Bioactivities Derived from Dry-Cured Ham Peptides: A Review
Abstract
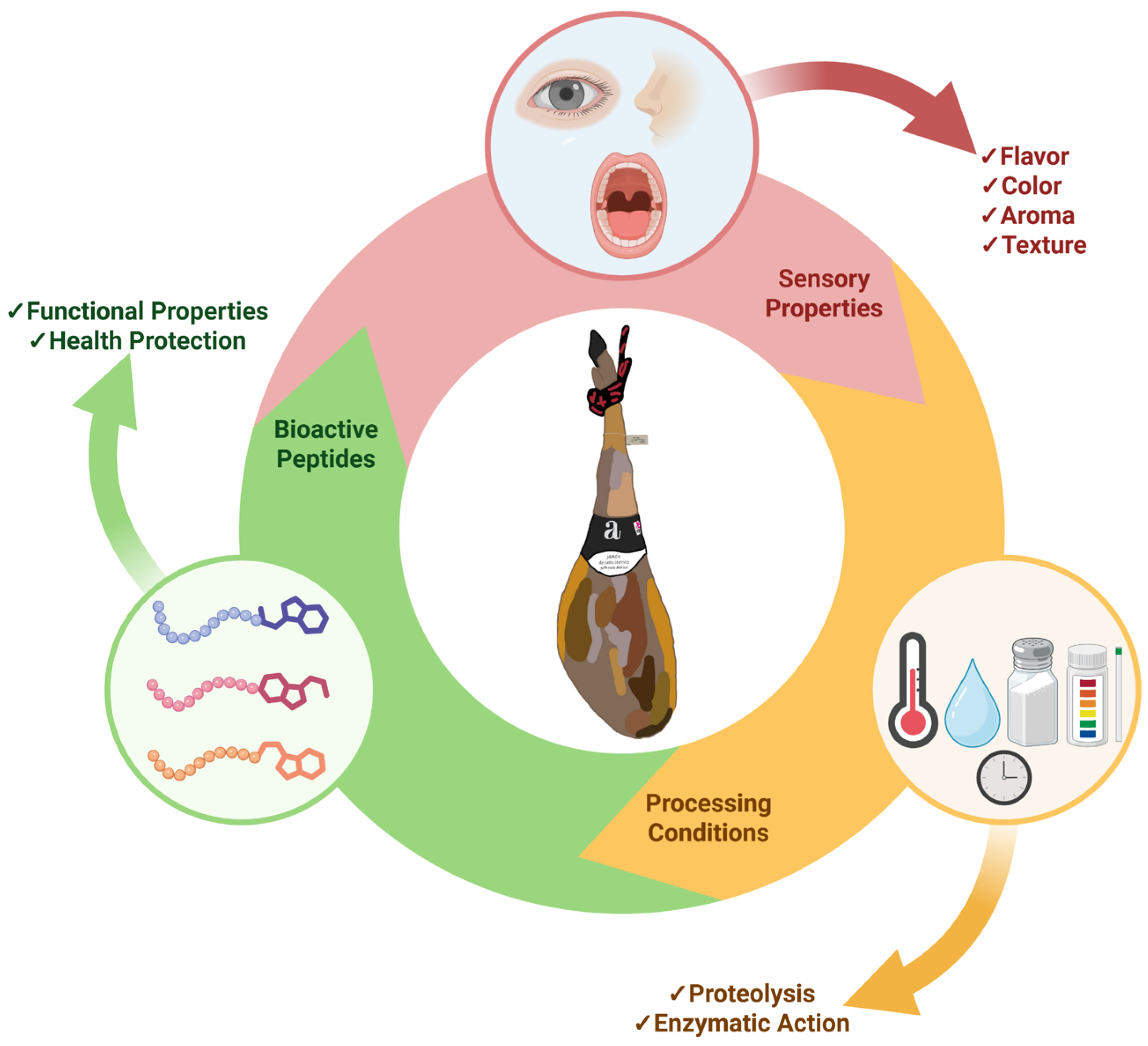
1. Introduction
2. Peptide Generation in Dry-Cured Ham
3. Bioactivity Associated with Peptides Derived from Dry-Cured Ham
3.1. Multifunctionality of Di- and Tripeptides Identified in Dry-Cured Ham
| Sequence | Mass (Da) | Protein of Origin | Described Bioactivity | References |
|---|---|---|---|---|
| AAP | 245.27 | Proteolyzed Polypeptides/Light Myosin | Antihypertensive | [40,79] |
| AKK | 345.44 | Titin (fragments) | Antihypertensive | [7,32,71] |
| ALM | / | / | Antihypertensive | [80] |
| DVK | 346.39 | Ubiquitin | Antihypertensive | [40,79] |
| EAK | 346.18 | Titin (fragments) | Antioxidant | [7,32] |
| EEE | 375.29 | Ubiquitin | Antioxidant | [40] |
| EEL | / | / | Antihypertensive | [42] |
| EGV | 275.28 | LIM Domain-Binding Protein 3 | Antioxidant | [40] |
| EKL | 388.49 | Myosin Light Chain | Immunomodulatory | [7,72,73] |
| ESV | / | / | Antihypertensive | [42] |
| LPK | 343.43 | Myosin | Antihypertensive | [79] |
| PAP | 297.33 | Titin | Antihypertensive | [7,32,71,79] |
| PFP | 359.42 | Myosin | Antidiabetic/Antiobesity | [14] |
| PPK | 357.42 | Titin/Myosin | Antihypertensive | [79] |
| SGL | 275.28 | Creatine Kinase | Antioxidant | [40,79] |
| SGP | 259.26 | Myosin Light Chain | Antihypertensive | [7,32,71] |
| SGV | 261.29 | Creatina quinasa | Antioxidant | [40,79] |
| STY | 369.39 | LIM Domain-Binding Protein 3 | Antidiabetic | [32,52] |
| TNP | 330.34 | Myosin Light Chain | Antihypertensive | [7,32,71] |
| VAP | 271.34 | Myosin | Antihypertensive | [40,79] |
| VDY | / | / | Antidiabetic | [14] |
| VPL | 271.34 | Troponin T | Antihypertensive | [40,79] |
| YPG | 335.35 | Myosin | Antidiabetic/Antiobesity | [14] |
| YPL | 391.46 | Myosin | Antidiabetic/Antiobesity | [14] |
3.2. Angiotensin-Converting Enzyme (ACE) Inhibitory Peptides
3.3. Antioxidant Peptides
3.4. Dipeptidyl Peptidase-IV Inhibitory Peptides
3.5. Anti-Inflammatory Peptides
3.6. Other Bioactivities Associated with Peptides Identified in Dry-Cured Ham
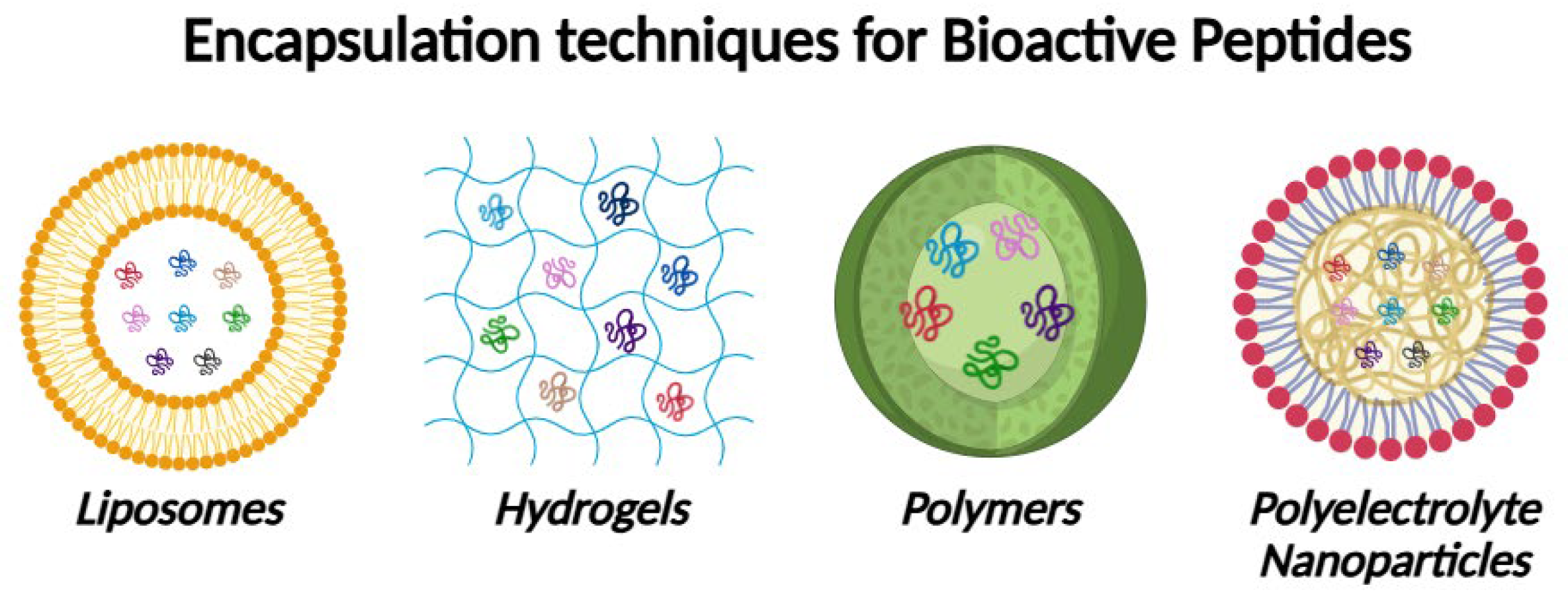
4. Use of Bioactive Peptides Derived from Dry-Cured Ham as Potential Nutraceuticals
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hersleth, M.; Næs, T.; Guerrero, L.; Claret, A.; Recchia, A.; Dinnella, C.; Monteleone, E. Consumer Perception of Dry-Cured Ham—A Cross-Cultural Study in Italy, Norway and Spain. J. Sens. Stud. 2013, 28, 450–466. [Google Scholar] [CrossRef]
- Zhou, G.H.; Zhao, G.M. Biochemical Changes during Processing of Traditional Jinhua Ham. Meat Sci. 2007, 77, 114–120. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Lu, S.; Wang, J.; Fu, H.; Gu, B.; Lyu, B.; Wang, Q. Changes in Proteolysis, Protein Oxidation, Flavor, Color and Texture of Dry-Cured Mutton Ham during Storage. LWT 2021, 149, 111860. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M.-C.; Mora, L. Bioactive Peptides Generated in the Processing of Dry-Cured Ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, A.; Calzada, J.; Gaya, P.; Nuñez, M. Proteolysis and Flavor Characteristics of Serrano Ham Processed under Different Ripening Temperature Conditions. J. Food Sci. 2015, 80, C2404–C2412. [Google Scholar] [CrossRef] [PubMed]
- Toldrá, F. The Role of Muscle Enzymes in Dry-Cured Meat Products with Different Drying Conditions. Trends Food Sci. Technol. 2006, 17, 164–168. [Google Scholar] [CrossRef]
- Mora, L.; Valero, M.L.; Sánchez del Pino, M.M.; Sentandreu, M.A.; Toldrá, F. Small Peptides Released from Muscle Glycolytic Enzymes during Dry-Cured Ham Processing. J. Proteom. 2011, 74, 442–450. [Google Scholar] [CrossRef]
- Toldrá, F.; Flores, M. The Role of Muscle Proteases and Lipases in Flavor Development During the Processing of Dry-Cured Ham. Crit. Rev. Food Sci. Nutr. 1998, 38, 331–352. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Gallego, M.; Mora, L. Bioactive Peptides in Meat and Meat Products. Meat Muscle Biol. 2023, 7, 16243. [Google Scholar] [CrossRef]
