Abstract
Plant by-products have gained significant attention due to their rich content in bioactive compounds, which exhibit promising antioxidant, antimicrobial, and antitumor properties. In European countries, vegetable waste generation ranged from 35 to 78 kg per capita in 2022, highlighting both the scale of the challenge and the potential for valorization. This review provides an overview of key studies investigating the potential of plant residues in biomedicine, highlighting their possible contents of antioxidant compounds, their antimicrobial and antitumor properties, as well as their applications in dermocosmetics and nutraceuticals. However, despite their potential, several challenges must be addressed, such as the standardization of extraction protocols, as bioactive compound profiles can vary with plant source, processing conditions, and storage methods. Effective segregation and storage protocols for household organic waste also require optimization to ensure the quality and usability of plant by-products in biomedicine. Emerging 4.0 technologies could help to identify suitable plant by-products for biomedicine, streamlining their selection process for high-value applications. Additionally, the transition from in vitro studies to clinical trials is hindered by gaps in the understanding of Absorption, Distribution, Metabolism, and Excretion (ADME) properties, as well as interaction and toxicity profiles. Nonetheless, environmental education and societal participation are crucial to enabling circular bioeconomy strategies and sustainable biomedical innovation.
1. Introduction
Currently, the pharmaceutical sector is showing a growing interest in the use of vegetable residues, primarily due to their richness in high-value-added bioactive molecules. These plant-derived residues, often generated during fruit and vegetable processing, include by-products such as pomace, seeds, leaves, pulp, peels, and stems. Far from being mere waste, these materials are recognized for their substantial content of carbohydrates, proteins, fibers, polyphenols, and lipids, compounds that exhibit significant nutritional and pharmacological potential [1,2,3,4]. An increasing number of studies have highlighted the diverse biological activities of these compounds, including anti-inflammatory, antioxidant, antibacterial, antiparasitic, and antifungal effects, positioning them as promising candidates for the development of novel therapeutic products [5,6]. Furthermore, these by-products have been successfully applied in the cosmetic industry, contributing to the formulation of skincare products and treatments that harness their natural bioactive potential. Beyond their conventional applications, vegetable residues are increasingly being investigated for advanced biomedical uses, such as their incorporation into tissue engineering scaffolds and drug delivery systems [7,8]. These innovative applications are driving a paradigm shift in how agro-industrial waste is perceived, transforming it from an environmental burden into a valuable source of functional materials. Simultaneously, the food industry is investing in the development of emerging technologies aimed at valorizing plant by-products to create novel food products with enhanced nutritional profiles [9,10,11]. This trend not only supports the principles of the circular economy but also aligns with current consumer demands for sustainable and health-promoting products.
Plant residues are generated by the processing of horticultural crops, and later, by agri-food industries and in the domestic sphere. According to the estimations made by the United Nations, the world’s population will increase from 8 billion at the beginning of 2024 to 9.7 billion in 2050 [12], which represents a 21% increase. Consequently, the rising demand for food involves the production of increased amounts of waste and by-products in fulfilling the needs of the population.
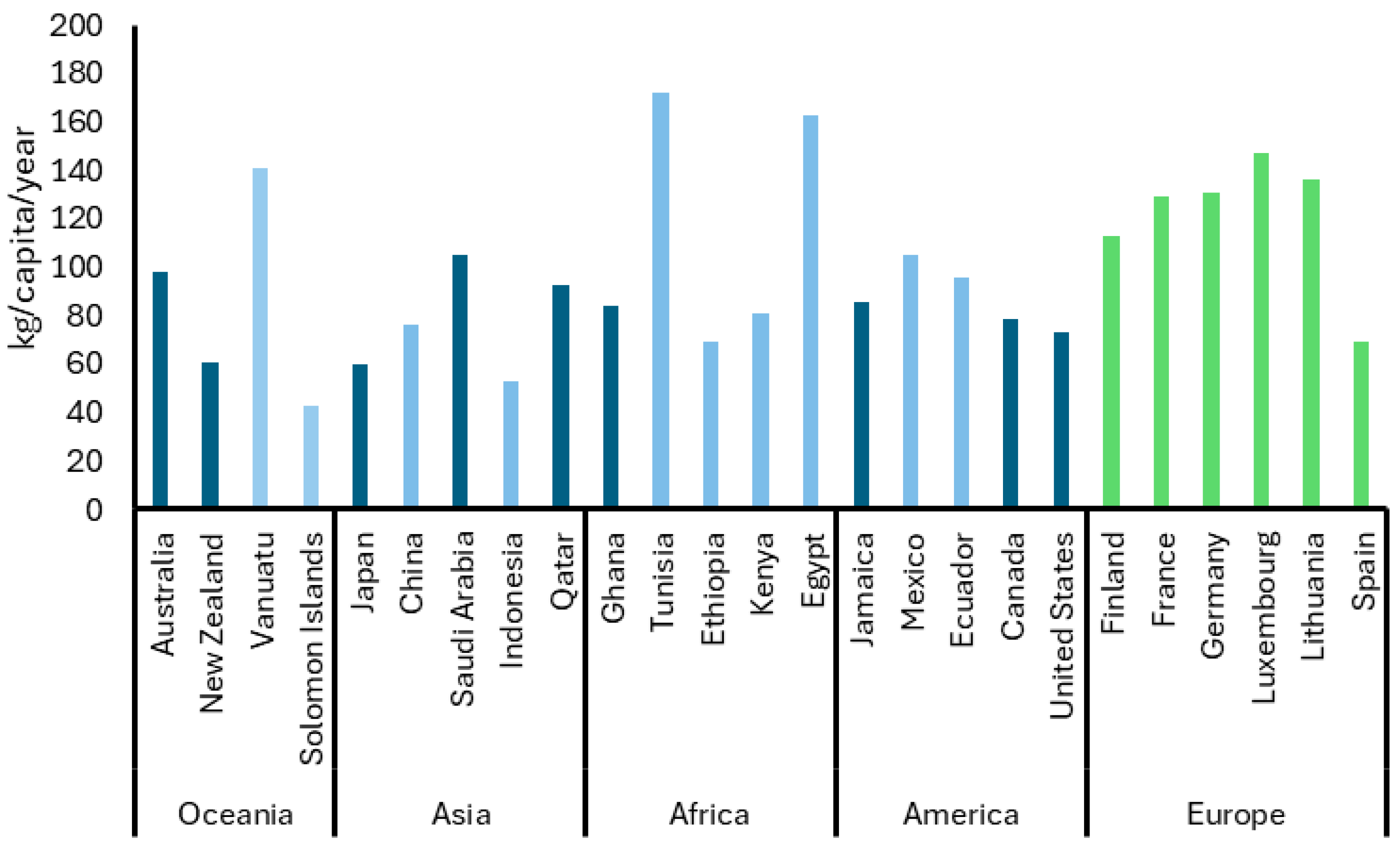
To provide insight into the scale of food waste generation, Figure 1 presents global data from 2022 on food waste per capita across different continents. It reveals significant disparities among continents. Countries such as Tunisia, Egypt, and Vanuatu report the highest values, though their estimates are based on medium-confidence data, which may limit the reliability of direct comparisons. In contrast, Australia, Saudi Arabia and Qatar show similarly high figures but with high confidence, suggesting stronger support for the reported values. European countries, represented with Eurostat data, show more moderate values clustered between 53 and 91 kg per capita, with relatively consistent patterns across the region [13]. Interestingly, although the United States, China, and Germany are the world’s three largest economies by Gross Domestic Product (GDP), their per-capita food waste levels do not show a consistent correlation with their economic performance. This suggests GDP alone does not explain food waste differences. Factors such as consumption habits, public policies, cultural values, and supply chain efficiency significantly influence food waste [14].
Figure 1.
Global domestic food waste generation (kg/capita/year) by continent and country in 2022. The figure uses a color scheme that distinguishes the level of confidence associated with each data point. High confidence (dark blue) estimates are nationally representative and methodologically robust, making them reliable for tracking food waste. Medium confidence (light blue) estimates are less representative, often based on localized data or adjusted figures. Eurostat data (green) follows standardized methods across European countries and corresponds to data published in 2024 with updates in 2025 [13,14].
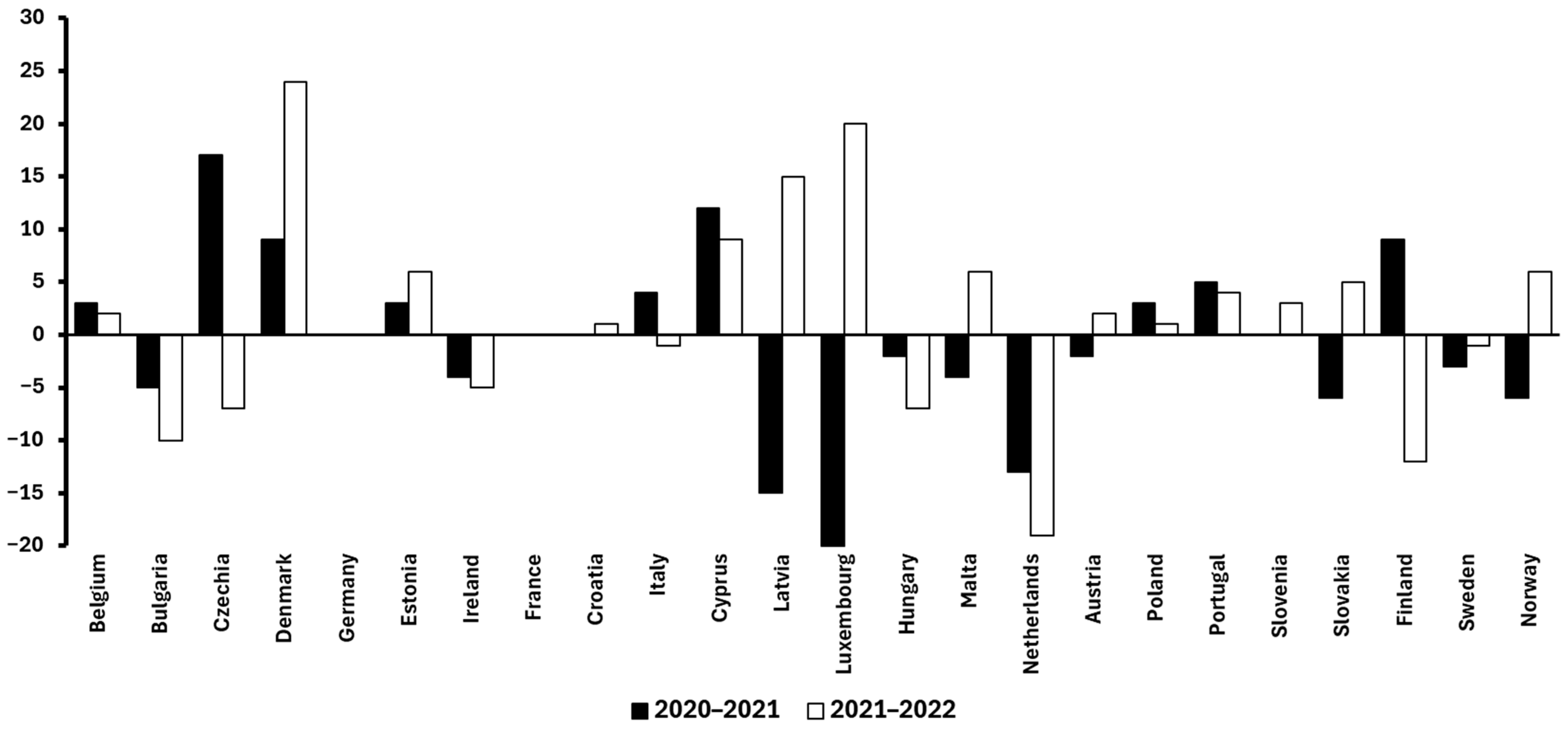
Building on this, it is also valuable to examine temporal trends within Europe to better understand how food waste is evolving in a high-income, highly regulated context. Between 2020 and 2022, per-capita food waste levels across European countries exhibited notable variation. For instance, Denmark and Cyprus showed marked increases of +33 kg and +21 kg per capita (2020–2022), respectively. In contrast, the Netherlands and Luxembourg experienced substantial reductions in the same period (–32 kg and –25 kg, respectively). Other countries such as Germany and Croatia maintained relatively stable figures, suggesting entrenched consumption and disposal patterns. Overall, the diversity of these trends highlights that even within a relatively homogeneous economic and regulatory area like the European Union (EU), national-level dynamics (including policy enforcement, public awareness campaigns, and food distribution systems) play a crucial role in shaping food waste outcomes [13].
It is also important to consider differences in vegetable availability and accessibility between countries, as these factors can directly impact food waste levels (Figure 1 and Figure 2). For example, Spain’s strong local vegetable production, supported by favorable climate and shorter supply chains, likely contributes to more stable availability and accessibility, which may help reduce waste through fresher produce and better inventory management. In contrast, countries such as the Netherlands and Luxembourg depend heavily on imports, leading to greater seasonal fluctuations and supply chain challenges that can increase food waste due to spoilage or overstocking. These structural differences in vegetable supply chains are reflected in the varying food waste trends observed across Europe, as shown in the data: countries with more localized production tend to have smaller increases or even reductions in waste, whereas those reliant on imports may experience greater volatility in waste generation. Interestingly, Germany (despite relying heavily on imported vegetables) maintained stable food waste levels from 2020 to 2022. This relative stability can be attributed to effective infrastructure, efficient logistics, and the implementation of a robust national strategy [13].
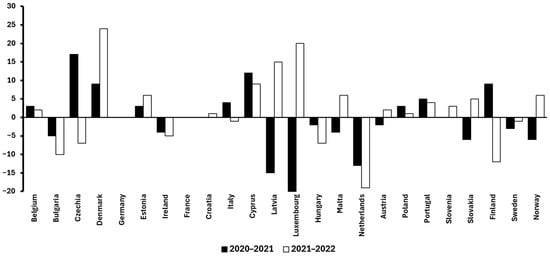
Figure 2.
Variation in food waste generation (kg/capita) across European countries from 2020 to 2022 [13].
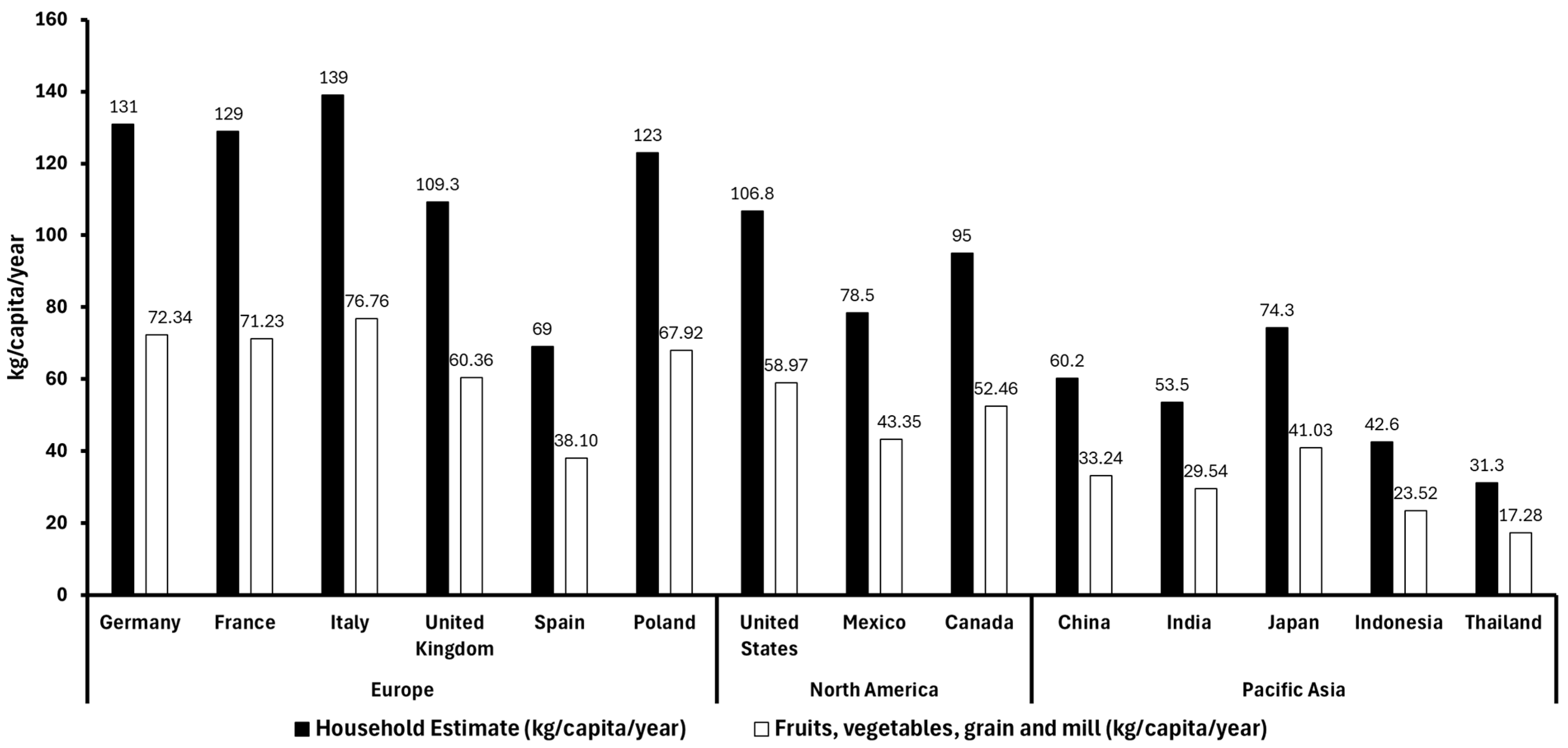
According to a pooled analysis conducted between 2010 and 2022, 55.22% of food waste in high-income countries is attributed to fruits, vegetables, and grain and mill products. Therefore, based on the database of global food waste statistics (Figure 1) [13,14], we have applied this percentage to estimate the proportion of food waste from fruits, vegetables, and grain and mill products in the Asia-Pacific, Europe, and North American regions (Figure 3). This suggests that plant-based waste contributes significantly to household food waste globally, with higher per-capita waste observed in countries with both strong local production and imports. For example, European countries like Italy and France, which produce large quantities of fruit, show high fruit waste per capita, while countries like Germany, relying more on imports, report lower fruit waste levels. In Asia, Indonesia and Thailand report lower fruit and vegetable waste levels mainly because of factors like lower overall consumption, more careful food use driven by economic constraints, and possibly less food surplus. Additionally, their food supply chains may be shorter or more localized in some areas, reducing spoilage before consumption. Cultural habits around minimizing waste and less access to overabundance in markets can also contribute to lower waste despite less production and reliance on imports [15].
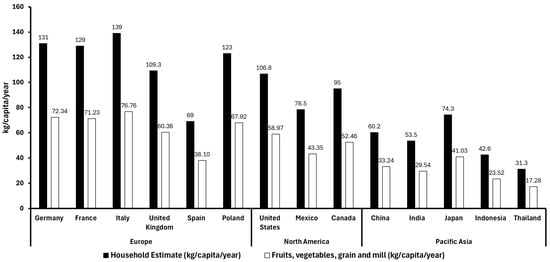
Figure 3.
Total food waste (kg/capita/year) (black bars) and fruit, vegetable, grain, and cereal residues (kg/capita/year) (white bars) in different countries of Europe, America, and Asia in 2022 [13,16].
