Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Location and Animal Management
2.3. Experimental Design
2.3.1. Experiment 1
2.3.2. Experiment 2
2.4. Artificial Insemination
2.5. Ultrasonography
2.6. Serum Collection and Analysis
2.7. Ovarian Sample Collection
2.8. Ovarian Transcriptome Analysis
2.9. Granulosa Cell and Luteal Cell Collection
2.10. Cell Counting Kit-8 (CCK-8) Assay
2.11. 5-Ethynyl-2′-Deoxyuridine (EdU) Assay
2.12. Terminal Transferase dUTP Nick-End Labeling (TUNEL) Assay
2.13. Reactive Oxygen Species (ROS) Assay
2.14. Mitochondrial Membrane Potential (MMP) Assay
2.15. Quantitative Real-Time PCR (qRT-PCR)
2.16. Western Blot Analysis
2.17. Statistical Analyses
3. Results
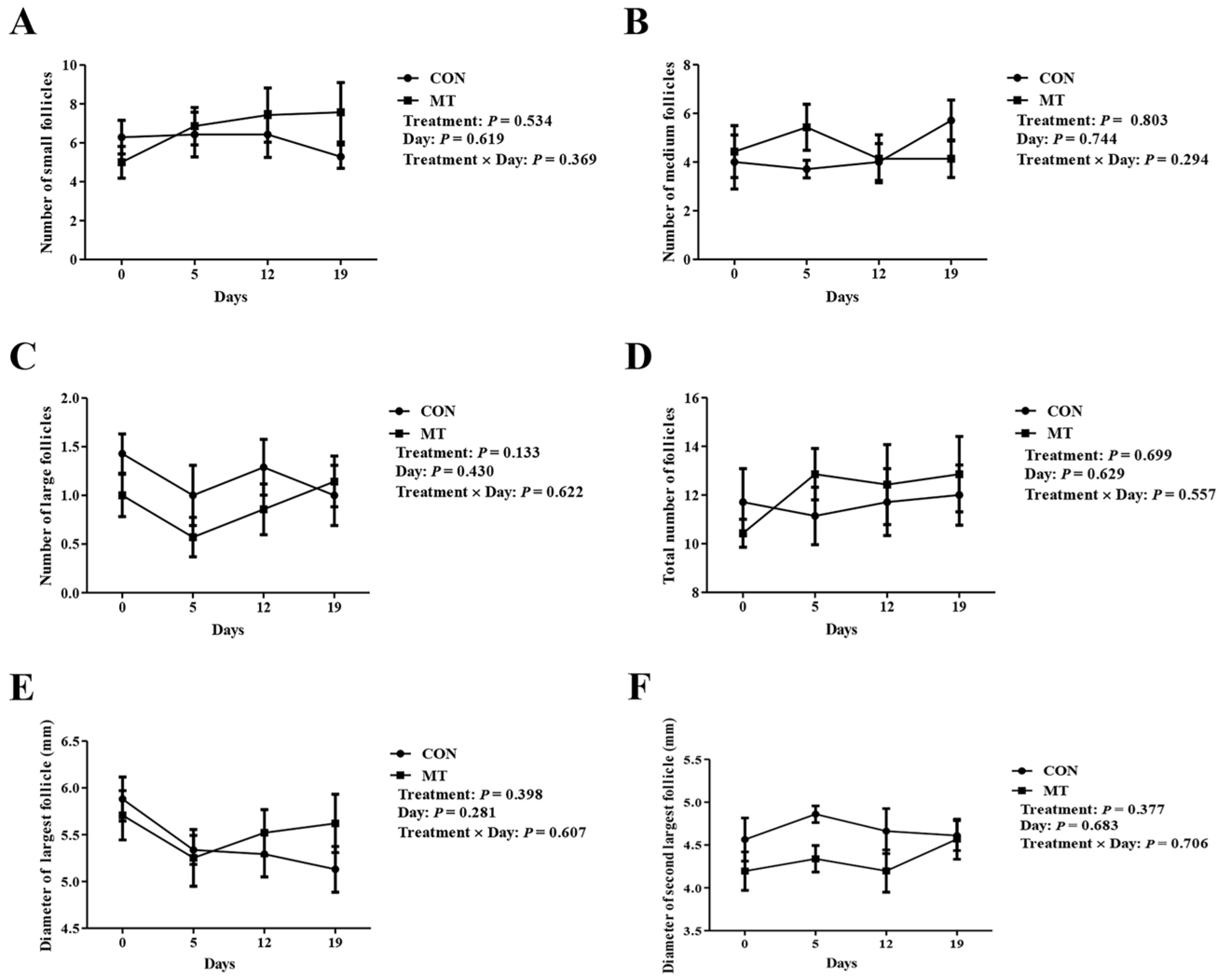
3.1. Effects of Melatonin on Hormonal Levels, Antioxidant Indices, and Ovarian Dynamics
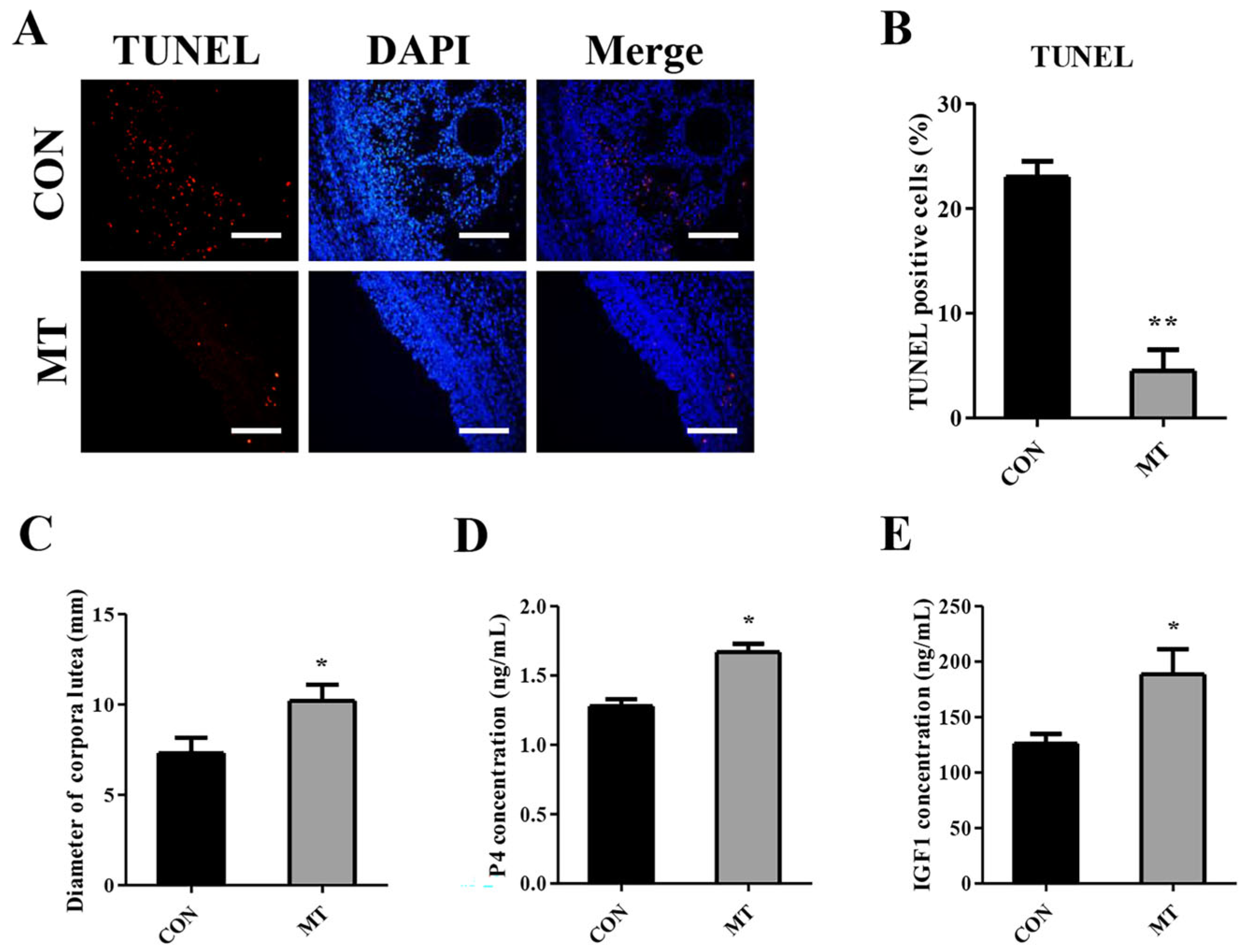
