Limited Chemopreventive Effects of Oral Administration of Polyphenol-60 from Green Tea in the MNU-Induced Rat Mammary Tumor Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Polyphenon-60 (PO-60)
2.3. Experimental Design
- (a)
- Rats from Group 1 (G1; n = 10) were intraperitoneally (i.p.) inoculated with a unique dose of 55 mg MNU/kg body weight (BW) and received regular food for rats (supplied by the Cantacuzino Institute, Bucharest, Romania) and tap water.
- (b)
- Rats from Group 2 (G2; n = 10) were inoculated i.p. with a unique dose of 55 mg MNU/kg BW and received regular food for rats and tap water supplemented with 0.5% PO-60, ad libitum.
- (c)
- Group 3 (G3; n = 10) was inoculated i.p. with a unique dose of saline and received regular food for rats and tap water supplemented with 0.5% PO-60, ad libitum.
- (d)
- Group 4 (G4; n = 10) was inoculated with a single dose of saline i.p. and received regular food for rats and tap water.
2.4. Necropsy and Histopathology
- (a)
- The percentage of the mammary tumor mass (MTM) compared with the final body weight (FBW) of the rat, using the formula MTM (g) × 100/FBW (g) = MTM (%);
- (b)
- The volume of tumoral mass (MTV) calculated using the formula recommended by Woditschka et al. (2008): 0.5 × tumor width × tumor length × tumor height [43];
- (c)
- Mammary tumor multiplicity (MTMp) for G1 and G2 (i.e., the average number of mammary tumors/rat).
2.5. Assays of Antioxidant Profile in Liver and Mammary Gland
2.6. Assays of Blood Biochemical Profile
2.7. Statistical Analysis
3. Results
3.1. Chemopreventive Effects of PO-60 on Mammary Tumor Development
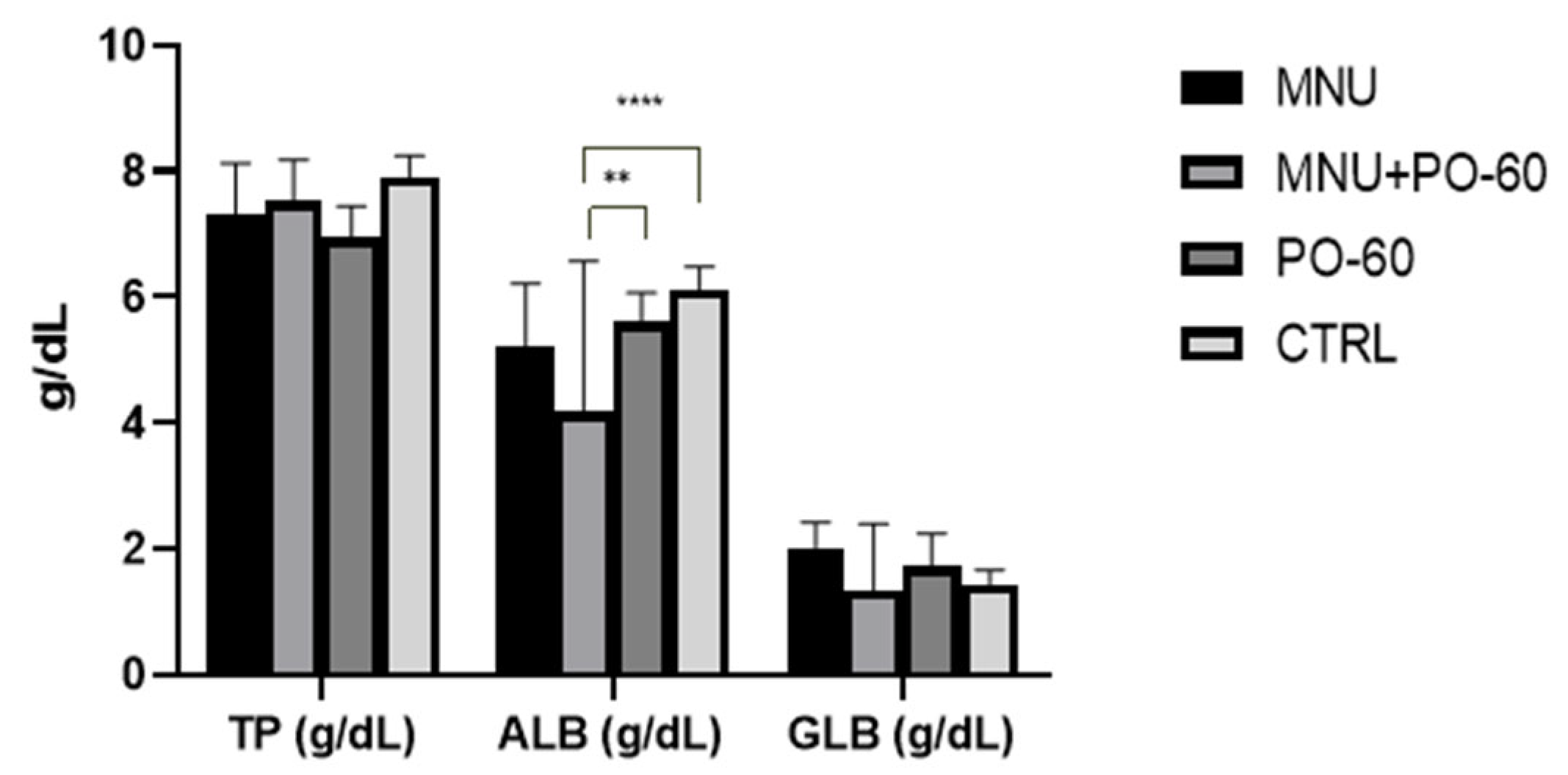
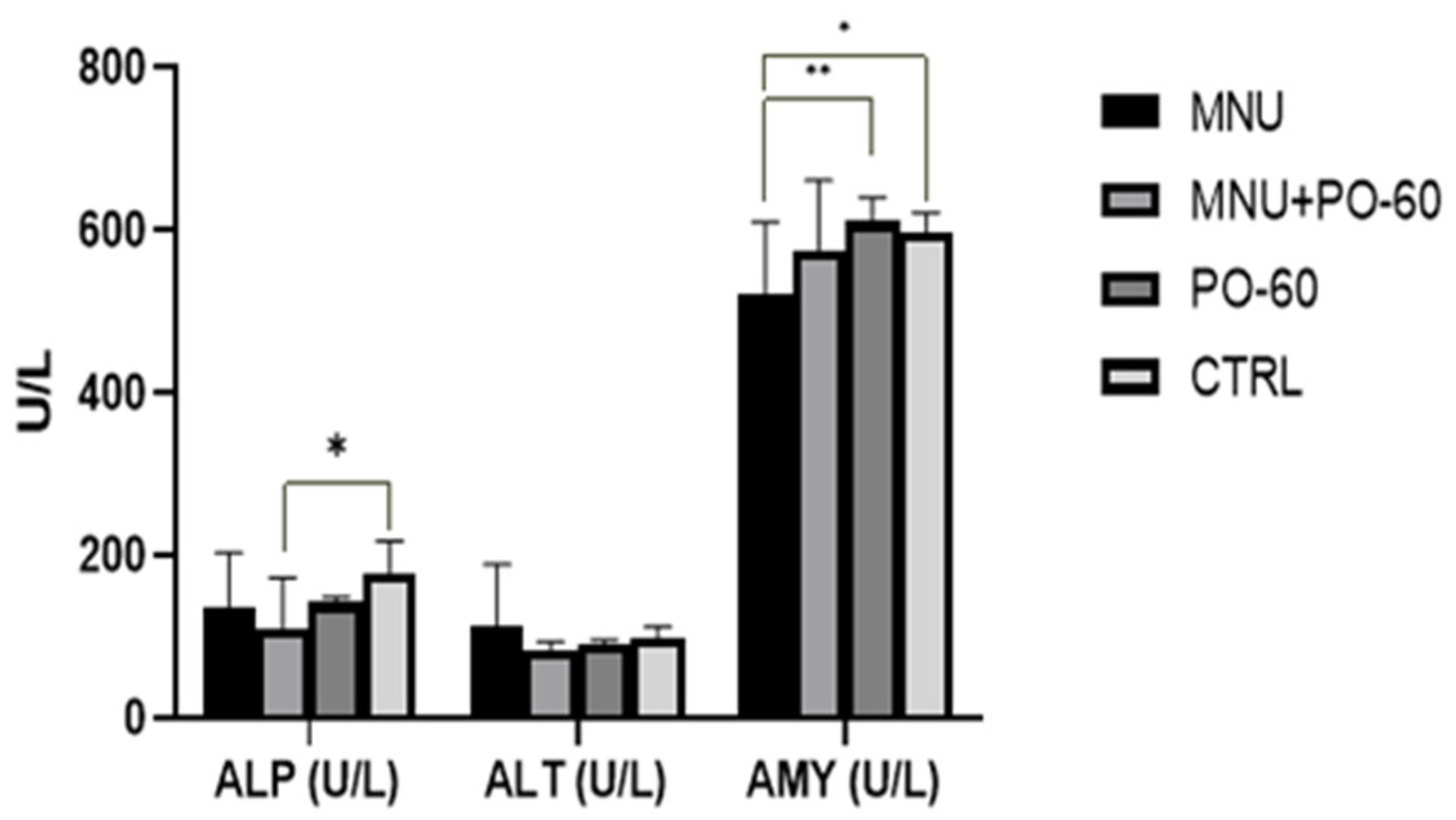
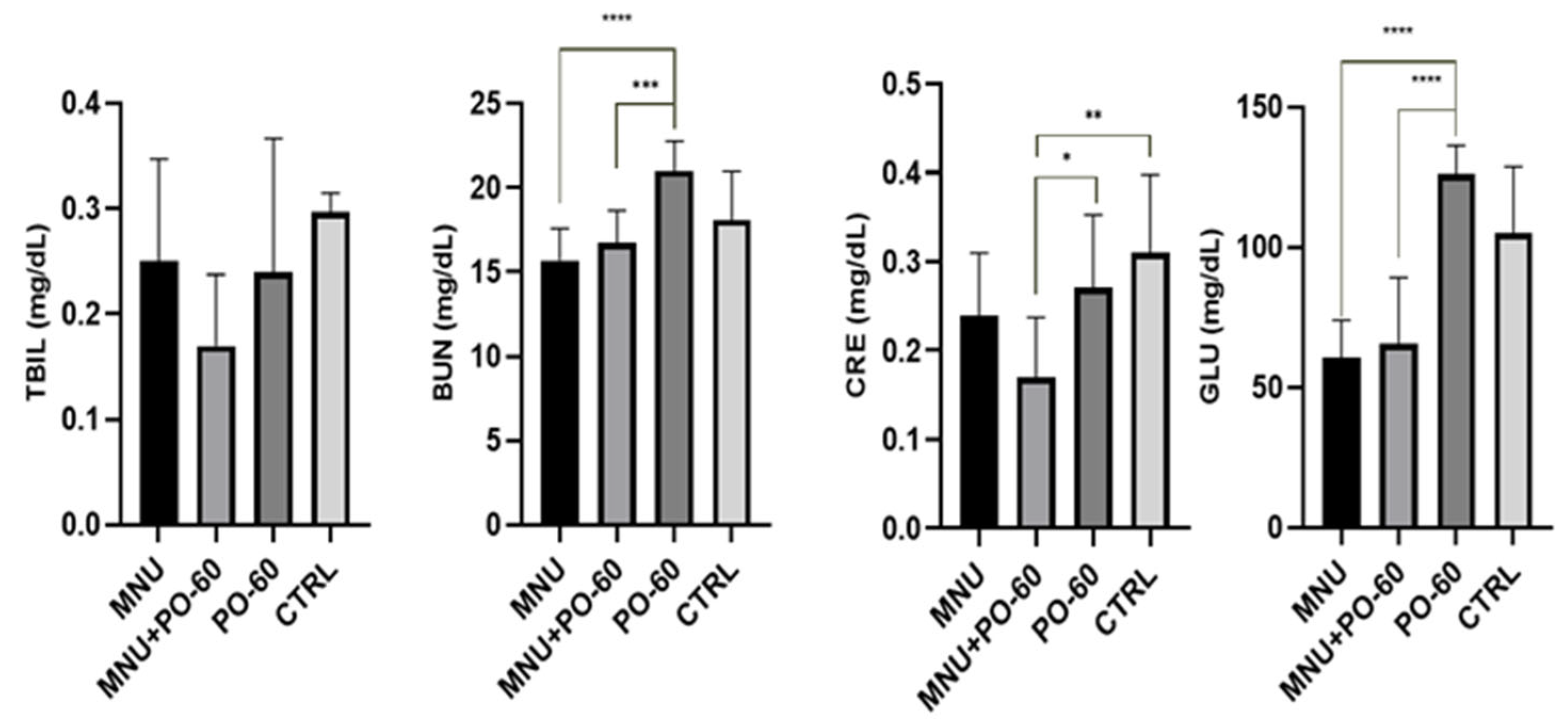
3.2. Blood Biochemical Profile in All Experimental Groups
3.2.1. Blood Proteins
3.2.2. The Determination of Antioxidant Enzyme Activity
3.2.3. Blood Biochemical Parameters
3.2.4. Blood Microminerals
3.3. The Effects of MNU and PO-60 on the Antioxidative Status in Tissues
3.3.1. Antioxidant Defense System in Rat Liver
3.3.2. Antioxidant Defense System in Rat Mammary Gland
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.L.; Fox, E.E.; Lai, D. Competing causes of death for women with breast cancer and change over time from 1975 to 2003. Am. J. Clin. Oncol. 2008, 31, 105–116. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Goldschmidt, M.H.; Peña, L.; Zappulli, V. Tumors of the mammary gland. Tumors Domest. Anim. 2016, 2016, 723–765. [Google Scholar] [CrossRef]