In this context, the idea of the circular economy arose with the aim of replacing “take–make–dispose” practices. The circular model is based on substituting constant production with a focus on achieving sufficiency, maximizing reusability, recycling what cannot be repurposed, fixing what is damaged, and remanufacturing what cannot be broken [17]. The circular economy minimizes the use of natural resource inputs and the generation of waste, contaminants, and carbon emissions [9,18,19]. In addition, Regulation (EC) 2008/98 on waste establishes the legal framework for waste management and promotes waste prevention, reuse, and recycling, encouraging the valorization of food by-products as raw materials rather than waste [20]. The directive distinguishes between food waste and by-products. The first one refers to edible or inedible residual materials that are no longer suitable for consumption or use and are typically discarded without further valorization. This includes leftovers, spoiled food, or unused ingredients from the supply chain. In contrast, by-products are secondary materials generated unintentionally during food processing or manufacturing. Although they are not primary products, they retain nutritional, functional, or economic value and can be repurposed for various applications [21,22]. Within this framework, the valorization of vegetable by-products aligns perfectly with the core principles of the circular economy, as it promotes the transformation of the vast amounts of food by-products generated worldwide, which were traditionally regarded as worthless, into valuable raw materials. This approach not only reduces the environmental impact but also adds economic value to the substantial volumes of by-products generated. Agro-industrial by-products such as fruit peels, seeds, stems, and pomace are now being re-evaluated as renewable sources of bioactive compounds with broad applicability. Plant by-products are being studied for their antimicrobial, anti-inflammatory, and antioxidant properties in pharmaceuticals [23]. In biomedicine, they help develop sustainable materials like scaffolds and drug delivery systems [24]. The cosmetic industry uses them for skincare products, while in nutrition, these compounds contribute to health-promoting functional foods and supplements [25]. This valorization strategy supports a shift towards more sustainable production systems, where environmental impact is minimized and material loops are closed. It also opens up new avenues for innovation, encouraging cross-sector collaboration between agriculture, food processing, biotechnology, and health sciences.
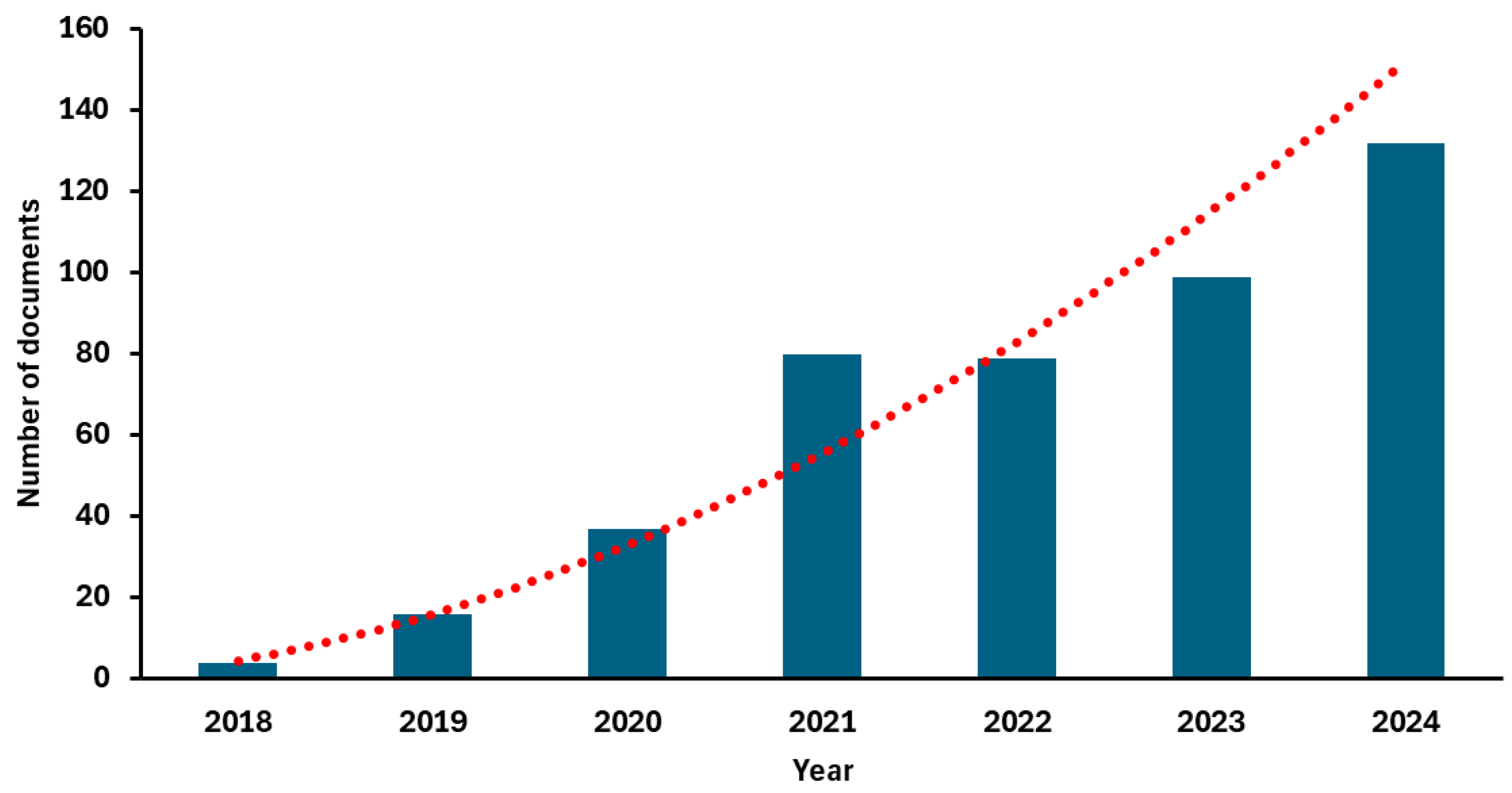
Accordingly, in recent years, this model has triggered an exponential growth in scientific research. As illustrated in Figure 4, the number of articles indexed in PubMed under the search terms “food by-products and circular economy” has increased significantly, reflecting the rising academic interest in this field [26].
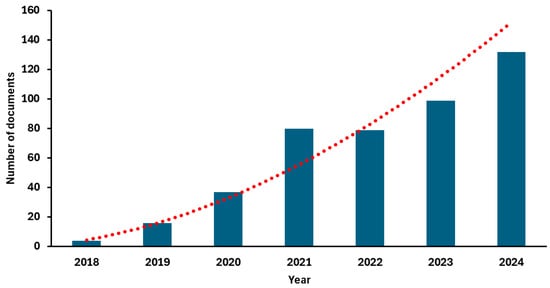
Figure 4.
Number of documents focused on food by-products published from 2018 to 2024 according to data collected from PubMed using “food by-products and circular economy” as keywords [26]. The red line represents the fitted power trendline, illustrating the accelerating growth in the number of publications over time.
With the goal of promoting research projects focused on the re-use of plant by-products, as well as the encouraging a circular economy, this review offers a comprehensive look at the potential health-related applications of these materials. The compiled information has allowed for performing a SWOT analysis (Strengths, Weaknesses, Opportunities, and Threats) focused on both the current situation and future perspectives on the application of agri-food plant by-products in biomedicine.
2. Materials and Methods
A systematic literature review was conducted using three major scientific databases: PubMed, Scopus, and Google Scholar, focusing on peer-reviewed articles published between 2013 and 2025. It was complemented by a search in the LENS patent database to capture innovations and intellectual property trends between 2013 and 2025.
To ensure broad and relevant coverage, a comprehensive set of keywords was used, including plant residues, plant by-products, circular economy, bioactive compounds, biomedical applications, antimicrobial, antifungal, antitumoral, dermocosmetics, biomaterials, prebiotics, nutraceuticals, phytochemicals, and green chemistry. These were supplemented with secondary terms such as sustainability, legislation, regulatory frameworks, economic feasibility, barriers, opportunities, innovation, green extraction methods, biorefineries, integral biorefineries, citizens education, and future perspectives.
Articles were selected following a two-stage screening process, beginning with title and abstract review, followed by full-text evaluation to ensure alignment with the scope of this review. Selection criteria emphasized scientific rigor, originality, and relevance to the valorization of plant residues in biomedical contexts. Only studies that clearly addressed the use or transformation of plant-derived by-products, such as peels, leaves, or stems, into bioactive compounds or functional materials for applications in health, medicine, or dermocosmetics were included. In particular, preference was given to experimental research (in vitro, in vivo, or clinical trials), systematic reviews, and meta-analyses that provided evidence-based insights into the therapeutic potential, mechanisms of action, or formulation strategies of these bioresources. Additionally, studies addressing broader dimensions such as technological challenges, regulatory frameworks, legislative developments, economic feasibility, and future implementation perspectives within the circular economy context were also prioritized, as they contribute to a more comprehensive understanding of the opportunities and limitations associated with the biomedical valorization of plant residues.
Research that did not specifically address the valorization of plant residues in biomedicine was excluded. Studies conducted in contexts outside biomedicine, such as those focusing solely on agriculture, were also excluded. The search was restricted to papers written in the English language.
3. Development Drivers of the Field
In recent years, there has been increasing interest in the efficient use of agricultural and food by-products as a means of improving sustainability, minimizing waste, and creating value-added products, which has stimulated scientific research (Figure 4) and technological advances [27]. In fact, there are several critical factors that have driven growth in the use of plant by-products, including increasing sustainability awareness, advances in material processing, and regulatory support.
Growing awareness of environmental sustainability is driving change across society and industries, encouraging practices that prioritize conserving resources. Sustainability aims to ensure that future generations have access to resources at least as abundant as those available today, preventing the depletion of natural resources. Current overconsumption represents an injustice to future generations, with negative impacts on the environment, economy, and society. Food waste, which leads to economic losses and worsens food insecurity, is a global issue with severe environmental consequences, such as greenhouse gas emissions [28]. In recent years, environmental sustainability awareness has grown significantly, with both young people and adults expressing deep concern over the planet’s future, especially in the context of climate change and environmental disasters [29]. In this context, there is an ever-increasing demand for products with highly valued properties (nutritional, healthcare, and cosmetic). Thus, fruit and vegetable by-products offer significant potential due to their abundance and their high levels of bioactive compounds [30].
The advancement in material processing technologies, including green emerging technologies for the extraction of bioactives (their strengths, limitations, and applications) and the development of integrated biorefineries, are detailed in Table S1 and Section 5. Conventional methods of extracting bioactive compounds from plant tissues include Soxhlet extraction, maceration, percolation, and hydro-distillation. Non-conventional, green, or emerging extraction methods include ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), fermentation-assisted extraction (FAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), pulse electric field-assisted extraction (PEFAE), supercritical water extraction (SWE), instant controlled pressure drop (détente instantanée contrôllée, DIC), supercritical liquid chromatography (SFC), pressurized hot water extraction (PHWE), and deep eutectic solvents (DESs). The main advantages of these methods over conventional ones are shorter extraction times, reduced solvent usage, higher yields, high-quality extracts, greater selectivity, better isolation, reduced environmental impact, lower energy consumption, and safer products [31,32,33].
Economic and political support has further advanced the circular economy, with public procurement driving innovation in resource conservation. By creating demand and offering incentives for research and development, it encourages companies to adopt more sustainable practices. Additionally, it fosters collaboration between suppliers and provides political support, further accelerating the transition to more sustainable and circular systems [34]. Programs within the European Union, such as Horizon Europe, Next-Generation EU, and LIFE, provide significant funding to support circular economy initiatives [35]. Globally, there are frameworks like the “2030 Agenda for Sustainable Development” adopted by the United Nations in 2015, which outlines 17 Sustainable Development Goals (SDGs), including goals focused on sustainable consumption, climate change, and environmental protection (SDGs 12, 13, 14, and 15) [36,37,38]. As circular economy practices expand, regulatory frameworks are essential to ensure safe and appropriate reuse of by-products. Previous studies questioned the safety of valorized food by-products due to limited research on their potential risks. Concerns arose because many studies focused only on their beneficial properties, without adequate safety evaluations, such as microbiological tests or contaminants analysis [39]. An example of this issue occurred in Italy in 2019 [40], where the improper use of olive pomace led to a legal case. The company failed to demonstrate its correct use as a fertilizer according to the requirements, highlighting the need for clear regulations to ensure the safe and proper use of food by-products.
Nowadays, in the European Union, food by-products are required to comply with strict safety regulations to ensure food safety and consumer protection. These include Regulation (EC) 178/2002, which sets the principles for food law; regulation (EC) 396/2005, which sets maximum pesticide residue levels in food and feed; or regulation (EC) 2073/2005, which defines microbiological criteria for food safety. These regulations ensure that foods, including vegetable by-products, comply with European food safety standards and are safe for consumer use [41]. Furthermore, in parallel, increasing Food and Drug Administration (FDA) approvals of natural products, driven by toxicity studies, highlight the growing confidence in the safety of plant-based compounds for biomedical applications, presenting these as low-toxicity alternatives to synthetic options [42].
Unfortunately, in developing countries, the implementation of circular economy practices, including the valorization of food by-products, faces challenges such as insufficient technological expertise, lack of public awareness, and weak enforcement of existing environmental laws. Although there is growing interest in turning vegetal by-products into valuable products, the overall lack of regulation and research impedes the country’s ability to fully embrace circular economy benefits [18].
4. The Possible Use of Plant By-Products in Biomedicine
4.1. Antimicrobial Agents
Over recent years, it has become well known that many microorganisms have developed resistance to a wide range of antibiotics, antifungals, and antiparasitics. Antimicrobial resistance (AMR) is a global public health crisis that threatens the effective prevention and treatment of a great number of infections caused by bacteria, viruses, fungi, and parasites [43,44].
4.1.1. Antibacterial Activity
Bacteria acquire and express resistance genes and then share those genes with other bacteria driving the resistance phenomenon (bacterial selection). The threat is most acute for synthetic antibacterial agents, which explains the strong interest in biologically active plant compounds as potential alternatives. In contrast to synthetic agents, plant-based extracts often contain dozens or even hundreds of antimicrobial constituents acting synergistically. This complex chemical diversity not only enhances antimicrobial efficacy but also reduces the likelihood of bacteria developing simultaneous resistance to all active components, thereby offering a more robust and sustainable approach to combating microbial infections [44,45].
Diverse studies from 2016 to 2020 (Table 1) concluded that different vegetable organs accumulate promising compounds with antimicrobial properties. What is more, vegetal residues such as peels, pulps, and leaves from pomegranate, orange, yellow lemon, ginger, onion, potato, garlic, tomato, and banana have demonstrated their effectiveness against bacterial infections caused by both Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Klebsiella spp., Salmonella spp., Shigella sonnei) and Gram-positive (Streptococcus spp., Enterococcus faecalis, Bacillus spp., Staphylococcus spp.) microorganisms, some of them able to cause major diseases difficult to treat [23,46,47,48,49,50,51]. Special attention should be paid to Staphylococcus aureus among these bacteria, because it is one of the most widespread bacterial pathogens. It causes a large number of skin infections and severe and invasive infections like pneumonia or endocarditis. S. aureus infections are particularly problematic due to their high prevalence, with the methicillin-resistant S. aureus (MRSA) the most important from the clinical point of view since it is a strain resistant to commonly used antibiotics, such as penicillin and methicillin [52]. Similarly, the number of E. coli strains resistant to commonly used antibiotics, such as amoxicillin or ciprofloxacin, is increasing, thus creating an urgent need to explore new treatment options, taking into account that the most common disease caused by this strain is the urinary tract infection [53].

Table 1.
Antibacterial effects of plant residues.
Table 1 summarizes the results of several studies focused on testing the effect of extracts obtained from different vegetal residues against diverse bacteria able to cause human diseases. The effectiveness of plant by-products as antimicrobial agents, however, can depend on several factors, including the plant variety and the chemical used to extract the bioactive compounds.
Plant variety was determinant in the case of pomegranate since the results differed when used peels from sweet, sour, or bittersweet varieties [23]. The best results were obtained with the bittersweet variety Piñón Tierno de Ojós 8 (PTO8) and with the acidic variety Hicaznar (HIC) (Table 1). The skin extracts from the PTO8 variety exhibited Minimum Inhibitory Concentrations (MICs) of 1.5 μg mL−1 and 68.5 μg mL−1 against B. subtilis and S. aureus, respectively. Moreover, MICs reached values of 12.5 μg mL−1 and 21.3 μg mL−1 against E. faecalis and Salmonella enterica, respectively, when applied to skin extracts of the HIC variety. This variety behaved as the most effective against S. aureus, achieving MIC values of 57.2 μg mL−1. In the case of tomato, Tiny Tim variety appeared as the most effective against S. aureus (MIC = 0.625 mg by-product mL−1), B. subtilis (MIC = 1.25 mg by-product mL−1), Listeria monocytogenes (MIC = 2.50 mg by-product mL−1), and Klebsiella pneumoniae (MIC = 2.50 mg by-product mL−1) among a total of six tested varieties (Table 1). This high effectiveness has been ascribed to the high levels of carotenoids and phenolic compounds (like ellagic acid and punicalagins) in the Tiny Tim variety. These compounds are known for their ability to disrupt microbial cell walls, membranes, or enzymatic activities [49].
As previously mentioned, the type of extractant can condition the antimicrobial activity displayed by a given plant residue. However, in some cases, high and similar antimicrobial activity has been found using different extractants, as in the case of ethanolic and aqueous extracts of banana fruit peels against multidrug-resistant bacteria [51]. In contrast, when researchers used banana leaves [48], a large difference was observed between methanolic extraction, which was highly effective, and extraction with hexane, without an antibacterial effect. Similarly, in most cases, distilled water was a more appropriate solvent than methanol for lemon and orange peels as antibacterial agents, except for K. pneumoniae, which behaved sensitively when researchers used methanolic extracts of lemon peel [50].
In a study conducted by Naqvi et al. [47], the antibacterial effects of ginger, garlic, onion, and potato peels were tested against four bacterial strains: E. coli, Bacillus cereus, B. megaterium, and S. aureus. The results showed that ginger peels exhibited the strongest inhibition against all bacterial strains, while onion peels did not show any inhibitory effect [47]. In a separate study, coffee mucilage was found to have a growth-inhibitory effect against B. cereus [55]. This residue, rich in chlorogenic acid, likely acts through membrane disruption, acidification of the bacterial cytoplasm, and possibly interference with cell wall synthesis. The mechanisms by which these compounds exert antimicrobial effects vary notably between Gram-positive and Gram-negative bacteria due to differences in their cell wall structures. Gram-positive bacteria (as B. cereus) possess a thick peptidoglycan layer, which can be targeted directly by certain phenolic compounds causing membrane destabilization and enzyme inhibition. Conversely, Gram-negative bacteria have an additional outer membrane composed of lipopolysaccharides, which acts as a barrier to many compounds, requiring antimicrobial agents to penetrate or disrupt this layer to exert effects [55,56]. Some plant-derived compounds, such as terpenes, have lipophilic properties that facilitate disruption of the outer membrane, increasing permeability and allowing other active substances to enter the bacterial cell. This differential mode of action highlights the importance of understanding bacterial physiology when developing plant-based antimicrobials [57].
In an attempt to characterize the molecule(s) responsible for the antibiotic effect, after a first extraction, fractions were separated and tested for whether the antimicrobial activity differed between those fractions and the original pure extract [46]. In numerous cases, the key bioactive compounds with antimicrobial activity belonged to the phenolic compounds. In fact, frequently, there was a positive relationship between the antioxidant and antimicrobial activities and the phenolic content [23,48,49]. Similarly, essential oils, such as those from the seed of Nigella sativa, are rich in terpenes and phenolic compounds, which are known to exhibit strong antimicrobial properties. In the case of N. sativa seed oil, when used alone, it showed promising antimicrobial effects. However, when combined with conventional antibiotics like augmentin, oxacillin, and cephalosporin, the bacterial inhibition was more rapid and stronger than when either N. sativa or the antibiotics were used individually. This suggests a synergistic effect, where the combination of N. sativa with antibiotics could help overcome bacterial resistance by potentially disrupting the bacterial cell wall. This disruption may enhance the antibiotics’ effectiveness, offering a potential solution to combating bacterial resistance through synergistic therapeutic strategies [54,58].