- Mora, L.; Escudero, E.; Toldrá, F. Characterization of the Peptide Profile in Spanish Teruel, Italian Parma and Belgian Dry-Cured Hams and Its Potential Bioactivity. Food Res. Int. 2016, 89, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Cutrell, S.; Alhomoud, I.S.; Mehta, A.; Talasaz, A.H.; Van Tassell, B.; Dixon, D.L. ACE-Inhibitors in Hypertension: A Historical Perspective and Current Insights. Curr. Hypertens. Rep. 2023, 25, 243–250. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Toldrá, F. Identification of Novel Antioxidant Peptides Generated in Spanish Dry-Cured Ham. Food Chem. 2013, 138, 1282–1288. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Env. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef]
- Mora, L.; González-Rogel, D.; Heres, A.; Toldrá, F. Iberian Dry-Cured Ham as a Potential Source of α-Glucosidase-Inhibitory Peptides. J. Funct. Foods 2020, 67, 103840. [Google Scholar] [CrossRef]
- Yang, Z.; Cui, Z.; Zhang, W. Isolation, Purification and Identification of Antibacterial Peptides from Jinhua Ham Broth and Molecular Simulation Analyses of Their Interaction with Bacterial Porins. Food Chem. 2025, 473, 143026. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Zhang, X.-L.; Xie, Q.-F. Purification and Identification of Anti-Inflammatory Peptides Derived from Simulated Gastrointestinal Digests of Velvet Antler Protein (Cervus Elaphus Linnaeus). J. Food Drug Anal. 2016, 24, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Toldrá, F. Perspectives in the Use of Peptidomics in Ham. Proteomics 2018, 18, 1700422. [Google Scholar] [CrossRef]
- Heres, A.; Saldaña, C.; Toldrá, F.; Mora, L. Identification of Dipeptides by MALDI-ToF Mass Spectrometry in Long-Processing Spanish Dry-Cured Ham. Food Chem. Mol. Sci. 2021, 3, 100048. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Comer, J.; Li, Y. Bioinformatics Approaches to Discovering Food-Derived Bioactive Peptides: Reviews and Perspectives. TrAC Trends Anal. Chem. 2023, 162, 117051. [Google Scholar] [CrossRef]
- Petrova, I.; Aasen, I.M.; Rustad, T.; Eikevik, T.M. Manufacture of Dry-Cured Ham: A Review. Part 1. Biochemical Changes during the Technological Process. Eur. Food Res. Technol. 2015, 241, 587–599. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhang, J.; Li, X.; Lin, Z.; Ma, C. Proteolysis, Protein Oxidation and Protease Activity in Dry-Cured Xuanwei Ham during the Salting Stages. Int. J. Food Sci. Technol. 2011, 46, 1370–1377. [Google Scholar] [CrossRef]
- Petrova, I.; Tolstorebrov, I.; Mora, L.; Toldrá, F.; Eikevik, T.M. Evolution of Proteolytic and Physico-Chemical Characteristics of Norwegian Dry-Cured Ham during Its Processing. Meat Sci. 2016, 121, 243–249. [Google Scholar] [CrossRef]
- Sárraga, C.; Gil, M.; García-Regueiro, J.A. Comparison of Calpain and Cathepsin (B, L and D) Activities during Dry-Cured Ham Processing from Heavy and Light Large White Pigs. J. Sci. Food Agric. 1993, 62, 71–75. [Google Scholar] [CrossRef]
- Grossi, A.B.; do Nascimento, E.S.P.; Cardoso, D.R.; Skibsted, L.H. Proteolysis Involvement in Zinc–Protoporphyrin IX Formation during Parma Ham Maturation. Food Res. Int. 2014, 56, 252–259. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, G.; Xu, X.; Zhang, W.; Li, C. Contribution of Cathepsin B and L to Endogenous Proteolysis in the Course of Modern Jinhua Ham Processing. Food Control 2022, 135, 108584. [Google Scholar] [CrossRef]
- Virgili, R.; Parolari, G.; Schivazappa, C.; Bordini, C.S.; Borri, M. Sensory and Texture Quality of Dry-Cured Ham as Affected by Endogenous Cathepsin B Activity and Muscle Composition. J. Food Sci. 1995, 60, 1183–1186. [Google Scholar] [CrossRef]
- Zhao, G.M.; Zhou, G.H.; Wang, Y.L.; Xu, X.L.; Huan, Y.J.; Wu, J.Q. Time-Related Changes in Cathepsin B and L Activities during Processing of Jinhua Ham as a Function of pH, Salt and Temperature. Meat Sci. 2005, 70, 381–388. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Gómez, B.; Pateiro, M.; Pérez-Santaescolástica, C.; López-Fernández, O.; Sarriés, M.V.; Lorenzo, J.M. Effect of NaCl Replacement by Other Chloride Salts on Physicochemical Parameters, Proteolysis and Lipolysis of Dry-Cured Foal “Cecina”. J. Food Sci. Technol. 2020, 57, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, G.; Pampalakis, G.; Diamandis, E.P. Functional Roles of Human Kallikrein-Related Peptidases. J. Biol. Chem. 2009, 284, 32989–32994. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, M.A.; Koistinen, K.M.; Fraser, P.D.; Toldrá, F.; Bramley, P.M. Naturally Generated Small Peptides Derived from Myofibrillar Proteins in Serrano Dry-Cured Ham. J. Agric. Food Chem. 2009, 57, 3228–3234. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Identification of Small Troponin T Peptides Generated in Dry-Cured Ham. Food Chem. 2010, 123, 691–697. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. The Relevance of Dipeptides and Tripeptides in the Bioactivity and Taste of Dry-Cured Ham. Food Prod. Process. Nutr. 2019, 1, 2. [Google Scholar] [CrossRef]
