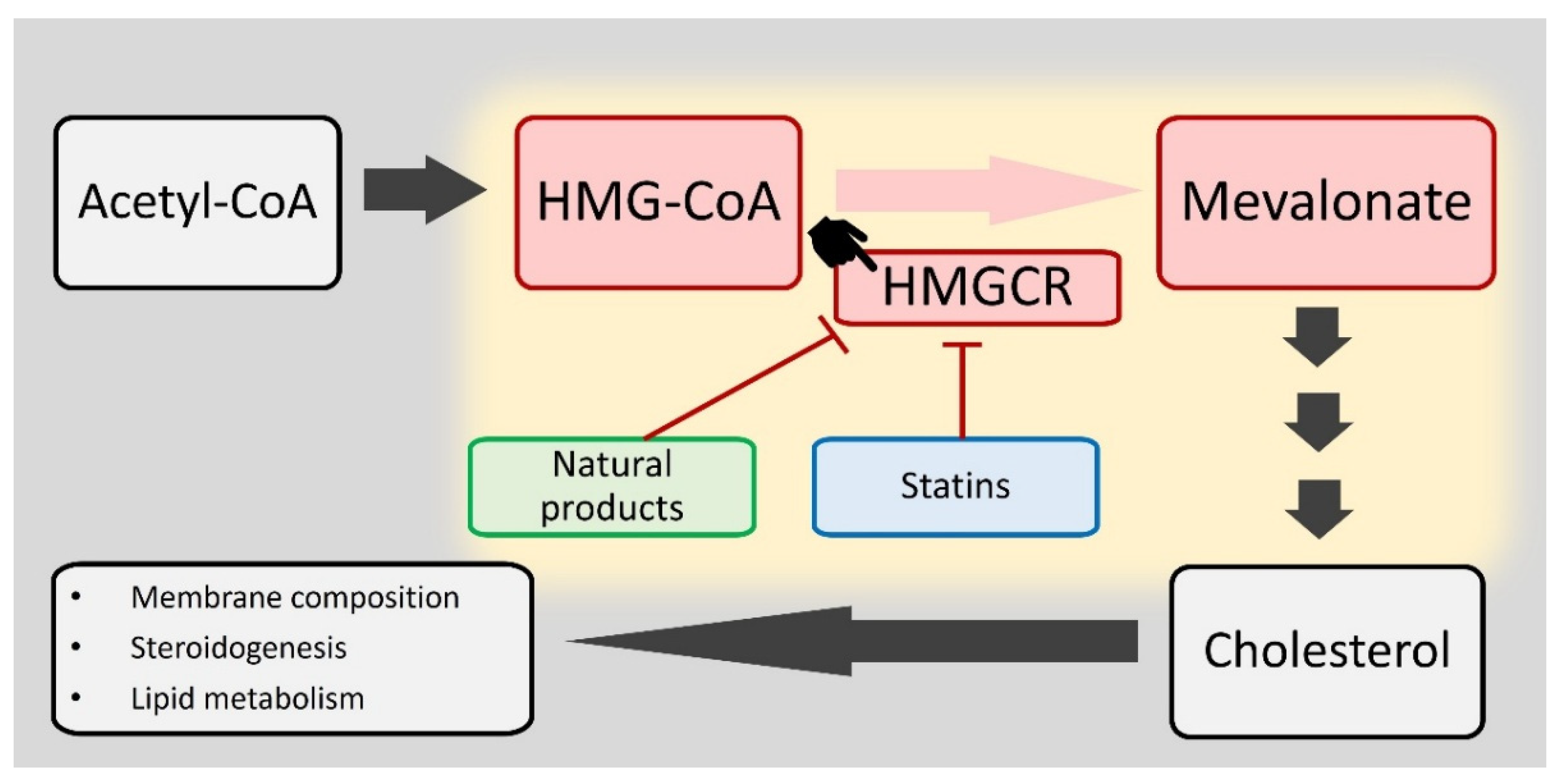
The Potential Therapeutic Applications of Natural Products in the Oxidative Stress-Related MVA Pathway: Focus on HMGCR
Abstract
1. Introduction
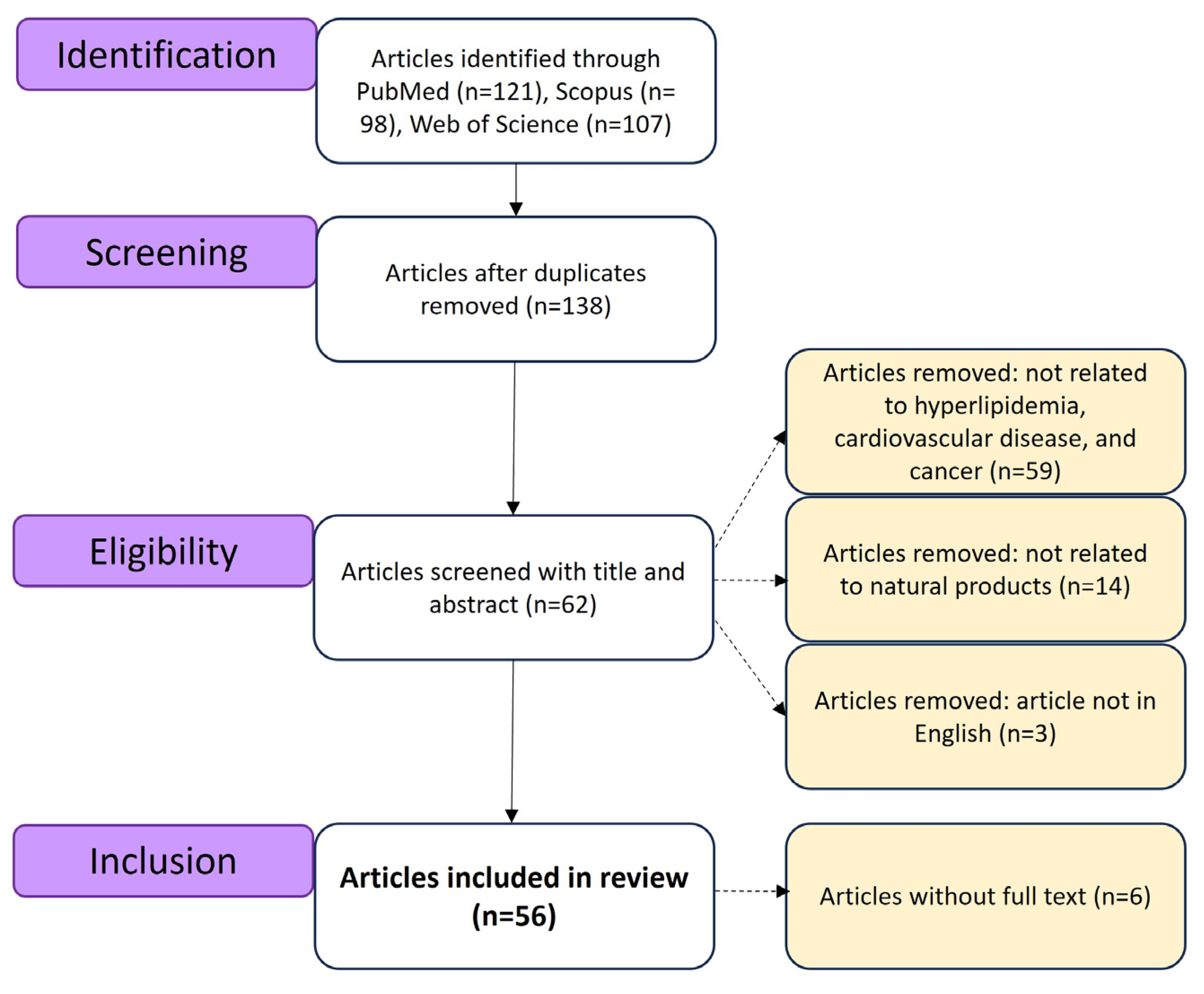
2. Methods
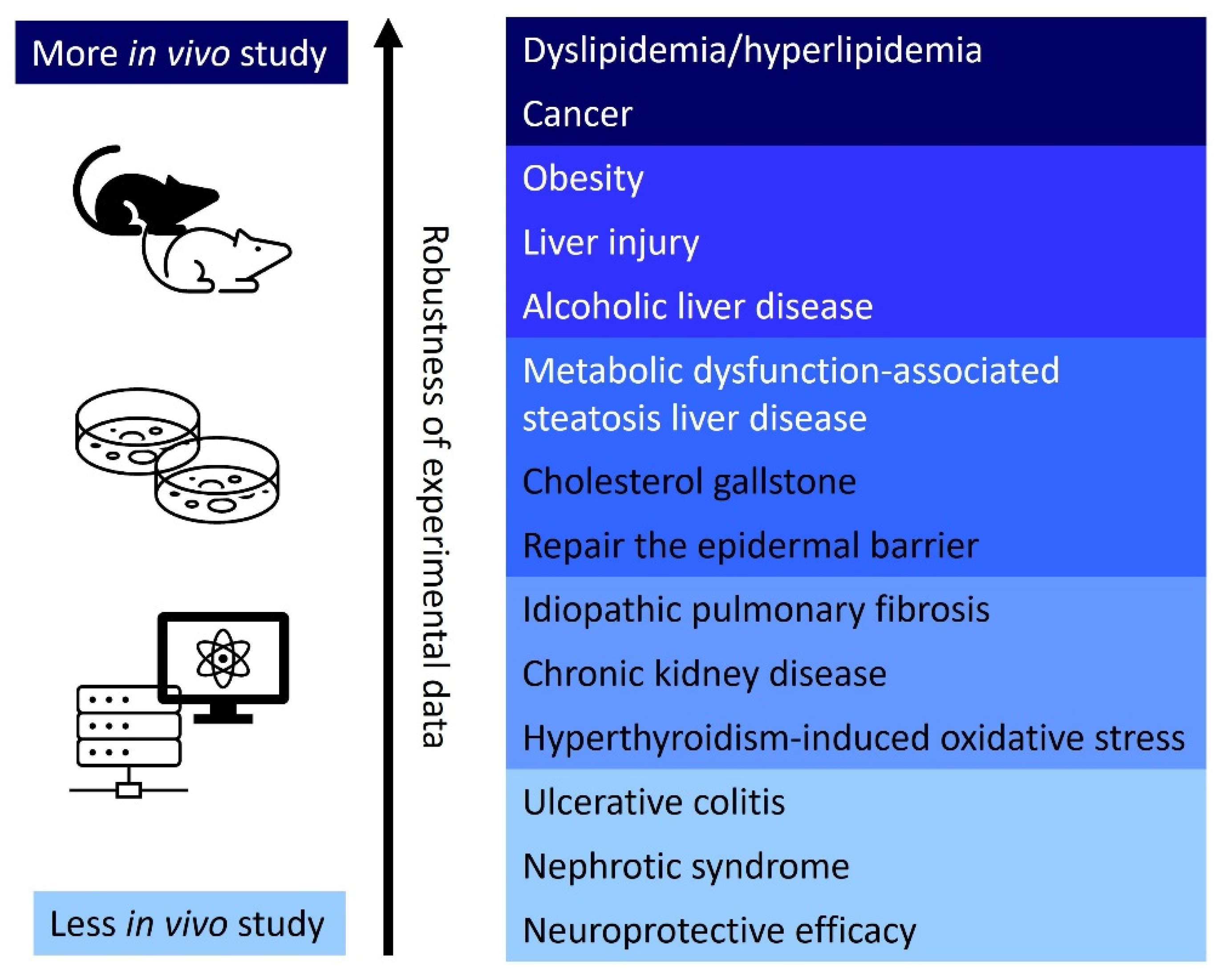
3. Therapeutic Applications
3.1. Dyslipidemia/Hyperlipidemia (Also Summarized in Table 1)
3.1.1. Modulation of HMGCR Expression by Flavonoids and Phenolic Compounds
3.1.2. Impact of Traditional Chinese Medicine (TCM) and Herbal Formulations on HMGCR
3.1.3. Role of Specific Natural Products in HMGCR Regulation
3.1.4. Influence of Food Processing and Fermentation on HMGCR-Related Effects
3.1.5. Modulation of HMGCR Through the AMPK Pathway
3.1.6. Synergistic Effects and Combinations
| Natural Product | Primary Bioactive Components | Dosage, Solvent for Extraction | Model System | HMGCR Effect | Proposed Mechanism | Reference |
|---|---|---|---|---|---|---|
| Coreopsis tinctoria Nutt. Extract | Luteolin, Marein, Naringenin, Chlorogenic Acid | Luteolin: 30 µM, marein: 10 µM, chlorogenic acid: 300 µM, naringenin: 200 µM | HepG2 cells (OA-induced) | Expression Downregulation | SREBP inhibition. | [18] |
| Cocoa Shell Ingredients (CSF/CSE) | Phenolic Compounds, Dietary Fiber | phenolic compounds (CSE: 271.4 mg 100 g−1, CSF: 43.8 mg 100 g−1), flour and aqueous extract | In vitro, hepG2 cells | Activity Inhibition | HMGCR inhibition may be linked to the phenolic compounds and dietary fiber. These compounds could interact with the HMGCR active site, reducing its activity. | [29] |
| Sanhua Jiangzhi Granules (SJG) | Complex Mixture of TCM Constituents | 10 μg/mL, methanol and water in a 4:1 ratio | Rat model (HF Diet) | Expression Downregulation | PPAR signaling pathway activation. | [20] |
| Quercetin (from Buckwheat) | Quercetin | 25, 50, 75, 100, 150, and 200 µg/mL, hexane, ethylacetate, and methanol | HepG2 cells | Expression Downregulation | Not fully elucidated; synergistic with simvastatin. | [23] |
| Forest Onion Extract (FOE) | Multiple—includes peptides | 35, 70, 105, 140, and 175 µg/mL, 96% ethanol | 3T3-L1 preadipocytes | Expression Downregulation | Inhibition of MAPK8, PPARG, HMGCR, CPT-1, and GLP-1 protein expressions. | [19] |
| Lactiplantibacillus plantarum SDJ09 | Cell Extracts, Metabolites, Heat-Inactivated Cells | 30 μg/mL, sterile water | HepG2 cells | Expression Downregulation | Reduction in lipid synthesis, upregulation of cholesterol excretion. | [30] |
| Alpiniae oxyphyllae Fructus (AOF) | Stigmasterol | 0, 1, 2, 4, 8, 16 µM | Cell experiments | N/A (not directly investigated the effect of AOF or its components on HMGCR expression or activity levels) | Upregulated the expression levels of ESR1 and PPARG to exert an anti-HUA effect. | [31] |
| Taxus chinensis var. mairei (AETC) | Active Compound and Osimertinib combination | 0.03 to 2 mg/mL, double-distilled water | Osimertinib-resistant cells and xenograft tumors in nude mice | Expression Downregulation | ERK/SREBP-2/HMGCR-mediated cholesterol biosynthesis and ROS levels. | [28] |
| Theabrownin from Qingzhuan tea (QTB) | Theabrownin extracted from Qingzhuan tea | 180 or 360 mg/kg/d, absolute ethanol, then distilled water | HFD-induced mice | Expression Downregulation | Upregulate the expression of ATGL, PPARα, FFAR2 and FFAR3, and inhibit the expression of LXRα, SREBP-1c, FAS and HMGCR genes. | [32] |
| Sinapic Acid | A Natural Source of Simple Phenolic Acids | 0.03% | HFD-induced obesity hamster | Expression Downregulation | Egulationg the activities of PPAR-γ, CPT-1, and CYP7A1. | [24] |
| DI-HET, hydroethanolic extract from Dillenia indica leaf | Naringenin, Catechin, Epicatechin, Shikimic Acid, Syringic Acid, Vanillic Acid, and Kaempferol | 5, 10, 20, 50, 100, 200, and 400 μg/mL, hydroethanolic extract | In vitro, HepG2 cells | Expression Downregulation | Activation of the SIRT-1/p-LKB-1/AMPK signaling pathway. | [33] |
| Protium heptaphyllum Gum Resin Extract (PHE) | Acidic Tetra- and Pentacyclic Triterpenoids | 10–200 µg mL−1, hydroalcoholic extraction process | Hepatocytes | Expression Downregulation, Activity Inhibition | Reduce cholesterol production and regulate the expression of proteins involved in its metabolism. | [34] |
| Virgin Camellia Seed Oil | Active Compounds in Vegetable Oil | 1.5 g/kg, squeezed using mall-pressed technologies | Sprague Dawley (SD) rats | Expression Downregulation | Modulating the AMPK-SREBP-signaling pathway. | [35] |
3.2. Cardiovascular Diseases (Also Summarized in Table 2)
3.2.1. Targeting HMGCR in Atherosclerosis
3.2.2. Modulation of HMGCR and Lipid Profiles by Natural Products