However, despite the promising antimicrobial potential of plant extracts, a major challenge lies in standardizing these complex mixtures to ensure consistent efficacy and safety. The variability in plant composition due to factors like geographic origin, harvest time, and extraction methods complicates reproducibility. Regulatory agencies face difficulties in approving plant-based antimicrobials because of these inconsistencies, lack of clear active ingredient identification, and the absence of standardized potency metrics. Potential solutions include the development of advanced analytical techniques for comprehensive chemical profiling, establishing reference standards, and harmonizing guidelines across jurisdictions. Moreover, combination therapies, as demonstrated with N. sativa and antibiotics, may accelerate regulatory acceptance by leveraging known pharmaceutical agents to enhance efficacy and reduce resistance risk [59].
In any case, there are some examples of patents related to the antimicrobial effect of plant by-products. For example, patent WO 2020/230044 A1, owned by the University of Parma (Italy) (https://www.lens.org/lens/patent/084-002-100-013-121/frontpage?l=en, accessed on 11 June 2025), describes the production of antimicrobials from vegetable waste against S. aureus, L. monocytogenes, Bacillus cereus, Salmonella spp., and E. coli, among others.
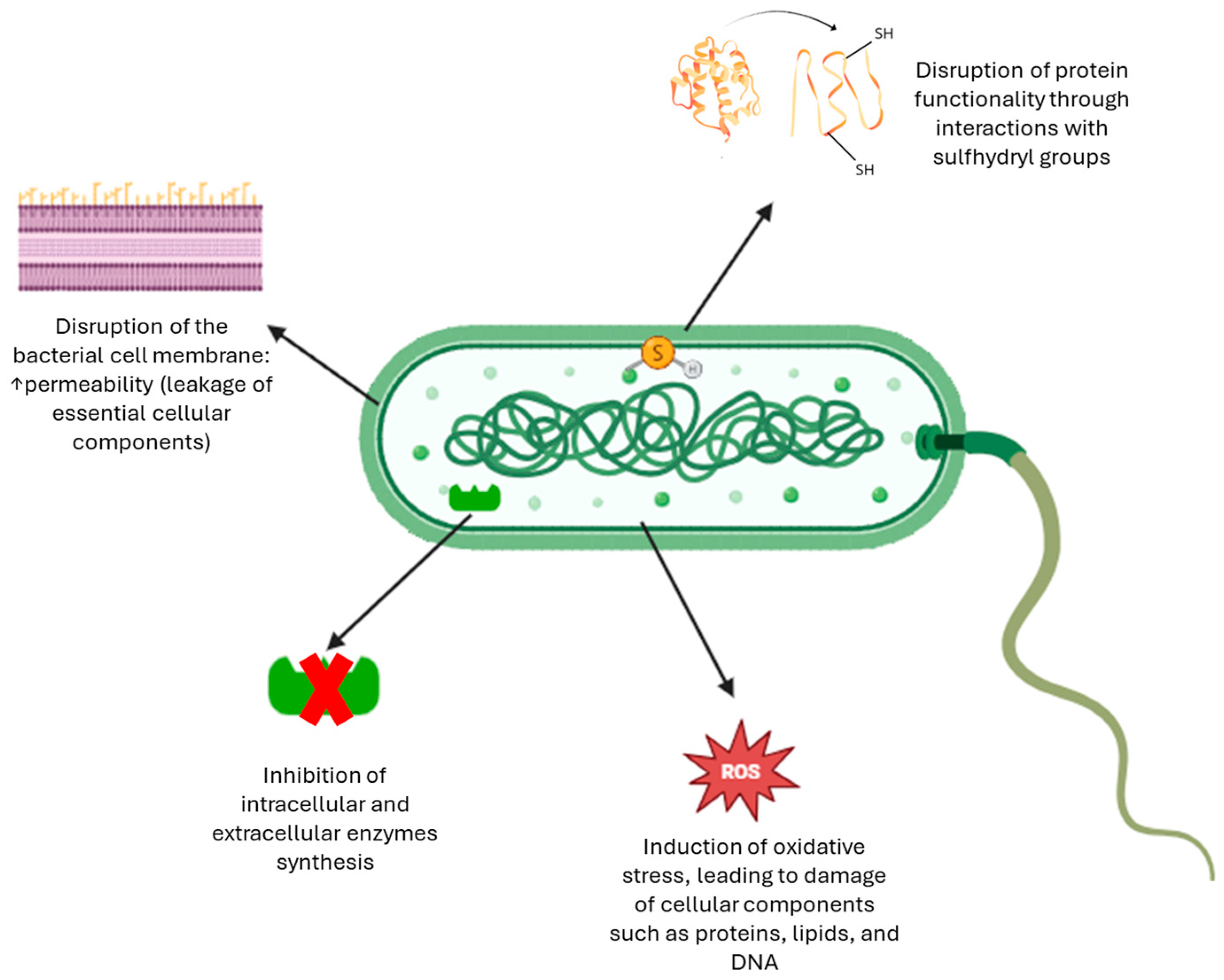
To sum up, Figure 5 illustrates the multifaceted antimicrobial effects of phenolic compounds, including their modes of action on bacterial membranes and intracellular targets [56].
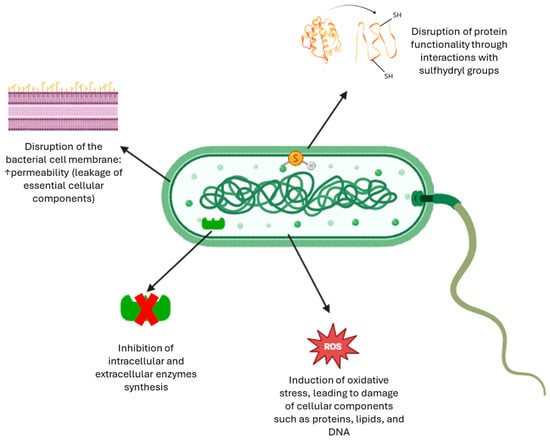
Figure 5.
Antibacterial mechanisms of phenolic compounds [56].
4.1.2. Antifungal Activity
Fungal infections have increased over the last decades. According to the Global Action Foundation for Fungal Infections, about 30 fungal species cause 99% of human fungal diseases, producing more than 1.6 million deaths each year in the world. Some fungal diseases are acute and severe, such as cryptococcal meningitis; others are recurrent, such as candida vaginitis or oral candidiasis in acquired immunodeficiency syndrome; and others become chronic, such as pulmonary aspergillosis or tinea capitis [60,61].
The uncontrolled use of antifungals, similar to what happens with antibiotics, coupled with the rapid adaptability of fungi to change, has led to decreased efficacy of pharmacological treatments as a consequence of the increased fungal resistance [62]. In addition, according to the World Health Organization (WHO), there are currently only four classes of antifungal drugs available and very few candidates in clinical development, which makes it urgent to find alternatives, including the potential use of plant residues as antifungal agents [63]. Table 2 presents the results of four studies focused on analyzing the efficacy of different organs (leaves, rhizomes, bulbs, fruits) of seven plants against four genera of fungi (Aspergillus, Fusarium, Trichophyton, Penicillium) which can be harmful to human health, either by causing a specific disease, such as keratitis or ringworm, or by producing and releasing toxins that can reach the consumer through the food chain [64].

Table 2.
Antifungal effects of plant residues.
The efficacy of plant residues as antifungal agents, however, depends on the extract fraction [51]. Pomegranate extracts [23], especially those obtained from the bittersweet variety PTO8, were highly effective in inhibiting the radial growth of Fusarium verticillioides (Table 2). The highest value of inhibition was achieved with the n-butanolic fraction (radial growth inhibition of 70%), whereas the hexane fraction with the highest polyphenol concentration and antioxidant activity surprisingly did not show the highest antifungal activity. The authors suggested that the n-butanolic fraction might contain antifungal compounds other than those usually reported in pomegranate peel extracts (punicalagins and ellagic acid). Considering that F. verticillioides is resistant to most of the antifungals currently used, such as azoles or amphotericin B, these results are promising in antifungal therapy [67]. In a similar way, banana leaf extracts applied in certain concentrations could be even more effective antifungal agents than nystatin, an antifungal drug currently used in medicine [48]. Banana leaf extracts likely contain tannins, flavonoids, saponins, phenolic acids, and alkaloids, which act together to inhibit fungal growth. Tannins disrupt cell walls, flavonoids reduce enzyme activity, saponins damage membranes, phenolic acids alter metabolism, and alkaloids may inhibit cell division, enhancing the antifungal effects of the extract. Moreover, ginger, garlic, and potato peel extracts showed medium positive inhibition against F. verticillioides, while onion peels exhibited slight positive inhibition [47]. This effect could be due to the presence of bioactive compounds such as gingerol [68,69] in ginger and allicin in garlic, which demonstrated antifungal properties [70]. In a clinical study, allicin was administered intravenously to mice before and after Trichosporon asahii infection. The results showed that allicin significantly improved survival and reduced the fungal burden in kidneys, the spleen, and the liver. Further analysis revealed that allicin affected fungal plasma membrane integrity by downregulating ergosterol biosynthesis genes and disrupting the cell wall by inhibiting glucan and chitin synthase. Additionally, allicin induced oxidative stress and mitochondrial dysfunction, leading to an energy deficit in the fungus, contributing to its antifungal effects [71].
The efficacy in vitro of aqueous extracts of mandarin and banana peels was tested in inhibiting the growth of mycotoxin-producing strains of fungi: A. flavus, A. tubingensis, and P. griseofulvum [65]. While mandarin peel appeared very effective in reducing the growth of P. griseofulvum (by around 90%), banana peel decreased the growth of P. griseofulvum, A. flavus, and A. tubingensis by 20%. This effect was dose-dependent, since an increase in the concentration of the residue from 5% to 10% (w:v) led to the percentage of inhibition for A. flavus reaching 80% with the mandarin residues and 40% with banana ones [66]. Additionally, a study published in 2024 achieved 80% inhibition of A. flavus growth using banana peels [72]. However, the results were not always positive with vegetable by-products: neither kiwi peel nor a mixture of mandarin and banana showed any inhibitory effect against those mycotoxin-producing fungi. Some studies attributed the antifungal activity to the cinnamic acid present in plants, the compound that inhibits the fungal cytochrome P450 CYP53A15, whose role is preventing the demethylation of lanosterol, a triterpenoid precursor of the sterol that makes up the fungal membrane [73].
While many studies have explored the antifungal activity of plant residues, few have focused on understanding the underlying mechanisms of action of these plant by-products. This lack of mechanistic insight makes it challenging to fully evaluate their potential as antifungal agents. Recent research has examined the antifungal potential of grape pomace extract (GPE), a by-product from wine production. Studies focusing on GPE’s effects on fungi, particularly the Saccharomyces cerevisiae yeast model, have identified key targets involved in its antifungal activity. GPE has been shown to disrupt the ergosterol biosynthesis pathway by inhibiting key enzymes, leading to the accumulation of toxic sterol intermediates and a compromised cell membrane. Additionally, GPE’s action in apoptosis regulation suggests that it may induce programmed cell death in yeast, although without the involvement of the Yca1 protein, which is typically a key regulator of apoptosis in S. cerevisiae. Moreover, GPE affects the cell wall integrity, particularly by interfering with the protein kinase C (Pkc1) signaling pathway, which is essential for maintaining cell wall structure and function [74].
Another study focused on polymethoxylated flavonoids (PMFs) from C. reticulata peel reveals complementary antifungal mechanisms. PMFs disrupt fungal cell membrane integrity by altering ion permeability, causing potassium (K+) to exit and sodium (Na+) to enter cells, leading to osmotic imbalance and cellular ageing. Additionally, PMFs impair cell wall strength by inhibiting chitin synthesis, a key structural component, resulting in weakened hyphae and cell lysis [75].
These findings provide valuable insights into how plant by-products may interfere with critical cellular processes in fungi, offering potential for the development of new antifungal agents targeting multiple pathways simultaneously.
4.1.3. Antiparasitic Activity
Parasitic infections are a global public health problem due to their high frequency in developing countries, their introduction into developed countries through people migration and travels, and their high morbidity [76]. The inexistence of vaccines to combat most of these diseases, and the fact that some drug treatments are becoming ineffective as a consequence of widespread resistances, underscore the necessity of prevention and finding new treatment strategies [77].
Cryptosporidiosis is responsible for 0.6–7.3% of diarrheal diseases in countries with modern sanitation systems and for an even higher percentage in areas with poor sanitation [78,79]. Current treatments are self-limiting, so as a new approach, some studies have tested plant by-products against this protozoo (Table 3). Pomegranate peel has proven to be effective against Cryptosporidium parvum in a murine model, with a reduction in oocyst shedding (oocysts 0.01 g−1 feces) of up to 100% [80]. Moreover, ethanolic extract from olive pomace has been demonstrated to be effective in a WST-1 assay against C. parvum (MIC = 250–500 µg mL−1). However, in this study, other plant by-products such as grape seed extract peels did not show any significant activity against this protozoo (MIC = 500–1000 µg mL−1) [81].
The protozoan parasite Trichomonas vaginalis is the causative agent of trichomoniasis, one of the most common non-viral sexually transmitted infections in humans. In contrast, T. foetus targets the gastrointestinal system of livestock, leading to trichomonosis, a condition marked by diarrhea and digestive disturbances [82]. The peel powder obtained from S. lycopersicum var. cerasiforme was effective in inhibiting the growth of different strains of human and animal trichomonas, achieving percentages of growth inhibition up to 70% for T. vaginalis G3, 70% for T. foetus C1, and 80% for T. foetus D1 [83].

Table 3.
Antiparasitic effects of plant residues.
Table 3.
Antiparasitic effects of plant residues.
| Plant | By-Product | Parasite Specie | Type of Extractant | Disease | Reference |
|---|---|---|---|---|---|
| Olive (Olea europaea) | Olive pomace | C. parvum | Deionized water, 70% aqueous ethanol or heptane | Criptosporidiosis | [81] |
| Pomegranate (P. granatum) | Peel powder | Aqueous | [80] | ||
| Cherry tomato (S. lycopersicum var. cerasiforme) | Peel powder | Trichomonas spp. | Dimethyl sulfoxide/water (1:1) | Trichomoniasis | [83] |
| Orange (C. sinensis) | Leaves | Promastigote forms of L. amazonensis | Hexane, ethyl acetate, dichloromethane/ethanol (1:1) or ethanol/water (7:3) | Leishmaniasis | [84] |
Leishmaniasis is among the top ten neglected tropical diseases (NTDa) with the highest incidence in the world. The disease is manifested in three main forms: visceral, cutaneous, and mucocutaneous. Leishmania amazonensis is associated with diffuse cutaneous leishmaniasis, a rare and severe form of the disease characterized by a widespread invasion of the skin and the gradual development of diverse lesions, including small, raised bumps, nodules, and thickened plaques. According to the WHO, this type of leishmaniasis is difficult to treat [85,86]. The antileishmanial potential of extracts obtained with dried leaves of Citrus sinensis demonstrated effectiveness against L. amazonensis promastigotes and intracellular amastigotes, with the hexane extract being the most effective (IC50 = 25.91 and 39.78 µg mL−1, respectively, against promastigotes and amastigotes) [84].
However, as commented previously for the potential antibacterial activity of vegetable residues, further research is needed to identify the active compounds in all of these by-products to gain deeper knowledge of the mechanism of action and to increase the therapeutic range. Moreover, considering the wide variety of parasitic infections and the lack of effective treatments, plant residues appear a promising tool for antiparasitic therapies.
4.2. Antitumoral Activity
Cancer is still one of the world’s principal causes of morbidity and mortality. The International Agency for Research on Cancer estimated that in the year 2020, approximately 19.3 million new cases of cancer would be diagnosed worldwide and the number of new cases will increase to 28 million per year by 2040 [87]. The malignancy of tumors poses a significant threat to an individual’s health. The prognosis for many patients with malignant tumors remains limited, largely due to the lack of highly effective therapeutic options [88].
In the history of natural compounds used in cancer therapy, ones that deserve special mention are the Vinca alkaloids, vincristine and vinblastine, which inhibit microtubule polymerization by binding to tubulin, causing cell cycle arrest in the metaphase, and triggering apoptosis, as well as the alkaloid paclitaxel (also known as Taxol®, Research Triangle Park, NC, USA), extracted from the bark of Taxus brevifolia. It stabilizes microtubules, preventing their depolymerization, which disrupts mitosis. It also triggers cell cycle arrest at the G2/M checkpoint, ultimately leading to programmed cell death [89]. In addition, emerging research highlights that many natural compounds exhibit cancer-selective properties, demonstrating antiproliferative, pro-apoptotic, and cytotoxic effects, specifically against malignant cells [90]. As an example, hesperetin, typically found in citrus peels, exerts its anticancer (colorectal cancer) effects by scavenging reactive oxygen species generation (ROS) and enhancing the activity of antioxidant enzymes like superoxide dismutase, glutathione reductase, and glutathione-S-transferase. Hesperetin also modulates the expression of key proteins such as TGF-β1, which regulates cell cycle progression and apoptosis. Additionally, it induces apoptosis by upregulating pro-apoptotic proteins like p53 and downregulating antiapoptotic proteins, while reducing the expression of the proliferation marker Ki67. These molecular actions collectively contribute to its anticarcinogenic properties [91]. Furthermore, the essential oils from the leaves of Vitex macrophylla exert their antitumoral effects (breast cancer) by interacting with various molecular pathways. The main component, citral, induces apoptosis by causing mitochondrial membrane depolarization, which leads to the release of cytochrome c and activation of caspases, triggering the intrinsic pathway of apoptosis. Additionally, citral disrupts microtubule dynamics by binding to tubulin, inhibiting its polymerization and leading to cytoskeletal destabilization. This results in alterations to the actin cytoskeleton, impairing cell motility and proliferation. Moreover, the essential oils regulate oxidative stress by increasing the production of ROS, which further contributes to cellular damage and death. These oils also exhibit chemopreventive properties by modulating signaling pathways such as AMPK/mTOR and by suppressing the activation of pro-inflammatory cytokines like TNF-α, contributing to the inhibition of tumor progression [92].
In that context, novel compounds obtained from vegetal residues are of growing interest. In fact, mango seed kernels, citrus peel oils, grape seeds and leaves, and pomegranate, apple, avocado, tomato, and potato peels have shown in vitro activity as potential antitumoral or chemopreventive agents (Table 4) [93,94,95,96,97,98,99,100,101,102,103,104,105,106,107].

Table 4.
Antitumoral effects of plant residues.
The extraction method of bioactive compounds is important and should be optimized, considering factors such as the type of solvent, fractions, solubility, or temperature. In a study with mango seed kernels, green pressurized-liquid extraction and dynamic maceration were used to evaluate in vitro bioactivity as antitumoral. Both evidenced antiproliferative activity against the human colon adenocarcinoma cell line HT-29; however, green pressurized-liquid extraction was more effective. Mangiferin is the major component in the sugar of mango seed kernel and exhibits significant antiproliferative activity through several molecular mechanisms: it protects cells from oxidative stress and DNA damage, downregulates inflammation, and induces apoptosis by inhibiting NF-κB activation. Additionally, mangiferin triggers cell cycle arrest and reduces proliferation in malignant cells. Other compounds like gallic acid and ellagic acid further enhance the extract’s effects by modulating apoptosis and cell cycle progression and inhibiting signaling pathways such as NF-κB and Akt. The synergistic interactions of these compounds contribute to the overall potent antiproliferative activity observed [93]. Efforts have been made to explore the therapeutic potential of mangiferin through clinical trials. However, its clinical use remains limited due to its low water and fat solubility, restricted absorption, and poor bioavailability, which hinder its oral efficacy. While trials have attempted to assess its benefits, these challenges have slowed progress in realizing its full potential. To address these issues, researchers are focusing on developing specific formulations to enhance its oral bioavailability, aiming to improve its clinical applications and effectiveness in treating various conditions [108].