- de Andrés, P.J.; Cáceres, S.; Illera, J.C.; Crespo, B.; Silván, G.; Queiroga, F.L.; Peña, L. Hormonal homologies between canine mammary cancer and human breast cancer in a series of cases. Vet. Sci. 2022, 9, 395. [Google Scholar] [CrossRef]
- Vazquez, E.; Lipovka, Y.; Cervantes-Arias, A.; Garibay-Escobar, A.; Haby, M.M.; Queiroga, F.L.; Velazquez, C. Canine mammary cancer: State of the art and future perspectives. Animals 2023, 13, 3147. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Yin, Y.C.; Wang, J.; Jiang, Y.F. Green tea compounds in breast cancer prevention and treatment. World J. Clin. Oncol. 2014, 5, 520. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Worley, D.R.; Goldschmidt, M.H. Tumors of the mammary gland. In Withrow and MacEwen’s Small Animal Clinical Oncology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 538–556. [Google Scholar]
- Tian, X.; Srinivasan, P.R.; Tajiknia, V.; Sanchez, S.; Uruchurtu, A.F.; Seyhan, A.A.; Carneiro, B.A.; De La Cruz, A.; Pinho-Schwermann, M.; George, A.; et al. Targeting apoptotic pathways for cancer therapy. J. Clin. Investig. 2024, 134, e179570. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Gal, A.F.; Stan, L.; Tăbăran, F.; Rugină, D.; Cătoi, A.F.; Andrei, S. Chemopreventive effects of propolis in the MNU-induced rat mammary tumor model. Oxid. Med. Cell Longev. 2020, 2020, 4014838. [Google Scholar] [CrossRef]
- Lecumberri, E.; Dupertuis, Y.M.; Miralbell, R.; Pichard, C. Green tea polyphenol epigallocatechin-3-gallate (EGCG) as adjuvant in cancer therapy. Clin. Nutr. 2013, 32, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Trisha, A.T.; Shakil, M.H.; Talukdar, S.; Rovina, K.; Huda, N.; Zzaman, W. Tea polyphenols and their preventive measures against cancer: Current trends and directions. Foods 2022, 11, 3349. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomedicine 2014, 10, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Wei, Y.; Yu, Y.; Shan, M.; Tang, Y.; Sun, Y. Green tea polyphenols inhibit malignant melanoma progression via regulating circ_MITF/miR-30e-3p/HDAC2 axis. Biotechnol. Appl. Biochem. 2022, 69, 808–821. [Google Scholar] [CrossRef]