3.2.3. Novel HMGCR Degraders for Enhanced Statin Therapy
3.2.4. Black Elderberry Extract May Improve HDL Function
3.2.5. Influence of the Intestinal Flora on Atherosclerosis
| Natural Product | Primary Bioactive Components | Dosage, Solvent for Extraction | Model System | HMGCR Effect | Proposed Mechanism | Reference |
|---|---|---|---|---|---|---|
| Tetrahydroxy stilbene glucoside (TSG) | N/A | 100 mg/kg/day | ApoE−/− mice | Expression Downregulation | Restores the expression of fatty acid metabolism-related genes. | [36] |
| Schipenindolene A (Spid A) | Indole diterpenoid | 0.01–20 μM, trace fermented extract | In vitro cell culture | Protein Degradation | ERAD pathway activation. | [39] |
| Arctium lappa leaves | Various (unspecified) | 12.5, 25, 50, 100, 200, and 400 μg/mL, 70% ethanol | Network pharmacology, in vitro and in vivo models | Did not directly measure HMGCR level | AMPK-mediated PPARG/LXRα pathway. | [37] |
| Flaxseed oil | A-linolenic acid ester of PS (ALA-PS) | flaxseed oil: 5% (w/w), ALA-PS: 3.3% (w/w) | ApoE-KO mice | Expression Downregulation | Modulatory effects on the expression levels of genes involved in lipid metabolism, including PPARα, HMGCR, and SREBPs. | [38] |
| Theabrownin from Qingzhuan tea (QTB) | Theabrownin extracted from Qingzhuan tea | 180 or 360 mg/kg/d, absolute ethanol, then distilled water | HFD-induced mice | Expression Downregulation | Possible to act as a prebiotic to prevent MASLD. Reduces the expression of the HMGCR. | [32] |
3.3. Cancer (Also Summarized in Table 3)
3.3.1. Targeting Cholesterol Synthesis in Cancer Therapy
| Natural Product | Dosage, Solvent for Extraction | Cancer Type | HMGCR Effect | Proposed Mechanism | Reference |
|---|---|---|---|---|---|
| Cepharanthine (CE) | 0.1–20 μM | Small Cell Lung Cancer (SCLC) | Expression Downregulation | Inhibition of cholesterol synthesis, direct binding to HMGCR and other enzymes | [43] |
| Taxus chinensis var. mairei (AETC) | 0.03 to 2 mg/mL, double-distilled water | EGFR-mutant NSCLC | Expression Downregulation | Regulation of ERK1/2, inhibition of cholesterol biosynthesis | [28] |
| Gypenoside L | 10 mg/kg or 20 mg/kg, 95% ethanol fraction | Hepatocellular Carcinoma (HCC) | Expression Downregulation | Targeting the SREBP2-HMGCS1 axis, regulating the mevalonate pathway | [44] |
| Carotenoids from Spondias mombin | 100 mg/kg and 200 mg/kg, n-hexane/acetone 1:1 (v/v) | Breast Cancer | Expression Downregulation | Hydrophobic interactions with key residues within the catalytic domain of HMGCR | [45] |
| Chinese Red Yeast Rice (RYR) | 0–300 μg/mL, methylene chloride | Prostate Cancer, Colon Cancer | No Significant Influence on Expression | Mechanisms beyond HMGCR are likely involved (related to pigments) | [46,47] |
3.3.2. Cepharanthine as a Small Cell Lung Cancer Inhibitor via HMGCR Modulation
3.3.3. Taxus chinensis var. mairei Extract (AETC) to Overcome Osimertinib Resistance in Non-Small Cell Lung Cancer (NSCLC)
3.3.4. Gypenoside L as a Hepatocellular Carcinoma Inhibitor via HMGCR Regulation
3.3.5. Carotenoids from Spondias mombin Demonstrate HMGCR Inhibition
3.3.6. Chinese Red Yeast Rice (RYR)
4. Mechanisms of HMGCR Modulation by Natural Products
4.1. HMGCR as a Target
4.2. HMGCR Activity Inhibition
4.3. HMGCR Expression Regulation (Also Summarized in Table 4)
4.3.1. Downregulation of HMGCR Expression
4.3.2. SREBP-2 Modulation
4.3.3. AMPK Activation
4.3.4. Other Mechanisms
| Natural Product/Extract | Effect on HMGCR Expression | Mechanism | Reference |
|---|---|---|---|
| Coreopsis tinctoria Nutt. extracts (luteolin, marein, NGN, CQA) | Downregulation | Reduce OA-induced oxidative stress and lipid accumulation | [18] |
| Aqueous extract of Taxus chinensis var. Mairei | Downregulation | ERK/SREBP-2/HMGCR-mediated cholesterol biosynthesis | [28] |
| Flavonoid-rich extract of Paulownia fortunei Flowers | Decrease | AMPK pathway | [60] |
| GINST (hydrolyzed ginseng extract) | Decrease | AMPKα activation | [59] |
| Sargassum fusiforme polysaccharide (SFP) co-administered with low-dose acarbose | Decrease in the expression of the HMGCR gene | Affecting the expression of HMGCR and SREBP-1c genes, restraining liver fat accumulation | [63] |
| Flaxseed oil (FO), a dietary oil rich in α-linolenic acid | Decreased protein expression | Decreased the protein expression of SREBP2, HMGCR, and LDLR while increasing the expression of CYP7A1 | [57] |
| Annatto-derived tocotrienol | Downregulated HMGCR gene expression | Downregulating HMGCR gene expression and inhibiting RhoA activation, leading to increased BMP-2 protein | [64] |
| Celastrus orbiculatus Thunb. | Upregulated HMGCR mRNA | LDL-R, SR-B1, CYP7A1 | [61] |