The agri-food industry generates tons of processing by-products that are being accumulated on the soil in adjacent areas. As an example, pomegranate peels [99] are the major by-products of the processing of pomegranate juice. A DPPH assay demonstrated that this peel has bioactive compounds (β-glucans) with antioxidant activity. Recent studies have reconfirmed their antitumoral properties [100]. Pomegranate peel compounds, such as ellagic acid and punicalagins, inhibit the STAT3 pathway, which regulates processes like angiogenesis, cell proliferation, and apoptosis, often dysregulated in cancer cells. Additionally, pomegranate compounds target the PI3K/Akt pathway, reducing cell survival and proliferation by inhibiting key proteins like Akt and mTOR. These actions help prevent tumor growth, metastasis, and resistance to cell death, making pomegranate a potential natural agent for cancer prevention and treatment [109,110,111]. The results from pre-clinical studies on pomegranate have been so promising that they have advanced to clinical trials. In clinical studies, pomegranate has been tested primarily in prostate cancer patients. They have measured the impact of pomegranate juice on oxidative stress biomarkers, such as 8-hydroxy-20-deoxyguanosine (8-OHdG), and its ability to delay the increase in prostate-specific antigen (PSA). One study showed that drinking pomegranate juice (equivalent to 570 mg of polyphenol gallic acid per day) after prostate surgery increased the mean time for PSA levels to double from 15 months to 54 months. These results are among the key findings that suggest pomegranate’s potential in prostate cancer treatment [111].
A study focused on grape seed proanthocyanidin extract [97] showed that this extract attenuated doxorubicin-induced ROS and prevent the cardiotoxicity of that antineoplasic due to its antioxidant capacity. Similarly, citrus peels, abundant in compounds with antioxidant activity, provide an additional shield against oxidative stress, thereby enhancing the overall cancer preventive and protective capacity within the body [94]. These natural sources could also be explored as adjuvant treatments in therapeutic settings, though this approach warrants further investigation through animal studies and subsequent clinical trials.
In other scenarios, with apple peel [101] and avocado seed [103], flavonoids and triterpenoids, respectively, displayed superior properties in not only antioxidation but also antitumor aspects, leading to the apoptosis of tumor cells. Likewise, diverse active compounds extracted from grapevine leaves have shown antihyperglycemic, antioxidant, anti-inflammatory, analgesic, and antipyretic properties [112]. In addition, in vitro studies have demonstrated effective cytotoxic activity of grapevine leaves against lung [113], breast [114], and leukemia [115] cancer cells. This effectiveness, however, can vary among grapevine cultivars and it is modulated by biotic and abiotic factors affecting this crop: elevated air temperatures and the association of grapevines with mycorrhizal fungi can enhance the cytotoxic activity of grapevine leaf extracts because both factors can induce the accumulation of phenolic compounds [95].
These promising results obtained in in vitro assays have recently been supported by preclinical studies performed with mice [102]. A nanoemulsion of carotenoids extracted from sweet potato peel reduced both the size and weight of tumors, although the inhibition efficiency of tumors in the animals was dependent on the type of carotenoid, the preparation method of carotenoid nanoemulsion, the dose applied, the administration length, and the cancer cell type. It was hypothesized that the effective inhibition of MCF-7 breast cancer cells could occur through a passive targeting effect and that the antitumor efficiency could be due to the synergistic effect of β-carotene and other carotenoids present in the nanoemulsion. These hopeful results emphasize the necessity of research in more advanced clinical phases. Building upon the compelling evidence of antitumoral activity in fruit and vegetable by-products, some studies delve deeper into their mechanisms of action (Table 4). That is the case of a study focused on Brazilian native fruits like açaí and camu-camu [116]. The diverse by-products of these fruits have been explored using different extractants, as shown in Table 4, each yielding distinct bioactive profiles and antitumoral activities. The hydroethanolic extract of açaí seeds demonstrated significant antitumoral effects on A549 lung cancer cells (dosage and period = 1.25–200 µg mL−1 for 48 h) by increasing antioxidant activity, inducing apoptosis, and halting the cell cycle in the G0/G1 phase, ultimately reducing cell viability by 72.07% [104]. The aqueous extract of açaí seeds also exhibited strong growth inhibition against NCI-H460 lung cancer cells (GI50 = 22 µg mL−1) while maintaining safety for normal cells (dosage and period = 0–125 μg mL−1 for 48 h) [105]. For camu-camu, its hydroethanolic seed extract (dosage and period = 100–900 μg mL−1 for 48 h) proved highly effective in reducing ROS generation and cisplatin-induced mutagenic damage. It selectively inhibited A549 lung cancer cell growth (GI50 = 251 µg mL−1) without harming normal cells, underscoring its dual antioxidant and cytotoxic capabilities [106]. The camu-camu seed water–ethanol–propanone extract (dosage and period = 100–900 μg mL−1 for 48 h) exhibited antioxidant, cytotoxic, and antiproliferative effects on the A549 lung cancer cell line (IC50 of 581.1 µg mL−1). Camu-camu’s anticancer effects are primarily driven by its polyphenolic compounds, such as methylvescalagin, proanthocyanidin A2, ellagic acid, and epicatechin. These compounds reduce reactive ROS generation, inhibit cancer cell proliferation, and induce apoptosis in cancer cells. They also possess anti-inflammatory properties, reducing TNF-α release and inhibiting NF-κB activation [107]. Examples of patents related to the use of fruit by-products as antitumor agents include US 2005/0147723 A1, which is owned by the Cornell Research Foundation (USA) (https://www.lens.org/lens/patent/027-550-960-192-801/frontpage?l=en, accessed on 11 June 2025), and CN 114558053 A, which is owned by the Hospital for People in the Jimo District of Qingdao City and the Qingdao University of Science and Technology (China) (https://www.lens.org/lens/patent/006-339-009-282-720/frontpage?l=en, accessed on 11 June 2025). The former, titled ‘Apple peel powder, methods of making, and uses thereof’, outlines a procedure for treating cancer in patients through the administration of apple peel powder. Cancer cell proliferation can also be avoided by bringing the cells into contact with the powder under conditions that effectively inhibit it. The second patent, titled ‘Waste fruit peel extract composition with effect of treating gastric cancer’, demonstrates the effectiveness of a mixture of guava, annona squamosa, and passion fruit peel extracts against gastric cancer.
4.3. Dermocosmetic Applications
Due to the growing interest in eco-labeled cosmetics, the use of biomolecules identified and isolated from plant residues opens up new perspectives on their sustainable application, contributing to the circular economy. In this line, almost a decade ago, it was proposed that we pursue the use of polyphenols obtained from vegetal residues in the cosmeceutical industry as chemopreventive agents for skin disorders because they possess anti-inflammatory, immunomodulatory, and antioxidant properties [25].
4.3.1. Photoprotection
The correct use of photoprotection can attenuate and mitigate skin damage caused by different types of radiation such as UV, HEVL, or IR-A [117]. Studies have demonstrated the efficacy of naturally occurring polyphenols, such as oleuropein from olive leaves, resveratrol from grape skin, or proanthocyanidins from grape seeds, against UV radiation-induced inflammation, oxidative stress, and DNA damage. Most of the natural polyphenols are pigments and they can absorb UV radiation, including the entire UVB spectrum and part of the UVC and UVA spectra [117].
Olive leaves, a residue produced during olive harvesting and tree pruning, are rich in polyphenolic compounds. Among these, oleuropein (OLE) is the most abundant and well-studied constituent, known for its effective photoprotective, antimutagenic, and antioxidant properties [118]. When comparing this OLE extract with the positive control of the study, resveratrol (RES), the results were very similar in terms of antioxidant activity (OLE EC50 =11.75 μg mL−1 and RES EC50 = 12.64 μg mL−1). When in vitro sun protection factor (SPF), UVA/UVB ratio, and λc (nm) values of developed formulations containing OLE, Polypodium leucotomos (PLE), and RES were studied, it was found that OLE showed a significant increase in SPF values from 22 for the control fraction to 42 and 56 for the concentrations of 3 and 5% of OLE. The results obtained for OLE were promising and more effective than those of the PLE extract. The antiphotomutagenesis effect was evaluated upon CD138 (ogg1) strain irradiation with solar simulated light (SSL). RES treatment led to an approximate 2.9-fold reduction in the number of SSL-induced CanR mutants compared to the control, while OLE achieved a 2.4-fold decrease. Notably, only OLE demonstrated effective photoprotective activity following SSL exposure lasting longer than two hours. In conclusion, oleuropein appeared the most effective active agent due to the combination of the three effects. These results can be used to formulate sunscreens or solar nutricosmetics containing this OLE extract, not only preventing immediate damage such as sunburn but also providing protection for DNA damage or skin ageing. It is an underexplored, promising, and sustainable compound [118].
4.3.2. Antiageing
Skin ageing is manifested in clinical features such as wrinkles, hyperpigmentation, or sagging skin as a result of changes in the functionality of skin components at the molecular level [118]. This is the case, for example, of elastase, an enzyme that hydrolyses elastin, a connective tissue protein that provides elasticity to tissues. High activity of this enzyme causes loss of the skin firmness and elasticity [119]. The same occurs with tyrosine, which is responsible for the synthesis of melanin in melanocytes, but, when there is an overproduction of melanin in the skin, pigmentary disorders occur, such as age spots and melasma. Numerous skin routines include antiageing products containing natural actives such as polyphenols [120].
In the agri-food industry, large quantities of by-products are generated, with the wine industry being one of the most important. Seeds, pulp, skins, leaves, stems, and wine lees contain bioactive compounds of interest, including polyphenols, oils, or lignins. Grape stem extracts inhibited tyrosinase and elastase, with this activity especially significant for extracts obtained from the Syrah grapevine variety: the inhibition percentages reached 53.83% and 98.02% for tyrosinase and elastase, respectively [121]. Therefore, these results support the idea that this by-product can be applied in cosmetic products to combat wrinkle and skin pigmentation disorders. Moreover, after applying shoot extracts of Thymus pulegioides, the tyrosinase activity was inhibited [122]. This herb was proposed as a functional food ingredient with important antioxidant, antiproliferative, and neuroprotective effects based on its content and profile of phenolic compounds.
4.3.3. Commercial Insights
The commercial landscape for plant-based dermocosmetic products has seen significant growth in recent years, driven by consumer demand for natural and sustainable alternatives. Unlike pharmaceutical products, dermocosmetics benefit from a less stringent regulatory framework, facilitating faster market entry and innovation [123]. However, ensuring the safety and efficacy of these products remains a critical challenge. As highlighted in recent regulatory reviews, including the 2013 EU Cosmetics Regulation, rigorous safety assessments must still be conducted, especially when using novel or less-characterized natural ingredients sourced from plant by-products. Moreover, there is a need for improved post-market surveillance and consumer reporting of adverse effects to fully understand the safety profile of these emerging dermocosmetic formulations [123].
Many companies are capitalizing on the bioactive properties of plant residues and by-products to develop formulations with antioxidant, antimicrobial, and anti-inflammatory benefits. Famous brands like Caudalie® (Bordeaux, France). and Apivita® (Athens, Greece). utilize grape seed extracts (an abundant winery residue) in several of their products, capitalizing on its rich polyphenol content to provide antioxidant protection and skin rejuvenation [124]. An illustrative example of the valorization of agricultural residues in the dermocosmetic sector is the Antcare project, part of the BIO4Africa technology catalogue. This initiative focuses on the upcycling of organic apple by-products, including seeds and peels from organic apple juice production, to create natural cosmetic ingredients. Through a sustainable process powered by renewable energy and employing recycled and recyclable packaging, the project produces apple paste rich in nourishing and antioxidant compounds, exemplifying circular economy principles and sustainable innovation in cosmetics [125,126].
However, despite the widespread use of botanical ingredients in the dermocosmetic industry, with brands such as Kiehl’s® (Paris, France), Origins® (Cambridge, MA, USA), L’Occitane® (Manosque, Provence, France), and Weleda® (Arlesheim, Suisse) incorporating plant-based ingredients, only a small fraction of these compounds are actually derived from agricultural or food-processing residues. Most commercial formulations still rely on cultivated or wild-harvested plant materials, thus missing the opportunity to valorize large volumes of bioactive-rich by-products.
In addition, various patents demonstrate the innovation potential of plant residues in dermocosmetics. For instance, patent CN117175533 discloses a combined plant extract for eliminating mites and acne, obtained through ultrasound-assisted water extraction and alcohol precipitation from sources such as Nepeta cataria, bamboo, celery seeds, and orange peels [127]. Similarly, patent CN100493711C discloses a cosmetic gel formulation with antioxidant, antiageing, and antibacterial effects that incorporates plant extracts and oils derived from botanical sources and plant residues, including grape seed oil [128].
In the coming years, plant residue-based dermocosmetics are expected to evolve beyond trend into a strategic pillar of sustainable innovation. However, to truly scale this model, the key will be fostering collaboration between the agri-food and cosmetic industries. By connecting producers of plant-based residues with companies specialized in green extraction and formulation, it becomes possible to transform by-products into high-value cosmetic ingredients. This approach not only reduces environmental impact but also creates economic value through circular economy principles, bridging sustainability and innovation in a tangible way [129]. To support this transition, strong and coherent regulatory frameworks will be essential, both to guarantee product safety and efficacy and to encourage the valorization of agricultural by-products through legal incentives, quality standards, and traceability requirements [130].
4.4. Other Applications
4.4.1. Human Microbiota and Probiotics
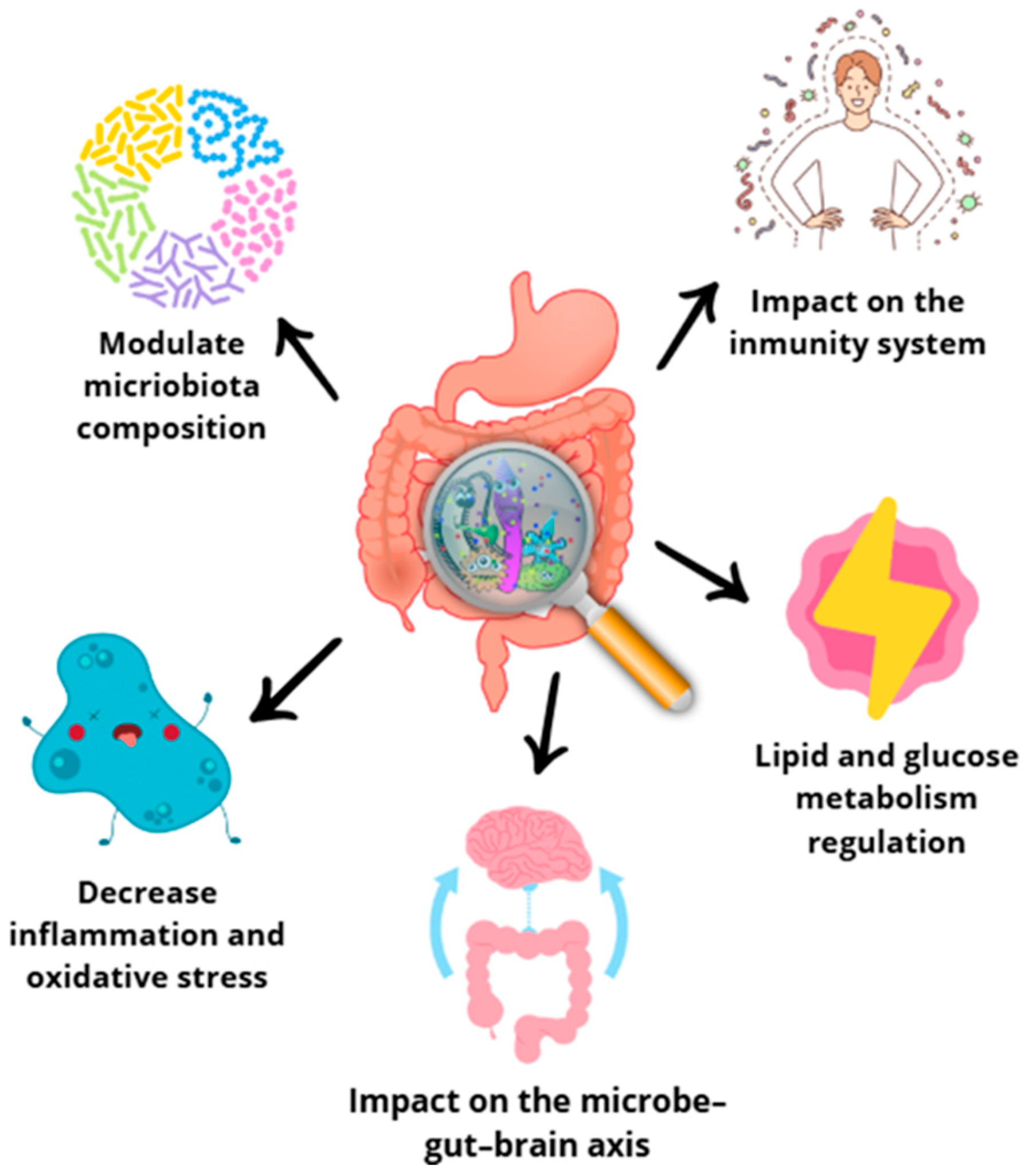
The human body hosts a remarkable diversity of microbes, constituting approximately 1–2% of its total weight [131]. Microorganisms colonize all surfaces of the human body, but the gut is the site particularly rich in microbiome, colonized by Lactobacillus spp., Clostridium spp., Bacteroides spp., Prevotella spp., and E. coli species. Diet assumes a predominant role in shaping the composition of the intestinal microbiota. The commensal microbiota actively participates in the fermentation of dietary components, which plays a crucial role in providing the host with essential nutrients and chemical signals that profoundly affect both immunity and metabolism (Figure 6). A noteworthy aspect is the presence of prebiotics among dietary substrates. Prebiotics are non-digestible food products that stimulate the growth of symbiotic bacterial species already present. This dynamic interplay between diet and the intestinal microbiota highlights the potential for dietary interventions to modulate microbial communities and support host health [132,133,134].
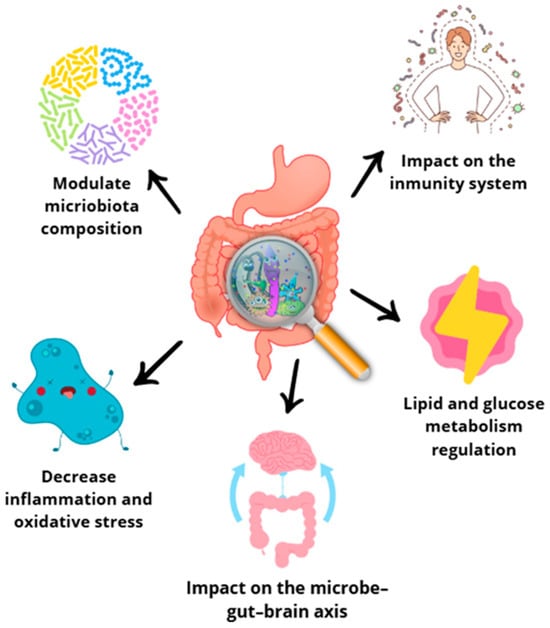
Figure 6.
Effects of polyphenols on gut microbiota [117,118,119].