- Golpour, M.; Ebrahimnejad, P.; Gatabi, Z.R.; Najafi, A.; Davoodi, A.; Khajavi, R.; Mousavi, T. Green tea-mediated synthesis of silver nanoparticles: Enhanced anti-cancer activity and reduced cytotoxicity melanoma and normal murine cell lines. Inorg. Chem. Commun. 2024, 161, 111989. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Dietary agents for prevention and treatment of lung cancer. Cancer Lett. 2015, 359, 155–164. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Pezzani, R.; Redaelli, M.; Zorzan, M.; Imran, M.; Ahmed Khalil, A.; Sharifi-Rad, J. Preclinical activities of epigallocatechin gallate in signaling pathways in cancer. Molecules 2020, 25, 467. [Google Scholar] [CrossRef]
- Thomas, P.; Dong, J. (−)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2021, 211, 105906. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Mobbili, G.; Galeazzi, R.; Armeni, T. Effect of epigallocatechin-3-gallate on EGFR signaling and migration in non-small cell lung cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.W.; Xia, J.; Cheng, B.H.; Gao, H.C.; Fu, L.W.; Luo, X.L. Tea polyphenol EGCG inhibited colorectal-cancer-cell proliferation and migration via downregulation of STAT3. Gastroenterol. Rep. 2021, 9, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights. Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Philips, B.J.; Coyle, C.H.; Morrisroe, S.N.; Chancellor, M.B.; Yoshimura, N. Induction of apoptosis in human bladder cancer cells by green tea catechins. Biomed. Res. 2009, 30, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Bonanni, B. A presurgical study of lecithin formulation of green tea extract in women with early breast cancer. Cancer Prev. Res. 2017, 10, 363–370. [Google Scholar] [CrossRef]
- Xiang, L.P.; Wang, A.; Ye, J.H.; Zheng, X.Q.; Polito, C.A.; Lu, J.L.; Liang, Y.R. Suppressive effects of tea catechins on breast cancer. Nutrients 2016, 8, 458. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria—A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (–)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells: Requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar] [CrossRef]
- Bigelow, R.L.H.; Cardelli, J.A. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene 2006, 25, 1922–1930. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic effects of green tea polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 2022, 24, 340. [Google Scholar] [CrossRef]