| Black raspberry extract | Downregulated Srebf2 and Hmgcr expressions | Upregulate and enhance Cyp7a1 and Abcg5 expressions | [62] |
4.4. Signaling Pathways Involvement (Also Summarized in Table 5)
4.4.1. SREBP Pathway
4.4.2. PPAR Pathway
4.4.3. AMPK Pathway
4.4.4. ERK1/2 Pathway
4.4.5. PI3K-Akt Pathway
4.4.6. mTOR Pathway
| Natural Product or Extract | Signaling Pathway(s) Involved | Effect on HMGCR | Reference |
|---|---|---|---|
| Sanhua Jiangzhi Granules | PPAR | Downregulation | [20] |
| Taxus chinensis var. Mairei Extract | ERK/SREBP-2 | Downregulation | [28] |
| Virgin Camellia Seed Oil | AMPK-SREBP | Downregulation | [35] |
| Unripe Rubus coreanus Extract and Ellagic acid | AMPK, SREBP-2 | Reduce HMGCR Activity | [58] |
| Red Raspberry Extract | PPAR | Downregulation | [65] |
| Flavonoid-rich Extract of Paulownia fortunei Flowers | AMPK | Decrease | [60] |
| Strawberry Methanolic Extract | AMPK | Inhibition | [66] |
| Gandi capsule | AMPK, PI3K-Akt | HMGCR identified as a potential target | [67] |
| Zexie Tang | FKBP38/mTOR/SREBPs | Downregulation | [56] |
4.5. Comparison or Combination with Statins or Other Therapies (Also Summarized in Table 6)
| Natural Inhibitor/Extract | Potency Compared to Statins | Key Mechanisms | Reference |
|---|---|---|---|
| Quercetin | Synergistic with Simvastatin | Reduces HMGCR expression, increases CYP7A1 expression. | [23] |
| Chlorogenic Acid-Enriched Extract from Eucommia ulmoides leaves | Lower IC50 than Simvastatin | Increases ABCA1, CYP7A1, and AMPKα2 mRNA expression, decreases SREBP2. | [71] |
| Curcuma Oil | Comparable to Ezetimibe (at high dose) | Modulates PPARα, LXRα, and associated genes in lipid metabolism and transport. | [72] |
| Forest Onion Extract | N/A | inhibits targeted protein expressions of MAPK8, PPARG, HMGCR, CPT-1, and GLP-1 in vitro in 3T3-L1 mouse cells. | [19] |
| Red Yeast Rice | N/A (In contrast to LV, neither RYR nor PF-RYR significantly altered the expression of HMGCR or SREBP-2) | Exhibit anticancer effects on colon cancer cells. May affect intracellular signaling pathways differently from purified crystallized LV. | [47] |
| Sargassum fusiforme polysaccharide (SFP) | N/A | Restores beneficial gut flora and activates IRS/PI3K/AKT signaling pathway (in combination with low-dose acarbose). Restrained liver fat accumulation via affecting the expression of HMGCR and SREBP-1c genes. | [63] |
5. Challenges and Future Directions
5.1. Research Gaps in Natural HMGCR Modulators
5.2. Challenges in Translating In Vitro Data to Clinical Application
5.3. Clinical Trial Suggestion
5.3.1. Hyperlipidemia
5.3.2. Cardiovascular Disease
5.3.3. Cancer
5.4. Need for Mechanistic Elucidation
5.5. Limitations in Natural Product Development
5.6. Bioavailability, Metabolism, and Safety Considerations
5.7. Potential Interactions with Conventional Medications
5.8. Batch-to-Batch Variability Challenges
5.9. Potential Risks of Natural Products
5.10. Priorities for Future Research
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP-binding cassette transporter A1 |
| ABCG5 | ATP-binding cassette transporter G5 |
| ACAT | Acyl CoA–cholesterol acyltransferase |
| AETC | Aqueous extract of Taxus chinensis var. Mairei |
| ALA-PS | α-linolenic acid ester of plant sterols |
| AMPK | AMP-activated protein kinase |
| AnTT | Annatto-derived tocotrienol |
| AOF | Alpiniae oxyphyllae Fructus |
| AS | Atherosclerosis |
| ATGL | Adipose triglyceride lipase |
| BEE | Black elderberry extract |
| BMP-2 | Bone morphogenetic protein-2 |
| CCL2 | Chemokine (C-C motif) ligand 2 |
| CE | Cepharanthine |
| CI | Combination index |
| CPT-1 | Carnitine palmitoyltransferase 1 |
| CQA | Chlorogenic acid |
| CRP | C-Reactive Protein |
| CSF/CSE | Two cocoa shell ingredients, a flour (CSF) and an aqueous extract (CSE) |
| CVD | Cardiovascular disease |
| CYP7A1 | Cholesterol 7α-hydroxylase |
| DI-HET | Hydroethanolic extract from Dillenia indica leaf |
| ERAD | Endoplasmic reticulum-associated degradation |
| ERK | Extracellular signal regulated kinase |
| ESR1 | Estrogen receptor 1 |
| FAS | Fatty acid synthase |
| FABP | Fatty-acid-binding proteins |
| FAT | Fatty-acid translocase |
| FATP | Fatty acid transport proteins |
| FDFT1 | Farnesyl-Diphosphate Farnesyltransferase 1 |
| FFAR | Free fatty acid receptors |
| FKBP38 | FK506-binding protein 8 |
| FOE | Forest onion extract |
| GINST | A hydrolyzed ginseng extract |
| GLP-1 | Glucagon-like peptide 1 |
| Gyp L | Gypenoside L |
| HCC | Hepatocellular carcinoma |
| HDL | High-density lipoprotein |
| HepG2 | Hepatocellular carcinoma cell line |
| HFD | High-fat diet |
| HMG-CoA | 3-Hydroxy-3-methylglutaryl-CoA |
