Aryl Hydrocarbon Receptor Is Required for Fasting-Induced Improvement of Gut Barrier Integrity in Caenorhabditis elegans
Abstract
1. Introduction
2. Materials and Methods
2.1. C. elegans Strains and Maintenance
2.2. Preparation of Inactivated Bacterial Food
2.3. Dietary Interventions
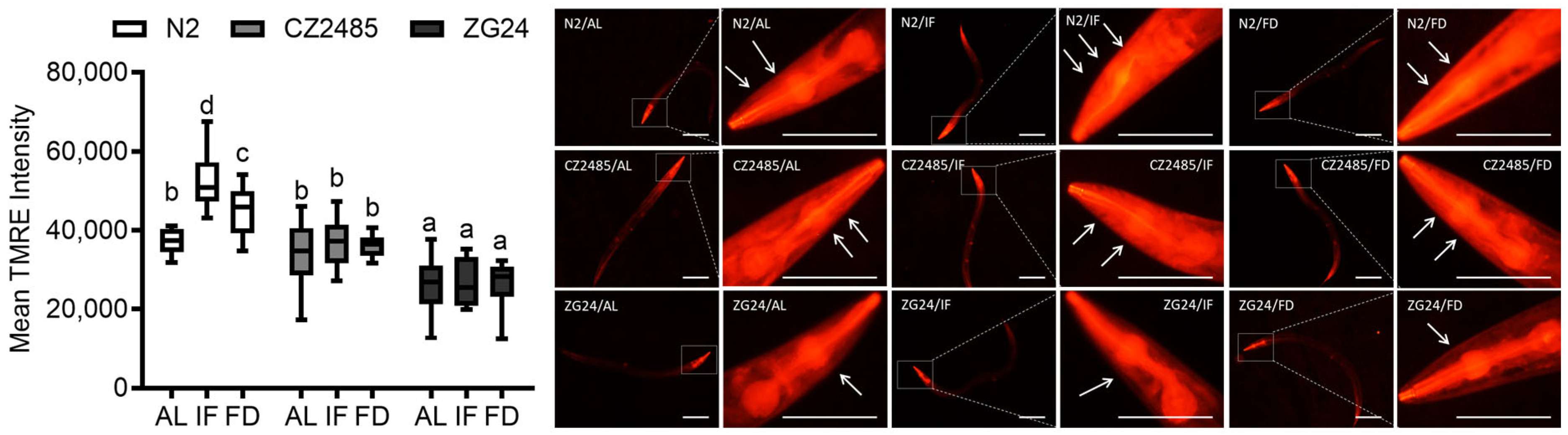
2.4. Tetramethylrhodamine (TMRE) Staining
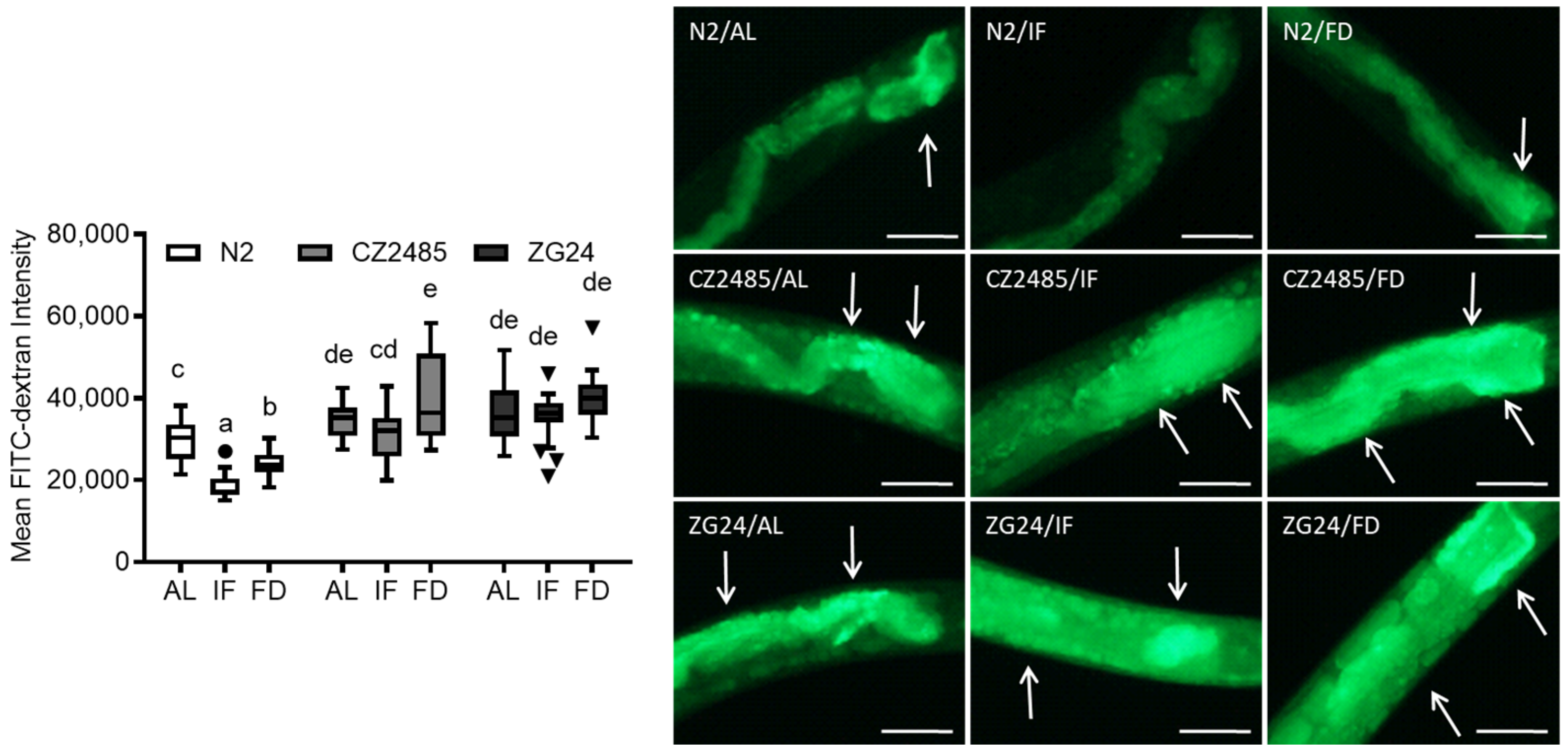
2.5. FITC-Dextran Uptake Assay
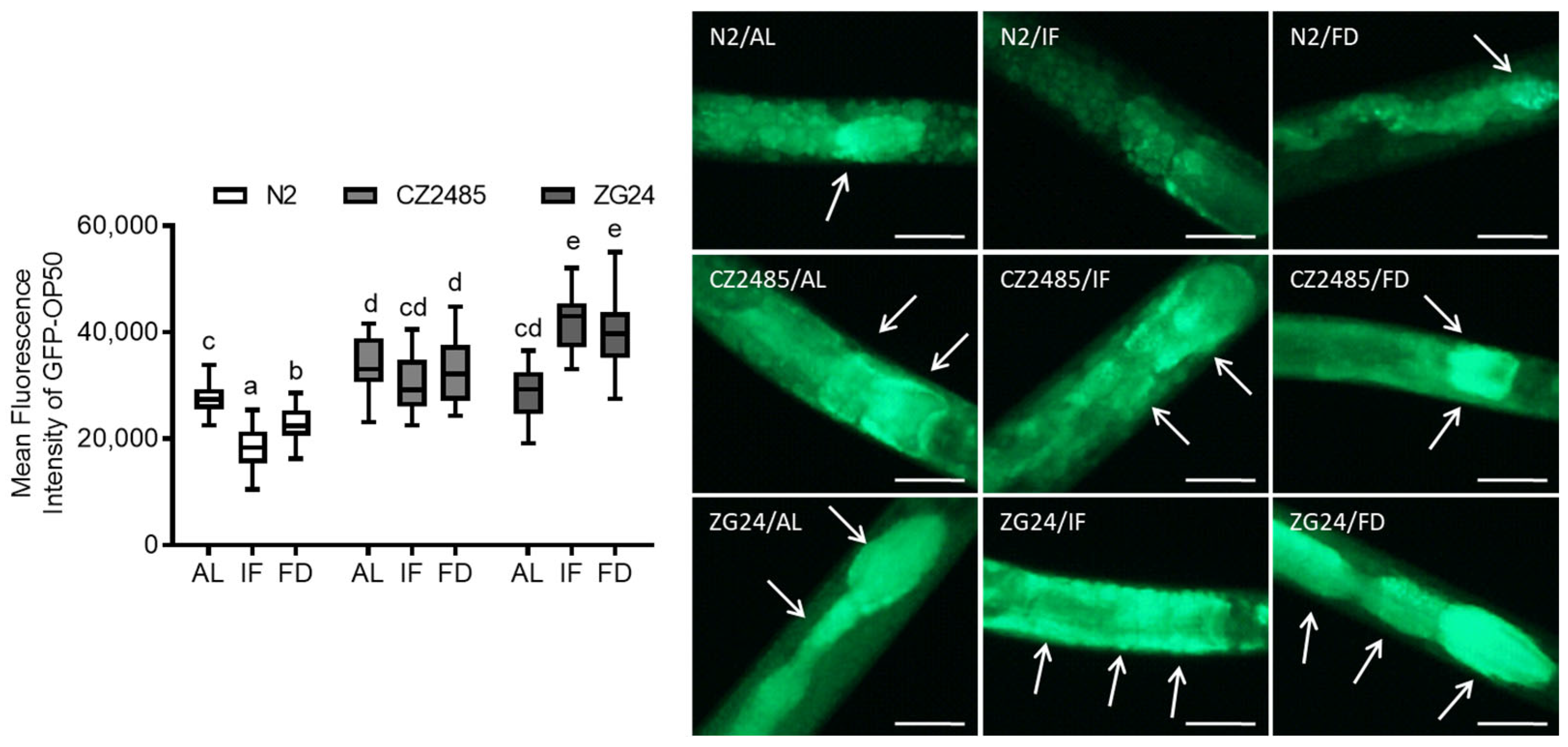
2.6. GFP-OP50 Bacterial Colonization Assay
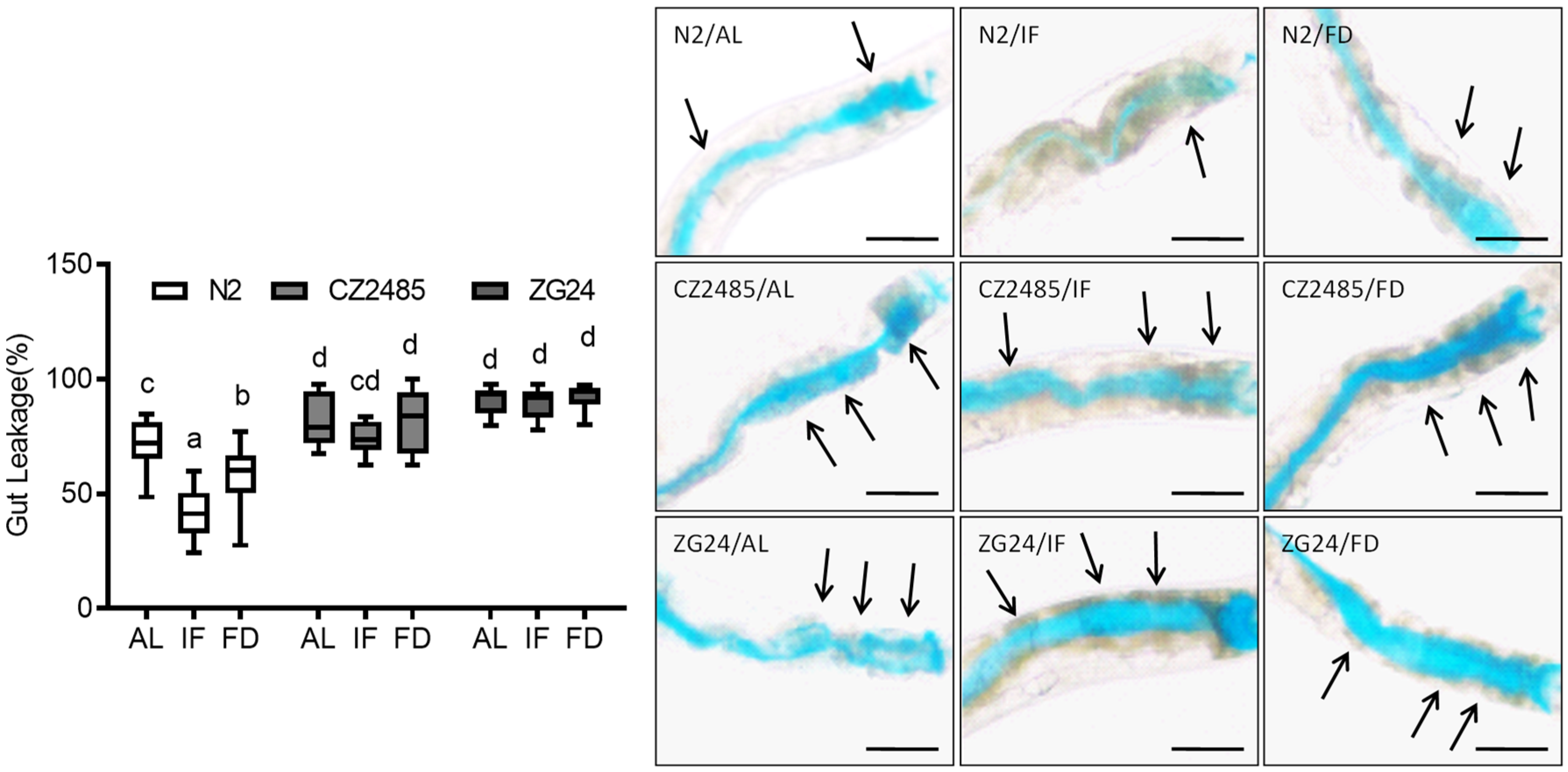
2.7. Smurf Assay
2.8. MitoSOX Staining
2.9. Statistical Analysis
3. Results
3.1. Fasting Reduces Intestinal Permeability in Wild-Type but Not in AHR Mutants
3.2. FITC-Dextran Assay Confirms Fasting-Induced Changes in Gut Permeability
3.3. GFP-OP50 Colonization Reveals Impaired Gut Defense in AHR Mutants Under Fasting
3.4. Fasting Enhances Mitochondrial Function in Wild-Type but Not in AHR Mutants
3.5. Fasting Reduces Mitochondrial ROS in Wild-Type but Not in AHR Mutants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C. elegans | Caenorhabditis elegans |
| AL | Ad libitum |
| IF | Intermittent fasting |
| FD | Complete food deprivation |
| AHR | Aryl hydrocarbon receptor |
References
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Odenwald, M.A.; Turner, J.R. Intestinal permeability defects: Is it time to treat? Clin. Gastroenterol. Hepatol. 2013, 11, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2017, 21, 455–466.e4. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Hemphill, D.; Jeejeebhoy, K. Oxidative stress and antioxidants in intestinal disease. Dig. Dis. 1998, 16, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.a.; Verspaget, H. oxidative stress as a pathogenic factor in inflammatory bowel disease—Radicals or ridiculous? Aliment. Pharmacol. Ther. 2002, 16, 1997–2015. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Lu, Y.; Lin, Y.; Xue, P.; Zhang, Y.; Wang, Y.; Yu, W.; Lin, X.; Yang, W.; Lv, C. Alternate day fasting enhances intestinal epithelial function during aging by regulating mitochondrial metabolism. Aging Cell 2025, 24, e70052. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, Q.; Zhao, B.; Zhang, J.; Zhao, W.; Li, Y.; Liu, R.; Liu, X.; Liu, Z. Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020, 32, 101535. [Google Scholar] [CrossRef] [PubMed]
- McGhee, J.D. The C. elegans intestine. In WormBook: The Online Review of C. elegans Biology [Internet]; WormBook: Pasadena, CA, USA, 2007. [Google Scholar]
- Xiao, R.; Chun, L.; Ronan, E.A.; Friedman, D.I.; Liu, J.; Xu, X.S. RNAi interrogation of dietary modulation of development, metabolism, behavior, and aging in C. elegans. Cell Rep. 2015, 11, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Microbiota and diabetes: An evolving relationship. Gut 2014, 63, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Meglio, P.D.; Gialitakis, M.; Duarte, J.H. The aryl hydrocarbon receptor: Multitasking in the immune system. Annu. Rev. Immunol. 2014, 32, 403–432. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Rannug, A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol. Rev. 2015, 67, 259–279. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Powell-Coffman, J.A. The Caenorhabditis elegans aryl hydrocarbon receptor, AHR-1, regulates neuronal development. Dev. Biol. 2004, 270, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.G.; Mouchiroud, L.; Frochaux, M.; Pandey, A.; Andreux, P.A.; Deplancke, B.; Auwerx, J. An evolutionarily conserved role for the aryl hydrocarbon receptor in the regulation of movement. PLoS Genet. 2014, 10, e1004673. [Google Scholar] [CrossRef] [PubMed]
- Aarnio, V.; Heikkinen, L.; Peltonen, J.; Goldsteins, G.; Lakso, M.; Wong, G. Transcriptional profiling reveals differential expression of a neuropeptide-like protein and pseudogenes in aryl hydrocarbon receptor-1 mutant Caenorhabditis elegans. Comp. Biochem. Physiol. Part D Genom. Proteom. 2014, 9, 40–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, H.; Zhai, Z.; Powell-Coffman, J.A. The Caenorhabditis elegans AHR-1 transcription complex controls expression of soluble guanylate cyclase genes in the URX neurons and regulates aggregation behavior. Dev. Biol. 2006, 298, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Kaeberlein, T.L.; Smith, E.D.; Tsuchiya, M.; Welton, K.L.; Thomas, J.H.; Fields, S.; Kennedy, B.K.; Kaeberlein, M. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 2006, 5, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.D.; Wilson, M.A.; Zhu, M.; Wolkow, C.A.; de Cabo, R.; Ingram, D.K.; Zou, S. Dietary deprivation extends lifespan in Caenorhabditis elegans. Aging Cell 2006, 5, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Honjoh, S.; Matsuda, M.; Hoshikawa, H.; Kishimoto, S.; Yamamoto, T.; Ebisuya, M.; Yamamoto, T.; Matsumoto, K.; Nishida, E. A fasting-responsive signaling pathway that extends life span in C. elegans. Cell Rep. 2013, 3, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef] [PubMed]
- Vieujean, S.; Caron, B.; Haghnejad, V.; Jouzeau, J.-Y.; Netter, P.; Heba, A.-C.; Ndiaye, N.C.; Moulin, D.; Barreto, G.; Danese, S. Impact of the exposome on the epigenome in inflammatory bowel disease patients and animal models. Int. J. Mol. Sci. 2022, 23, 7611. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Eckers, A.; Jakob, S.; Heiss, C.; Haarmann-Stemmann, T.; Goy, C.; Brinkmann, V.; Cortese-Krott, M.M.; Sansone, R.; Esser, C.; Ale-Agha, N. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 2016, 6, 19618. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.J.; Suh, J.H.; Lee, S.H.; Poulsen, K.L.; An, Y.A.; Moorthy, B.; Hartig, S.M.; Moore, D.D.; Kim, K.H. Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice. Mol. Metab. 2023, 72, 101717. [Google Scholar] [CrossRef] [PubMed]
- Latchney, S.E.; Hein, A.M.; O’Banion, M.K.; DiCicco-Bloom, E.; Opanashuk, L.A. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J. Neurochem. 2013, 125, 430–445. [Google Scholar] [CrossRef] [PubMed]
- López-Lluch, G.; Navas, P. Calorie restriction as an intervention in ageing. J. Physiol. 2016, 594, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Catterson, J.H.; Khericha, M.; Dyson, M.C.; Vincent, A.J.; Callard, R.; Haveron, S.M.; Rajasingam, A.; Ahmad, M.; Partridge, L. Short-term, intermittent fasting induces long-lasting gut health and TOR-independent lifespan extension. Curr. Biol. 2018, 28, 1714–1724.e4. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Rahman, M.S.; Sohag, A.A.M.; Dash, R.; Hossain, K.S.; Farjana, M.; Uddin, M.J. Intermittent fasting, a possible priming tool for host defense against SARS-CoV-2 infection: Crosstalk among calorie restriction, autophagy and immune response. Immunol. Lett. 2020, 226, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Muku, G.E.; Murray, I.A.; Espín, J.C.; Perdew, G.H. Urolithin A is a dietary microbiota-derived human aryl hydrocarbon receptor antagonist. Metabolites 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Moon, Y. Aryl Hydrocarbon Receptor Is Required for Fasting-Induced Improvement of Gut Barrier Integrity in Caenorhabditis elegans. Antioxidants 2025, 14, 905. https://doi.org/10.3390/antiox14080905
Sun J, Moon Y. Aryl Hydrocarbon Receptor Is Required for Fasting-Induced Improvement of Gut Barrier Integrity in Caenorhabditis elegans. Antioxidants. 2025; 14(8):905. https://doi.org/10.3390/antiox14080905
Chicago/Turabian StyleSun, Junjie, and Yuseok Moon. 2025. "Aryl Hydrocarbon Receptor Is Required for Fasting-Induced Improvement of Gut Barrier Integrity in Caenorhabditis elegans" Antioxidants 14, no. 8: 905. https://doi.org/10.3390/antiox14080905
APA StyleSun, J., & Moon, Y. (2025). Aryl Hydrocarbon Receptor Is Required for Fasting-Induced Improvement of Gut Barrier Integrity in Caenorhabditis elegans. Antioxidants, 14(8), 905. https://doi.org/10.3390/antiox14080905