- Shim, J.H.; Su, Z.Y.; Chae, J.I.; Kim, D.J.; Zhu, F.; Ma, W.Y.; Dong, Z. Epigallocatechin gallate suppresses lung cancer cell growth through Ras–GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev. Res. 2010, 3, 670–679. [Google Scholar] [CrossRef]
- Lee, W.J.; Shim, J.Y.; Zhu, B.T. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005, 68, 1018–1030. [Google Scholar] [CrossRef]
- Alsamri, H.; El Hasasna, H.; Al Dhaheri, Y.; Eid, A.H.; Attoub, S.; Iratni, R. Carnosol, a natural polyphenol, inhibits migration, metastasis, and tumor growth of breast cancer via a ROS-dependent proteasome degradation of STAT3. Front. Oncol. 2019, 9, 743. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Cardiff, R.D.; Wellings, S.R. The comparative pathology of human and mouse mammary glands. J. Mammary Gland. Biol. Neoplasia 1999, 4, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.F.; Andrei, S.; Cernea, C.; Taulescu, M.; Catoi, C. Effects of astaxanthin supplementation on chemically induced tumorigenesis in Wistar rats. Acta Vet. Scand. 2012, 54, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.Y.; Lee, S.H.; Kim, H.H.; Kim, S.M.; Shin, M.J.; Kim, N.; Gong, G. Evaluation of tumor angiogenesis with a second-generation US contrast medium in a rat breast tumor model. Korean J. Radiol. 2008, 9, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Malicka, I.; Siewierska, K.; Pula, B.; Kobierzycki, C.; Haus, D.; Paslawska, U.; Wozniewski, M. The effect of physical training on the N-methyl-N-nitrosourea-induced mammary carcinogenesis of Sprague–Dawley rats. Exp. Biol. Med. 2015, 240, 1408–1415. [Google Scholar] [CrossRef]
- Shen, C.L.; Brackee, G.; Song, X.; Tomison, M.D.; Finckbone, V.; Mitchell, K.T.; Tang, L.; Ming-Chien Chyu, D.; Dunn, M.; Wang, J.S. Safety Evaluation of Green Tea Polyphenols Consumption in Middle—Aged Ovariectomized Rat Model. J. Food Sci. 2017, 82, 2192–2205. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, L.; Shen, C.L.; Wang, J.S. Green tea polyphenols boost gut-microbiota-dependent mitochondrial TCA and urea cycles in Sprague–Dawley rats. J. Nutr. Biochem. 2020, 81, 108395. [Google Scholar] [CrossRef]