| HMGCR | HMG-CoA reductase |
| HMGCS1 | 3-Hydroxy-3-methylglutaryl-CoA synthase 1 |
| HSD11B1 | 11β-Hydroxysteroid dehydrogenase 1 |
| HSYA | Hydroxysafflor yellow A |
| HUA | Hyperuricemia |
| IDI1 | Isopentenyl-diphosphate delta-isomerase |
| IRS | Insulin receptor substrate |
| LDL | Low-density lipoprotein |
| LDL-C | Low-density lipoprotein cholesterol |
| LDLR | LDL receptor |
| LV | Lovastatin |
| LXRα | Liver X receptor α |
| MAPK8 | Mitogen-activated protein kinase 8 |
| MASLD | Metabolic dysfunction-associated steatosis liver disease |
| mTOR | Mammalian target of rapamycin |
| MVA | Mevalonate pathway |
| NGN | Naringenin |
| NOS2 | Nitric oxide synthase 2 |
| OA | Oleic acid |
| OS | Overall survival |
| ORR | Objective response rate |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PFS | Progression-free survival |
| PHE | Protium heptaphyllum Gum Resin Extract |
| PI3K-Akt | PI3K (phosphatidylinositol 3-kinase) and Akt (protein kinase B) |
| p-LKB-1 | Phospho-liver kinase B1 |
| PPAR | Peroxisome proliferator-activated receptors |
| PTGS2 | Prostaglandin G/H synthase 2 |
| QTB | Theabrownin from Qingzhuan tea |
| RAD | Radix Angelica dahuricae |
| RCT | Randomized controlled trials |
| ROS | Reactive oxygen species |
| RYR | Red yeast rice |
| SCLC | Small cell lung cancer |
| SFP | Sargassum fusiforme polysaccharide |
| SIRT-1 | Sirtuin 1 |
| SJG | Sanhua Jiangzhi Granules |
| SLXG | Shugan Lidan Xiaoshi Granules |
| Spid A | Schipenindolene A |
| SQLE | Squalene epoxidase |
| SR-B1 | Scavenger receptor class B type 1 |
| SREBP-2 | Sterol regulatory element-binding protein 2 |
| TC | Total cholesterol |
| TCM | Traditional Chinese Medicine |
| TG | Triglyceride |
| TSG | Tetrahydroxy stilbene glucoside |
| UME | Ulmus macrocarpa Hance |
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Neshat, S.; Rezaei, A.; Farid, A.; Sarallah, R.; Javanshir, S.; Ahmadian, S.; Chatrnour, G.; Daneii, P.; Heshmat-Ghahdarijani, K. The tangled web of dyslipidemia and cancer: Is there any association? J. Res. Med. Sci. 2022, 27, 93. [Google Scholar] [CrossRef]
- Vekic, J.; Stromsnes, K.; Mazzalai, S.; Zeljkovic, A.; Rizzo, M.; Gambini, J. Oxidative Stress, Atherogenic Dyslipidemia, and Cardiovascular Risk. Biomedicines 2023, 11, 2897. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Istvan, E.S.; Deisenhofer, J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001, 292, 1160–1164. [Google Scholar] [CrossRef]
- Sever, P.S.; Dahlöf, B.; Poulter, N.R.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.T.; et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): A multicentre randomised controlled trial. Lancet 2003, 361, 1149–1158. [Google Scholar] [CrossRef]
- Sever, P.S.; Poulter, N.R.; Dahlof, B.; Wedel, H.; Beevers, G.; Caulfield, M.; Collins, R.; Kjeldsen, S.E.; Kristinsson, A.; McInnes, G.; et al. The Anglo-Scandinavian Cardiac Outcomes Trial lipid lowering arm: Extended observations 2 years after trial closure. Eur. Heart J. 2008, 29, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef]
- Gomez Sandoval, Y.H.; Braganza, M.V.; Daskalopoulou, S.S. Statin discontinuation in high-risk patients: A systematic review of the evidence. Curr. Pharm. Des. 2011, 17, 3669–3689. [Google Scholar] [CrossRef]
- Chaudhuri, A. Frontiers in Lipid Lowering Therapy: To Statins and Beyond. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 798–799. [Google Scholar] [CrossRef]
- Mulder, K.C.; Mulinari, F.; Franco, O.L.; Soares, M.S.; Magalhães, B.S.; Parachin, N.S. Lovastatin production: From molecular basis to industrial process optimization. Biotechnol. Adv. 2015, 33, 648–665. [Google Scholar] [CrossRef]
- Alberts, A.W.; Chen, J.; Kuron, G.; Hunt, V.; Huff, J.; Hoffman, C.; Rothrock, J.; Lopez, M.; Joshua, H.; Harris, E.; et al. Mevinolin: A highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc. Natl. Acad. Sci. USA 1980, 77, 3957–3961. [Google Scholar] [CrossRef] [PubMed]
- Adel Mehraban, M.S.; Tabatabaei-Malazy, O.; Rahimi, R.; Daniali, M.; Khashayar, P.; Larijani, B. Targeting dyslipidemia by herbal medicines: A systematic review of meta-analyses. J. Ethnopharmacol. 2021, 280, 114407. [Google Scholar] [CrossRef]
- Hanis, N.; Ismail, N.A.; Ali, E.Z. Systematic review on effectiveness of flavonoids against hypercholesterolemia: Insights from in-silico, in-vitro, and in-vivo studies. Food Chem. Adv. 2025, 7, 100981. [Google Scholar] [CrossRef]