- Correas, N.H.; Martínez, A.R.; Abellán, A.; Sánchez, H.P.; Tejada, L. Curing Strategies and Bioactive Peptide Generation in Ham: In Vitro Digestion and in Silico Evaluation. Food Chem. 2025, 484, 144360. [Google Scholar] [CrossRef] [PubMed]
- Hernández Correas, N.; Abellán, A.; Cayuela, J.M.; Bande-De León, C.; Tejada, L. Effect of Overripening on the Physico-Chemical and Sensory Characteristics of Boneless, Salt-Reduced Iberian Dry-Cured Ham. Foods 2024, 13, 1588. [Google Scholar] [CrossRef] [PubMed]
- Hernandez Correas, N.; Abellán Guillén, A.; Muñoz Rosique, B.; Bande De León, C.M.; Gómez, R.; Tejada, L. Overripening and Increased Temperature: Alternative Strategies to Enhance Peptide Production and Bioactivity in Salt-Reduced Boneless Cured Iberian Hams. Appl. Food Res. 2024, 4, 100639. [Google Scholar] [CrossRef]
- Leonardo Betiol, L.F.; Evangelista, R.R.; Ribeiro Sanches, M.A.; Basso, R.C.; Gullón, B.; Lorenzo, J.M.; Carla da Silva Barretto, A.; Romero, J.T. Influence of Temperature and Chemical Composition on Water Sorption Isotherms for Dry-Cured Ham. LWT 2020, 123, 109112. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Cittadini, A.; Bermúdez, R.; Munekata, P.E.; Domínguez, R. Influence of Partial Replacement of NaCl with KCl, CaCl2 and MgCl2 on Proteolysis, Lipolysis and Sensory Properties during the Manufacture of Dry-Cured Lacón. Food Control 2015, 55, 90–96. [Google Scholar] [CrossRef]
- Muñoz-Rosique, B.; Hernández-Correas, N.; Abellán, A.; Bueno, E.; Gómez, R.; Tejada, L. Influence of Pig Genetic Line and Salt Reduction on Peptide Production and Bioactivity of Dry-Cured Hams. Foods 2023, 12, 1022. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the Antioxidant Peptide AEEEYPDL and Its Quantification in Spanish Dry-Cured Ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Tian, W.; Li, M.-Y.; Liu, Y.-X.; Zhao, G.-M. Separation and Identification of Peptides from Dry-Cured Jinhua Ham. Int. J. Food Prop. 2017, 20, S2980–S2989. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Aristoy, M.C.; Fraser, P.D.; Toldrá, F. Peptides Naturally Generated from Ubiquitin-60S Ribosomal Protein as Potential Biomarkers of Dry-Cured Ham Processing Time. Food Control 2015, 48, 102–107. [Google Scholar] [CrossRef]
- Hao, L.; Gao, X.; Zhou, T.; Cao, J.; Sun, Y.; Dang, Y.; Pan, D. Angiotensin I-Converting Enzyme (ACE) Inhibitory and Antioxidant Activity of Umami Peptides after In Vitro Gastrointestinal Digestion. J. Agric. Food Chem. 2020, 68, 8232–8241. [Google Scholar] [CrossRef] [PubMed]
- Montoro-García, S.; Velasco-Soria, Á.; Mora, L.; Carazo-Díaz, C.; Prieto-Merino, D.; Avellaneda, A.; Miranzo, D.; Casas-Pina, T.; Toldrá, F.; Abellán-Alemán, J. Beneficial Impact of Pork Dry-Cured Ham Consumption on Blood Pressure and Cardiometabolic Markers in Individuals with Cardiovascular Risk. Nutrients 2022, 14, 298. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Xing, L.; Hao, Y.; Yang, Z.; Teng, S.; Wei, L.; Zhang, W. The Anti-Inflammatory Effects of Dry-Cured Ham Derived Peptides in RAW264.7 Macrophage Cells. J. Funct. Foods 2021, 87, 104827. [Google Scholar] [CrossRef]
- Escudero, E.; Mora, L.; Fraser, P.D.; Aristoy, M.-C.; Arihara, K.; Toldrá, F. Purification and Identification of Antihypertensive Peptides in Spanish Dry-Cured Ham. J. Proteom. 2013, 78, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Aristoy, M.-C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive Effect and Antioxidant Activity of Peptide Fractions Extracted from Spanish Dry-Cured Ham. Meat Sci. 2012, 91, 306–311. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Zhang, W.-G.; Zhou, G.-H.; Xu, X.-L.; Kang, Z.-L.; Yin, Y. Isolation and Identification of Antioxidant Peptides from Jinhua Ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef]