Polyphenol consumption may be related to the abundance and diversity of the gut microbiota [135]. Table 5 summarizes the conclusions of diverse studies [136,137,138], which have demonstrated that fruit peels from yellow watermelon, honeydew, papaya, orange, olive, apple, banana, and passion fruit exert beneficial effects on the human microbiota, as they contain a reasonably high amount of potential prebiotic compounds such as polyphenols (inulin, cellulose, hemicellulose, fructans, lignin, or pectin).

Table 5.
Vegetal residues with promising properties as prebiotics.
Recently, the polysaccharide extract obtained from yellow watermelon peel showed high efficacy in stimulating the growth of probiotics, specifically Lactobacillus rhamnosus and Bifidobacterium bifidum, and its effectiveness was higher than that shown by other peel extracts from honeydew and papaya. Furthermore, polysaccharide extracts from watermelon and honeydew peels demonstrated robust resilience under acidic gastric conditions and digestion by α-amylase in the small intestine. This resilience positions them as promising prebiotic candidates for further research and potential application in the development of functional foods and nutraceutical products [138].
The growing interest in this topic is attested to by the research projects that are being developed—among them, ‘Birbizi’, developed at the Public University of Navarra (Spain)—and based on good innovative practices for reusing whey and underused by-products from agroindustry. Among the vegetable residues worth mentioning are orange peel and aqueous juice obtained from the residue remaining after extracting oil from the olives. The final drinkable products obtained are rich in beneficial bacteria for the microbiota, vitamin C, and antioxidants [136]. In the same line, the addition of apple and banana fibers to fiber-enriched skim yogurts preserves the viability of various probiotic strains (Bifidobacterium animalis subsp. lactis Bl04, HN019, B94, and Lactobacillus acidophilus L10) for four weeks of cold storage. These fiber-enriched yogurts had enhanced levels of short-chain fatty acids and polyunsaturated fatty acids [137]. All these findings suggest the potential application of these fruit by-products in developing new probiotic yogurts with enhanced nutritional value.
4.4.2. Sugar Replacement for Diabetics
The International Diabetic Federation Atlas [139] reports that 10.5% of the adult population from 20 to 79 years are diabetic and most of them (90%) suffer from type 2 diabetes, characterized by insulin resistance and hyperglycemia. Assays performed with diabetic rats fed with cupcakes made with either sucrose or a powder made from stevia leaves (SLP), banana peels (BPP), and carrot leaves (CLP) showed promising results. The diabetic rats fed with cupcakes including the vegetable residues showed an improved lipid profile in comparison with the group fed with sucrose-made cupcakes. Concretely, the consumption of cupcakes fortified with SLP, BBP, and CLP led to a decrease the levels of total cholesterol, glucose, triglyceride, glucose, and low-density lipoproteins (LDLs). This was particularly noticeable in cupcakes supplemented with a mixture of CLP and SLP. There was also a significant increase in high-density lipoprotein (HDL) levels for diabetic groups treated with BPP, CLP, SLP, and their mixtures. As diabetic people must avoid high-fat and high-sugar food, the substitution of sucrose by other vegetable residues may represent a good alternative to eating some sweet foods, avoiding the negative effects of sucrose [140].
A similar approach was taken in a recent clinical study using a nutraceutical derived from thinned nectarines (immature fruits discarded during agricultural thinning). These nectarines are rich in abscisic acid, a compound involved in glucose regulation. In a 12-week trial with type 2 diabetic patients, supplementation with the nectarine extract significantly reduced fasting blood glucose levels. Notably, the lower dose also decreased insulin levels by nearly 30%, suggesting improved insulin sensitivity. This highlights the potential of fruit residues not only as sugar substitutes but also as bioactive tools to support glycemic control in diabetic populations [141].
In addition, recent studies have explored the use of dried apple pomace as a sugar substitute in some sweet food products. The replacement of sucrose in chocolate with this plant-based residue significantly enhanced both the antioxidant activity and the total phenolic content of the product [142]. Similarly, biscuits enriched with 20% apple pomace exhibited a significantly lower glycemic index compared to control biscuits. These findings highlight how a common fruit residue can be valorized to simultaneously reduce the sugar content and enhance the functional antioxidant profile of sweet products, aligning with both health and sustainability goals [143].
4.4.3. Tissue Engineering
In the field of tissue engineering, biodegradable polymers derived from natural and synthetic plant by-products have shown great potential for developing scaffolds that aid in tissue regeneration. Natural polymers that can be derived from agricultural by-products offer an eco-friendly alternative for scaffold materials. These by-products can be processed into polymers with desirable properties for promoting tissue growth and healing, making them an increasingly valuable resource in tissue engineering [144,145].
A key example is the use of lignin, a major component of plant cell walls. Lignin has antioxidant, antimicrobial, and immunomodulatory properties, making it useful in tissue engineering. It has been used in creating nanofibers, hydrogels, and other materials for regenerating bone, liver, kidney, and neural tissues. Additionally, lignin can improve the mechanical properties of scaffolds and promote the regeneration of specific cells, such as osteoblasts and hepatocytes [24].
Moreover, pectin extracted from citrus peel by-products has shown promise in hydrogel formation to support soft tissue regeneration. A study developed green hydrogels using pectin from orange peels, demonstrating their potential as carrier systems for analgesics and antibiotics, facilitating drug delivery and promoting tissue healing [146].
4.4.4. Drug Delivery Systems
Drug delivery systems have significantly advanced the way medications are administered, providing targeted and controlled release to improve therapeutic outcomes and reduce side effects [147].
Among the materials used in these systems, plant-derived by-products offer a promising alternative to synthetic polymers. For example, polysaccharides such as cellulose, pectin, and starch are frequently used to form nanoparticles, microgels, and hydrogels for drug encapsulation. These polysaccharides can be extracted from various plant by-products, such as the peels of some fruits (citrus peels for pectin), woody biomass (for cellulose), and corn or potato by-products (for starch). These plant-based materials not only offer biocompatibility but also break down into harmless by-products like glucose or xylose, which are naturally metabolized by the body [8,24,148].
5. Economic and Technical Impact
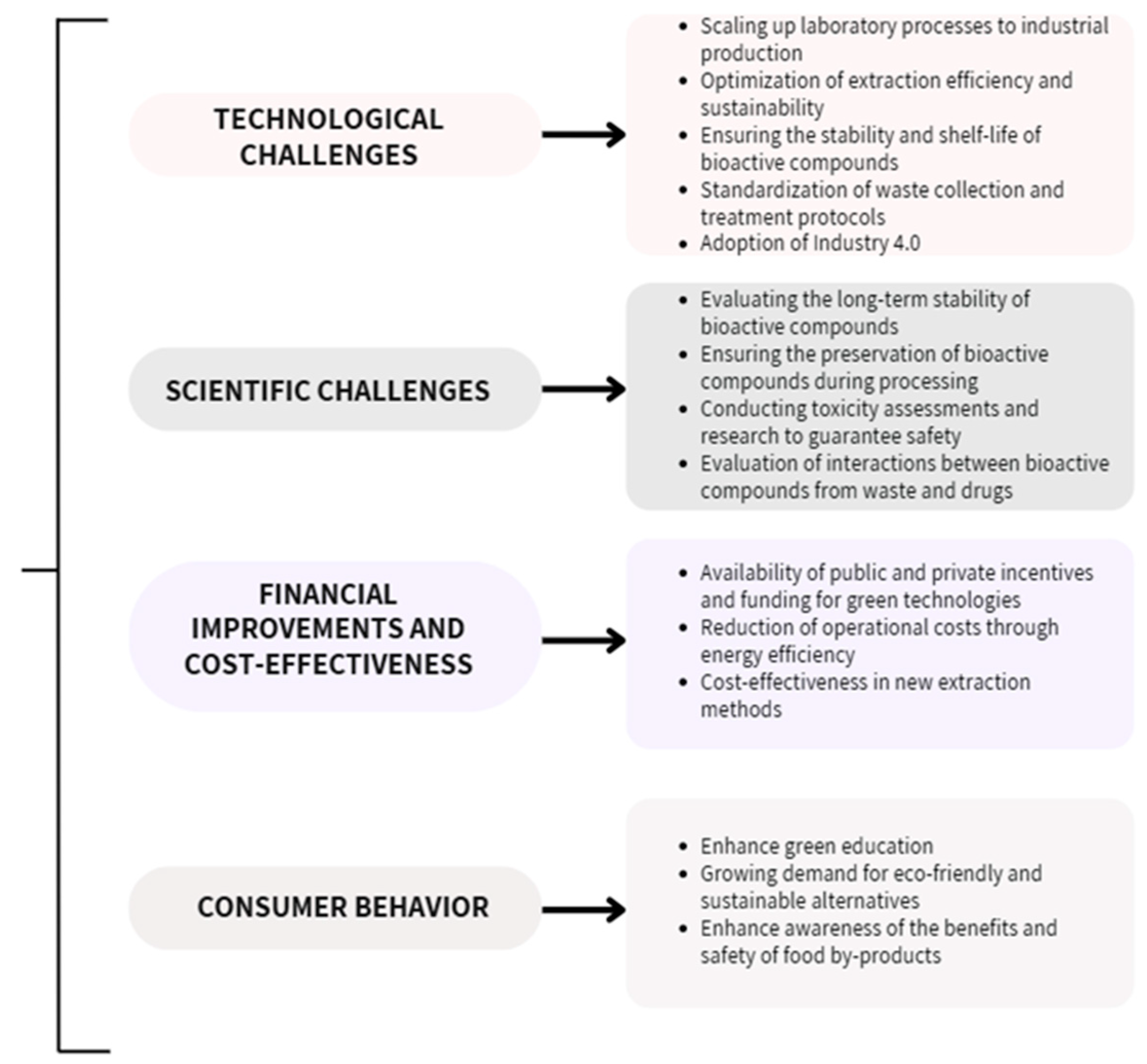
The valorization of vegetal by-products offers substantial economic benefits by transforming plant by-products into valuable resources, aligning with sustainability and circular economy principles. However, the process faces significant technical challenges, such as optimizing extraction methods and ensuring product quality at scale. Addressing these challenges requires investment in research, infrastructure, and efficient energy use to achieve cost-effectiveness and widespread commercial success. The authors have presented their perspective on these challenges and have summarized these key aspects in a comprehensive figure (Figure 7) [149].
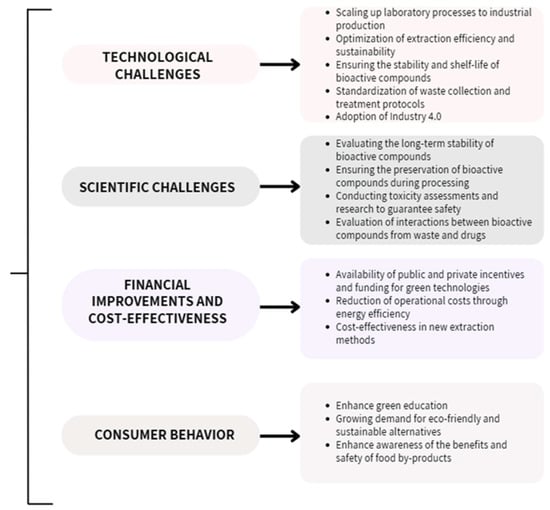
Figure 7.
Technological and scientific challenges, economic impacts, and consumer attitude involved in the valorization of plant by-products [149].
From an economic perspective, the successful valorization of food by-products is closely tied to the financial viability of these practices. While European consumers are increasingly inclined toward greener alternatives, the acceptance of items derived from food by-products has historically been limited. However, this trend is gradually evolving due to the strengthening of food safety regulations [40,41] and an increasing number of research studies and toxicity assessments that enhance consumer trust [42]. Thus, economic incentives and funding opportunities are critical in accelerating this transition and fostering investment in green technologies. These financial supports, alongside increasing consumer acceptance and trust in the benefits and safety of by-product-derived items, are expected to drive higher demand and facilitate market integration. In the context of a circular economy, the plant by-products generated by a company can be transformed from a problem to be solved to a new business opportunity. As a result, this evolution directly impacts the scalability of by-product valorization [150,151].
In the food industry, by-products must not only be nutritionally beneficial but also safe for human consumption [150]. This requires preserving the integrity of bioactive compounds throughout processing, ensuring food safety and optimizing shelf life without compromising product quality. To achieve this, advanced tools like high-performance liquid chromatography (HPLC), mass spectrometry, and nuclear magnetic resonance (NMR) are essential for monitoring the purity and concentration of bioactive compounds throughout the valorization process [152]. Ensuring that these methods are efficiently integrated into production workflows is essential to achieving both technical precision and economic feasibility, particularly as the scale of production increases.
Technological advances supporting the extraction, purification, and commercialization of bioactives from plant by-products are a key factor in making the valorization of plant by-products in biomedicine possible. Over the past five years, there have been significant advancements in the technologies surrounding the commercialization of bioactives from food residues. This includes the development of the advanced extraction techniques mentioned and explained in Table S1, as well as integrated biorefineries. Combining some of these emerging extraction methods (SFE and UAE, for example) can increase the overall efficiency and extract yield, ensuring enough material is available for pharmacological testing. However, these novel extraction protocols and methodologies have limitations and present challenges. Two such limitations are the variability in the composition of plant by-products and the difficulty of scaling up extraction methodologies to high Technology Readiness Levels (TRLs). Once extracted, the stability and activity of bioactive compounds can decrease, so measurements to prevent this are needed. These include optimizing storage conditions, using stabilizing agents, and performing encapsulation (e.g., nanoencapsulation, liposomal encapsulation, spray drying) [153]. This includes the development of advanced extraction techniques and integrated biorefineries. Linked to the advancement of sophisticated extraction techniques and biorefineries, technological progress has led to novel food applications. These applications facilitate the incorporation of bioactive compounds derived from plant by-products into functional foods, nutraceuticals, and food supplements. This development considerably expands their commercial potential. As an example, the combination of green extraction techniques and a biorefinery approach has been shown to be effective in valorizing coffee by-products as bioactive food ingredients and nutraceuticals [154].
Conventional methods of extracting bioactive compounds from plant tissues include Soxhlet extraction, maceration, percolation, and hydro-distillation. Non-conventional, green, or emerging extraction methods include ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), enzyme-assisted extraction (EAE), fermentation-assisted extraction (FAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), pulse electric field-assisted extraction (PEFAE), supercritical water extraction (SWE), instant controlled pressure drop (détente instantanée contrôllée, DIC), supercritical liquid chromatography (SFC), pressurized hot water extraction (PHWE), and deep eutectic solvents (DESs). The main advantages of these methods over conventional ones are high-quality extracts, greater selectivity, better isolation, reduced environmental impact, lower energy consumption, and safer products [33]. Each of the advanced extraction techniques mentioned has its own particularities, strengths, and limitations, which are summarized in Table S1.
Biorefineries have the capacity to produce a wide range of pharmaceutical products, including drugs, nutraceuticals, and other health-related products. However, there are some challenges that must be overcome, such as scaling up biological processes for large-scale production, ensuring commercial viability, and adhering to strict regulatory compliance and rigorous quality control to guarantee product safety and efficacy [155]. Technological advances in this area have led to the development of integrated biorefineries. The key distinction between a biorefinery and an integrated biorefinery lies in the extent of product diversification and the technologies employed. While a typical biorefinery focuses on the conversion of biomass into a range of products, an integrated biorefinery uses a broader range of technologies and feedstocks to produce a wider spectrum of valuable products [156]. Indeed, integrated biorefineries have the potential to enable the co-production of biofuels and pharmaceutical compounds through biomass valorization, thus delivering an innovative interdisciplinary solution to the global demands for sustainable energy and advanced therapeutics [155]. Despite this promising perspective, implementing biorefineries to extract and valorize plant by-products for pharmaceutical and cosmetic applications faces several real-world challenges. These include high costs, regulatory hurdles, and low consumer awareness [157,158]. The technological limitations that we have identified are due to the complexity of processing and scaling up and the need to maintain product quality. From an economic perspective, the feasibility of such a venture would depend on various factors, including feedstock costs, product prices, and investment costs. It should be noted that there are inherent uncertainties associated with new technologies and products. Regulatory barriers involve navigating complex regulations and securing necessary permits. Investment challenges are typically associated with the need for substantial upfront costs and the potential for long payback periods. Furthermore, nowadays, that the production of bioproducts with added value for the pharmaceutical industries is efficiently achieved through algae biotechnology applied in biorefineries [157].
A report by the EU in 2018 [159] identified 803 biorefineries within the EU that produced bio-based chemicals, liquid biofuels, composites, and fibers. However, few of them integrated the production of bio-based products (chemicals and/or composites) and bio-based energy (biofuels and/or other types of energy derived from biomass). In most cases, the predominant type of feedstock was agricultural, except in Finland, Sweden, and Portugal, where the most abundant feedstock came from forestry. Ten facilities in France, six in the Netherlands, five in Spain, three in Germany, and three in Ireland employed marine-derived feedstock (including macro/microalgae). More recently, a study commissioned by CEPI (the Confederation of European Paper Industries) in 2024 (https://www.cepi.org/wp-content/uploads/2024/09/Cepi_BioRefineries_Executive-Summary_2024.pdf, accessed on 11 June 2025) revealed the swift growth of the biorefinery sector in Europe. Biorefineries produce environmentally friendly alternatives to materials, chemicals, fuels, energy, food and feed, and pharmaceuticals and cosmetics, which are currently mostly supplied by Europe’s large petrochemical sector.
EU policies support the development of projects focused on smart, integrated energy-driven biorefineries for the co-production of advanced biofuels, biochemicals, and biomaterials (https://ec.europa.eu/info/funding-tenders/opportunities/portal/screen/opportunities/topic-details/horizon-cl5-2024-d3-02-03, accessed on 11 June 2025). One such project is SCALE, developed between 2021 and 2025, which aims to build and operate a flagship plant producing high-nutrient ingredients derived from untapped microalgal diversity for the food, food supplement, feed, and cosmetics sectors through economically sound and environmentally friendly processes. Coordinated by Microphyt (Baillargues, France), the project has been integrated by a wide consortium of participants (research centers and industries) from various countries, including France, Norway, Spain, the United Kingdom, and Belgium.
Moreover, several industrial-scale ventures and patented processes have recently advanced the valorization of plant by-products into bioactives. Arbiom (Paris, France/Durham, NC, USA) operates a continuous fermentation pilot to transform wood and agricultural residues into single-cell protein (SylPro®, Arbiom, Paris, France), integrating biomass valorization and regulatory scale-up [160]. Andritz offers its Turbex™ (Höri, Switzerland) extraction system, a commercial low-pressure, green extraction solution that processes fruit peels, and brewers’ bagasse to produce functional food ingredients [161]. Recent patents further highlight industrial innovation in extracting bioactives from plant by-products. WO 2021/119826 A1 [162] describes a method for enriching polyphenols from various plant residues, and patent US 10034910 B2 targets the extraction of flavan-3-ols from Chardonnay grape seed [163].
6. Opportunities and Remaining Challenges
The valorization of vegetable residues represents a promising and sustainable strategy that aligns with the principles of the circular economy. In a recent review [164], a list of plant-derived bioactive compounds whose therapeutic application has already been approved is provided. The years of approval range from 1827, when morphine extracted from Papaver somniferum was approved for use as an analgesic, to 2021, when samidorphan extracted from the same plant was approved for use in cases of schizophrenia and bipolar disorder. However, despite this promising outlook, there are still many weaknesses in the process of plant by-product recovery in the biomedical field (Table 6).

Table 6.
SWOT analysis of the situation.