- Cheung, B.; Sikand, G.; Dineen, E.H.; Malik, S.; Barseghian El-Farra, A. Lipid-Lowering Nutraceuticals for an Integrative Approach to Dyslipidemia. J. Clin. Med. 2023, 12, 3414. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Zhao, L.S.; Liu, R.F.; Kang, Y.T.; Chen, Y.Y.; Xiao, Y.C.; Zhang, L.L.; Cheng, X.R. Effects of Four Main Active Flavonoids of Coreopsis tinctoria Nutt. on Oleic Acid-Induced Lipid Metabolism and Oxidative Stress in HepG2 Cells. Discov. Med. 2025, 37, 372–382. [Google Scholar] [CrossRef]
- Permatasari, H.K.; Abshori, N.F.; Syahputra, R.A.; Harahap, U.; Amalia, N.; Kumalawati, D.A.; Mayulu, N.; Taslim, N.A.; Tallei, T.E.; Tjandrawinata, R.R.; et al. Novel Functional Food Properties of Forest Onion (Eleutherine bulbosa Merr.) Phytochemicals for Treating Metabolic Syndrome: New Insights from a Combined Computational and In Vitro Approach. Nutrients 2024, 16, 1441. [Google Scholar] [CrossRef]
- Wei, J.; Lv, Q.; Luan, F.; Zhang, X.; Guo, D.; Zhai, B.; Chen, S.; Zou, J.; Shi, Y. Exploration of potential mechanism of Sanhua Jiangzhi granules for the treatment of hyperlipidemia based on network pharmacology and experimental verification. Fitoterapia 2024, 179, 106271. [Google Scholar] [CrossRef]
- Han, H.J.; Song, X.; Yadav, D.; Hwang, M.S.; Lee, J.H.; Lee, C.H.; Kim, T.H.; Lee, J.J.; Kwon, J. Ulmus macrocarpa Hance modulates lipid metabolism in hyperlipidemia via activation of AMPK pathway. PLoS ONE 2019, 14, e0217112. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Tang, Z.; Song, Z.; Cao, F.; Shi, X.; Xie, P.; Wei, P.; Li, M. Radix Angelica dahuricae extract ameliorates oestrogen deficiency-induced dyslipidaemia in ovariectomized (OVX) rats by modulating the gut microbiota and bile acid signalling. Phytomedicine 2022, 107, 154440. [Google Scholar] [CrossRef]
- Bhat, S.; Majeed, Y.; Yatoo, G.N.; Hassan, S.; Khan, T.; Sofi, P.A.; Ganai, B.A.; Fazili, K.M.; Zargar, S.M. Unravelling effects of phytochemicals from buckwheat on cholesterol metabolism and lipid accumulation in HepG2 cells and its validation through gene expression analysis. Mol. Biol. Rep. 2024, 51, 759. [Google Scholar] [CrossRef]
- Wang, K.; Liang, C.; Cao, W.; Luo, G.; Zhong, S.; Zeng, Z.; Dai, L.; Song, J.L. Dietary sinapic acid attenuated high-fat diet-induced lipid metabolism and oxidative stress in male Syrian hamsters. J. Food Biochem. 2022, 46, e14203. [Google Scholar] [CrossRef]
- Lupo, M.G.; Macchi, C.; Marchianò, S.; Cristofani, R.; Greco, M.F.; Dall’Acqua, S.; Chen, H.; Sirtori, C.R.; Corsini, A.; Ruscica, M.; et al. Differential effects of red yeast rice, Berberis aristata and Morus alba extracts on PCSK9 and LDL uptake. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Guo, M.; Xie, J.B.; Xiao, M.Y.; Qi, Y.S.; Duan, Y.; Li, F.F.; Piao, X.L. Effects of heat-processed Gynostemma pentaphyllum on high-fat diet-fed mice of obesity and functional analysis on network pharmacology and molecular docking strategy. J. Ethnopharmacol. 2022, 294, 115335. [Google Scholar] [CrossRef]
- Lee, M.S.; Kim, J.S.; Cho, S.M.; Lee, S.O.; Kim, S.H.; Lee, H.J. Fermented Rhus verniciflua Stokes Extract Exerts an Antihepatic Lipogenic Effect in Oleic-Acid-Induced HepG2 Cells via Upregulation of AMP-Activated Protein Kinase. J. Agric. Food Chem. 2015, 63, 7270–7276. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, G.C.; Xiang, Y.; Liu, Y.; Wang, H.; Zhao, F.; Shu, Q. Taxus chinensis var. mairei (Lemée et Lévl) Cheng et L.K. Fu overcomes the resistance to osimertinib in EGFR-mutant non-small-cell lung cancer via suppression of ERK1/2-related cholesterol biosynthesis. J. Ethnopharmacol. 2024, 334, 118586. [Google Scholar] [CrossRef]
- Braojos, C.; Rebollo-Hernanz, M.; Cañas, S.; Aguilera, Y.; Gil-Ramírez, A.; Benítez, V.; Martín-Cabrejas, M.A. Cocoa shell ingredients improve their lipid-lowering properties under simulated digestion: In vitro and HepG2 cells study. Food Res. Int. 2024, 196, 115037. [Google Scholar] [CrossRef]
- Yang, B.; Wang, W.; Jian, C.; Lv, B.; He, H.; Wang, M.; Li, S.; Guo, Y. Screening of the Lipid-Lowering Probiotic Lactiplantibacillus Plantarum SDJ09 and its Anti-Obesity Mechanism. Appl. Biochem. Biotechnol. 2025, 197, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Zhang, R.; Gao, J.; Wu, M.; Wang, J.; Sheng, J.; Sun, P. The Potential Mechanism of Alpiniae oxyphyllae Fructus Against Hyperuricemia: An Integration of Network Pharmacology, Molecular Docking, Molecular Dynamics Simulation, and In Vitro Experiments. Nutrients 2024, 17, 71. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, C.; Lei, Y.; Ye, D.; Wang, L.; Xiong, F.; Wu, H.; He, Q.; Zhou, H.; Li, L.; et al. Theabrownin from Qingzhuan tea prevents high-fat diet-induced MASLD via regulating intestinal microbiota. Biomed. Pharmacother. 2024, 174, 116582. [Google Scholar] [CrossRef]
