Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
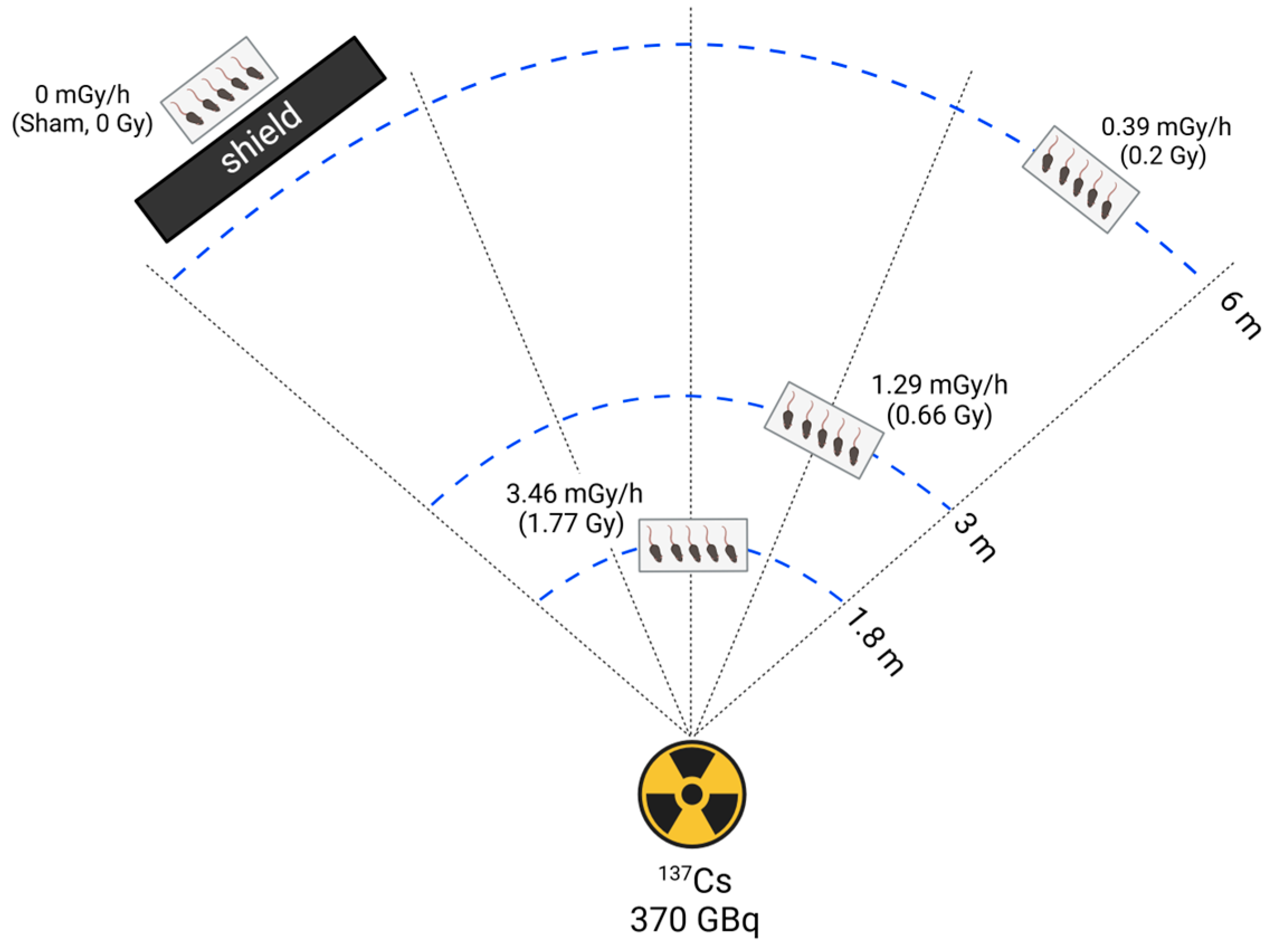
2.2. Radiation Exposure
2.3. Chemicals
2.4. Testis and Epididymis Isolation and Histological Staining
2.4.1. Hematoxylin and Eosin (H&E) Staining
2.4.2. Sirius Red Staining
2.4.3. Masson’s Trichrome Staining
2.5. TUNEL Staining
2.6. Comet Assay
2.7. Measurement of Total Free Radical Activity
2.8. Isolation of Total RNA and Reverse Transcriptase–Polymerase Chain Reaction (RT-PCR)
2.9. Statistical Analysis
3. Results
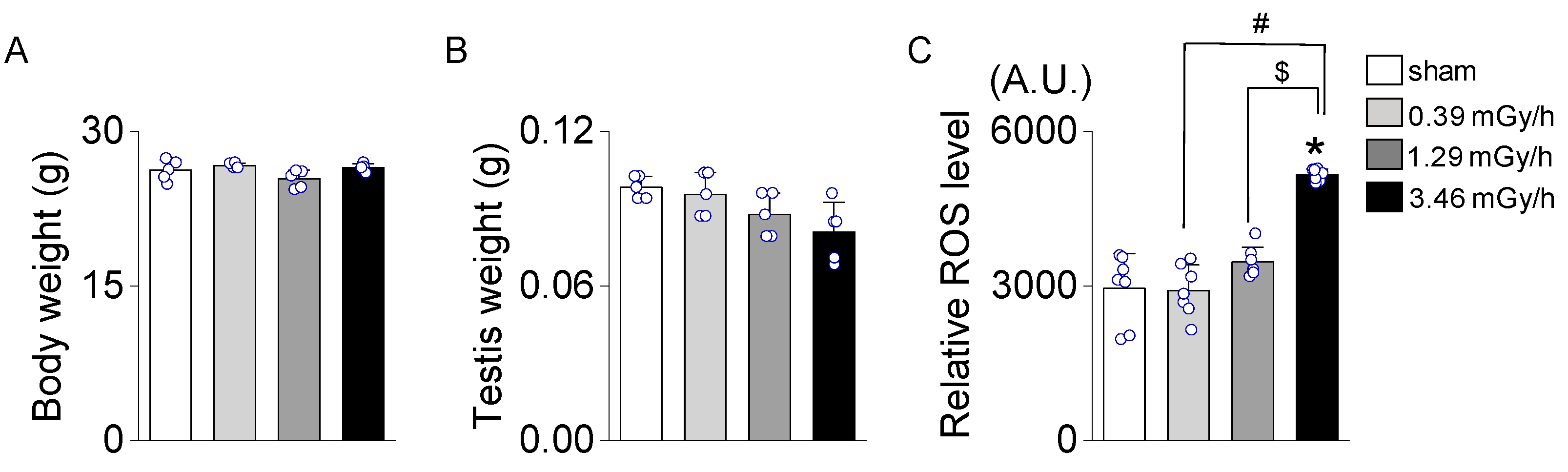
3.1. Effect of Low-Dose-Rate (LDR) Radiation on Testicular Oxidative Stress
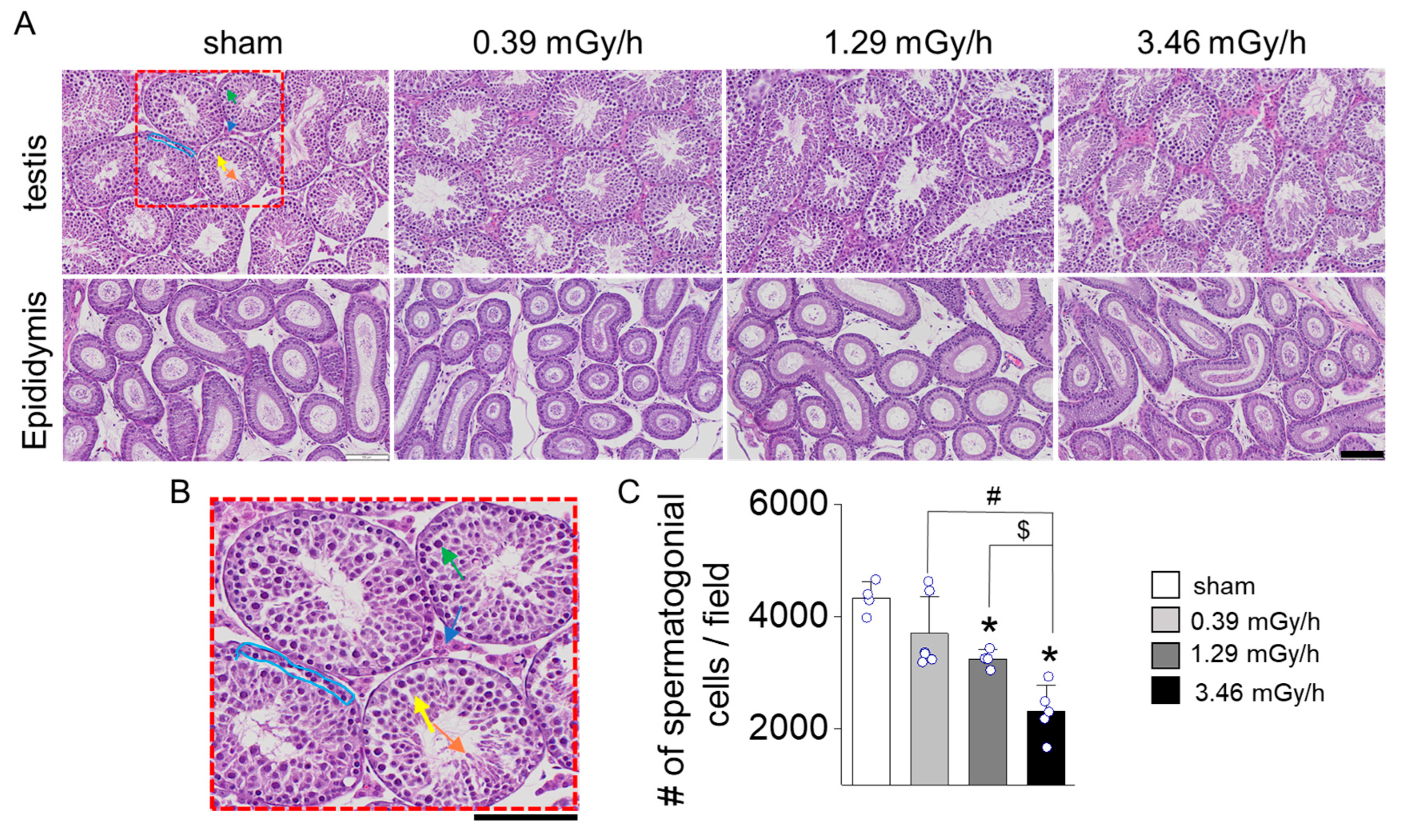
3.2. Histopathological Analysis of Testicular Tissue
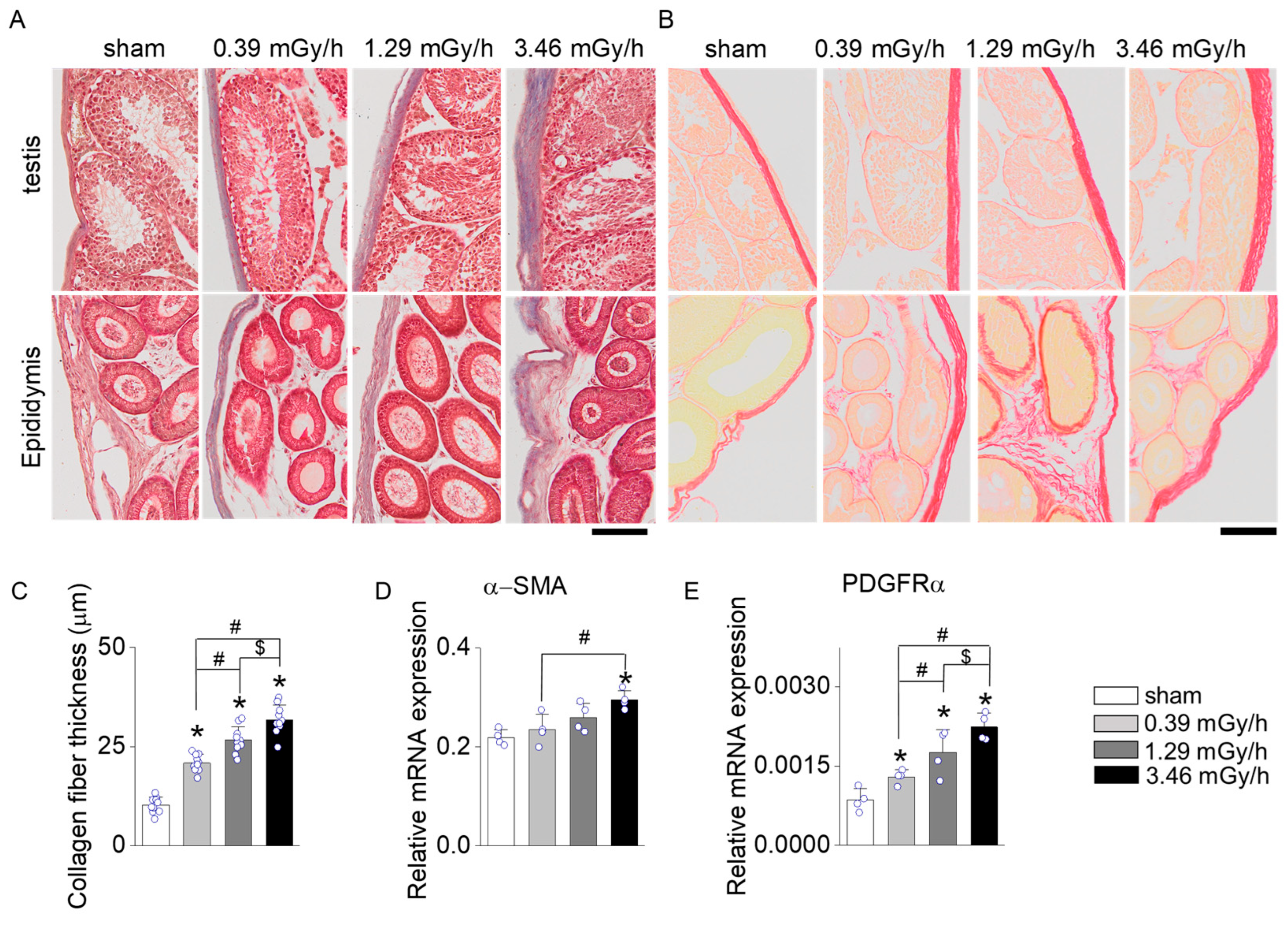
3.3. Fibrosis Assessment Following LDR Radiation Exposure
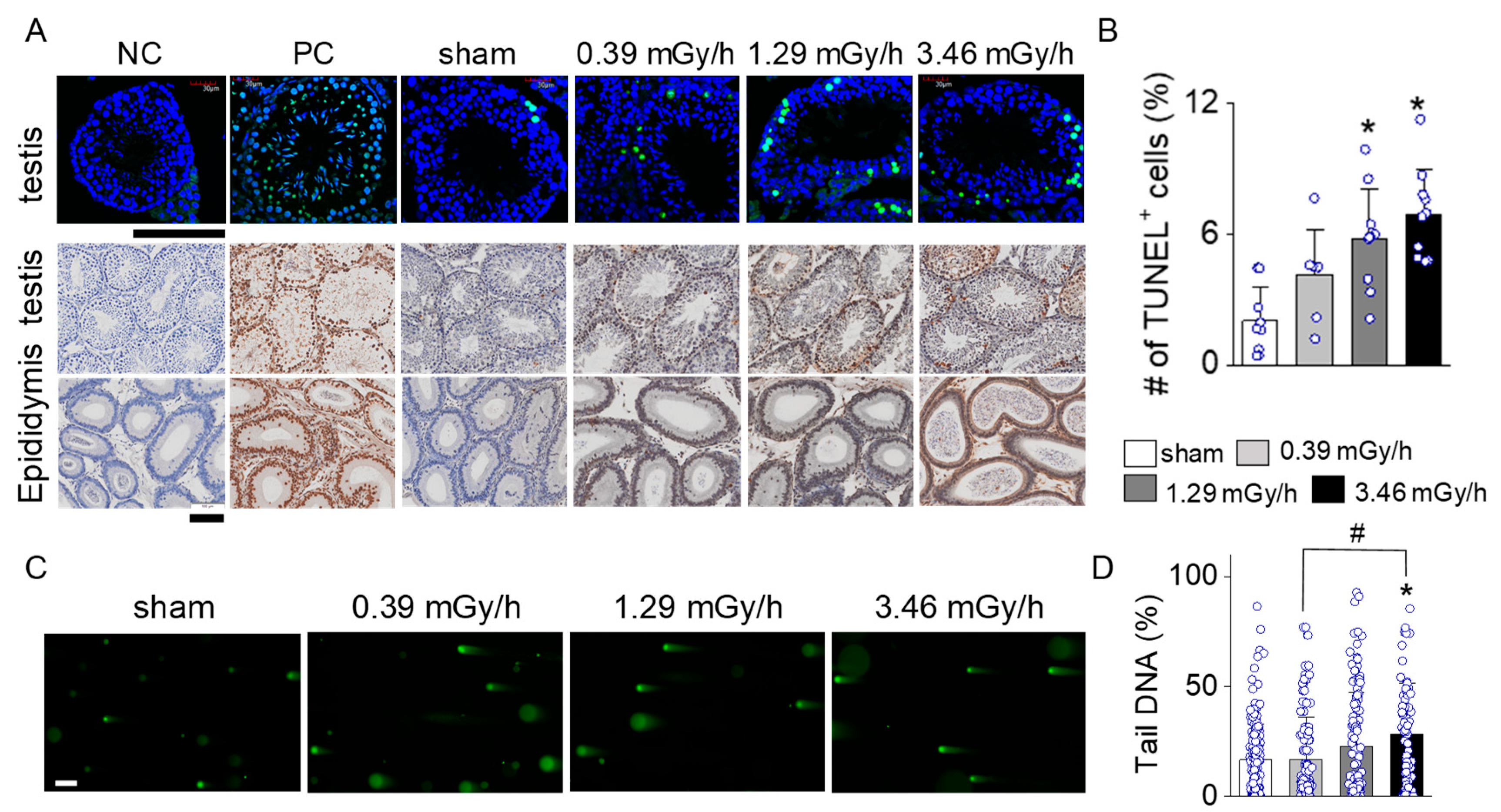
3.4. Increase in Apoptotic Signal in LDR-Exposed Experimental Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LDR | Low Dose Rate |
| PDGFR | Platelet-Derived Growth Factor Receptor |
| ROS | Reactive Oxygen Species |
| SMA | Smooth Muscle Actin |
References
- Hall, E.J. Weiss lecture. The dose-rate factor in radiation biology. Int. J. Radiat. Biol. 1991, 59, 595–610. [Google Scholar] [CrossRef]