- Patil, P.; Mandal, S.; Tomar, S.K.; Anand, S. Food Protein-Derived Bioactive Peptides in Management of Type 2 Diabetes. Eur. J. Nutr. 2015, 54, 863–880. [Google Scholar] [CrossRef]
- Castellano, P.; Mora, L.; Escudero, E.; Vignolo, G.; Aznar, R.; Toldrá, F. Antilisterial Peptides from Spanish Dry-Cured Hams: Purification and Identification. Food Microbiol. 2016, 59, 133–141. [Google Scholar] [CrossRef]
- Montoro-García, S.; Zafrilla-Rentero, M.P.; Celdrán-de Haro, F.M.; Piñero-de Armas, J.J.; Toldrá, F.; Tejada-Portero, L.; Abellán-Alemán, J. Effects of Dry-Cured Ham Rich in Bioactive Peptides on Cardiovascular Health: A Randomized Controlled Trial. J. Funct. Foods 2017, 38, 160–167. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Gallego, M.; Aristoy, M.-C.; Toldrá, F. Dipeptidyl Peptidase IV Inhibitory Peptides Generated in Spanish Dry-Cured Ham. Meat Sci. 2014, 96, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Evaluation of the Potential of Dietary Proteins as Precursors of Dipeptidyl Peptidase (DPP)-IV Inhibitors by an in Silico Approach. J. Funct. Foods 2012, 4, 403–422. [Google Scholar] [CrossRef]
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Antihypertensive Potential of Sweet Ala-Ala Dipeptide and Its Quantitation in Dry-Cured Ham at Different Processing Conditions. J. Funct. Foods 2021, 87, 104818. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Hayes, M.; Reig, M.; Toldrá, F. Peptides with Potential Cardioprotective Effects Derived from Dry-Cured Ham Byproducts. J. Agric. Food Chem. 2019, 67, 1115–1126. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Toldrá, F. Degradation of Myosin Heavy Chain and Its Potential as a Source of Natural Bioactive Peptides in Dry-Cured Ham. Food Biosci. 2019, 30, 100416. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ohno, M.; Motoyama, T.; Ito, S.; Kawarasaki, Y. Analyzing a Dipeptide Library to Identify Human Dipeptidyl Peptidase IV Inhibitor. Food Chem. 2015, 175, 66–73. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Lin, F.; Cai, F.; Luo, B.; Gu, R.; Ahmed, S.; Long, C. Variation of Microbiological and Biochemical Profiles of Laowo Dry-Cured Ham, an Indigenous Fermented Food, during Ripening by GC-TOF-MS and UPLC-QTOF-MS. J. Agric. Food Chem. 2020, 68, 8925–8935. [Google Scholar] [CrossRef]
- Lan, V.T.T.; Ito, K.; Ito, S.; Kawarasaki, Y. Trp-Arg-Xaa Tripeptides Act as Uncompetitive-Type Inhibitors of Human Dipeptidyl Peptidase IV. Peptides 2014, 54, 166–170. [Google Scholar] [CrossRef]
- Heres, A.; Yokoyama, I.; Gallego, M.; Toldrá, F.; Arihara, K.; Mora, L. Impact of Oxidation on the Cardioprotective Properties of the Bioactive Dipeptide AW in Dry-Cured Ham. Food Res. Int. 2022, 162, 112128. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, M.; Wang, Q.; Dong, J.; Lu, S.; Lyu, B.; Ma, X. Antioxidant Activities of Peptides Derived from Mutton Ham, Xuanwei Ham and Jinhua Ham. Food Res. Int. 2021, 142, 110195. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Mora, L.; Toldrá, F. Inhibition of 3-Hydroxy-3-Methyl-Glutaryl-Coenzyme A Reductase Enzyme by Dipeptides Identified in Dry-Cured Ham. Food Prod. Process. Nutr. 2021, 3, 18. [Google Scholar] [CrossRef]
- Heres, A.; Gallego, M.; Mora, L.; Toldrá, F. Identification and Quantitation of Bioactive and Taste-Related Dipeptides in Low-Salt Dry-Cured Ham. Int. J. Mol. Sci. 2022, 23, 2507. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of Peptide Substrates and Inhibitors of Angiotensin-Converting Enzyme. Importance of the COOH-Terminal Dipeptide Sequence. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Y.; Zhou, X.; Li, C.; Liu, Y. Changes in the Extent and Products of In Vitro Protein Digestion during the Ripening Periods of Chinese Dry-Cured Hams. Meat Sci. 2021, 171, 108290. [Google Scholar] [CrossRef]
- Mora, L.; Sentandreu, M.A.; Toldrá, F. Intense Degradation of Myosin Light Chain Isoforms in Spanish Dry-Cured Ham. J. Agric. Food Chem. 2011, 59, 3884–3892. [Google Scholar] [CrossRef]
- van Platerink, C.J.; Janssen, H.-G.M.; Haverkamp, J. Application of At-Line Two-Dimensional Liquid Chromatography–Mass Spectrometry for Identification of Small Hydrophilic Angiotensin I-Inhibiting Peptides in Milk Hydrolysates. Anal. Bioanal. Chem. 2008, 391, 299–307. [Google Scholar] [CrossRef]