- Poornima, M.S.; Sindhu, G.; Billu, A.; Sruthi, C.R.; Nisha, P.; Gogoi, P.; Baishya, G.; Raghu, K.G. Pretreatment of hydroethanolic extract of Dillenia indica L. attenuates oleic acid induced NAFLD in HepG2 cells via modulating SIRT-1/p-LKB-1/AMPK, HMGCR & PPAR-α signaling pathways. J. Ethnopharmacol. 2022, 292, 115237. [Google Scholar] [CrossRef]
- Mannino, G.; Iovino, P.; Lauria, A.; Genova, T.; Asteggiano, A.; Notarbartolo, M.; Porcu, A.; Serio, G.; Chinigò, G.; Occhipinti, A.; et al. Bioactive Triterpenes of Protium heptaphyllum Gum Resin Extract Display Cholesterol-Lowering Potential. Int. J. Mol. Sci. 2021, 22, 2664. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, G.; Ma, L.; Chen, B.; Zhang, D.; Gao, J.; Deng, S.; Chen, Y. Virgin Camellia Seed Oil Improves Glycolipid Metabolism in the Kidney of High Fat-Fed Rats through AMPK-SREBP Pathway. Nutrients 2023, 15, 4888. [Google Scholar] [CrossRef]
- Li, M.; Meng, Y.; Hong, X.; Chai, H.; Huang, J.; Wang, F.; Zhang, W.; Wang, J.; Liu, Q.; Xu, Y. Anti-atherosclerotic effect of tetrahydroxy stilbene glucoside via dual-targeting of hepatic lipid metabolisms and aortic M2 macrophage polarization in ApoE(-/-) mice. J. Pharm. Biomed. Anal. 2024, 248, 116338. [Google Scholar] [CrossRef]
- Wang, M.; Cui, B.; Gong, M.; Liu, Q.; Zhuo, X.; Lv, J.; Yang, L.; Liu, X.; Wang, Z.; Dai, L. Arctium lappa leaves based on network pharmacology and experimental validation attenuate atherosclerosis by targeting the AMPK-mediated PPARG/LXRα pathway. Biomed. Pharmacother. 2022, 153, 113503. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Yan, P.; Chen, L.; Luo, C.; Gao, H.; Deng, Q.; Zheng, M.; Shi, Y.; Liu, L. Flaxseed Oil Containing α -Linolenic Acid Ester of Plant Sterol Improved Atherosclerosis in ApoE Deficient Mice. Oxid. Med. Cell Longev. 2015, 2015, 958217. [Google Scholar] [CrossRef]
- Su, X.Z.; Zhang, L.F.; Hu, K.; An, Y.; Zhang, Q.P.; Tang, J.W.; Yan, B.C.; Li, X.R.; Cai, J.; Li, X.N.; et al. Discovery of Natural Potent HMG-CoA Reductase Degraders for Lowering Cholesterol. Angew. Chem. Int. Ed. Engl. 2024, 63, e202313859. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Chiu, C.C.; Hung, S.W.; Liu, J.Y.; Wang, Y.C.; Lv, Q.; Hsu, C.C.; Huang, Y.W.; Huang, W.C.; Chuang, H.L.; et al. Effects of plant- and animal-based high-fat diets on lipid storage and distribution in environmental bacteria-colonized gnotobiotic mice. Biochem. Biophys. Res. Commun. 2017, 493, 1075–1081. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, L.; Zhao, M.; Ai, Y.; Liao, W.; Wan, L.; Liu, Q.; Li, S.; Zeng, J.; Ma, X.; et al. Targeting lipid metabolism with natural products: A novel strategy for gastrointestinal cancer therapy. Phytother. Res. 2023, 37, 2036–2050. [Google Scholar] [CrossRef]
- Zhao, F.; Ding, Z.; Chen, M.; Ji, M.; Li, F. Cepharanthine as an effective small cell lung cancer inhibitor: Integrated insights from network pharmacology, RNA sequencing, and experimental validation. Front. Pharmacol. 2024, 15, 1517386. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.Y.; Pei, W.J.; Li, S.; Li, F.F.; Xie, P.; Luo, H.T.; Hyun Yoo, H.; Piao, X.L. Gypenoside L inhibits hepatocellular carcinoma by targeting the SREBP2-HMGCS1 axis and enhancing immune response. Bioorg. Chem. 2024, 150, 107539. [Google Scholar] [CrossRef] [PubMed]
- Metibemu, D.S.; Akinloye, O.A.; Akamo, A.J.; Okoye, J.O.; Omotuyi, I.O. In-silico HMG-CoA reductase-inhibitory and in-vivo anti-lipidaemic/anticancer effects of carotenoids from Spondias mombin. J. Pharm. Pharmacol. 2021, 73, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Seeram, N.P.; Zhang, Y.; Heber, D. Chinese red yeast rice versus lovastatin effects on prostate cancer cells with and without androgen receptor overexpression. J. Med. Food 2008, 11, 657–666. [Google Scholar] [CrossRef]
- Hong, M.Y.; Seeram, N.P.; Zhang, Y.; Heber, D. Anticancer effects of Chinese red yeast rice versus monacolin K alone on colon cancer cells. J. Nutr. Biochem. 2008, 19, 448–458. [Google Scholar] [CrossRef]
- Sangande, F.; Agustini, K.; Budipramana, K. Antihyperlipidemic mechanisms of a formula containing Curcuma xanthorrhiza, Sechium edule, and Syzigium polyanthum: In silico and in vitro studies. Comput. Biol. Chem. 2023, 105, 107907. [Google Scholar] [CrossRef]
- Yu, H.C.; Bai, Q.R.; Guo, J.J.; Chen, M.Y.; Wang, L.; Tong, F.C.; Zhang, S.L.; Wu, J. Elucidating hydroxysafflor yellow A’s multi-target mechanisms against alcoholic liver disease through integrative pharmacology. Phytomedicine 2024, 134, 155956. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhou, T.; Chen, Z.; Wang, W.; Liu, B.; Li, Y. Investigating the potential mechanism and therapeutic effects of SLXG for cholesterol gallstone treatment. Phytomedicine 2024, 132, 155886. [Google Scholar] [CrossRef]