- Mirzaie-Joniani, H.; Eriksson, D.; Sheikholvaezin, A.; Johansson, A.; Lofroth, P.O.; Johansson, L.; Stigbrand, T. Apoptosis induced by low-dose and low-dose-rate radiation. Cancer 2002, 94, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Adjemian, S.; Oltean, T.; Martens, S.; Wiernicki, B.; Goossens, V.; Vanden Berghe, T.; Cappe, B.; Ladik, M.; Riquet, F.B.; Heyndrickx, L.; et al. Ionizing radiation results in a mixture of cellular outcomes including mitotic catastrophe, senescence, methuosis, and iron-dependent cell death. Cell Death Dis. 2020, 11, 1003. [Google Scholar] [CrossRef]
- Gong, E.J.; Shin, I.S.; Son, T.G.; Yang, K.; Heo, K.; Kim, J.S. Low-dose-rate radiation exposure leads to testicular damage with decreases in DNMT1 and HDAC1 in the murine testis. J. Radiat. Res. 2014, 55, 54–60. [Google Scholar] [CrossRef]
- Averbeck, D. Low-Dose Non-Targeted Effects and Mitochondrial Control. Int. J. Mol. Sci. 2023, 24, 11460. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, D.; Rodriguez-Lafrasse, C. Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts. Int. J. Mol. Sci. 2021, 22, 11047. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ye, Y.; Gao, Y.; Xu, Q.; Su, M.; Sun, S.; Xu, W.; Fu, Q.; Wang, A.; Hu, S. Low-Dose Ionizing Radiation and Male Reproductive Immunity: Elucidating Subtle Modulations and Long-Term Health Implications. Int. J. Mol. Sci. 2025, 26, 2269. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Shalet, S.M. Spermatogenesis after cancer treatment: Damage and recovery. J. Natl. Cancer Inst. Monogr. 2005, 34, 12–17. [Google Scholar] [CrossRef]