- Sentandreu, M.A.; Stoeva, S.; Aristoy, M.A.; Laib, K.; Voelter, W.; Toldra, E. Identification of Small Peptides Generated in Spanish Dry-Cured Ham. J. Food Sci. 2003, 68, 64–69. [Google Scholar] [CrossRef]
- Inoue, N.; Nagao, K.; Sakata, K.; Yamano, N.; Gunawardena, P.E.R.; Han, S.-Y.; Matsui, T.; Nakamori, T.; Furuta, H.; Takamatsu, K.; et al. Screening of Soy Protein-Derived Hypotriglyceridemic Di-Peptides in Vitro and in Vivo. Lipids Health Dis. 2011, 10, 85. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Aristoy, M.C.; Toldrá, F. Titin-Derived Peptides as Processing Time Markers in Dry-Cured Ham. Food Chem. 2015, 167, 326–339. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.K.; Shastri, V.; Madhu, M.K.; Sharma, V.K. Prediction of Anti-Inflammatory Proteins/Peptides: An Insilico Approach. J. Transl. Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Manavalan, B.; Shin, T.H.; Kim, M.O.; Lee, G. AIPpred: Sequence-Based Prediction of Anti-Inflammatory Peptides Using Random Forest. Front. Pharmacol. 2018, 9, 276. [Google Scholar] [CrossRef]
- Dhanda, S.; Singh, J.; Singh, H. Hydrolysis of Various Bioactive Peptides by Goat Brain Dipeptidylpeptidase-III Homologue. Cell Biochem. Funct. 2008, 26, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Inoue, Y.; Arima, J.; Kumagai, Y.; Usuki, H.; Kawakami, K.; Kimura, M.; Mukaihara, T. Production of Dipeptidyl Peptidase IV Inhibitory Peptides from Defatted Rice Bran. Food Chem. 2012, 134, 797–802. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Mooney, C.; Shields, D.C.; FitzGerald, R.J. In Silico Approaches to Predict the Potential of Milk Protein-Derived Peptides as Dipeptidyl Peptidase IV (DPP-IV) Inhibitors. Peptides 2014, 57, 43–51. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Howard, A. Meat Proteome as Source of Functional Biopeptides. Food Res. Int. 2013, 54, 1021–1032. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Potential DPP IV Inhibitory Peptides from Dry-Cured Pork Loins after Hydrolysis: An In Vitro and In Silico Study. Curr. Issues Mol. Biol. 2021, 43, 1335–1349. [Google Scholar] [CrossRef]
- Zhu, X.; Sun-Waterhouse, D.; Tao, Q.; Li, W.; Shu, D.; Cui, C. The Enhanced Serotonin (5-HT) Synthesis and Anti-Oxidative Roles of Trp Oligopeptide in Combating Anxious Depression C57BL/6 Mice. J. Funct. Foods 2020, 67, 103859. [Google Scholar] [CrossRef]
- Dellafiora, L.; Paolella, S.; Dall’Asta, C.; Dossena, A.; Cozzini, P.; Galaverna, G. Hybrid in Silico/in Vitro Approach for the Identification of Angiotensin I Converting Enzyme Inhibitory Peptides from Parma Dry-Cured Ham. J. Agric. Food Chem. 2015, 63, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Heres, A.; Mora, L.; Toldrá, F. Bioactive and Sensory Di- and Tripeptides Generated during Dry-Curing of Pork Meat. Int. J. Mol. Sci. 2023, 24, 1574. [Google Scholar] [CrossRef]
- Liu, X.; Hu, Q.; Shen, Y.; Wu, Y.; Gao, L.; Xu, X.; Hao, G. Research Progress on Antioxidant Peptides from Fish By-Products: Purification, Identification, and Structure–Activity Relationship. Metabolites 2024, 14, 561. [Google Scholar] [CrossRef]
- Chen, C.; Chi, Y.-J.; Zhao, M.-Y.; Lv, L. Purification and Identification of Antioxidant Peptides from Egg White Protein Hydrolysate. Amino. Acids 2012, 43, 457–466. [Google Scholar] [CrossRef]
- Mirzaei, M.; Aminlari, M.; Hosseini, E. Antioxidant, ACE-Inhibitory and Antimicrobial Activities of Kluyveromyces Marxianus Protein Hydrolysates and Their Peptide Fractions. Funct. Foods Health Dis. 2016, 6, 425–439. [Google Scholar] [CrossRef]
- Liu, W.-Y.; Zhang, J.-T.; Miyakawa, T.; Li, G.-M.; Gu, R.-Z.; Tanokura, M. Antioxidant Properties and Inhibition of Angiotensin-Converting Enzyme by Highly Active Peptides from Wheat Gluten. Sci. Rep. 2021, 11, 5206. [Google Scholar] [CrossRef] [PubMed]
- Escudero, E.; Mora, L.; Toldrá, F. Stability of ACE Inhibitory Ham Peptides against Heat Treatment and in Vitro Digestion. Food Chem. 2014, 161, 305–311. [Google Scholar] [CrossRef]
- Wang, W.; Yang, W.; Dai, Y.; Liu, J.; Chen, Z.-Y. Production of Food-Derived Bioactive Peptides with Potential Application in the Management of Diabetes and Obesity: A Review. J. Agric. Food Chem. 2023, 71, 5917–5943. [Google Scholar] [CrossRef] [PubMed]
- CV Pharmacology|Angiotensin Converting Enzyme (ACE) Inhibitors. Available online: https://cvpharmacology.com/vasodilator/ace (accessed on 10 July 2025).