The first issue of note is the role played by the solvents used to produce the different by-product extracts. The biological effect of plant by-products seems to be closely related to the type of solvent used. The most commonly used solvents are ethanol, methanol, water, or mixtures of these solvents. The mechanism of action underlying this biological effect is therefore solvent-dependent. This means that the observed biological activity is not only a reflection of the inherent properties of the plant but also the result of the specific compounds extracted, which vary depending on the solvent used [169]. Standardization in obtaining plant extracts is therefore a crucial aspect to ensure consistent replication of the effects observed in vitro or in vivo in a subsequent medical application. Such standardization, however, needs much improvement to date. The application of rigorous quality control procedures, supported by precise legislation, is key to ensuring the safety and efficacy of products obtained from natural sources [170]. Another essential aspect is to have a detailed profile of the extracted compounds using different solvents, as well as their concentration and the presence of possible impurities. This will ensure a better understanding of the biological effects, allow more reliable and reproducible studies, and enhance the safety and effectiveness of the plant compounds obtained. The application of modern techniques such as high-throughput screening and computational tools can make it possible to test a massive number of extracts for compounds with therapeutic potential in a short time [170]. On the other hand, it is highly desirable that the production of plant extracts is carried out in the most environmentally sustainable way possible, which supports the importance of research into emerging ‘green’ extraction technologies [171].
Research aimed at discovering the biochemical and molecular mechanisms underlying the action of active compounds extracted from plants has mostly been carried out using compounds isolated individually from individual plants. However, it has been shown that the therapeutic activity of isolated compounds may be lower than that of the extract from which they were obtained [172]. This is due to possible synergistic effects between several of the compounds included in the extract. Possible interactions between different compounds present in the same extract and synergistic, additive, or antagonistic effects have been detected between bioactive compounds extracted from plants and medicines, with the consequence that the pharmacokinetics and pharmacodynamics of medicines may be altered [153,165]. For instance, mandarin and lemon peels are rich in flavonoids such as hesperidin and naringenin (Table 2 and Table 4), which can inhibit cytochrome P450 enzymes (CYP3A4), potentially increasing the bioavailability of drugs like statins or certain chemotherapeutics [173]. Banana peels (Table 1) are a source of catecholamines like dopamine, which could interact with medications affecting the neurotransmitter balance, such as antidepressants [174]. Enzyme assays can study enzymes like CYP450 to understand compound metabolism and identify potential interactions [167]. However, not all interactions are detrimental; some can even be beneficial. For example, grape seed extract (Table 4) has shown synergy with chemotherapy drugs, enhancing their anticancer effects and potentially reducing the toxicity of treatments like cisplatin.
To date, fewer natural products have entered clinical trials than synthetic compounds. However, tested bioactive products tend to show more favorable results in early clinical phases, mainly due to lower toxicity profiles than synthetic compounds [42]. A very relevant aspect to obtain crucial information on the behavior of plant bioactive compounds in the body is the study of ADME properties. After consumption, the absorption of phytochemicals can be influenced by the pH of the gastrointestinal tract, their distribution and ability to reach target sites can be modified by their affinity for plasma proteins, and gut microbiota or phase II metabolism can transform these compounds, potentially inactivating them or altering their activity. Fernandez-Ochoa et al. [167] concluded that in vitro assays will provide a comprehensive understanding of how bioactive compounds from a plant by-product extract behave in the digestive system, supporting the transition to in vivo studies and clinical trials.
The importance of standardizing the extraction of plant extracts has been discussed above, but this is not the only aspect in need of standardization. Storage conditions at waste production sites, transport to the processing site, transit time, and handling practices can lead to degradation or loss of bioactive compounds. In other words, transport and logistics need to be supported by standardized protocols to ensure the consistency and efficacy of the material [5]. Fortunately, cold-chain monitoring systems and wireless Internet of Things (IoT) technologies, such as radiofrequency identification (RFID), allow for tracking the movement of plant by-products in real time, providing valuable data on environmental conditions (temperature, humidity, etc.) during transit [175,176].
Throughout this presentation, we have made clear the great importance of standardizing processes and protocols in order to convert plant by-products into a source of bioactive compounds with biomedical potential. However, there is a key factor that is difficult to standardize: the composition and quality of the residues generated. Even in cases where certain medicinal plants are used to extract bioactive compounds, there are aspects that are difficult to control, such as the age of the plant at the time of harvesting (very difficult to know in perennial plants) and the growing conditions (highly variable in plants from the field, where they have been exposed to a changing climate and have grown on different soils with different nutritional inputs, for example). The species or variety within a plant genus, the organ where the active compound is synthesized or stored, and the specific time in the plant’s life cycle are also aspects that lead to variability in the composition and levels of bioactive compounds [164]. Along the agri-food chain, these aspects may be fairly well known at the level of the primary sector that grows the plants (farmers), less well known in industrial processes that work with the raw material provided by the primary sector (juice companies, for example, who may use the same trusted suppliers), and completely unknown at the household and catering levels. In the latter areas, moreover, organic residue recycling does not currently discriminate between animal and vegetable residues, nor is it prepared for the specific recycling of certain vegetable residues of interest due to the properties of the bioactive compounds they can store. Even if animal residues are separated from vegetable ones, plant by-products will consist of random mixtures of different plants, which will vary throughout the year depending on the seasonal products. In addition, in the domestic environment, the mixture of plant by-products includes raw material and material that has undergone different cooking techniques (boiling, frying, etc.) that can modify the properties of the active compounds. In addition, the conditions of temporary storage of household waste (time, temperature, humidity), both at home and in urban containers, can also alter these properties and accelerate the microbial spoilage of by-products [150]. Current household waste collection systems must be restructured to enable the reuse of plant-based residues for bio-health applications. This requires staff training in the hospitality sector and early civic education to foster proper recycling habits, aligning with the environmental education goals of the European Union (European Higher Education Area) [177]. There are concrete examples of the efforts made in citizens’ education. The EU-funded SchoolFood4Change project (https://schoolfood4change.eu/, accessed on 12 June 2025) develops innovative solutions and locally adaptable good practices for schools, ultimately aiming to achieve the ambitious goal of enabling community-wide food system change. Moreover, the Agro2Circular project (https://agro2circular.eu, accessed on 12 June 2025), coordinated by CETEC in Spain and comprising several universities, industries, and financial entities, encourages citizens to participate in the regular and selective collection of agri-food waste. This waste is then transported to the National Technological Centre for Canning, where its active ingredients are extracted for use in nutritional formulas, cosmetics, food, and other high-value products. The project aims to promote acceptance of the circular economy in local communities by raising awareness and providing information to citizens, as well as collecting waste.
Once these residues have been collected, the application of Food Industry 4.0 technologies, such as Artificial Intelligence, data science, bioinformatics, smart sensors, robotics, or digital twins would allow the transformation of plant residues into revalued products [168]. We propose that these technologies, if their cost-effectiveness and scalability are positive, should be used to carry out an initial screening to check their quality and, based on the results, to decide on their final use. VCG.AI (https://vcg.ai/, accessed on 11 June 2025) is a company that uses intelligent algorithms to match by-products with proven, value-adding technologies and high-demand markets. It delivers solutions that maximize economic and environmental benefits. The company developed the ‘Data-driven food waste valorization for a large food retail chain’ project to extract lycopene and pectin from fruit and vegetable waste in order to manufacture pigments, dyes, cocoa, sugar confectionery, and non-distilled fermented beverages.
Poorer-quality batches of residues could be diverted to composting for application as fertilizer, for example. However, today, this proposition is more feasible in developed countries, which possess state-of-the-art machinery and processes that can transform by-products into valuable products [178], as well as strong regulatory frameworks and financial incentives [179]. In contrast, developing countries often face significant challenges in the area of by-product recycling due to their limited access to advanced technologies. Nowadays, there are numerous documents focused on the regulation of herbal medicinal products, but the legislation dealing with plant-based by-products is very scarce. Moreover, the regulation of the utilization of plant by-products in biomedicine or nutrition can also vary across countries [170].
Despite the significant limitations that remain to be overcome, the use of plant by-products, whether in biomedicine or nutrition, presents significant potential for advancing both fields. Not only are these by-products cheap and highly available, but they also offer an invaluable and sustainable resource for developing new therapeutic solutions to combat devastating diseases such as those discussed in this article [180]. Favorable laws and the availability of both public and private funding encourage their application, particularly through investments in research and scalability. The growing market for these resources could generate new employment opportunities and companies could also benefit by selling their plant by-products to third parties, further expanding the value chain.
7. Conclusions
The valorization of vegetable residues represents a promising and sustainable strategy that aligns with the principles of the circular economy, turning large amounts of agro-industrial by-products into valuable raw materials for diverse applications in fields such as biomedicine, pharmaceuticals, nutrition, and dermocosmetics.
Despite ongoing weaknesses and challenges, such as the lack of standardized extraction and processing methods, the field holds considerable strengths and opportunities. Advances in green extraction methods have significantly improved the efficiency and quality of bioactive compounds obtained from these residues, and the development and implementation of several biorefineries demonstrate the practical feasibility of these technologies. Moreover, consumer acceptance is gradually increasing, driven by stricter safety regulations, growing awareness of sustainability, educational programs promoting best practices, and an expanding body of scientific evidence supporting the benefits of plant-based bioactives.
These combined efforts pave the way for a more sustainable and innovative future, unlocking the full potential of vegetable residues across multiple industries. This not only enhances resource efficiency and promotes zero-waste goals but also offers a pathway to more resilient and health-conscious production systems in the context of circular bioeconomy models.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antiox14080942/s1, Table S1: Strengths, limitations and applications of the main innovative-green-emerging extraction methods. Examples of bioactive compounds extracted from plant by-products applying the mentioned emerging methods. References [181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198] are cited in the supplementary materials.
Author Contributions
Conceptualization, C.S. and N.G.; investigation, S.E.-N. and T.V.; supervision, D.P., C.S. and N.G.; writing—original draft preparation, S.E.-N.; writing—review and editing, D.P., C.S. and N.G.; funding acquisition, C.S. and N.G.; project administration, C.S. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
Silvia Estarriaga was the recipient of a grant from Asociación de Amigos of the University of Navarra (Strategy 2025: University and Sustainability). This research was funded by the European Union: Next-Generation EU and executed within the framework of the Recovery, Transformation and Resilience Plan (Ministry of Science, Innovation and Universities of Spanish Government, Agenda 2030 Navarra Government).
Data Availability Statement
No data was used for the research described in the article.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Richter, J.K.; Ek, P.; Gu, B.-J.; Ganjyal, G.M. Utilization of Food Processing by-Products in Extrusion Processing: A Review. Front. Sustain. Food Syst. 2021, 4, 603751. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Berenguer, C.V.; Andrade, C.F.P.; Câmara, J.S. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Appl. Sci. 2022, 12, 2747. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Delerue-Matos, C.; Grosso, C. Unveiling the Potential of Agrifood by-Products: A Comprehensive Review of Phytochemicals, Bioactivities and Industrial Applications. Waste Biomass Valor. 2024, 16, 2715–2748. [Google Scholar] [CrossRef]
- Aqilah, N.M.N.; Rovina, K.; Felicia, W.X.L.; Vonnie, J.M. A Review on the Potential Bioactive Components in Fruits and Vegetable Wastes as Value-Added Products in the Food Industry. Molecules 2023, 28, 2631. [Google Scholar] [CrossRef]
- Ghosal, K.; Ghosh, S. Biodegradable Polymers from Lignocellulosic Biomass and Synthetic Plastic Waste: An Emerging Alternative for Biomedical Applications. Mater. Sci. Eng. R Rep. 2023, 156, 100761. [Google Scholar] [CrossRef]
- Huang, L.; Huang, X.-H.; Yang, X.; Hu, J.-Q.; Zhu, Y.-Z.; Yan, P.-Y.; Xie, Y. Novel Nano-Drug Delivery System for Natural Products and Their Application. Pharmacol. Res. 2024, 201, 107100. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Mora, Z.V.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell Peppers (Capsicum annum L.) Losses and Wastes: Source for Food and Pharmaceutical Applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant Secondary Metabolites: An Opportunity for Circular Economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Osorio, L.; Flórez-López, E.; Grande-Tovar, C.D. The Potential of Selected Agri-Food Loss and Waste to Contribute to a Circular Economy: Applications in the Food, Cosmetic and Pharmaceutical Industries. Molecules 2021, 26, 515. [Google Scholar] [CrossRef]
- Population|United Nations. Available online: https://www.un.org/en/global-issues/population (accessed on 5 October 2024).
- European Commission. Food Waste and Food Waste Prevention by Nace Rev. 2 Activity-Tonnes of Fresh Mass; European Union: Brussels, Belgium, 2024. [Google Scholar]
- United Nations Environment Programme (UNEP). Food Waste Index Report 2024. Think Eat Save: Tracking Progress to Halve Global Food Waste; UNEP & WRAP: Nairobi, Kenya, 2024; Available online: https://www.unep.org/resources/publication/food-waste-index-report-2024 (accessed on 30 July 2025).
- Varjani, S.; Vyas, S.; Su, J.; Siddiqui, M.A.; Qin, Z.H.; Miao, Y.; Liu, Z.; Ethiraj, S.; Mou, J.H.; Lin, C.S.K. Nexus of Food Waste and Climate Change Framework: Unravelling the Links between Impacts, Projections, and Emissions. Environ. Pollut. 2024, 344, 123387. [Google Scholar] [CrossRef]
- Krah, C.Y.; Bahramian, M.; Hynds, P.; Priyadarshini, A. Household Food Waste Generation in High-Income Countries: A Scoping Review and Pooled Analysis between 2010 and 2022. J. Clean. Prod. 2024, 471, 143375. [Google Scholar] [CrossRef]
- Linder, M.; Williander, M. Circular Business Model Innovation: Inherent Uncertainties. Bus. Strategy Environ. 2015, 26, 182–196. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mahmud, S.; Acet, D.H. Circular Economy Model for Developing Countries: Evidence from Bangladesh. Heliyon 2022, 8, e09530. [Google Scholar] [CrossRef]
- Blomsma, F.; Brennan, G. The Emergence of Circular Economy: A New Framing around Prolonging Resource Productivity. J. Ind. Ecol. 2017, 21, 603–614. [Google Scholar] [CrossRef]
- Tlais, A.Z.A.; Fiorino, G.M.; Polo, A.; Filannino, P.; Di Cagno, R. High-Value Compounds in Fruit, Vegetable and Cereal Byproducts: An Overview of Potential Sustainable Reuse and Exploitation. Molecules 2020, 25, 2987. [Google Scholar] [CrossRef]
- Jaouhari, Y.; Travaglia, F.; Giovannelli, L.; Picco, A.; Oz, E.; Oz, F.; Bordiga, M. From Industrial Food Waste to Bioactive Ingredients: A Review on the Sustainable Management and Transformation of Plant-Derived Food Waste. Foods 2023, 12, 2183. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Zinoviadou, K.G.; Papageorgiou, M.; Rocha, J.M. Advances on the Valorisation and Functionalization of by-Products and Wastes from Cereal-Based Processing Industry. Foods 2020, 9, 1243. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Burgos, E.C.; Burgos-Hernandez, A.; Noguera-Artiaga, L.; Kacaniova, M.; Hernandez-Garcia, F.; Cardenas-Lopez, J.L.; Carbonell-Barrachina, A.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Sharma, H.; Safdar, M.; Lee, J.; Kim, W.; Park, S.; Jeong, H.E.; Kim, J. Micro/Nanoengineered Agricultural by-Products for Biomedical and Environmental Applications. Environ. Res. 2024, 250, 118490. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as Active Ingredients for Cosmetic Products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Ng, S.-L.; Wong, F.-M. Recent Developments in Research on Food Waste and the Circular Economy. Biomass 2024, 4, 472–489. [Google Scholar] [CrossRef]
- Rațu, R.N.; Veleșcu, I.D.; Stoica, F.; Usturoi, A.; Arsenoaia, V.N.; Crivei, I.C.; Postolache, A.N.; Lipșa, F.D.; Filipov, F.; Florea, A.M.; et al. Application of Agri-Food by-Products in the Food Industry. Agriculture 2023, 13, 1559. [Google Scholar] [CrossRef]
- Attiq, S.; Chau, K.Y.; Bashir, S.; Habib, M.D.; Azam, R.I.; Wong, W.K. Sustainability of Household Food Waste Reduction: A Fresh Insight on Youth’s Emotional and Cognitive Behaviors. Int. J. Environ. Res. Public Health 2021, 18, 7013. [Google Scholar] [CrossRef]
- Calculli, C.; D’Uggento, A.M.; Labarile, A.; Ribecco, N. Evaluating People’s Awareness About Climate Changes and Environmental Issues: A Case Study. J. Clean. Prod. 2021, 324, 129244. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and Vegetable Waste Management: Conventional and Emerging Approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Kumar Gupta, R.; Ae Ali, E.; Abd El Gawad, F.; Mecheal Daood, V.; Sabry, H.; Karunanithi, S.; Prakash Srivastav, P. Valorization of Fruits and Vegetables Waste Byproducts for Development of Sustainable Food Packaging Applications. Waste Manag. Bull. 2024, 2, 21–40. [Google Scholar] [CrossRef]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current Technologies and Uses for Fruit and Vegetable Wastes in a Sustainable System: A Review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef]
- Yadav, N.; Patel, P.; Kashyap, P.; Tamboli, R.; Chandel, S.; Santosh, R. Kirtane Conventional Extraction Methods Versus Novel Extraction Methods of Bioactive Compounds from Some Medicinal Plants. Int. J. Green Pharm. 2024, 16, 1–6. [Google Scholar]
- Meili, R.; Stucki, T. Money Matters: The Role of Money as a Regional and Corporate Financial Resource for Circular Economy Transition at Firm-Level. Res. Policy 2023, 52, 104884. [Google Scholar] [CrossRef]
- Financing the Circular Economy. Available online: https://circulareconomy.europa.eu/platform/en/financing-circular-economy (accessed on 9 October 2024).
- Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sdgs.un.org/2030agenda (accessed on 5 November 2024).
