, 439–445. [Google Scholar]
- Mingoti, G.Z.; Castro, V.S.D.C.; Méo, S.C.; Sá Barretto, L.S.; Garcia, J.M. The effects of macromolecular and serum supplements and oxygen tension during bovine in vitro procedures on kinetics of oocyte maturation and embryo development. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 361–367. [Google Scholar] [CrossRef]
- Gómez, M.C.; Pope, C.E.; Ricks, D.M.; Lyons, J.; Dumas, C.; Dresser, B.L. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod. Fertil. Dev. 2009, 21, 76–82. [Google Scholar] [CrossRef]
- El-Sayed, A.; Hoelker, M.; Rings, F.; Salilew, D.; Jennen, D.; Tholen, E.; Sirard, M.-A.; Schellander, K.; Tesfaye, D. Large-scale transcriptional analysis of bovine embryo biopsies in relation to pregnancy success after transfer to recipients. Physiol. Genom. 2006, 28, 84–96. [Google Scholar] [CrossRef]
- Machado, G.M.; Ferreira, A.R.; Pivato, I.; Fidelis, A.; Spricigo, J.F.; Paulini, F.; Lucci, C.M.; Franco, M.M.; Dode, M.A. Post-hatching development of in vitro bovine embryos from day 7 to 14 in vivo versus in vitro. Mol. Reprod. Dev. 2013, 80, 936–947. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).