- Abachi, S.; Bazinet, L.; Beaulieu, L. Antihypertensive and Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Fish as Potential Cardioprotective Compounds. Mar. Drugs 2019, 17, 613. [Google Scholar] [CrossRef]
- Rico-Campà, A.; Sayón-Orea, C.; Martínez-González, M.Á.; Ruiz-Canela, M.; Ruiz-Estigarribia, L.; de la Fuente-Arrillaga, C.; Toledo, E.; Bes-Rastrollo, M. Cured Ham Consumption and Incidence of Hypertension: The “Seguimiento Universidad de Navarra” (SUN) Cohort. Med. Clínica 2020, 155, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Xiao, S.; Chen, X.; Wang, B.; Hu, Y.; Wang, J. Separation, Enrichment and Cytoprotection of Antioxidant Peptides from Xuanwei Ham Using Aqueous Two-Phase Extraction. Food Chem. 2024, 446, 138600. [Google Scholar] [CrossRef]
- Xie, R.; Xiao, S.; Ma, D.; Wang, B.; Chen, G.; Xiang, J.; Wang, J. Protective Mechanism of Antioxidant Peptides Derived from Dry-Cured Ham against Ultraviolet A-Induced Oxidative Damage in HaCat Cells. Food Biosci. 2024, 62, 105394. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Li, R.; Wang, Y.; Huang, L. Identification and Characterization of Antioxidant Peptides from Chinese Dry-Cured Mutton Ham. J. Sci. Food Agric. 2020, 100, 1246–1255. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Toldrá, F.; Teng, S.; Yin, Y.; Zhang, W. The Stability of Dry-Cured Ham Derived Peptides and Its Anti-Inflammatory Effect in RAW264.7 Macrophage Cells. Int. J. Food Sci. Technol. 2023, 58, 1575–1585. [Google Scholar] [CrossRef]
- Zhu, C.-Z.; Zhang, W.-G.; Zhou, G.-H.; Xu, X.-L. Identification of Antioxidant Peptides of Jinhua Ham Generated in the Products and through the Simulated Gastrointestinal Digestion System. J. Sci. Food Agric. 2016, 96, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Mora, L.; Reig, M.; Toldrá, F. Stability of the Potent Antioxidant Peptide SNAAC Identified from Spanish Dry-Cured Ham. Food Res. Int. 2018, 105, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Mora, L.; Escudero, E.; Fraser, P.; Aristoy, M.-C.; Toldrá, F. Proteomic Identification of Antioxidant Peptides from 400 to 2500 Da Generated in Spanish Dry-Cured Ham Contained in a Size-Exclusion Chromatography Fraction. Food Res. Int. 2014, 56, 68–76. [Google Scholar] [CrossRef]
- Xing, L.; Fu, L.; Hao, Y.; Zhang, W. Dry-Cured Ham-Derived Peptide (Asp–Leu–Glu–Glu) Exerts Cytoprotective Capacity in Human Intestinal Epithelial Caco-2 Cells. Antioxidants 2021, 10, 1354. [Google Scholar] [CrossRef]
- Zhu, Z.; Xu, Z.; Li, Y.; Fan, Y.; Zhou, Y.; Song, K.; Meng, L. Antioxidant Function and Application of Plant-Derived Peptides. Antioxidants 2024, 13, 1203. [Google Scholar] [CrossRef]
- Kęska, P.; Stadnik, J. Dipeptidyl Peptidase IV Inhibitory Peptides Generated in Dry-Cured Pork Loin during Aging and Gastrointestinal Digestion. Nutrients 2022, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Xing, L.; Wang, Z.; Cai, J.; Toldrá, F.; Zhang, W. Study on the Anti-Inflammatory Activity of the Porcine Bone Collagen Peptides Prepared by Ultrasound-Assisted Enzymatic Hydrolysis. Ultrason. Sonochem. 2023, 101, 106697. [Google Scholar] [CrossRef]
- Hu, Y.; Xiao, S.; Zhou, G.; Chen, X.; Wang, B.; Wang, J. Bioactive Peptides in Dry-Cured Ham: A Comprehensive Review of Preparation Methods, Metabolic Stability, Safety, Health Benefits, and Regulatory Frameworks. Food Res. Int. 2024, 186, 114367. [Google Scholar] [CrossRef]
- Lindström, J.; Virtanen, S.M. 11—Functional Foods and Prevention of Diabetes. In Functional Foods, 2nd ed.; Saarela, M., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Oxford, UK, 2011; pp. 261–276. ISBN 978-1-84569-690-0. [Google Scholar]
- Sahingil, D.; Gokce, Y.; Celikbicak, O.; Hayaloglu, A.A. ACE-Inhibitory Activities of Peptide Fractions (<3 kDa) and Identification of Peptide Sequence by MALDI-ToF-MS in Model Cheeses Incorporating Different Lactobacillus Species. J. Food Compos. Anal. 2022, 110, 104579. [Google Scholar] [CrossRef]
- Nutraceutical Proteins and Peptides in Health and Disease|Yoshinori. Available online: https://www.taylorfrancis.com/books/mono/10.1201/9781420028836/nutraceutical-proteins-peptides-health-disease-fereidoon-shahidi-yoshinori-mine (accessed on 10 July 2025).
- Sharma, M.; Vidhya, C.S.; Ojha, K.; Yashwanth, B.S.; Singh, B.; Gupta, S.; Pandey, S.K. The Role of Functional Foods and Nutraceuticals in Disease Prevention and Health Promotion. Eur. J. Nutr. Food Saf. 2024, 16, 61–83. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Lin, Y.; Bao, H.; Miao, S. Recent Progress in Microencapsulation of Active Peptides—Wall Material, Preparation, and Application: A Review. Foods 2023, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sun, J.; Chandrapala, J.; Majzoobi, M.; Brennan, C.; Zeng, X.-A.; Sun, B. Recent Progress of Food-Derived Bioactive Peptides: Extraction, Purification, Function, and Encapsulation. Food Front. 2024, 5, 1240–1264. [Google Scholar] [CrossRef]
- Sun, X.; Okagu, O.D.; Udenigwe, C.C. Chapter 15—Encapsulation Technology for Protection and Delivery of Bioactive Peptides. In Biologically Active Peptides; Toldrá, F., Wu, J., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 331–356. ISBN 978-0-12-821389-6. [Google Scholar]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef]