- Mukherjee, P.K.; Das, B.; Bhardwaj, P.K.; Tampha, S.; Singh, H.K.; Chanu, L.D.; Sharma, N.; Devi, S.I. Socio-Economic Sustainability with Circular Economy—An Alternative Approach. Sci. Total Environ. 2023, 904, 166630. [Google Scholar] [CrossRef]
- Rashwan, A.K.; Bai, H.; Osman, A.I.; Eltohamy, K.M.; Chen, Z.; Younis, H.A.; Al-Fatesh, A.; Rooney, D.W.; Yap, P.-S. Recycling Food and Agriculture by-Products to Mitigate Climate Change: A Review. Environ. Chem. Lett. 2023, 21, 3351–3375. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-Products and Food Wastes: Are They Safe Enough for Their Valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Zarbà, C.; Chinnici, G.; La Via, G.; Bracco, S.; Pecorino, B.; D’Amico, M. Regulatory Elements on the Circular Economy: Driving into the Agri-Food System. Sustainability 2021, 13, 8350. [Google Scholar] [CrossRef]
- Knežević, N.; Ranilović, J.; Cvetković, T.; Grbavac, S.; Palfi, M. Vegetable by-Products in the European Food Legislative Framework. Croat. J. Food Technol. Biotechnol. Nutr. 2021, 16, 115–122. [Google Scholar] [CrossRef]
- Domingo-Fernández, D.; Gadiya, Y.; Preto, A.J.; Krettler, C.A.; Mubeen, S.; Allen, A.; Healey, D.; Colluru, V. Natural Products Have Increased Rates of Clinical Trial Success Throughout the Drug Development Process. J. Nat. Prod. 2024, 87, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The Scope of the Antimicrobial Resistance Challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Arip, M.; Selvaraja, M.; Mogana, R.; Tan, L.F.; Leong, M.Y.; Tan, P.L.; Yap, V.L.; Chinnapan, S.; Tat, N.C.; Abdullah, M.; et al. Review on Plant-Based Management in Combating Antimicrobial Resistance-Mechanistic Perspective. Front. Pharmacol. 2022, 13, 879495. [Google Scholar] [CrossRef]
- Al-Mqbali, L.R.A.; Hossain, M.A. Cytotoxic and Antimicrobial Potential of Different Varieties of Ripe Banana Used Traditionally to Treat Ulcers. Toxicol. Rep. 2019, 6, 1086–1090. [Google Scholar] [CrossRef]
- Naqvi, S.A.; Irfan, A.; Zahoor, A.F.; Zafar, M.; Maria, A.; Chand, A.; Ashfaq, S. Determination of Antimicrobial and Antioxidant Potential of Agro-Waste Peels. An. Acad. Bras. Cienc. 2020, 92, e20181103. [Google Scholar] [CrossRef]
- Harith, S.S.; Yasim, N.H.M.; Harun, A.; Wan Omar, W.S.A.; Musa, M.S. Phytochemical Screening, Antifungal and Antibacterial Activities of Musa acuminata Plant. Malays. J. Anal. Sci. 2018, 22, 452–457. [Google Scholar] [CrossRef]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and Antioxidant Properties of Tomato Processing Byproducts and Their Correlation with the Biochemical Composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Saleem, M.; Saeed, M.T. Potential Application of Waste Fruit Peels (Orange, Yellow Lemon and Banana) as Wide Range Natural Antimicrobial Agent. J. King Saud Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Evbuomwan, L.; Jacob, I.B.; Onodje, G.O.; Patrick, C. Evaluating the Antibacterial Activity of Musa acuminata (Banana) Fruit Peels against Multidrug Resistant Bacterial Isolates. Int. J. Novel Res. Life Sci. 2018, 5, 26–31. [Google Scholar]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and Virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, S.J.; Choe, H.S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. Biomed Res. Int. 2018, 2018, 14. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Ibrahim, H.I.M. A Molecular Insight into the Synergistic Mechanism of Nigella sativa (Black cumin) With β-Lactam Antibiotics against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Appl. Sci. 2021, 11, 3206. [Google Scholar] [CrossRef]
- Chaves-Ulate, C.; Rodríguez-Sánchez, C.; Arias-Echandi, M.L.; Esquivel, P. Antimicrobial Activities of Phenolic Extracts of Coffee Mucilage. NFS J. 2023, 31, 50–56. [Google Scholar] [CrossRef]
- Chen, X.; Lan, W.; Xie, J. Natural Phenolic Compounds: Antimicrobial Properties, Antimicrobial Mechanisms, and Potential Utilization in the Preservation of Aquatic Products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef]
- Berida, T.I.; Adekunle, Y.A.; Dada-Adegbola, H.; Kdimy, A.; Roy, S.; Sarker, S.D. Plant Antibacterials: The Challenges and Opportunities. Heliyon 2024, 10, e31145. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Haq, A.; Siddiqi, M.; Batool, S.Z.; Islam, A.; Khan, A.; Khan, D.; Khan, S.; Khan, H.; Shah, A.A.; Hasan, F.; et al. Comprehensive Investigation on the Synergistic Antibacterial Activities of Jatropha curcas Pressed Cake and Seed Oil in Combination with Antibiotics. AMB Express 2019, 9, 67. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Steixner, S. The Changing Epidemiology of Fungal Infections. Mol. Asp. Med. 2023, 94, 101215. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Evolution, Mechanisms and Impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Osset-Trénor, P.; Pascual-Ahuir, A.; Proft, M. Fungal Drug Response and Antimicrobial Resistance. J. Fungi 2023, 9, 565. [Google Scholar] [CrossRef]
- Xu, J. Assessing Global Fungal Threats to Humans. mLife 2022, 1, 223–240. [Google Scholar] [CrossRef]
- Maiza, M. Antifungal Activity of Organic Residues of Plant Origin; University of Navarra: Pamplona, Spain, 2022; (Unpublished Results). [Google Scholar]
- Romero, A. Antifungal Activity of Domestic Vegetable Waste; University of Navarra: Pamplona, Spain, 2023; (Unpublished Results). [Google Scholar]
- Herkert, P.F.; Al-Hatmi, A.M.S.; de Oliveira Salvador, G.L.; Muro, M.D.; Pinheiro, R.L.; Nucci, M.; Queiroz-Telles, F.; de Hoog, G.S.; Meis, J.F. Molecular Characterization and Antifungal Susceptibility of Clinical Fusarium Species from Brazil. Front. Microbiol. 2019, 10, 737. [Google Scholar] [CrossRef]
- Xi, K.-Y.; Xiong, S.-J.; Li, G.; Guo, C.-Q.; Zhou, J.; Ma, J.-W.; Yin, J.-L.; Liu, Y.-Q.; Zhu, Y.-X. Antifungal Activity of Ginger Rhizome Extract against Fusarium solani. Horticulturae 2022, 8, 983. [Google Scholar] [CrossRef]
- Rashed, H.H.; Al-Hussaini, I.M.; Al-Marzoqi, A.H. Evaluation of the Inhibitory Effect of Gingerol on Bacteria and Fungi Isolated from the Vagina. Med. J. Babylon. 2024, 21, 85–93. [Google Scholar] [CrossRef]
- Leontiev, R.; Hohaus, N.; Jacob, C.; Gruhlke, M.C.H.; Slusarenko, A.J. A Comparison of the Antibacterial and Antifungal Activities of Thiosulfinate Analogues of Allicin. Sci. Rep. 2018, 8, 6763. [Google Scholar] [CrossRef]
- Yang, X.; Bai, S.; Wu, J.; Fan, Y.; Zou, Y.; Xia, Z.; Ao, J.; Chen, T.; Zhang, M.; Yang, R. Antifungal Activity and Potential Action Mechanism of Allicin against Trichosporon asahii. Microbiol. Spectr. 2023, 11, e0090723. [Google Scholar] [CrossRef]
- Hashem, A.H.; Saied, E.; Ali, O.M.; Selim, S.; Al Jaouni, S.K.; Elkady, F.M.; El-Sayyad, G.S. Pomegranate Peel Extract Stabilized Selenium Nanoparticles Synthesis: Promising Antimicrobial Potential, Antioxidant Activity, Biocompatibility, and Hemocompatibility. Appl. Biochem. Biotechnol. 2023, 195, 5753–5776. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Sánchez-Hernández, E.; Noversa, J.; Cunha, A.; Cortez, I.; Marques, G.; Martín-Ramos, P.; Oliveira, R. Antifungal Activity of Plant Waste Extracts against Phytopathogenic Fungi: Allium sativum Peels Extract as a Promising Product Targeting the Fungal Plasma Membrane and Cell Wall. Horticulturae 2023, 9, 136. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, D.; He, M.; Pan, S.; Yao, X.; Xu, X. Antifungal Action and Inhibitory Mechanism of Polymethoxylated Flavones from Citrus reticulata Blanco Peel against Aspergillus niger. Food Control 2014, 35, 354–359. [Google Scholar] [CrossRef]
- Steverding, D. The Spreading of Parasites by Human Migratory Activities. Virulence 2020, 11, 1177–1191. [Google Scholar] [CrossRef]
- Koger-Pease, C.; Perera, D.J.; Ndao, M. Recent Advances in the Development of Adenovirus-Vectored Vaccines for Parasitic Infections. Pharmaceuticals 2023, 16, 334. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Karanis, P. Cryptosporidium and Cryptosporidiosis: The Perspective from the Gulf Countries. Int. J. Environ. Res. Public Health 2020, 17, 6824. [Google Scholar] [CrossRef]
- Marie, C.; Petri, W. Cryptosporidiosis. Available online: https://www.msdmanuals.com/professional/infectious-diseases/intestinal-protozoa-and-microsporidia/cryptosporidiosis (accessed on 5 October 2024).
- Al-Mathal, E.M.; Alsalem, A.M. Pomegranate (Punica granatum) Peel Is Effective in a Murine Model of Experimental Cryptosporidium parvum. Exp. Parasitol. 2012, 131, 350–357. [Google Scholar] [CrossRef]
- Teichmann, K.; Kuliberda, M.; Schatzmayr, G.; Pacher, T.; Zitterl-Eglseer, K.; Joachim, A.; Hadacek, F. In Vitro Inhibitory Effects of Plant-Derived by-Products against Cryptosporidium parvum. Parasite 2016, 23, 41. [Google Scholar] [CrossRef]
- Vilela, R.C.; Benchimol, M. Trichomonas vaginalis and Tritrichomonas foetus: Interaction with Fibroblasts and Muscle Cells-New Insights into Parasite-Mediated Host Cell Cytotoxicity. Mem. Inst. Oswaldo Cruz 2012, 107, 720–727. [Google Scholar] [CrossRef]
- Friedman, M.; Tam, C.C.; Kim, J.H.; Escobar, S.; Gong, S.; Liu, M.; Mao, X.Y.; Do, C.; Kuang, I.; Boateng, K.; et al. Anti-Parasitic Activity of Cherry Tomato Peel Powders. Foods 2021, 10, 230. [Google Scholar] [CrossRef]
- Garcia, A.R.; Amaral, A.C.F.; Azevedo, M.M.B.; Corte-Real, S.; Lopes, R.C.; Alviano, C.S.; Pinheiro, A.S.; Vermelho, A.B.; Rodrigues, I.A. Cytotoxicity and Anti-Leishmania amazonensis Activity of Citrus sinensis Leaf Extracts. Pharm. Biol. 2017, 55, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Scorza, B.M.; Carvalho, E.M.; Wilson, M.E. Cutaneous Manifestations of Human and Murine Leishmaniasis. Int. J. Mol. Sci. 2017, 18, 1296. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; De Castro Gomes, C.M.; Laurenti, M.D.; Corbett, C.E.P. Immunopathogenic Competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American Cutaneous Leishmaniasis. Parasite Immunol. 2009, 31, 423–431. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer. 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.-Y. Exploring Treatment Options in Cancer: Tumor Treatment Strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C. Natural Bioactive Compounds, Vegetal Extracts and Modern Pharmaceutical Formulations: New Insights into the Anti-Cancer Mechanism of Action. Anti-Cancer Agents Med. Chem. 2020, 20, 1754–1755. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.K.; El-Kalaawy, A.M.; Abd El-Twab, S.M.; Alblihed, M.A.; Ahmed, O.M. Hesperetin and Capecitabine Abate 1,2 Dimethylhydrazine-Induced Colon Carcinogenesis in Wistar Rats Via Suppressing Oxidative Stress and Enhancing Antioxidant, Anti-Inflammatory and Apoptotic Actions. Life 2023, 13, 984. [Google Scholar] [CrossRef]
- Colone, M.; Maggi, F.; Rakotosaona, R.; Stringaro, A. Vepris macrophylla Essential Oil Produces Notable Antiproliferative Activity and Morphological Alterations in Human Breast Adenocarcinoma Cells. Appl. Sci. 2021, 11, 4369. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Morantes, S.J.; Sánchez-Camargo, A.d.P.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. An Integrated Approach for the Valorization of Mango Seed Kernel: Efficient Extraction Solvent Selection, Phytochemical Profiling and Antiproliferative Activity Assessment. Food Res. Int. 2019, 126, 108616. [Google Scholar] [CrossRef]
- Nair, S.A.; Sr, R.K.; Nair, A.S.; Baby, S. Citrus Peels Prevent Cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef]
- Torres, N.; Plano, D.; Antolín, M.C.; Sanmartín, C.; Domínguez-Fernández, M.; De Peña, M.-P.; Encío, I.; Goicoechea, N. Potential Biomedical Reuse of Vegetative Residuals from Mycorrhized Grapevines Subjected to Warming. Arch. Agron. Soil Sci. 2019, 65, 1341–1353. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and Cytotoxic Effects of Grape Pomace and Grape Seed Extracts on Colorectal Cancer Cell Lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Ramachandran, S.; Waypa, G.B.; Yin, J.J.; Li, C.Q.; Han, M.; Huang, H.H.; Sillard, W.W.; Vanden Hoek, T.L.; et al. Grape Seed Proanthocyanidins Ameliorate Doxorubicin-Induced Cardiotoxicity. Am. J. Chin. Med. 2010, 38, 569–584. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, Q.; Lu, W.; Shen, J.; Zhou, D.; Wang, Y.; Gao, S.; Wang, Z. Grape Seed Procyanidin B2 Promotes the Autophagy and Apoptosis in Colorectal Cancer Cells Via Regulating Pi3k/Akt Signaling Pathway. Onco Targets Ther. 2019, 12, 4109–4118. [Google Scholar] [CrossRef]
- Fazio, A.; Iacopetta, D.; La Torre, C.; Ceramella, J.; Muià, N.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Finding Solutions for Agricultural Wastes: Antioxidant and Antitumor Properties of Pomegranate Akko Peel Extracts and β-Glucan Recovery. Food Funct. 2018, 9, 6618–6631. [Google Scholar] [CrossRef]
- Abdelrahman, K.N.; Abdel Ghany, A.G.A.; Saber, R.A.; Osman, A.; Sitohy, B.; Sitohy, M. Anthocyanins from Pomegranate Peel (Punica granatum), Chili Pepper Fruit (Capsicum annuum), and Bougainvillea Flowers (Bougainvillea spectabilis) with Multiple Biofunctions: Antibacterial, Antioxidant, and Anticancer. Heliyon 2024, 10, e32222. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Yang, X.; Zhu, Y.; Zhang, M. Evaluation of Antioxidative and Antitumor Activities of Extracted Flavonoids from Pink Lady Apples in Human Colon and Breast Cancer Cell Lines. Food Funct. 2015, 6, 3789–3798. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chen, B.H. A Comparative Study on Inhibition of Breast Cancer Cells and Tumors in Mice by Carotenoid Extract and Nanoemulsion Prepared from Sweet Potato (Ipomoea batatas L.) Peel. Pharmaceutics 2022, 14, 980. [Google Scholar] [CrossRef]
- Abubakar, A.N.F.; Achmadi, S.S.; Suparto, I.H. Triterpenoid of Avocado (Persea americana) Seed and Its Cytotoxic Activity toward Breast MCF-7 and Liver HepG2 Cancer Cells. Asian Pac. J. Trop. Biomed. 2017, 7, 397–400. [Google Scholar] [CrossRef]
- Martinez, R.M.; Guimarães, D.d.A.B.; Berniz, C.R.; Abreu, J.P.d.; Rocha, A.P.M.d.; Moura, R.S.d.; Resende, A.C.; Teodoro, A.J. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef]
- Barros, L.; Calhelha, R.C.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Santos, E.A.; Regis, W.C.B.; Ferreira, I.C.F.R. The Powerful in Vitro Bioactivity of Euterpe oleracea Mart. Seeds and Related Phenolic Compounds. Ind. Crops Prod. 2015, 76, 318–322. [Google Scholar] [CrossRef]
- Carmo, M.A.V.D.; Fidelis, M.; Pressete, C.G.; Marques, M.J.; Castro-Gamero, A.M.; Myoda, T.; Granato, D.; Azevedo, L. Hydroalcoholic Myrciaria dubia (Camu-Camu) Seed Extracts Prevent Chromosome Damage and Act as Antioxidant and Cytotoxic Agents. Food Res. Int. 2019, 125, 108551. [Google Scholar] [CrossRef]
- Fidelis, M.; do Carmo, M.A.V.; da Cruz, T.M.; Azevedo, L.; Myoda, T.; Miranda Furtado, M.; Boscacci Marques, M.; Sant’Ana, A.S.; Inês Genovese, M.; Young Oh, W.; et al. Camu-Camu Seed (Myrciaria dubia)–from Side Stream to Anantioxidant, Antihyperglycemic, Antiproliferative, Antimicrobial, Antihemolytic, Anti-Inflammatory, and Antihypertensive Ingredient. Food Chem. 2020, 310, 125909. [Google Scholar] [CrossRef]
- Zivković, J.; Kumar, K.A.; Rushendran, R.; Ilango, K.; Fahmy, N.M.; El-Nashar, H.A.S.; El-Shazly, M.; Ezzat, S.M.; Melgar-Lalanne, G.; Romero-Montero, A.; et al. Pharmacological Properties of Mangiferin: Bioavailability, Mechanisms of Action and Clinical Perspectives. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 763–781. [Google Scholar] [CrossRef]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and Ellagic Acid Demonstrate Antimutagenic Activity and Inhibition of Benzo[a]Pyrene Induced DNA Adducts. Biomed. Res. Int. 2014, 2014, 467465. [Google Scholar] [CrossRef]
- Subkorn, P.; Norkaew, C.; Deesrisak, K.; Tanyong, D. Punicalagin, a Pomegranate Compound, Induces Apoptosis and Autophagy in Acute Leukemia. PeerJ 2021, 9, e12303. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Akash, S.; Hossain, M.E.; Tumpa, A.A.; Abrar Ishtiaque, G.M.; Ahmed, L.; Rauf, A.; Khalil, A.A.; Al Abdulmonem, W.; et al. Pomegranate-Specific Natural Compounds as Onco-Preventive and Onco-Therapeutic Compounds: Comparison with Conventional Drugs Acting on the Same Molecular Mechanisms. Heliyon 2023, 9, e18090. [Google Scholar] [CrossRef]
- Aouey, B.; Samet, A.M.; Fetoui, H.; Simmonds, M.S.J.; Bouaziz, M. Anti-Oxidant, Anti-Inflammatory, Analgesic and Antipyretic Activities of Grapevine Leaf Extract (Vitis vinifera) in Mice and Identification of Its Active Constituents by Lc–Ms/Ms Analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef]
- Abed, A.H.; Harb, J.; Khasib, S.; Saad, B. Invitro Assessment of Cytotoxic, Antioxidant and Antimicrobial Activities of Leaves from Two Grape Varieties Collected from Arid and Temperate Regions in Palestine. QScience Connect 2015, 4. [Google Scholar] [CrossRef]
- Esfahanian, Z.; Behbahani, M.; Shanehsaz, M.; Hessami, M.J.; Nejatian, M.A. Evaluation of Anticancer Activity of Fruit and Leave Extracts from Virus Infected and Healthy Cultivars of Vitis vinifera. Cell J. 2013, 15, 116–123. [Google Scholar]
- Handoussa, H.; Hanafi, R.; Eddiasty, I.; El-Gendy, M.; El Khatib, A.; Linscheid, M.; Mahran, L.; Ayoub, N. Anti-Inflammatory and Cytotoxic Activities of Dietary Phenolics Isolated from Corchorus olitorius and Vitis vinifera. J. Funct. Foods. 2013, 5, 1204–1216. [Google Scholar] [CrossRef]
- Machado, A.P.d.F.; Alves, M.d.R.; Nascimento, R.d.P.d.; Reguengo, L.M.; Marostica Junior, M.R. Antiproliferative Effects and Main Molecular Mechanisms of Brazilian Native Fruits and Their by-Products on Lung Cancer. Food Res. Int. 2022, 162, 111953. [Google Scholar] [CrossRef]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection Assessment of Olive (Olea europaea L.) Leaves Extract Standardized to Oleuropein: In Vitro and in Silico Approach for Improved Sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Baumann, L.; Bernstein, E.F.; Weiss, A.S.; Bates, D.; Humphrey, S.; Silberberg, M.; Daniels, R. Clinical Relevance of Elastin in the Structure and Function of Skin. Aesthet. Surg. J. Open Forum 2021, 3, ojab019. [Google Scholar] [CrossRef]
- Hassan, M.; Shahzadi, S.; Kloczkowski, A. Tyrosinase Inhibitors Naturally Present in Plants and Synthetic Modifications of These Natural Products as Anti-Melanogenic Agents: A Review. Molecules 2023, 28, 378. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential Application of Grape (Vitis vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Taghouti, M.; Martins-Gomes, C.; Schäfer, J.; Félix, L.M.; Santos, J.A.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Thymus pulegioides L. As a Rich Source of Antioxidant, Anti-Proliferative and Neuroprotective Phenolic Compounds. Food Funct. 2018, 9, 3617–3629. [Google Scholar] [CrossRef]
- Vieira, D.; Duarte, J.; Vieira, P.; Gonçalves, M.B.S.; Figueiras, A.; Lohani, A.; Veiga, F.; Mascarenhas-Melo, F. Regulation and Safety of Cosmetics: Pre- and Post-Market Considerations for Adverse Events and Environmental Impacts. Cosmetics 2024, 11, 184. [Google Scholar] [CrossRef]
- Yarovaya, L.; Waranuch, N.; Wisuitiprot, W.; Khunkitti, W. Clinical Study of Asian Skin Changes after Application of a Sunscreen Formulation Containing Grape Seed Extract. J. Cosmet. Dermatol. 2022, 21, 4523–4535. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; Viretto, A.; Thevenon, M.-F.; Brancheriau, L. Sustainable Biocomposites from Renewable Resources in West Africa: A Review. J. Renew. Mater. 2025, 2164–6341. [Google Scholar] [CrossRef]
- Bio4Africa Antcare. Available online: https://www.bio4africa.eu/technologies/technology-catalogue/antcare/ (accessed on 11 June 2025).
