A Systematic Review of Genetic Variants in Glutathione S-Transferase Genes and Their Dual Role in SARS-CoV-2 Pathogenesis: From Acute Respiratory Complications to Long COVID
Abstract
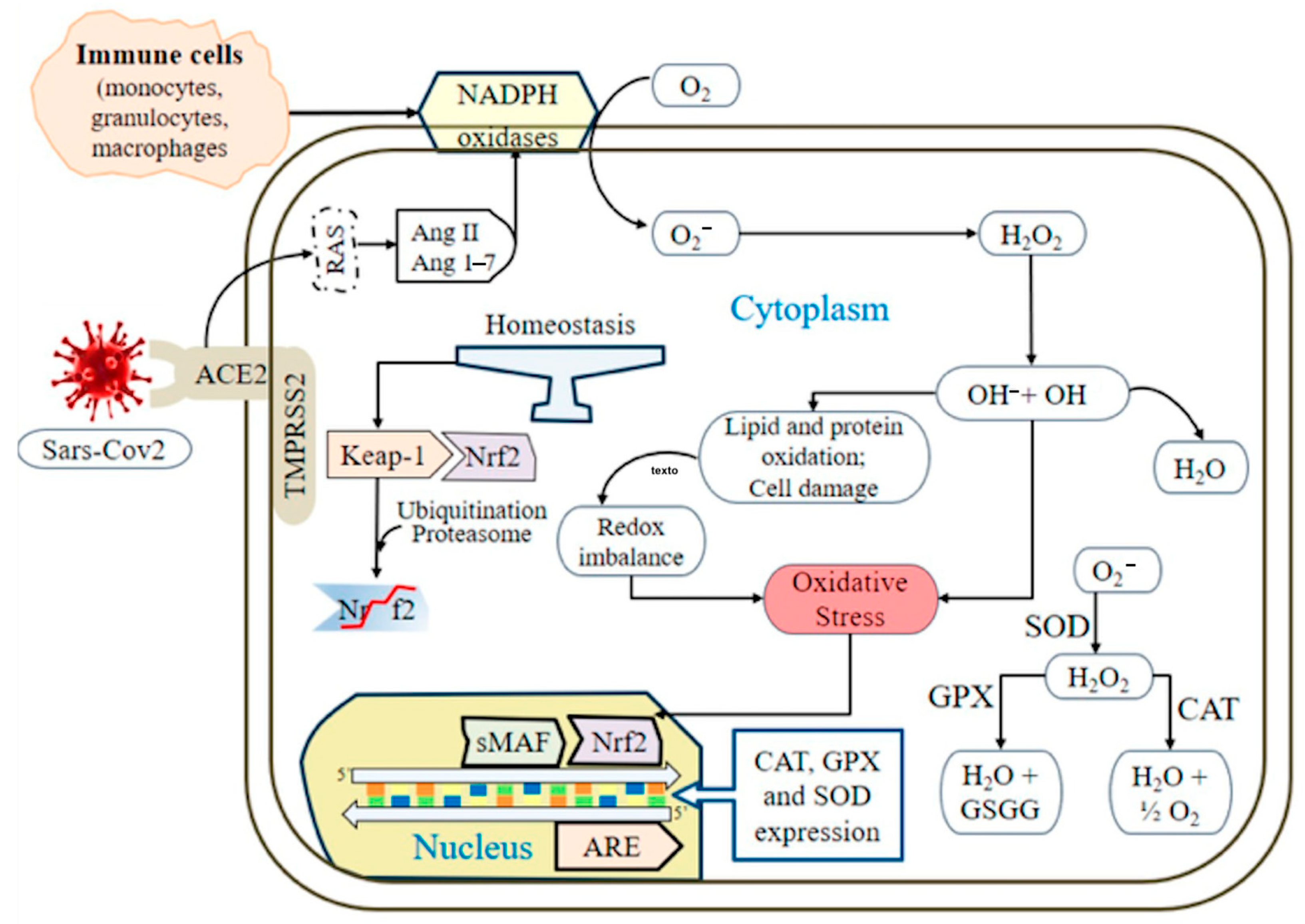
1. Introduction
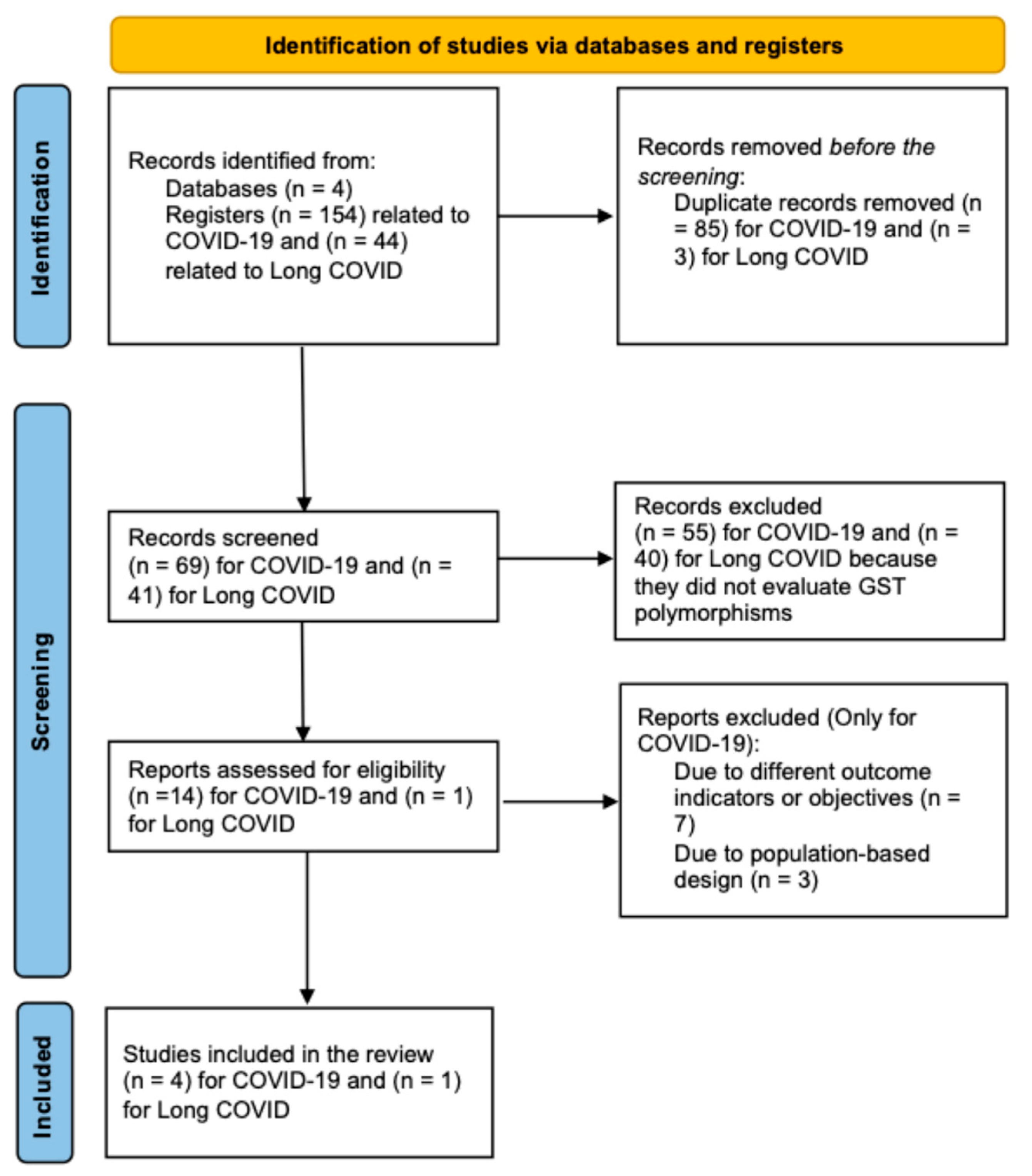
2. Materials and Methods
3. Results
3.1. GST Polymorphisms and COVID-19 Studies
3.2. GST Polymorphisms and Long COVID Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| OS | Oxidative stress |
| RONS | Nitrogen species |
| GSH | Glutathione |
| GST | Glutathione S-transferases |
| GPx | Glutathione S-transferases |
| O2‒ | Superoxide anion |
| SOD | Superoxide dismutase |
| H2O2 | Hydrogen peroxide |
| Fe2+ | Iron |
| HO– | Hydroxyl radical |
| HClO‒ | Hypochlorous acid |
| CAT | Catalase |
| ROS | Reactive oxygen species |
| WHO | World Health Organization |
| COVID-19 | Coronavirus disease |
| ARDS | Respiratory distress syndrome |
| ACE2 | Angiotensin-converting enzyme 2 |
| TMPRSS2 | Type 2 transmembrane serine protease |
| ACE | Angiotensin-converting enzyme |
| Ang I | Angiotensin I |
| Ang II | Angiotensin II |
| Ang 1-7 | Angiotensin 1-7 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MEDLINE | National Library of Medicine, Bethesda, MD |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| NICE | National Institute for Health and Care Excellence |
| AI | Artificial intelligence |
| GSTT1‒/‒ | GSTT1 null genotype |
| GSTM1‒/‒ | GSTM1 null genotype |
| GSSG | Oxidized glutathione |
| TBARs | Thiobarbituric acid reactive substances |
| Nrf2 | Nuclear factor-erythroid 2-related factor 2 |
| sMAF | Small MAF |
| ONOO‒ | peroxynitrite |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| MDA | malondialdehyde |
References