- Salindeho, N.; Mokolensang, J.F.; Manu, L.; Taslim, N.A.; Nurkolis, F.; Gunawan, W.B.; Yusuf, M.; Mayulu, N.; Tsopmo, A. Fish Scale Rich in Functional Compounds and Peptides: A Potential Nutraceutical to Overcome Undernutrition. Front. Nutr. 2022, 9, 1072370. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V. Nutraceuticals and Pharmaceuticals from Marine Fish and Invertebrates. Mar. Drugs 2021, 19, 401. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Cai, Q.-L.; Gu, Z.-Y.; Song, T.-Y. Novel ACE-Inhibiting Peptides from Soybean Protein Hydrolysates by Peptidomics Combined with in Silico Analysis and Their Inhibitory Effects on Proliferation and Migration of Ang II-Induced VSMCs. Food Med. Homol. 2024, 1, 9420023. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, H.; Niu, H.; Wu, R. Peptidomic Analyses: The Progress in Enrichment and Identification of Endogenous Peptides. TrAC Trends Anal. Chem. 2020, 125, 115835. [Google Scholar] [CrossRef]
- Dong, Z.-H.; Pan, R.-Y.; Ren, G.-Y.; Zhou, M.; Zhang, B.; Fan, J.-L.; Qiu, Z.-J. A Novel Antidiabetic Peptide GPAGAP from Andrias davidianus Collagen Hydrolysates: Screening, Action Mechanism Prediction and Improving Insulin Resistance in HepG2 Cells. Food Med. Homol. 2024, 1, 9420010. [Google Scholar] [CrossRef]

| Sequence | Mass (Da) | Protein of Origin | Described Bioactivity | References |
|---|---|---|---|---|
| AA | 204.23 | Myosin7590 | Antihypertensive/Antidiabetic | [32,52,54,55,56] |
| AD | 204.18 | Myosin Light Chain | Antidiabetic | [14,57] |
| AL | 188.21 | Myosin | Immunomodulatory/Antidiabetic | [10,16,18,52,58] |
| AS | 176.08 | Actin | Antidiabetic | [59,60] |
| AW | 246.3 | Myosin | Antioxidant/Antihypertensive | [54,61] |
| AY | 252.27 | LIM Domain-Binding Protein 3 | Antioxidant | [32] |
| DA | 204.18 | Actin/Myosin | Anti-inflammatory/Antihypertensive/Lipid-Metabolism-Modulating Activity | [18,33,61,62,63] |
| DG | 190.15 | Myosin | Anti-inflammatory/Antihypertensive/Antioxidant | [33,61,62,64] |
| DL | 246.28 | Creatine Kinase | Antihypertensive | [55] |
| EA | 218.21 | Titin/Myoglobin | Antihypertensive/Antidiabetic | [14,52,55,62,65] |
| EE | 276.24 | Titin (fragments) | Anti-inflammatory/Antihypertensive | [33,61,64,66] |
| EF | 278.35 | Ubiquitin-60S | Antihypertensive | [32,67] |
| EK | 275.3 | Myosin | Antihypertensive/Antidiabetic | [55] |
| EL | 244.3 | Enolase | Antioxidant/Antihypertensive | [7,32,42] |
| ES | 234.21 | Titin | Anti-inflammatory/Antihypertensive/Antioxidant | [33,61,62,64] |
| EV | 277.24 | Myosin | Antyhypertensive | [42,58] |
| EW | 333.34 | Myosin | Antihypertensive | [14] |
| GA | 146.15 | Titin | Anti-inflammatory/Antidiabetic/Antihypertensive | [14,18,32,33,53,55,61,62,63,64] |
| GP | 172.2 | Titin | Antihypertensive/Antidiabetic | [32,52,55,61,64] |
| IE | 260.29 | α-enolase | Antihypertensive/Antidiabetic | [14,33,68] |
| IF | 278.35 | Ubiquitin-60S | Antihypertensive | [14] |
| II | 244.33 | Glyceraldehyde-3-Phosphate Dehydrogenase | Antidiabetic | [32,67,69] |
| IL | 244.33 | Enolase | Antidiabetic | [32,67,69] |
| IP | 244.3 | Troponin T | Antidiabetic | [32] |
| IV | 230.3 | Myosin | Antidiabetic | [32,67,69] |
| KA | 217.27 | Ubiquitin-60S | Antihypertensive/Antidiabetic Lipid-Metabolism-Modulating Activity | [7,32,52,55,56,70,71] |
| KP | 243.3 | Titina | Antidiabetic/Antihypertensive Antioxidant | [7,32,55] |
| LA | 202.25 | Creatine Kinase | Antihypertensive/Antidiabetic | [55] |
| LI | 244.33 | Myosin | Antidiabetic | [7,32,69] |
| LL | 244.33 | Lactate Dehydrogenase | Antidiabetic/Immunomodulatory | [7,32,67,69,72,73] |
| LR | 287.36 | Myosin Light Chain | Antihypertensive Brain Health-Promoting and Neuron-Related Activities | [7,10,32,40,56,67] |
| NR | 289.29 | Creatine Kinase | Antihypertensive | [32,67] |
| PE | 244.25 | Titin/Myosin | Antidiabetic | [14,74] |
| PG | 172.18 | Actin | Antidiabetic | [32,53,61] |
| PL | 228.3 | Creatine Kinase | Antidiabetic/Antihypertensive | [14,52,55] |
| PP | 212.25 | Myosin | Antihypertensive/Antidiabetic | [14,55,66,68,75] |
| QN | 260.25 | Myosin | Antidiabetic | [59,60] |
| RG | 246.28 | Troponin T | Antihypertensive/Antidiabetic | [55] |
| RL | 287.36 | LIM Domain-Binding Protein 3 | Antihypertensive/Immunomodulatory | [7,42,72,73] |
| RP | 271.32 | Myosin | Antihypertensive | [32,55,56] |
| SI | 246.3 | α-enolasa | Antidiabetic | [14,57] |
| SV | 232.3 | Titin | Antidiabetic | [32] |
| VD | 232.23 | Antidiabetic | [14,33] | |
| VE | 246.26 | Titin | Antihypertensive/Antidiabetic | [14,55,57,66,68] |
| VF | 262.30 | Myosin Light Chain | Antihypertensive/Antidiabetic | [14,18,58,63,64] |
| VG | 174.20 | Myosin | Anti-inflammatory/Antihypertensive/Immunomodulatory | [33,61,62] |
| VH | 254.29 | Troponin T | Immunomodulatory | [10,16,18,52] |
| VK | 245.32 | Myosin Light Chain | Lipid-Metabolism-Modulating Activity | [7,55,70,71] |
| VV | 216.28 | LIM Domain-Binding Protein 3; β-enolasa | Antidiabetic | [14,76] |
| VY | 280.32 | Enolase | Antihypertensive/Antioxidant Brain Health-Promoting and Neuron-Related Activities | [7,10,32,40,55,56] |