- Kovacs, G.T.; Stern, K. Reproductive aspects of cancer treatment: An update. Med. J. Aust. 1999, 170, 495–497. [Google Scholar] [CrossRef]
- Uemura, I.; Takahashi-Suzuki, N.; Kuroda, S.; Kumagai, K.; Tsutsumi, Y.; Anderson, D.; Satoh, T.; Yamashiro, H.; Miura, T.; Yamauchi, K.; et al. Effects of low-dose rate radiation on immune and epigenetic regulation of the mouse testes. Radiat. Prot. Dosim. 2024, 200, 1620–1624. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Z.; He, Y. Radiation-induced fibrosis: Mechanisms and therapeutic strategies from an immune microenvironment perspective. Immunology 2024, 172, 533–546. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, C.; Song, B.; Zhang, S.; Chen, C.; Li, C.; Zhang, S. Tissue fibrosis induced by radiotherapy: Current understanding of the molecular mechanisms, diagnosis and therapeutic advances. J. Transl. Med. 2023, 21, 708. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, P.; Chen, W.; Chen, J.; Liu, C.; Zhang, H. Testicular fibrosis pathology, diagnosis, pathogenesis, and treatment: A perspective on related diseases. Andrology 2024. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Bae, M.J.; Kang, M.K.; Kim, H.; Kang, Y.R.; Jo, W.S.; Lee, C.G.; Jung, B.; Lee, J.; Moon, C.; et al. Possible association of G6PC2 and MUC6 induced by low-dose-rate irradiation in mouse intestine with inflammatory bowel disease. Mol. Med. Rep. 2024, 30, 127. [Google Scholar] [CrossRef] [PubMed]