- Shan, C. Combined Plant Extract for Getting Rid of Mites and Acne and Preparation Method and Use Thereof. US 2022/0110997 A1, 3 May 2021. [Google Scholar]
- Atabay, Ş. Anti-Inflammatory, Anti-Allergic, Anti-Bacterial, Anti-Aging and Anti-Oxidant Effective Multifunctional and Nanotechnological Gel Content and Production Method for the Facial Contour. WO 2022/235238 A2, 26 April 2022. [Google Scholar]
- Gomez-Molina, M.; Albaladejo-Marico, L.; Yepes-Molina, L.; Nicolas-Espinosa, J.; Navarro-León, E.; Garcia-Ibañez, P.; Carvajal, M. Exploring Phenolic Compounds in Crop by-Products for Cosmetic Efficacy. Int. J. Mol. Sci. 2024, 25, 5884. [Google Scholar] [CrossRef]
- Ariyanta, H.A.; Sholeha, N.A.; Fatriasari, W. Current and Future Outlook of Research on Renewable Cosmetics Derived from Biomass. Chem. Biodivers. 2025, 22, e202402249. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. [Google Scholar] [CrossRef]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the Normal Gut Microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.-W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Zhang, L.; Wang, M.; Chang, W. The Interaction of Polyphenols and the Gut Microbiota in Neurodegenerative Diseases. Nutrients 2022, 14, 5373. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Chan, M.; Zhang, P.; Ng, K. Modulation of the Human Gut Microbiota by Phenolics and Phenolic Fiber-Rich Foods. Compreh. Rev. Food Sci. Food Saf. 2020, 19, 1268–1298. [Google Scholar] [CrossRef]
- Birbizi, A. Project of Innovative Best Practices for the Reuse of Whey and Underutilized Agro-Industrial Waste. Available online: https://www.birbizi.com/ (accessed on 9 July 2025).
- do Espírito Santo, A.P.; Cartolano, N.S.; Silva, T.F.; Soares, F.A.S.M.; Gioielli, L.A.; Perego, P.; Converti, A.; Oliveira, M.N. Fibers from Fruit by-Products Enhance Probiotic Viability and Fatty Acid Profile and Increase Cla Content in Yoghurts. Int. J. Food Microbiol. 2012, 154, 135–144. [Google Scholar] [CrossRef]
- Hao, C.L.; Esah, E.M.; Tajarudin, H.A.; Akter, B.; Salleh, R.M. Effect of Potential Prebiotics from Selected Fruits Peel on the Growth of Probiotics. J. Food Process. Preserv. 2021, 45, e15581. [Google Scholar] [CrossRef]
- Federation, I.D. Diabetes Facts & Figures. Available online: https://idf.org/about-diabetes/diabetes-facts-figures/ (accessed on 9 July 2025).
- El-Waseif, M.; Saed, B.; El-Behairy, S.; Ali, H.; Elkhadragy, M.; Yehia, H.; Farouk, A. The Valorization of Agro-Wastes and Stevia Leaves as a Sugar Replacer in Cupcake Formulas: Histological and in Vivo Studies on Diabetic Rats. Sustainability 2023, 15, 9126. [Google Scholar] [CrossRef]
- Schiano, E.; Piccolo, V.; Novellino, E.; Maisto, M.; Iannuzzo, F.; Summa, V.; Tenore, G.C. Thinned Nectarines, an Agro-Food Waste with Antidiabetic Potential: Hplc-Hesi-Ms/Ms Phenolic Characterization and in Vitro Evaluation of Their Beneficial Activities. Foods 2022, 11, 1010. [Google Scholar] [CrossRef]
- Büker, M.; Angın, P.; Nurman, N.; Rasouli Pirouzian, H.; Akdeniz, E.; Toker, O.S.; Sagdic, O.; Tamtürk, F. Effects of Apple Pomace as a Sucrose Substitute on the Quality Characteristics of Compound Chocolate and Spread. J. Food Process. Preserv. 2021, 45, e15773. [Google Scholar] [CrossRef]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the Glycemic Index of Short Dough Biscuits by Using Apple Pomace as a Functional Ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Behrooznia, Z.; Nourmohammadi, J. Polysaccharide-Based Materials as an Eco-Friendly Alternative in Biomedical, Environmental, and Food Packaging. Giant 2024, 19, 100301. [Google Scholar] [CrossRef]
- Ansari, M.M.; Heo, Y.; Do, K.; Ghosh, M.; Son, Y.-O. Nanocellulose Derived from Agricultural Biowaste by-Products–Sustainable Synthesis, Biocompatibility, Biomedical Applications, and Future Perspectives: A Review. Carbohydr. Polym. Technol. Appl. 2024, 8, 100529. [Google Scholar] [CrossRef]
- Hamed, R.; Magamseh, K.H.; Al-Shalabi, E.; Hammad, A.; Abu-Sini, M.; Abulebdah, D.H.; Tarawneh, O.; Sunoqrot, S. Green Hydrogels Prepared from Pectin Extracted from Orange Peels as a Potential Carrier for Dermal Delivery Systems. ACS Omega 2025, 10, 17182–17200. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in Drug Delivery Systems, Challenges and Future Directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Novoselov, K.S.; Liang, F.; Meng, J.; Ho, S.-H.; Zhao, T.; Zhou, H.; Ahmad, A.; Zhu, Y.; et al. Bioresource Upgrade for Sustainable Energy, Environment, and Biomedicine. Nano-Micro Lett. 2023, 15, 35. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of Fruit and Vegetable Waste to Produce Value-Added Products: Conventional Utilization and Emerging Opportunities-a Review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing by-Products in the Eu: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical Success and Risk Factors for Circular Business Models Valorising Agricultural Waste and by-Products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S. Nmr-Based Chromatography Readouts: Indispensable Tools to “Translate” Analytical Features into Molecular Structures. Cells 2022, 11, 3526. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, M.; Pantina, V.D.; Venezia, V.; Modica, C.; Lo Iacono, M.; Bianca, P.; Bozzari, G.; Angeloro, F.; Cozzolino, V.; Stassi, G.; et al. Exploring the Antitumorigenic Properties of Agro-Food Byproducts: A Comprehensive Scientific Review. Pharmacol. Res. 2025, 216, 107740. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Gil-Ramírez, A.; Benítez, V.; Cañas, S.; Braojos, C.; Martin-Cabrejas, M.A. Biorefinery and Stepwise Strategies for Valorizing Coffee by-Products as Bioactive Food Ingredients and Nutraceuticals. Appl. Sci. 2023, 13, 8326. [Google Scholar] [CrossRef]
- Liu, T.; He, M.; Shi, R.; Yin, H.; Luo, W. Biofuel–Pharmaceutical Co-Production in Integrated Biorefineries: Strategies, Challenges, and Sustainability. Fermentation 2025, 11, 312. [Google Scholar] [CrossRef]
- Barathi, A.; Dagwar, P.P.; Kundu, D.; Rajendran, K.; Jacob, S. Biorefineries and Waste Valorization in Integrated Biorefinery Concepts and Applications. In Biotechnological Applications in Industrial Waste Valorization; Kumar, V., Verma, P., Eds.; Springer Nature: Singapore, 2025; pp. 1–21. [Google Scholar]
- Samoraj, M.; Çalış, D.; Trzaska, K.; Mironiuk, M.; Chojnacka, K. Advancements in Algal Biorefineries for Sustainable Agriculture: Biofuels, High-Value Products, and Environmental Solutions. Biocatal. Agric. Biotechnol. 2024, 58, 103224. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H. Barriers to Commercial Deployment of Biorefineries: A Multi-Faceted Review of Obstacles across the Innovation Chain. Heliyon 2024, 10, e32649. [Google Scholar] [CrossRef]
- Parisi, C. Biorefineries Distribution in the Eu; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- Holt, D.A.; Aldrich, C.G. Evaluation of Torula Yeast as a Protein Source in Extruded Feline Diets. J. Anim. Sci. 2022, 100, skac327. [Google Scholar] [CrossRef]
- Cravotto, G.; Mariatti, F.; Gunjevic, V.; Secondo, M.; Villa, M.; Parolin, J.; Cavaglià, G. Pilot Scale Cavitational Reactors and Other Enabling Technologies to Design the Industrial Recovery of Polyphenols from Agro-Food by-Products, a Technical and Economical Overview. Foods 2018, 7, 130. [Google Scholar] [CrossRef]
- Rothstein, S.; Akhtar, T.; Casaretto, J.; Bozzo, G.; Perrin, C.; Parry, C. Extracts Enriched with Polyphenolic Compounds and Related Methods. WO 2021/119826 A1, 18 December 2020. [Google Scholar]
- Ianiro Teodoro, T.; Fisher Laurel, A. Chardonnay Grape Seed Extract. US 10034910 B2, 29 May 2015. [Google Scholar]
- Vieira, S.F.; Reis, R.L.; Ferreira, H.; Neves, N.M. Plant-Derived Bioactive Compounds as Key Players in the Modulation of Immune-Related Conditions. Phytochem. Rev. 2025, 24, 343–460. [Google Scholar] [CrossRef]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Zaky, A.A.; Akram, M.U.; Rybak, K.; Witrowa-Rajchert, D.; Nowacka, M. Bioactive Compounds from Plants and by-Products: Novel Extraction Methods, Applications, and Limitations. AIMS Mol. Sci. 2024, 11, 150–188. [Google Scholar] [CrossRef]
- Fernández-Ochoa, Á.; Cádiz-Gurrea, M.L.; Fernández-Moreno, P.; Rojas-García, A.; Arráez-Román, D.; Segura-Carretero, A. Recent Analytical Approaches for the Study of Bioavailability and Metabolism of Bioactive Phenolic Compounds. Molecules 2022, 27, 777. [Google Scholar] [CrossRef]
- Aït-Kaddour, A.; Hassoun, A.; Tarchi, I.; Loudiyi, M.; Boukria, O.; Cahyana, Y.; Ozogul, F.; Khwaldia, K. Transforming Plant-Based Waste and by-Products into Valuable Products Using Various “Food Industry 4.0” Enabling Technologies: A Literature Review. Sci. Total Environ. 2024, 955, 176872. [Google Scholar] [CrossRef]
- Angeloni, C.; Malaguti, M.; Prata, C.; Freschi, M.; Barbalace, M.C.; Hrelia, S. Mechanisms Underlying Neurodegenerative Disorders and Potential Neuroprotective Activity of Agrifood by-Products. Antioxidants 2022, 12, 94. [Google Scholar] [CrossRef] [PubMed]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Rana, L.; Kumar, M.; Rajput, J.; Kumar, N.; Sow, S.; Kumar, S.; Kumar, A.; Singh, S.N.; Jha, C.K.; Singh, A.K.; et al. Nexus between Nanotechnology and Agricultural Production Systems: Challenges and Future Prospects. Discov. Appl. Sci. 2024, 6, 555. [Google Scholar] [CrossRef]
- Shilpa, V.S.; Shams, R.; Dash, K.K.; Pandey, V.K.; Dar, A.H.; Ayaz Mukarram, S.; Harsányi, E.; Kovács, B. Phytochemical Properties, Extraction, and Pharmacological Benefits of Naringin: A Review. Molecules 2023, 28, 5623. [Google Scholar] [CrossRef]
- Šeremet, D.; Durgo, K.; Komljenović, A.; Antolić, M.; Mandura Jarić, A.; Huđek Turković, A.; Komes, D.; Šantek, B. Red Beetroot and Banana Peels as Value-Added Ingredients: Assessment of Biological Activity and Preparation of Functional Edible Films. Polymers 2022, 14, 4724. [Google Scholar] [CrossRef] [PubMed]
- Cil, A.Y.; Abdurahman, D.; Cil, I. Internet of Things Enabled Real Time Cold Chain Monitoring in a Container Port. J. Shipp. Trade 2022, 7, 9. [Google Scholar] [CrossRef]
- Ruiz-Garcia, L.; Lunadei, L. Monitoring Cold Chain Logistics by Means of Rfid; InTech: Houston, TX, USA, 2010. [Google Scholar]
- Dlouhá, J.; Glavič, P.; Barton, A. Higher Education in Central European Countries–Critical Factors for Sustainability Transition. J. Clean. Prod. 2017, 151, 670–684. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef]
- Ronie, M.E.; Abdul Aziz, A.H.; Kobun, R.; Pindi, W.; Roslan, J.; Putra, N.R.; Mamat, H. Unveiling the Potential Applications of Plant by-Products in Food–a Review. Waste Manag. Bull. 2024, 2, 183–203. [Google Scholar] [CrossRef]
- Ingrao, C.; Arcidiacono, C.; Bezama, A.; Ioppolo, G.; Winans, K.; Koutinas, A.; Gallego-Schmid, A. Sustainability Issues of by-Product and Waste Management Systems, to Produce Building Material Commodities: A Comprehensive Review of Findings from a Virtual Special Issue. Resour. Conserv. Recycl. 2019, 146, 358–365. [Google Scholar] [CrossRef]
- Chaudhary, N.; Dangi, P.; Chaudhary, V.; Dewan, A.; Sharma, S.P.; Poonia, A.; Kumar, M. A Review on Instant Controlled Pressure Drop Technology—A Strategic Tool for Extraction of Bioactive Compounds. Int. J. Food Sci. Technol. 2022, 57, e1–e11. [Google Scholar] [CrossRef]
- Dhiman, S.; Thakur, B.; Kaur, S.; Ahuja, M.; Gantayat, S.; Sarkar, S.; Singh, R.; Tripathi, M. Closing the Loop: Technological Innovations in Food Waste Valorisation for Global Sustainability. Discover Sustain. 2025, 6, 258. [Google Scholar] [CrossRef]
- Ferraz, L.P.; Silva, E.K. Pulsed Electric Field-Assisted Extraction Techniques for Obtaining Vegetable Oils and Essential Oils: Recent Progress and Opportunities for the Food Industry. Sep. Purif. Technol. 2025, 354, 128833. [Google Scholar] [CrossRef]
- Herzyk, F.; Piłakowska-Pietras, D.; Korzeniowska, M. Supercritical Extraction Techniques for Obtaining Biologically Active Substances from a Variety of Plant Byproducts. Foods 2024, 13, 1713. [Google Scholar] [CrossRef]
- Shawky, E.; Gibbons, S.; Selim, D.A. Bio-Sourcing from Byproducts: A Comprehensive Review of Bioactive Molecules in Agri-Food Waste (Afw) Streams for Valorization and Sustainable Applications. Bioresour. Technol. 2025, 431, 132640. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Franquesa, A.; Montemurro, M.; Casertano, M.; Fogliano, V. The Food by-Products Bioprocess Wheel: A Guidance Tool for the Food Industry. Trends Food Sci. Technol. 2024, 152, 104652. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M.; Milovanovic, M.G.; Zerajic, S.; Gajic, D.G. Optimization of Ultrasound-Assisted Extraction and Encapsulation of Antioxidants from Orange Peels in Alginate-Chitosan Microparticles. Antioxidants 2022, 11, 297. [Google Scholar] [CrossRef]
- Vargas-Serna, C.L.; Ochoa-Martínez, C.I.; Vélez-Pasos, C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae 2022, 8, 791. [Google Scholar] [CrossRef]
- Aresta, A.; De Vietro, N.; Cotugno, P.; Pierri, C.L.; Trisolini, L.; Zambonin, C. Supercritical Fluid Extraction of Bioactive Components from Apple Peels and Their Modulation of Complex I Activity in Isolated Mitochondria. Antioxidants 2024, 13, 307. [Google Scholar] [CrossRef]
- García, P.; Bustamante, A.; Echeverría, F.; Encina, C.; Palma, M.; Sanhueza, L.; Sambra, V.; Pando, M.E.; Jiménez, P. A Feasible Approach to Developing Fiber-Enriched Bread Using Pomegranate Peel Powder: Assessing Its Nutritional Composition and Glycemic Index. Foods 2023, 12, 2798. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhu, S.; Wan, W.; Yu, M.; Zeng, X.A.; Deng, Z. Pulsed Electric Field-Assisted Extraction of Hesperidin from Tangerine Peel and Its Technological Optimization through Response Surface Methodology. J. Sci. Food Agric. 2024, 104, 6687–6695. [Google Scholar] [CrossRef]
- Amulya, P.R.; Ul Islam, R. Optimization of Enzyme-Assisted Extraction of Anthocyanins from Eggplant (Solanum melongena L.) Peel. Food Chem. 2023, 18, 100643. [Google Scholar] [CrossRef]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Torres-Leon, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Recovery of Ellagic Acid from Mexican Rambutan Peel by Solid-State Fermentation-Assisted Extraction. Food Bioprod. Process. 2022, 134, 86–94. [Google Scholar] [CrossRef]
- Singh, P.P.; Saldaña, M.D.A. Subcritical Water Extraction of Phenolic Compounds from Potato Peel. Food Res. Int. 2011, 44, 2452–2458. [Google Scholar] [CrossRef]
- Ranjbar, N.; Eikani, M.H.; Javanmard, M.; Golmohammad, F. Impact of Instant Controlled Pressure Drop on Phenolic Compounds Extraction from Pomegranate Peel. Innov. Food Sci. Emerg. Technol. 2016, 37, 177–183. [Google Scholar] [CrossRef]
- Li, S.; Lambros, T.; Wang, Z.; Goodnow, R.; Ho, C.-T. Efficient and Scalable Method in Isolation of Polymethoxyflavones from Orange Peel Extract by Supercritical Fluid Chromatography. J. Chromatogr. B 2007, 846, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, H.; Kaya, M.; Berktas, S.; Basyigit, B.; Cam, M. Pressurised Hot Water Extraction of Phenolic Compounds with a Focus on Eriocitrin and Hesperidin from Lemon Peel. Int. J. Food Sci. Technol. 2023, 58, 2060–2066. [Google Scholar] [CrossRef]
- Gomez-Urios, C.; Siroli, L.; Grassi, S.; Patrignani, F.; Blesa, J.; Lanciotti, R.; Frígola, A.; Iametti, S.; Esteve, M.J.; Di Nunzio, M. Sustainable Valorization of Citrus by-Products: Natural Deep Eutectic Solvents for Bioactive Extraction and Biological Applications of Citrus Sinensis Peel. Eur. Food Res. Technol. 2025, 251, 1965–1980. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).