- Chen, N.; Chu, Y.; Su, S.; Zhang, Q.; Zhang, L. Network Pharmacology and Molecular Docking Validation to Explore the Pharmacological Mechanism of Zhuling Decoction against Nephrotic Syndrome. Curr. Pharm. Des. 2024, 30, 2244–2256. [Google Scholar] [CrossRef]
- Sakarwal, A.; Sen, K.; Ram, H.; Chowdhury, S.; Kashyap, P.; Shukla, S.D.; Panwar, A. Neuroprotective Efficacy of Phytoconstituents of Methanolic Shoots Extract of Calligonum polygonoides L. in Hypercholesterolemia-associated Neurodegenerations. Endocr. Metab. Immune Disord. Drug Targets 2025, 25, 152–172. [Google Scholar] [CrossRef]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle(®) Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef]
- Navarro-González, I.; Pérez-Sánchez, H.; Martín-Pozuelo, G.; García-Alonso, J.; Periago, M.J. The inhibitory effects of bioactive compounds of tomato juice binding to hepatic HMGCR: In vivo study and molecular modelling. PLoS ONE 2014, 9, e83968. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Shafie, N.H.; Mohd Esa, N.; Shafie, S.R.; Bahari, H.; Abdullah, M.A. Mikania micrantha Extract Inhibits HMG-CoA Reductase and ACAT2 and Ameliorates Hypercholesterolemia and Lipid Peroxidation in High Cholesterol-Fed Rats. Nutrients 2020, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, E.W.; Gao, G.; Du, Y.; Wang, M.; Wang, H.; Wang, P.; Qiao, Y.; Su, Y.; Xu, J.; et al. Zexie Tang targeting FKBP38/mTOR/SREBPs pathway improves hyperlipidemia. J. Ethnopharmacol. 2022, 290, 115101. [Google Scholar] [CrossRef]
- Ren, J.; Zuo, J.; Yin, B.; Huang, D.; Wen, R.; Pei, H.; Liu, J.; Zhang, Y.; Zhu, S.; Zhen, S.; et al. Flaxseed Oil Alleviates PFOS-Induced Liver Injury by Regulating Hepatic Cholesterol Metabolism. J. Agric. Food Chem. 2024, 72, 23465–23477. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, E.S.; Jang, G.; Na, J.R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Regulates AMPK, SREBP-2, HMGCR, and INSIG-1 Signaling and Cholesterol Metabolism In Vitro and In Vivo. Nutrients 2020, 12, 610. [Google Scholar] [CrossRef]
- Han, J.S.; Sung, J.H.; Lee, S.K. Inhibition of Cholesterol Synthesis in HepG2 Cells by GINST-Decreasing HMG-CoA Reductase Expression Via AMP-Activated Protein Kinase. J. Food Sci. 2017, 82, 2700–2705. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Sun, J.; Cheng, C.; Feng, Z.; Jiang, H.; Yang, W. Flavonoid-Rich Extract of Paulownia fortunei Flowers Attenuates Diet-Induced Hyperlipidemia, Hepatic Steatosis and Insulin Resistance in Obesity Mice by AMPK Pathway. Nutrients 2017, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Si, Y.; Yao, S.; Yang, N.; Song, G.; Sang, H.; Zu, D.; Xu, X.; Wang, J.; Qin, S. Celastrus orbiculatus Thunb. decreases athero-susceptibility in lipoproteins and the aorta of guinea pigs fed high fat diet. Lipids 2013, 48, 619–631. [Google Scholar] [CrossRef]
- Lim, T.; Lee, K.; Kim, R.H.; Cha, K.H.; Koo, S.Y.; Moon, E.C.; Hwang, K.T. Black raspberry extract can lower serum LDL cholesterol via modulation of gut microbial composition and serum bile acid profile in rats fed trimethylamine-N-oxide with a high-fat diet. Food Sci. Biotechnol. 2022, 31, 1041–1051. [Google Scholar] [CrossRef]
- Li, Z.R.; Jia, R.B.; Wu, J.; Lin, L.; Ou, Z.R.; Liao, B.; Zhang, L.; Zhang, X.; Song, G.; Zhao, M. Sargassum fusiforme polysaccharide partly replaces acarbose against type 2 diabetes in rats. Int. J. Biol. Macromol. 2021, 170, 447–458. [Google Scholar] [CrossRef]
- Wan Hasan, W.N.; Chin, K.Y.; Abd Ghafar, N.; Soelaiman, I.N. Annatto-Derived Tocotrienol Promotes Mineralization of MC3T3-E1 Cells by Enhancing BMP-2 Protein Expression via Inhibiting RhoA Activation and HMG-CoA Reductase Gene Expression. Drug Des. Devel Ther. 2020, 14, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Sun, H.; Tang, M.; Zhao, J.; Zhang, Z.; Sun, X.; He, S. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019, 133, 110796. [Google Scholar] [CrossRef] [PubMed]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid Accumulation in HepG2 Cells Is Attenuated by Strawberry Extract through AMPK Activation. Nutrients 2017, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ye, Q.; Huang, X.; Xu, A.; Liu, Y.; Qi, J.; Zhang, H.; Zhang, J. Revealing active components, action targets and molecular mechanism of Gandi capsule for treating diabetic nephropathy based on network pharmacology strategy. BMC Complement. Med. Ther. 2020, 20, 362. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug synergism: Its detection and applications. J. Pharmacol. Exp. Ther. 2001, 298, 865–872. [Google Scholar] [CrossRef]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [CrossRef]