- Siregar, A.S.; Nyiramana, M.M.; Kim, E.-J.; Shin, E.-J.; Kim, C.-W.; Lee, D.; Hong, S.-G.; Han, J.; Kang, D. TRPV1 Is Associated with Testicular Apoptosis in Mice. J. Anim. Reprod. Biotechnol. 2019, 34, 311–317. [Google Scholar] [CrossRef]
- Kulms, D.; Zeise, E.; Poppelmann, B.; Schwarz, T. DNA damage, death receptor activation and reactive oxygen species contribute to ultraviolet radiation-induced apoptosis in an essential and independent way. Oncogene 2002, 21, 5844–5851. [Google Scholar] [CrossRef]
- Fang, F.; Gong, P.S.; Zhao, H.G.; Bi, Y.J.; Zhao, G.; Gong, S.L.; Wang, Z.C. Mitochondrial modulation of apoptosis induced by low-dose radiation in mouse testicular cells. Biomed. Environ. Sci. 2013, 26, 820–830. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, S.; Wang, Z.; Li, Y.; Guo, W.; Lin, C.; Gong, S.; Li, C.; Wang, G.; Cai, L. Repetitive exposures to low-dose X-rays attenuate testicular apoptotic cell death in streptozotocin-induced diabetes rats. Toxicol. Lett. 2010, 192, 356–364. [Google Scholar] [CrossRef]
- Sharma, P.; Parmar, J.; Verma, P.; Goyal, P.K. Radiation induced oxidative stress and its toxicity in testes of mice and their prevention by Tinospora cordifolia extract. J. Reprod. Health Med. 2015, 1, 64–75. [Google Scholar] [CrossRef]