| WK | 319.4 | β-enolase | Antidiabetic | [14,76] |
| YA | 252.27 | Myosin | Antihypertensive/Antidiabetic | [59,60,77] |
| Sequence | Mass (Da) | Protein of Origin | Validation Methodology | Antihypertensive Activity | Reference |
|---|---|---|---|---|---|
| AAATP | 429.5 | Allantoicase (metabolic enzyme) | In vitro (ACE inhibition), in vivo (SHR* rat) | IC50 = 100 µM PAS* decrease = –25.6 mmHg (8 h) | [45] |
| AAPLAP | 538.6 | Heavy chain of myosin XV | In vitro (ACE inhibition) | IC50 = 14.38 µM | [86] |
| AMNPP | 528.6 | Myosin 3 (heavy chain) | In vitro (ACE inhibition) | IC50 = 304.5 µM | [86] |
| ASGPINFT | 805.9 | Myosin regulatory light chain 2 | In vitro (ACE inhibition) | IC50 = 975 µM | [45] |
| DVITGA | 574.6 | Light chain of myosin | In vitro (ACE inhibition) | IC50 = 900 µM | [45] |
| FNMPLTIRITPGSKA | 1646.0 | Fragment of myofibrillar protein | In vitro (ACE inhibition) | >70% inhibition at 170 µM | [55] |
| GGVPGG | 442.5 | Elastin | In vitro (ACE inhibition) | 79.9% inhibition at 1 mol/L | [55] |
| GVVPL | 483.6 | Heavy chain of myosin | In vitro (ACE inhibition) | IC50 = 956 µM | [80] |
| HCNKKYRSEM | 1295.5 | Heavy chain of dynein 3 | In vitro (ACE inhibition) | >70% inhibition at 170 µM | [55] |
| IAGRP | 512.6 | Titin (myofibrillar protein) | In vitro (ACE inhibition) | IC50 = 25.94 µM | [86] |
| IKLPP | 566.7 | Myosin IXb (heavy chain) | In vitro (ACE inhibition) | IC50 = 193.9 µM | [86] |
| KAAAAP | 527.6 | Light chain 3 of myosin | In vitro (ACE inhibition) | IC50 = 19.79 µM | [86] |
| KAAAATP | 628.7 | PR-domain zinc finger protein 2 | In vitro (ACE inhibition) | IC50 = 25.64 µM | [86] |
| KPGRP | 553.7 | Titin | In vitro (ACE inhibition) | IC50 = 67.08 µM | [86] |
| KPVAAP | 581.7 | Myosin | In vitro (ACE inhibition) | IC50 = 12.37 µM | [86] |
| KVLPG | 512.7 | Phosphoglycerate kinase 1 | In vitro (ACE inhibition) | IC50 = 265.4 µM | [86] |
| PAPPK | 508.6 | Light chain 1/3 of myosin | In vitro (ACE inhibition) | IC50 = 199.6 µM | [86] |
| PSNPP | 510.5 | Titin | In vitro (ACE inhibition) | IC50 = 192.8 µM | [86] |
| SFVTT | 553.6 | Glyceraldehyde-3-phosphate dehydrogenase | In vitro (ACE inhibition) | IC50 = 395 µM | [80] |
| TGLKP | 514.6 | Aspartate aminotransferase | In vitro (ACE inhibition) | IC50 = 51.57 µM | [86] |
| TKYRVP | 762.9 | Titin | In vitro (ACE inhibition) | >70% inhibition at 170 µM | [55] |
| TSNRYHSYPWG | 1367.4 | Protein kinase | In vitro (ACE inhibition) | >70% inhibition at 170 µM | [55] |
| Sequence | Mass (Da) | Protein of Origin | Validation Methodology | Antioxidant Activity | Reference |
|---|---|---|---|---|---|
| AEEEYPDL | 1109 | Creatine kinase (muscle) | In vitro (ABTS capture, ORAC) | ABTS: 1474 nmol TEAC/mg; ORAC: 960 nmol TE/mg (high antioxidant activity) | [39] |
| APPAAPPASGWPPTR | 1743 | / | Cell model (HaCaT + UVA) | Protected HaCaT cells from oxidative damage caused by UVA, increasing their survival and antioxidant response | [91,92] |
| APYMM | 611.76 | / | In vitro (ABTS) | ABTS: 0.12 mg/mL | [93] |
| DLEE | 576 | / | In vitro (ABTS, ORAC); cell model (Caco-2) | ABTS: 148 μmol TE/g; ORAC: 1032 μmol TE/g (similar to glutathione); reduced intracellular ROS and activated the Nrf2-Keap1 pathway | [94] |
| DPLPPGWEIK | 1331 | E3 ubiquitin ligase | Cell model (HaCaT keratinocytes + UVA) | Increased the viability of UVA-damaged HaCaT cells (~10–15% vs. control), activating the Nrf2-Keap1 antioxidant pathway | [91,92] |
| FLKMN | 652 | Myosin light chain | In vitro (DPPH) | DPPH: 65% inhibition at 1 mg/mL; OH−:60% at 1 mg/mL | [95] |
| FWIEE | 706.84 | / | In vitro (ABTS) | ABTS: 0.23 mg/mL | [93] |
| GKFNV | 564 | / | In vitro (DPPH) | DPPH: 92.7% inhibition at 1 mg/mL | [47,95] |
| LPGGGHGDL | 822 | / | In vitro (O-) | OH−: 85% at 1 mg/mL | [95] |
| LPGGGT | 501 | / | In vitro (DPPH) | DPPH: 65% inhibition at 1 mg/mL; OH−: 60% at 1 mg/mL | [95] |
| MWTD | 551.61 | / | In vitro (ABTS) | ABTS: 0.4 mg/mL | [93] |
| SAGNPN | 640 | Integrin α-3 (muscle) | In vitro (DPPH) | DPPH: 50% inhibition at 1.5 mg/mL (moderate antioxidant activity) | [12] |
| SNAAC | 555 | Heavy myosin chain | In vitro (DPPH, reducing power) | DPPH: 95.7% at 3 mg/mL; FRAP: Abs_700 = 1.7 at 1 mg/mL (potent antioxidant) | [96,97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Correas, N.; Liceaga, A.M.; Abellán, A.; Muñoz-Rosique, B.; Tejada, L. Bioactivities Derived from Dry-Cured Ham Peptides: A Review. Antioxidants 2025, 14, 1011. https://doi.org/10.3390/antiox14081011
Hernández Correas N, Liceaga AM, Abellán A, Muñoz-Rosique B, Tejada L. Bioactivities Derived from Dry-Cured Ham Peptides: A Review. Antioxidants. 2025; 14(8):1011. https://doi.org/10.3390/antiox14081011
Chicago/Turabian StyleHernández Correas, Noelia, Andrea M. Liceaga, Adela Abellán, Beatriz Muñoz-Rosique, and Luis Tejada. 2025. "Bioactivities Derived from Dry-Cured Ham Peptides: A Review" Antioxidants 14, no. 8: 1011. https://doi.org/10.3390/antiox14081011
APA StyleHernández Correas, N., Liceaga, A. M., Abellán, A., Muñoz-Rosique, B., & Tejada, L. (2025). Bioactivities Derived from Dry-Cured Ham Peptides: A Review. Antioxidants, 14(8), 1011. https://doi.org/10.3390/antiox14081011