- Thompson, H.J. Methods for the induction of mammary carcinogenesis in the rat using either 7, 12-Dimethylbenz [α] anthracene or 1-Methyl-1-Nitrosourea. In Methods in Mammary Gland Biology and Breast Cancer Research; Springer US: Boston, MA, USA, 2000; pp. 19–29. [Google Scholar]
- Woditschka, S.; Haag, J.D.; Mau, B.; Lubet, R.A.; Gould, M.N. Chemopreventive effects of celecoxib are limited to hormonally responsive mammary carcinomas in the neu-induced retroviral rat model. Breast Cancer Res. 2008, 10, 1–9. [Google Scholar] [CrossRef]
- Russo, J.; Russo, I.H. Atlas and histologic classification of tumors of the rat mammary gland. J. Mammary Gland. Biol. Neoplasia 2000, 5, 187–200. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Ciobanu, L.; Tantau, M.; Valean, S.; Parau, A.; Bedecean, I.; Mîrleneanu, R.; Berce, C.; Catoi, C.; Taulescu, M. Rifaximin modulates 5-fluorouracil-induced gastrointestinal mucositis in rats. Eur. Rev. Med. Pharmacol. 2016, 20, 4993–5001. [Google Scholar]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semiquantification, localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Gibson-Corley, K.N.; Olivier, A.K.; Meyerholz, D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013, 50, 1007–1015. [Google Scholar] [CrossRef]
- Matei-Latiu, M.-C.; Gal, A.F.; Rus, V.; Buza, V.; Martonos, C.; Latiu, C.; Stefanut, L.-C. Intestinal dysbiosis in rats: Interaction between amoxicillin and probiotics, a histological and immunohistochemical evaluation. Nutrients 2023, 15, 1105. [Google Scholar] [CrossRef]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Lim, J.T.; Moriwaki, H.; Kopelovich, L.; Weinstein, I.B. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin. Cancer Res. 2005, 11, 2735–2746. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sherbiny, I.M.; ElMissiry, M.A.; Ali, D.A.; AbdElhakim, E. Polyphenon-E encapsulated into chitosan nanoparticles inhibited proliferation and growth of Ehrlich solid tumor in mice. Egypt. J. Basic Appl. Sci. 2018, 5, 110–120. [Google Scholar] [CrossRef]
- Stuart, E.C.; Scandlyn, M.J.; Rosengren, R.J. Role of epigallocatechin gallate (EGCG) in the treatment of breast and prostate cancer. Life Sci. 2006, 79, 2329–2336. [Google Scholar] [CrossRef]
- Rizzi, F.; Naponelli, V.; Silva, A.; Modernelli, A.; Ramazzina, I.; Bonacini, M.; Bettuzzi, S. Polyphenon E®, a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis 2014, 35, 828–839. [Google Scholar] [CrossRef]