- Rakici, S.Y.; Irfan, G.A.; Levent, T.; Hatice, S.N.; Mercantepe, T. Pelvic Radiation-Induced Testicular Damage: An Experimental Study at 1 Gray. Syst. Biol. Reprod. Med. 2020, 66, 89–98. [Google Scholar] [CrossRef]
- Azmoonfar, R.; Mirzaei, F.; Najafi, M.; Varkeshi, M.; Ghazikhanlousani, K.; Momeni, S.; Saber, K. Radiation-induced Testicular Damage in Mice: Protective Effects of Apigenin Revealed by Histopathological Evaluation. Curr. Radiopharm. 2024, 17, 238–246. [Google Scholar] [CrossRef]
- Xu, R.; Shen, S.; Wang, D.; Ye, J.; Song, S.; Wang, Z.; Yue, Z. The role of HIF-1α-mediated autophagy in ionizing radiation-induced testicular injury. J. Mol. Histol. 2023, 54, 439–451. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J. TGF-beta signaling and the fibrotic response. FASEB J. 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Karam, H.M.; Shaaban, E.A.; Safar, M.M.; El-Yamany, M.F. MitoQ ameliorates testicular damage induced by gamma irradiation in rats: Modulation of mitochondrial apoptosis and steroidogenesis. Life Sci. 2019, 232, 116655. [Google Scholar] [CrossRef]
- Canbay, A.; Friedman, S.; Gores, G. Apoptosis: The nexus of liver injury and fibrosis. Hepatology 2004, 39, 273–278. [Google Scholar] [CrossRef]
- Kuwano, K.; Hagimoto, N.; Nakanishi, Y. The role of apoptosis in pulmonary fibrosis. Histol. Histopathol. 2004, 19, 867–881. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Garabuczi, E.v.; Joós, G.; Tsay, G.; Sarang, Z. Impaired Clearance of Apoptotic Cells in Chronic Inflammatory Diseases: Therapeutic Implications. Front. Immunol. 2014, 5, 354. [Google Scholar] [CrossRef]