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Norouzi, P.; Aazami, H.; Moosavi-Movahedi, A.A. Review on oxidative stress relation on COVID-19: Biomolecular and bioanalytical approach. Int. J. Biol. Macromol. 2021, 189, 802–818. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef] [PubMed]
- Nissar, S.; Sameer, A.S.; Rasool, R.; Chowdri, N.A.; Rashid, F. Glutathione S Transferases: Biochemistry, Polymorphism and Role in Colorectal Carcinogenesis. J. Carcinog. Amp. Mutagen. 2017, 8, 287. [Google Scholar] [CrossRef]
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.D.; Townsend, D.M. Glutathione-s-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.F.; Fernandes, P.A.; Ramos, M.J. Mammalian cytosolic glutathione transferases. Curr. Protein Pept. Sci. 2008, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Hayes, J.D. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab. Rev. 2011, 43, 92–137. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.; Aninat, C. The glutathione transferase kappa family. Drug Metab. Rev. 2011, 43, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Mazari, A.M.A.; Zhang, L.; Ye, Z.W.; Zhang, J.; Tew, K.D.; Townsend, D.M. The Multifaceted Role of Glutathione S-Transferases in Health and Disease. Biomolecules 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Labarrere, C.A.; Kassab, G.S. Glutathione deficiency in the pathogenesis of SARS-CoV-2 infection and its effects upon the host immune response in severe COVID-19 disease. Front. Microbiol. 2022, 13, 979719. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, K.; Zhang, Y.; Gu, Z.; Huang, C. Neutrophils in COVID-19: Recent insights and advances. Virol. J. 2023, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- PAHO|Pan American Health Organization; WHO. La OMS Caracteriza a COVID-19 Como Una Pandemia. Available online: https://www.paho.org/es/noticias/11-3-2020-oms-caracteriza-covid-19-como-pandemia (accessed on 1 April 2025).