- Lee, M.H.; Han, D.W.; Hyon, S.H.; Park, J.C. Apoptosis of human fibrosarcoma HT-1080 cells by epigallocatechin-3-O-gallate via induction of p53 and caspases as well as suppression of Bcl-2 and phosphorylated nuclear factor-κB. Apoptosis 2011, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Nigam, N.; George, J.; Srivastava, S.; Shukla, Y. Induction of apoptosis by tea polyphenols mediated through mitochondrial cell death pathway in mouse skin tumors. Cancer Biol. Ther. 2008, 8, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.M.; Baliga, M.S.; Katiyar, S.K. Epigallocatechin-3-gallate induces apoptosis in estrogen receptor–negative human breast carcinoma cells via modulation in protein expression of p53 and Bax and caspase-3 activation. Mol. Cancer Ther. 2005, 4, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Lamy, S.; Gingras, D.; Béliveau, R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002, 62, 381–385. [Google Scholar]
- Suzuki, Y.; Tsubono, Y.; Nakaya, N.; Koizumi, Y.; Tsuji, I. Green tea and the risk of breast cancer: Pooled analysis of two prospective studies in Japan. Br. J. Cancer 2004, 90, 1361–1363. [Google Scholar] [CrossRef]
- Tao, M.H.; Liu, D.K.; Gao, L.F.; Jin, F. Relationship between tea drinking and breast cancer in women. Tumor 2002, 22, 176–180. [Google Scholar]
- Shrubsole, M.J.; Lu, W.; Chen, Z.; Shu, X.O.; Zheng, Y.; Dai, Q.; Zheng, W. Drinking green tea modestly reduces breast cancer risk. J. Nutr. 2009, 139, 310–316. [Google Scholar] [CrossRef]
- Key, T.J.; Sharp, G.B.; Appleby, P.N.; Beral, V.; Goodman, M.T.; Soda, M.; Mabuchi, K. Soya foods and breast cancer risk: A prospective study in Hiroshima and Nagasaki, Japan. Br. J. Cancer 1999, 81, 1248–1256. [Google Scholar] [CrossRef]
- Nagano, J.; Kono, S.; Preston, D.L.; Mabuchi, K. A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki (Japan). Cancer Causes Control 2001, 12, 501–508. [Google Scholar] [CrossRef]