- Peng, W.; Kepsch, A.; Kracht, T.O.; Hasan, H.; Wijayarathna, R.; Wahle, E.; Pleuger, C.; Bhushan, S.; Günther, S.; Kauerhof, A.C.; et al. Activin A and CCR2 regulate macrophage function in testicular fibrosis caused by experimental autoimmune orchitis. Cell. Mol. Life Sci. 2022, 79, 602. [Google Scholar] [CrossRef] [PubMed]
- Arida, D.A.; Osman, A.; Nour El-Deen, A.E.-S.; Elzeiny, D.; Elyamany, M.I.; Youssef, O.M. Nanoparticulate versus conventional losartan: Protective effects against CCl4-induced testicular oxidative stress and fibrosis in rats. J. Hazard. Mater. Adv. 2025, 18, 100692. [Google Scholar] [CrossRef]
- Pilling, D.; Martinez, T.C.; Gomer, R.H. Inhibition of CCl4-induced liver inflammation and fibrosis by a NEU3 inhibitor. PLoS ONE 2024, 19, e0308060. [Google Scholar] [CrossRef] [PubMed]
- Banan Khojasteh, S.M.; Javanmard Khameneh, R. Sophora pachycarpa Root Extract has Ameliorative Effects on Testicular Injury but is Hepatotoxic in Carbon Tetrachloride Intoxicated Rats. J. Med. Plants By-Products 2024, 13, 447–452. [Google Scholar] [CrossRef]
- Sung Dae, K.; Eun Ji, G.; Minji, B.; Kwangmo, Y.; Joong Sun, K. Bioassay in BALB/c mice exposed to low dose rate radiation. J. Radiat. Prot. Res. 2012, 37, 159–165. [Google Scholar] [CrossRef][Green Version]