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506, Erratum in Lancet 2020, 395, 496. https://doi.org/10.1016/S0140-6736(20)30252-X. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943, Erratum in JAMA Intern Med. 2020, 180, 1031. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, B.V.; Popova, E.N.; Prikhodko, A.S.; Grebenchikov, O.A.; Zinovkina, L.A.; Zinovkin, R.A. COVID-19 and Oxidative Stress. Biochemistry 2020, 85, 1543–1553. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.G.; de Brito, C.A.; Dos Reis, V.M.S.; Sato, M.N.; Pereira, N.Z. SARS-CoV-2 and Other Respiratory Viruses: What Does Oxidative Stress Have to Do with It? Oxid. Med. Cell. Longev. 2020, 2020, 8844280. [Google Scholar] [CrossRef] [PubMed]
- Komaravelli, N.; Casola, A. Respiratory Viral Infections and Subversion of Cellular Antioxidant Defenses. J. Pharmacogenomics Pharmacoproteomics 2014, 5, 1000141. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Fara, A.; Mitrev, Z.; Rosalia, R.A.; Assas, B.M. Cytokine storm and COVID-19: A chronicle of pro-inflammatory cytokines. Open Biol. 2020, 10, 200160. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, D.; Guo, W.; Cao, Y.; Huang, D.; Wang, H.; Wang, T.; Zhang, X.; Chen, H.; Yu, H.; et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Investig. 2020, 130, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Polonikov, A. Endogenous Deficiency of Glutathione as the Most Likely Cause of Serious Manifestations and Death in COVID-19 Patients. ACS Infect. Dis. 2020, 6, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Post COVID-19 Condition (Long COVID). Available online: https://www.who.int/europe/news-room/fact-sheets/item/post-covid-19-condition (accessed on 1 April 2025).