- Wu, A.H.; Yu, M.C.; Tseng, C.C.; Hankin, J.; Pike, M.C. Green tea and risk of breast cancer in Asian Americans. Int. J. Cancer 2003, 106, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Nakachi, K.; Matsuyama, S.; Miyake, S.; Suganuma, M.; Imai, K. Preventive effects of drinking green tea on cancer and cardiovascular disease: Epidemiological evidence for multiple targeting prevention. Biofactors 2000, 13, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.W.; Makey, K.L.; Tucker, K.B.; Chinchar, E.; Mao, X.; Pei, I.; Miele, L. EGCG, a major green tea catechin suppresses breast tumor angiogenesis and growth via inhibiting the activation of HIF-1α and NFκB, and VEGF expression. Vasc. Cell 2013, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Robien, K.; Wang, R.; Van Den Berg, D.J.; Koh, W.P.; Yu, M.C. Green tea intake, MTHFR/TYMS genotype and breast cancer risk: The Singapore Chinese Health Study. Carcinogenesis 2008, 29, 1967–1972. [Google Scholar] [CrossRef]
- Sameni, M.; Moin, K.; Sloane, B.F. Imaging proteolysis by living human breast cancer cells. Neoplasia 2000, 2, 496–504. [Google Scholar] [CrossRef]
- Badr, M.O.; Edrees, N.M.; Abdallah, A.A.; Hashem, M.A.; El-Deen, N.A.; Neamat-Allah, A.N.; Ismail, H.T. Biochemical and antioxidant effect of doxorubicin hydrochloride and propolis on N-methyl-N-nitrosourea (MNU) induced adenocarcinoma in rats. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2015, 72. [Google Scholar] [CrossRef]
- Shreya, S.; Grosset, C.F.; Jain, B.P. Unfolded protein response signaling in liver disorders: A 2023 updated review. Int. J. Mol. Sci. 2023, 24, 14066. [Google Scholar] [CrossRef]
- Ballesteros-Ramírez, R.; Lasso, P.; Urueña, C.; Saturno, J.; Fiorentino, S. Assessment of acute and chronic toxicity in Wistar rats (Rattus norvegicus) and New Zealand rabbits (Oryctolagus cuniculus) of an enriched polyphenol extract obtained from Caesalpinia spinosa. J. Toxicol. 2024, 2024, 3769933. [Google Scholar] [CrossRef]
- Ratanasrimetha, P.; Workeneh, B.T.; Seethapathy, H. Sodium and potassium dysregulation in the patient with cancer. Adv. Chronic Kidney Dis. 2022, 29, 171–179. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Elko, E.A.; Cunniff, B.; Seward, D.J.; Chia, S.B.; Aboushousha, R.; van de Wetering, C.; Janssen-Heininger, Y.M. Peroxiredoxins and beyond; redox systems regulating lung physiology and disease. Antioxid. Redox Signal 2019, 31, 1070–1091. [Google Scholar] [CrossRef]