- Shin, E.; Lee, S.; Kang, H.; Kim, J.; Kim, K.; Youn, H.; Jin, Y.W.; Seo, S.; Youn, B. Organ-Specific Effects of Low Dose Radiation Exposure: A Comprehensive Review. Front. Genet. 2020, 11, 566244. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-J.; Prayoga, A.H.; Ha, J.; Kang, D.G.; Yang, J.; Kang, S.; Kim, J.-M.; Ahn, B.; Cao, D.L.; Yun, S.P.; et al. Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis. Antioxidants 2025, 14, 1028. https://doi.org/10.3390/antiox14081028
Kim E-J, Prayoga AH, Ha J, Kang DG, Yang J, Kang S, Kim J-M, Ahn B, Cao DL, Yun SP, et al. Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis. Antioxidants. 2025; 14(8):1028. https://doi.org/10.3390/antiox14081028
Chicago/Turabian StyleKim, Eun-Jin, Anjas Happy Prayoga, Jina Ha, Deok Gyeong Kang, Jinsung Yang, Sohi Kang, Jin-Mok Kim, Byeonggyu Ahn, Dang Long Cao, Seung Pil Yun, and et al. 2025. "Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis" Antioxidants 14, no. 8: 1028. https://doi.org/10.3390/antiox14081028
APA StyleKim, E.-J., Prayoga, A. H., Ha, J., Kang, D. G., Yang, J., Kang, S., Kim, J.-M., Ahn, B., Cao, D. L., Yun, S. P., Lee, B. H., Kim, J.-S., & Kang, D. (2025). Cumulative Low-Dose-Rate Radiation Induces Oxidative Stress, Apoptosis, and Fibrosis in Mouse Testis. Antioxidants, 14(8), 1028. https://doi.org/10.3390/antiox14081028