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J.Ž. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, C.; Giraldo Ramirez, N.D.; De La Hoz Castro, A.D.; Vidal Vargas, C.G.; Pacheco, H.A.; Fernández Sánchez, D.; González Salazar, L.V.; Romero Otta, S.S.; Vergara Jaimes, S.; Bolivar Ospina, J.F.; et al. Association of dead space fraction to mortality in patients with COVID-19-related ARDS: A historical cohort observational study. Med. Intensiv. 2024, 48, 639–645. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Mohamadian, M.; Chiti, H.; Shoghli, A.; Biglari, S.; Parsamanesh, N.; Esmaeilzadeh, A. COVID-19: Virology, biology and novel laboratory diagnosis. J. Gene Med. 2021, 23, e3303. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Sharma, L.; Chang, D. Pathophysiology and clinical management of coronavirus disease (COVID-19): A mini-review. Front. Immunol. 2023, 14, 1116131. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Balshem, H.; Helfand, M.; Schünemann, H.J.; Oxman, A.D.; Kunz, R.; Brozek, J.; Vist, G.E.; Falck-Ytter, Y.; Meerpohl, J.; Norris, S.; et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011, 64, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Manterola, C.; Zavando, D. Grupo MINCIR. Cómo interpretar los “Niveles de Evidencia” en los diferentes escenarios clínicos. Rev. Chil. Cirugia 2009, 61, 582–595. [Google Scholar] [CrossRef]
- Abbas, M.; Verma, S.; Verma, S.; Siddiqui, S.; Khan, F.H.; Raza, S.T.; Siddiqi, Z.; Eba, A.; Mahdi, F. Association of GSTM1 and GSTT1 gene polymorphisms with COVID-19 susceptibility and its outcome. J. Med. Virol. 2021, 93, 5446–5451. [Google Scholar] [CrossRef] [PubMed]
- Orlewska, K.; Klusek, J.; Zarębska-Michaluk, D.; Kocańda, K.; Oblap, R.; Cedro, A.; Witczak, B.; Klusek, J.; Śliwczyński, A.; Orlewska, E. Association between Glutathione S-Transferases Gene Variants and COVID-19 Severity in Previously Vaccinated and Unvaccinated Polish Patients with Confirmed SARS-CoV-2 Infection. Int. J. Environ. Res. Public Health 2023, 20, 3752. [Google Scholar] [CrossRef] [PubMed]
- Coric, V.; Milosevic, I.; Djukic, T.; Bukumiric, Z.; Savic-Radojevic, A.; Matic, M.; Jerotic, D.; Todorovic, N.; Asanin, M.; Ercegovac, M.; et al. GSTP1 and GSTM3 Variant Alleles Affect Susceptibility and Severity of COVID-19. Front. Mol. Biosci. 2021, 8, 747493. [Google Scholar] [CrossRef] [PubMed]
- Djukic, T.; Stevanovic, G.; Coric, V.; Bukumiric, Z.; Pljesa-Ercegovac, M.; Matic, M.; Jerotic, D.; Todorovic, N.; Asanin, M.; Ercegovac, M.; et al. GSTO1, GSTO2 and ACE2 Polymorphisms Modify Susceptibility to Developing COVID-19. J. Pers. Med. 2022, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Ercegovac, M.; Asanin, M.; Savic-Radojevic, A.; Ranin, J.; Matic, M.; Djukic, T.; Coric, V.; Jerotic, D.; Todorovic, N.; Milosevic, I.; et al. Antioxidant Genetic Profile Modifies Probability of Developing Neurological Sequelae in Long-COVID. Antioxidants 2022, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Craddock, V.; Mahajan, A.; Spikes, L.; Krishnamachary, B.; Ram, A.K.; Kumar, A.; Chen, L.; Chalise, P.; Dhillon, N.K. Persistent circulation of soluble and extracellular vesicle-linked Spike protein in individuals with postacute sequelae of COVID-19. J. Med. Virol. 2023, 95, e28568. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tan, Y.; Li, Z.; Hu, L.; Chen, Y.; Zhu, S.; Hu, J.; Huai, T.; Li, M.; Zhang, G.; et al. Pulmonary redox imbalance drives early fibroproliferative response in moderate/severe coronavirus disease-19 acute respiratory distress syndrome and impacts long-term lung abnormalities. Ann. Intensive Care 2024, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Valente Coronel, P.M.; Luiz Soares Basilio, D.C.; Teixeira Espinoça, I.; Souza de Souza, K.F.; Miranda Campos, N.; Seiji Nakano Ota, R.; Paredes-Gamero, E.J.; Wilhelm Filho, D.; Coimbra Motta-Castro, A.R.; Trentin Perdomo, R.; et al. Involvement of oxidative stress in post-acute sequelae of COVID-19: Clinical implications. Redox Rep. 2025, 30, 2471738. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Study Design | Country | Disease | Sample | Studied Genes | Results | Evidence Level | Grade of Recommendation | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbas, M | 2021 | Cohort | India | COVID-19 | 269 patients with COVID-19 confirmed by RT-PCR with mild (n = 149) and severe (n = 120) infection | GSTM1/GSTT1 |
| 2+ Low certainty | D | [40] |
| Orlewska, K | 2023 | Cases and controls | Poland | COVID-19 | 176 patients with confirmed SARS-CoV-2 infection. | GSTM1/GSTT1/GSTP1 |
| 2+ Low certainty | D | [41] |
| Coric, V | 2021 | Cases and controls | Serbia | COVID-19 | 459 Caucasian patients: 207 with RT-PCR-confirmed COVID-19 > 18 years and 252 without IgG and IgM antibodies for SARS-CoV-2. | GSTP1/GSTM3/GSTM1/GSTT1/GSTA1 |
| 2+ Low certainty | D | [42] |
| Djukic, T | 2022 | Cases and controls | Serbia | COVID-19 | 491 Caucasian patients: 255 with COVID-19 confirmed by RT-PCR > 18 years and 236 without IgG and IgM antibodies for SARS-CoV-2. | GSTO1, GSTO2 |
| 2+ Low certainty | D | [43] |
| Ercegovac, M | 2022 | Cohort | Serbia | Long COVID | 167 patients recruited 3 months after COVID-19 | GSTM1/GSTT1/GSTO1/GSTP1 |
| 2+ Low certainty | D | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas Sánchez, V.; Chávez Pacheco, J.L.; Palacios Arreola, M.I.; Sierra-Vargas, M.P.; Colín Godinez, L.A.; Ahumada Topete, V.H.; Fernández Plata, R.; Higuera-Iglesias, A.; Lara-Lemus, R.; Aquino-Gálvez, A.; et al. A Systematic Review of Genetic Variants in Glutathione S-Transferase Genes and Their Dual Role in SARS-CoV-2 Pathogenesis: From Acute Respiratory Complications to Long COVID. Antioxidants 2025, 14, 912. https://doi.org/10.3390/antiox14080912
Villegas Sánchez V, Chávez Pacheco JL, Palacios Arreola MI, Sierra-Vargas MP, Colín Godinez LA, Ahumada Topete VH, Fernández Plata R, Higuera-Iglesias A, Lara-Lemus R, Aquino-Gálvez A, et al. A Systematic Review of Genetic Variants in Glutathione S-Transferase Genes and Their Dual Role in SARS-CoV-2 Pathogenesis: From Acute Respiratory Complications to Long COVID. Antioxidants. 2025; 14(8):912. https://doi.org/10.3390/antiox14080912
Chicago/Turabian StyleVillegas Sánchez, Valeria, Juan Luis Chávez Pacheco, Margarita Isabel Palacios Arreola, Martha Patricia Sierra-Vargas, Luz Adriana Colín Godinez, Víctor Hugo Ahumada Topete, Rosario Fernández Plata, Anjarath Higuera-Iglesias, Roberto Lara-Lemus, Arnoldo Aquino-Gálvez, and et al. 2025. "A Systematic Review of Genetic Variants in Glutathione S-Transferase Genes and Their Dual Role in SARS-CoV-2 Pathogenesis: From Acute Respiratory Complications to Long COVID" Antioxidants 14, no. 8: 912. https://doi.org/10.3390/antiox14080912
APA StyleVillegas Sánchez, V., Chávez Pacheco, J. L., Palacios Arreola, M. I., Sierra-Vargas, M. P., Colín Godinez, L. A., Ahumada Topete, V. H., Fernández Plata, R., Higuera-Iglesias, A., Lara-Lemus, R., Aquino-Gálvez, A., Torres-Espíndola, L. M., & Castillejos-López, M. (2025). A Systematic Review of Genetic Variants in Glutathione S-Transferase Genes and Their Dual Role in SARS-CoV-2 Pathogenesis: From Acute Respiratory Complications to Long COVID. Antioxidants, 14(8), 912. https://doi.org/10.3390/antiox14080912