- Dumitraş, D.A.; Dreanca, A.I.; Pall, E.; Gal, A.F.; Rus, V.; Morohoschi, A.G.; Andrei, S. Inhibition of tumor growth and modulation of antioxidant activity of rhodoxanthin isolated from Taxus baccata aril against B16F10 murine malignant melanoma. Antioxidants 2022, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Gönenç, A.; Özkan, Y.; Torun, M.; Şimşek, B. Plasma malondialdehyde (MDA) levels in breast and lung cancer patients. J. Clin. Pharm. Ther. 2001, 26, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Dharmajaya, R.; Sari, D.K. Malondialdehyde value as radical oxidative marker and endogenous antioxidant value analysis in brain tumor. Ann. Med. Surg. 2022, 77, 103231. [Google Scholar] [CrossRef] [PubMed]
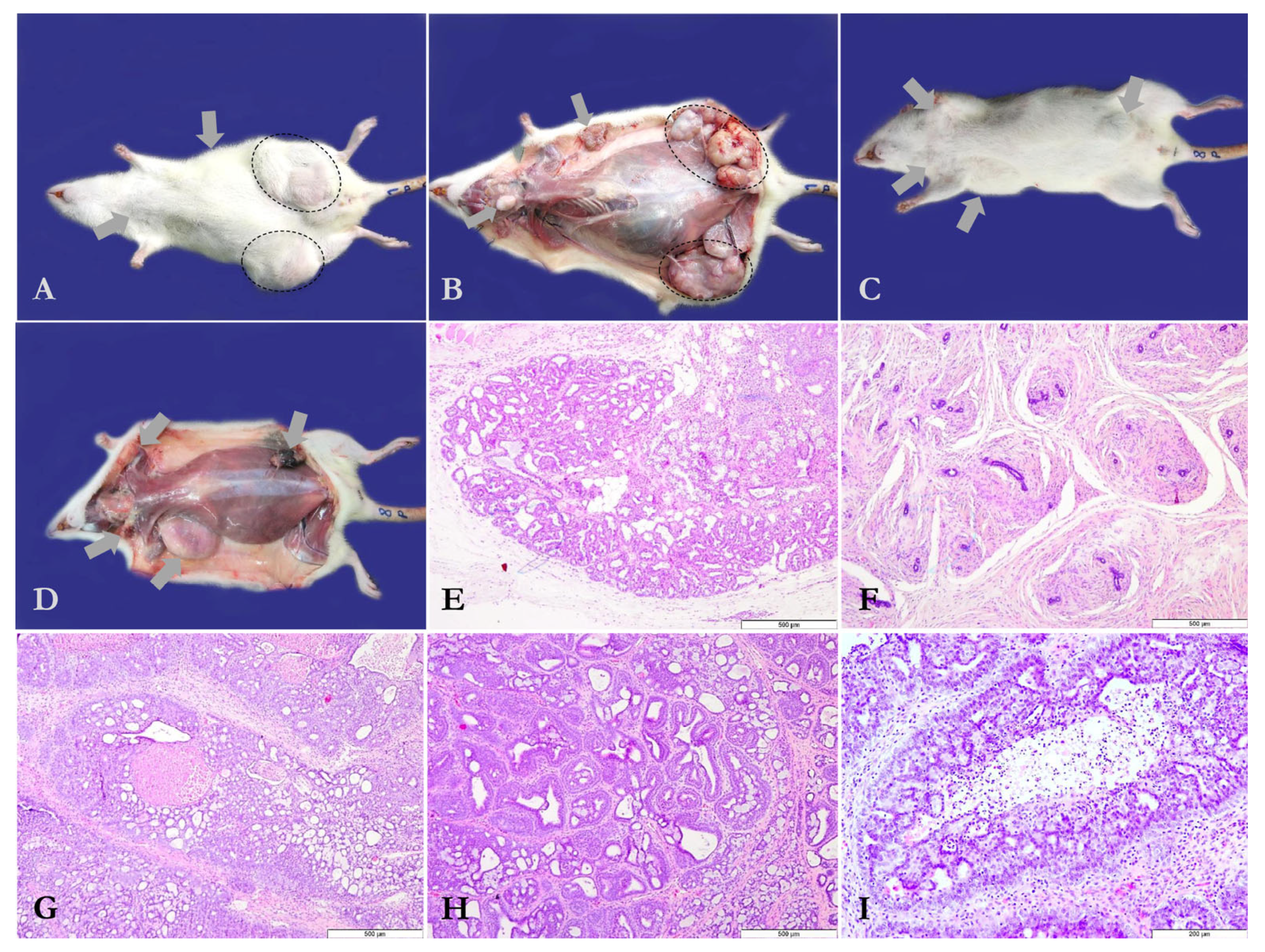

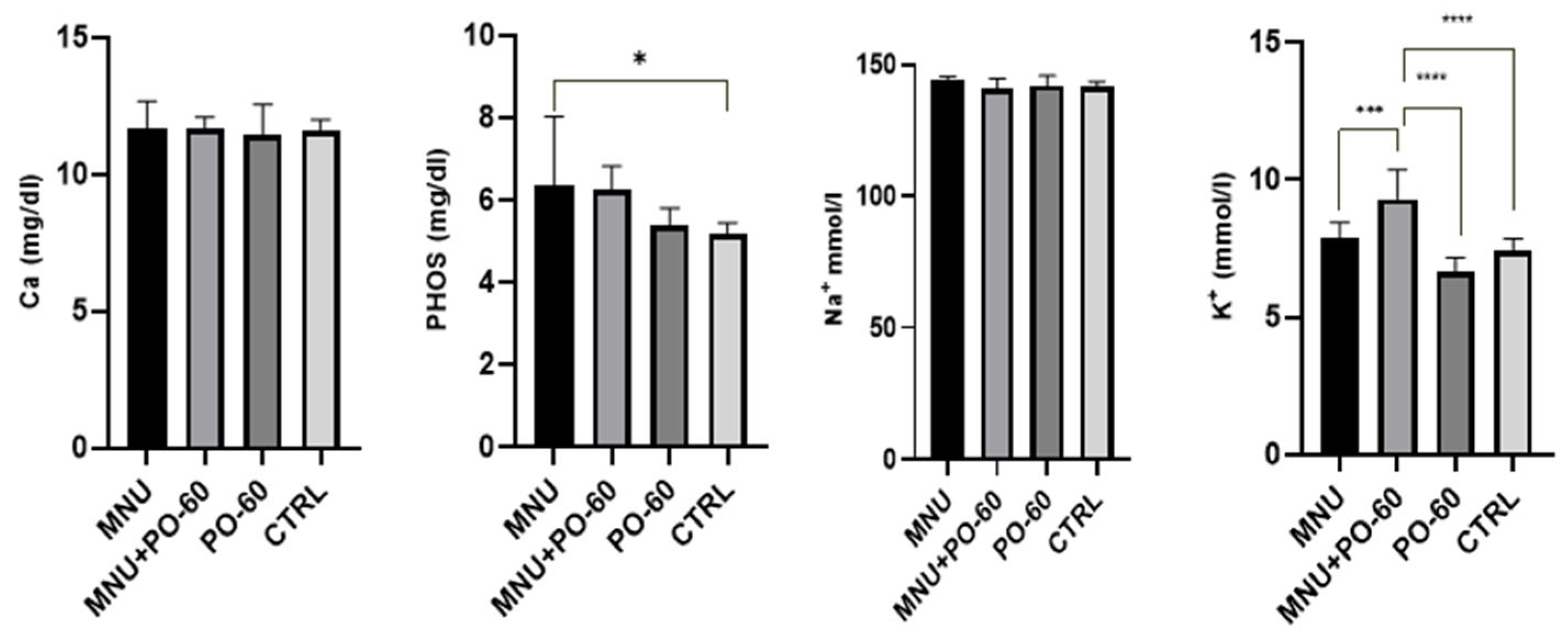
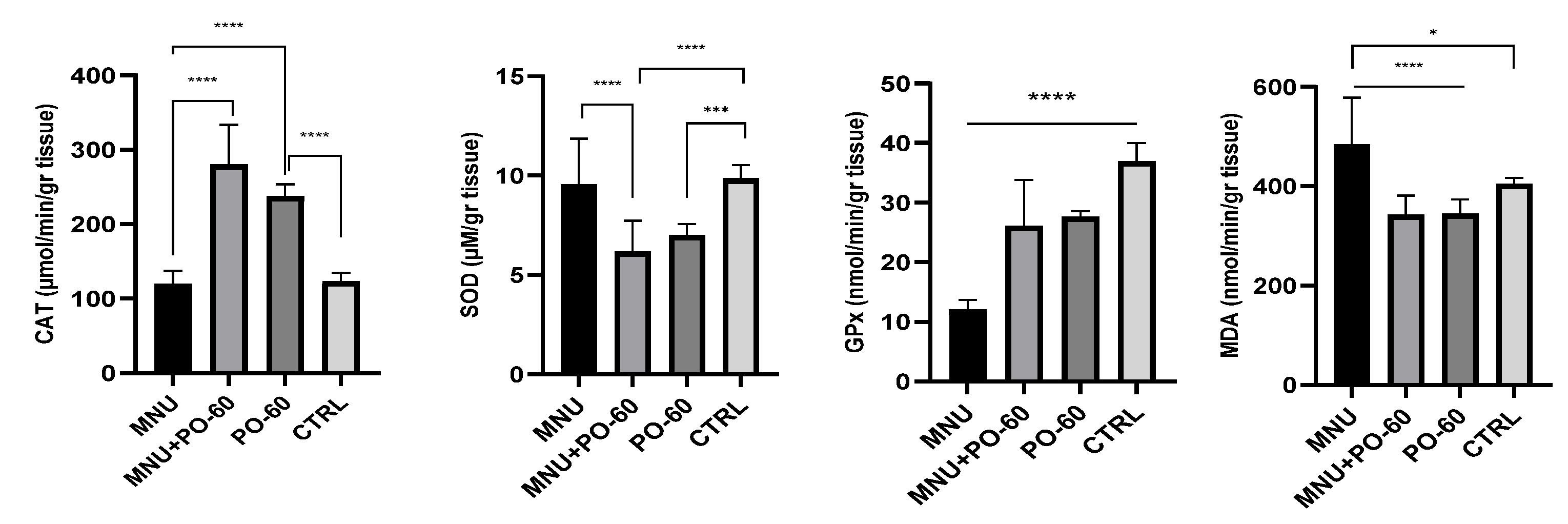
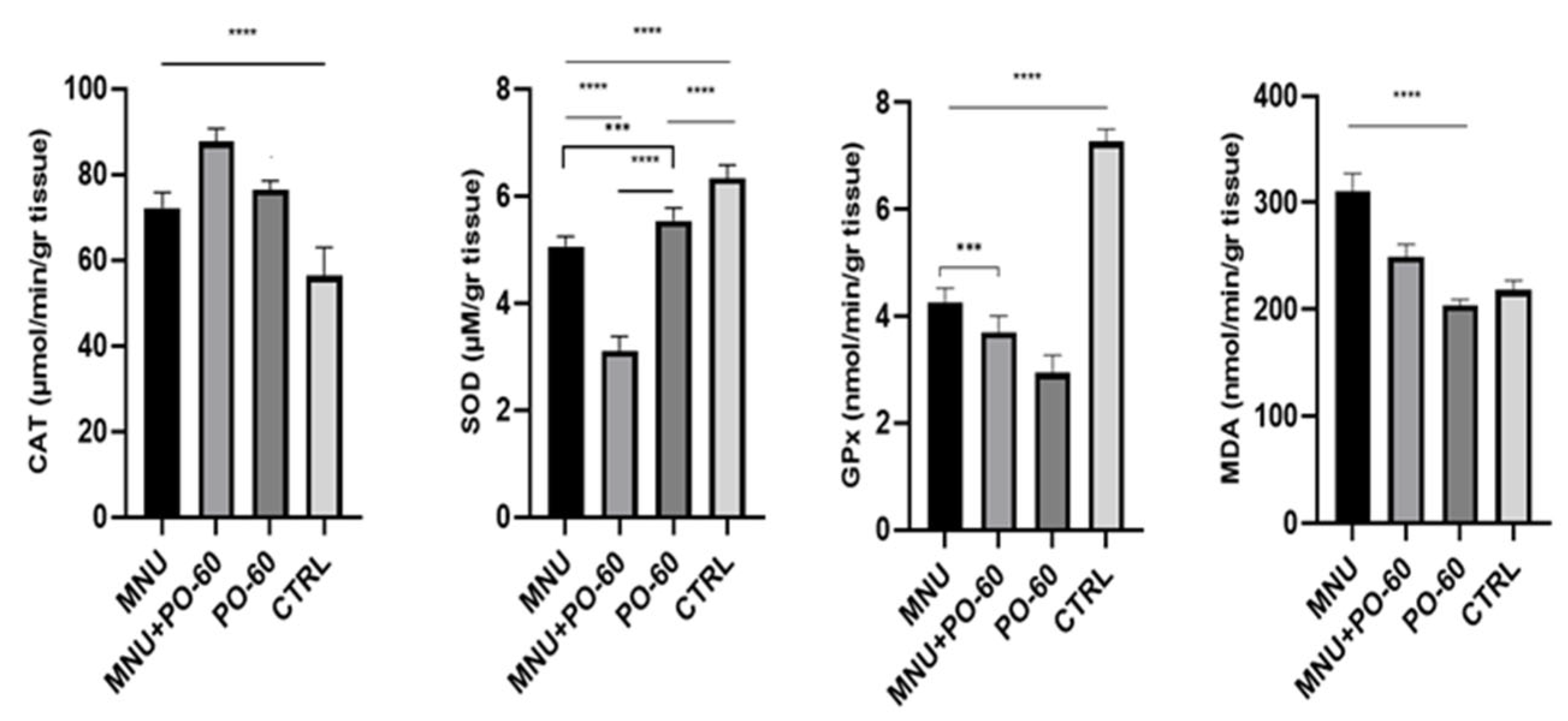
| Experimental Group | MTMp 1 | Average MTM 2 Relative to FBW 3 (%) | Average MTV 4 | TNMT-G 5 | Non-Mammary Tumors/Group | Semi-Quantitative Tumor Scores (Median IQR), Range 0–9 |
|---|---|---|---|---|---|---|
| Group 1 | 3.7 ± 2.7 | 9.0 ± 10.8 | 16.6 ± 33.3 | 37 | 4 | 7 (1–9) |
| Group 2 | 3.1 ± 2.8 ns1 | 6.8 ± 10.5 ns1 | 13.8 ± 44.6 ns1 | 31 ns1 | 5 | 7 (0–9) ns2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gal, A.F.; Rugină, D.; Dumitraș, D.A.; Tabaran, A.-F.; Matei-Lațiu, M.-C.; Andrei, S.M. Limited Chemopreventive Effects of Oral Administration of Polyphenol-60 from Green Tea in the MNU-Induced Rat Mammary Tumor Model. Antioxidants 2025, 14, 1009. https://doi.org/10.3390/antiox14081009
Gal AF, Rugină D, Dumitraș DA, Tabaran A-F, Matei-Lațiu M-C, Andrei SM. Limited Chemopreventive Effects of Oral Administration of Polyphenol-60 from Green Tea in the MNU-Induced Rat Mammary Tumor Model. Antioxidants. 2025; 14(8):1009. https://doi.org/10.3390/antiox14081009
Chicago/Turabian StyleGal, Adrian Florin, Dumitrița Rugină, Daria Antonia Dumitraș, Alexandru-Flaviu Tabaran, Maria-Cătălina Matei-Lațiu, and Sanda Maria Andrei. 2025. "Limited Chemopreventive Effects of Oral Administration of Polyphenol-60 from Green Tea in the MNU-Induced Rat Mammary Tumor Model" Antioxidants 14, no. 8: 1009. https://doi.org/10.3390/antiox14081009
APA StyleGal, A. F., Rugină, D., Dumitraș, D. A., Tabaran, A.-F., Matei-Lațiu, M.-C., & Andrei, S. M. (2025). Limited Chemopreventive Effects of Oral Administration of Polyphenol-60 from Green Tea in the MNU-Induced Rat Mammary Tumor Model. Antioxidants, 14(8), 1009. https://doi.org/10.3390/antiox14081009