- Hao, S.; Xiao, Y.; Lin, Y.; Mo, Z.; Chen, Y.; Peng, X.; Xiang, C.; Li, Y.; Li, W. Chlorogenic acid-enriched extract from Eucommia ulmoides leaves inhibits hepatic lipid accumulation through regulation of cholesterol metabolism in HepG2 cells. Pharm. Biol. 2016, 54, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Jain, M.; Misra, A.; Khanna, V.; Rana, M.; Prakash, P.; Malasoni, R.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M.K. Curcuma oil ameliorates hyperlipidaemia and associated deleterious effects in golden Syrian hamsters. Br. J. Nutr. 2013, 110, 437–446. [Google Scholar] [CrossRef]
- Lalu, M.M.; Montroy, J.; Begley, C.G.; Bubela, T.; Hunniford, V.; Ripsman, D.; Wesch, N.; Kimmelman, J.; Macleod, M.; Moher, D.; et al. Identifying and understanding factors that affect the translation of therapies from the laboratory to patients: A study protocol. F1000Research 2020, 9, 485. [Google Scholar] [CrossRef]
- Contopoulos-Ioannidis, D.G.; Ntzani, E.; Ioannidis, J.P. Translation of highly promising basic science research into clinical applications. Am. J. Med. 2003, 114, 477–484. [Google Scholar] [CrossRef]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef]
- Lorian, V. Differences between in vitro and in vivo studies. Antimicrob. Agents Chemother. 1988, 32, 1600–1601. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic Effects of Plant Derivatives and Conventional Chemotherapeutic Agents: An Update on the Cancer Perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Aspects Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Williamson, E.M. Synergy and other interactions in phytomedicines. Phytomedicine 2001, 8, 401–409. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef]
- Yusuf, S.; Collins, R.; Peto, R. Why do we need some large, simple randomized trials? Statist. Med. 1984, 3, 409–420. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Guedes, L.; Reis, P.; Machuqueiro, M.; Ressaissi, A.; Pacheco, R.; Serralheiro, M.L. Bioactivities of Centaurium erythraea (Gentianaceae) Decoctions: Antioxidant Activity, Enzyme Inhibition and Docking Studies. Molecules 2019, 24, 3795. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Butler, M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004, 67, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.; Aprotosoaie, A.C.; Trifan, A.; Xiao, J. Flavonoids as modulators of metabolic enzymes and drug transporters. Ann. N. Y. Acad. Sci. 2017, 1398, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Eggertsen, R.; Andreasson, A.; Andrén, L. Effects of treatment with a commercially available St John’s Wort product (Movina) on cholesterol levels in patients with hypercholesterolemia treated with simvastatin. Scand. J. Prim. Health Care 2007, 25, 154–159. [Google Scholar] [CrossRef]
- Lilja, J.J.; Kivistö, K.T.; Neuvonen, P.J. Grapefruit juice-simvastatin interaction: Effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin. Pharmacol. Ther. 1998, 64, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Gurley, B.J. Clinically Relevant Herb–Drug Interactions: A 30-Year Historical Assessment. J. Diet. Suppl. 2025, 22, 78–104. [Google Scholar] [CrossRef]
- Klein-Junior, L.C.; de Souza, M.R.; Viaene, J.; Bresolin, T.M.B.; de Gasper, A.L.; Henriques, A.T.; Heyden, Y.V. Quality Control of Herbal Medicines: From Traditional Techniques to State-of-the-art Approaches. Planta Med. 2021, 87, 964–988. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Halbert, S.C.; French, B.; Gordon, R.Y.; Farrar, J.T.; Schmitz, K.; Morris, P.B.; Thompson, P.D.; Rader, D.J.; Becker, D.J. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am. J. Cardiol. 2010, 105, 198–204. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, Y.-N. The Potential Therapeutic Applications of Natural Products in the Oxidative Stress-Related MVA Pathway: Focus on HMGCR. Antioxidants 2025, 14, 1001. https://doi.org/10.3390/antiox14081001
Teng Y-N. The Potential Therapeutic Applications of Natural Products in the Oxidative Stress-Related MVA Pathway: Focus on HMGCR. Antioxidants. 2025; 14(8):1001. https://doi.org/10.3390/antiox14081001
Chicago/Turabian StyleTeng, Yu-Ning. 2025. "The Potential Therapeutic Applications of Natural Products in the Oxidative Stress-Related MVA Pathway: Focus on HMGCR" Antioxidants 14, no. 8: 1001. https://doi.org/10.3390/antiox14081001
APA StyleTeng, Y.-N. (2025). The Potential Therapeutic Applications of Natural Products in the Oxidative Stress-Related MVA Pathway: Focus on HMGCR. Antioxidants, 14(8), 1001. https://doi.org/10.3390/antiox14081001