3.2. Effects of Melatonin on Ovarian Apoptosis and Corpus Luteum Development
3.3. Effects of Melatonin on Reproductive Performance
3.4. Effect of MT Implantation on Reproductive Performance of Ewes in a Field Test
3.5. Effects of Melatonin on the Ovarian Transcriptome
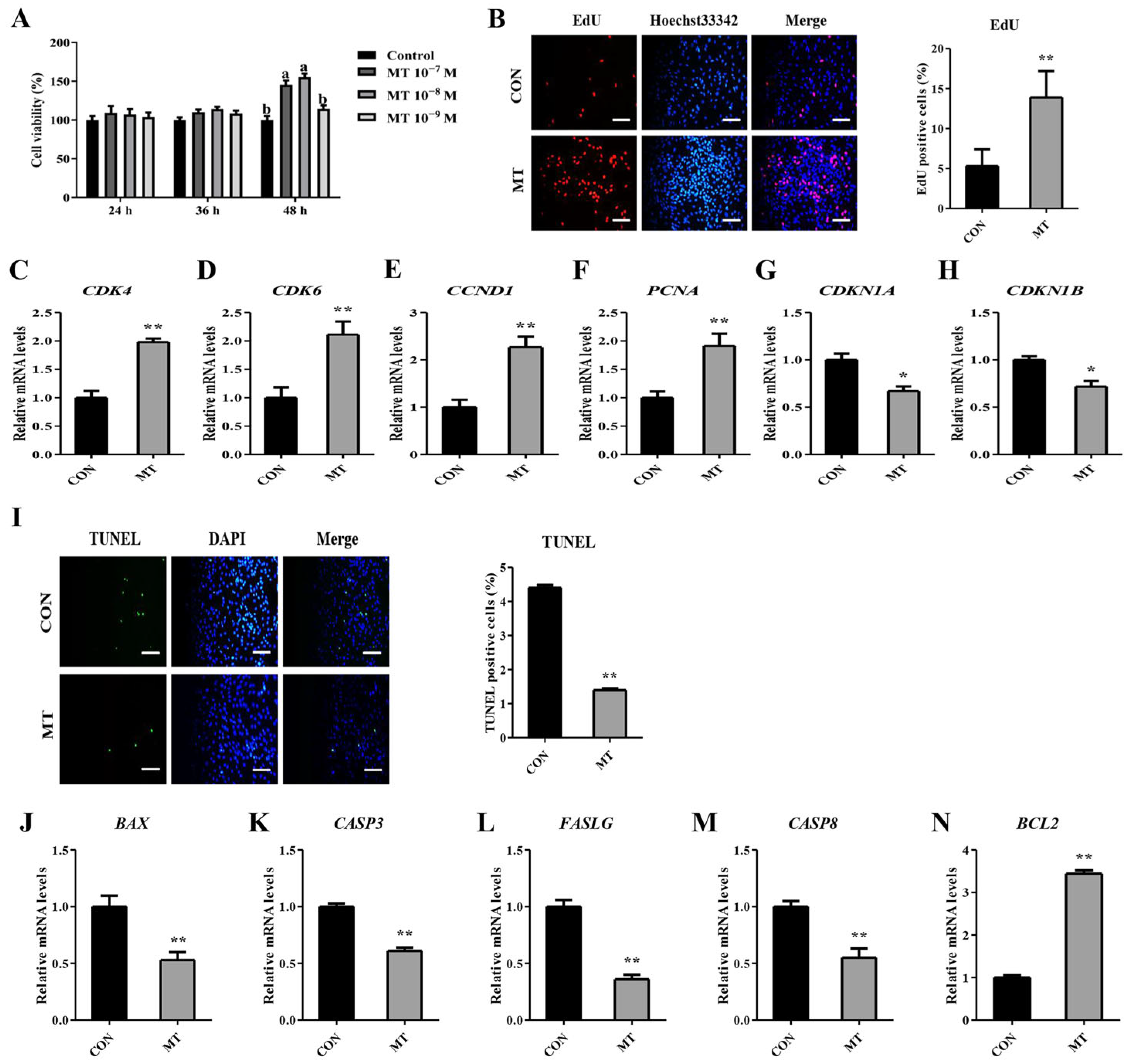
3.6. Melatonin Stimulates Proliferation While Suppressing Apoptosis in Cultured Granulosa and Luteal Cells
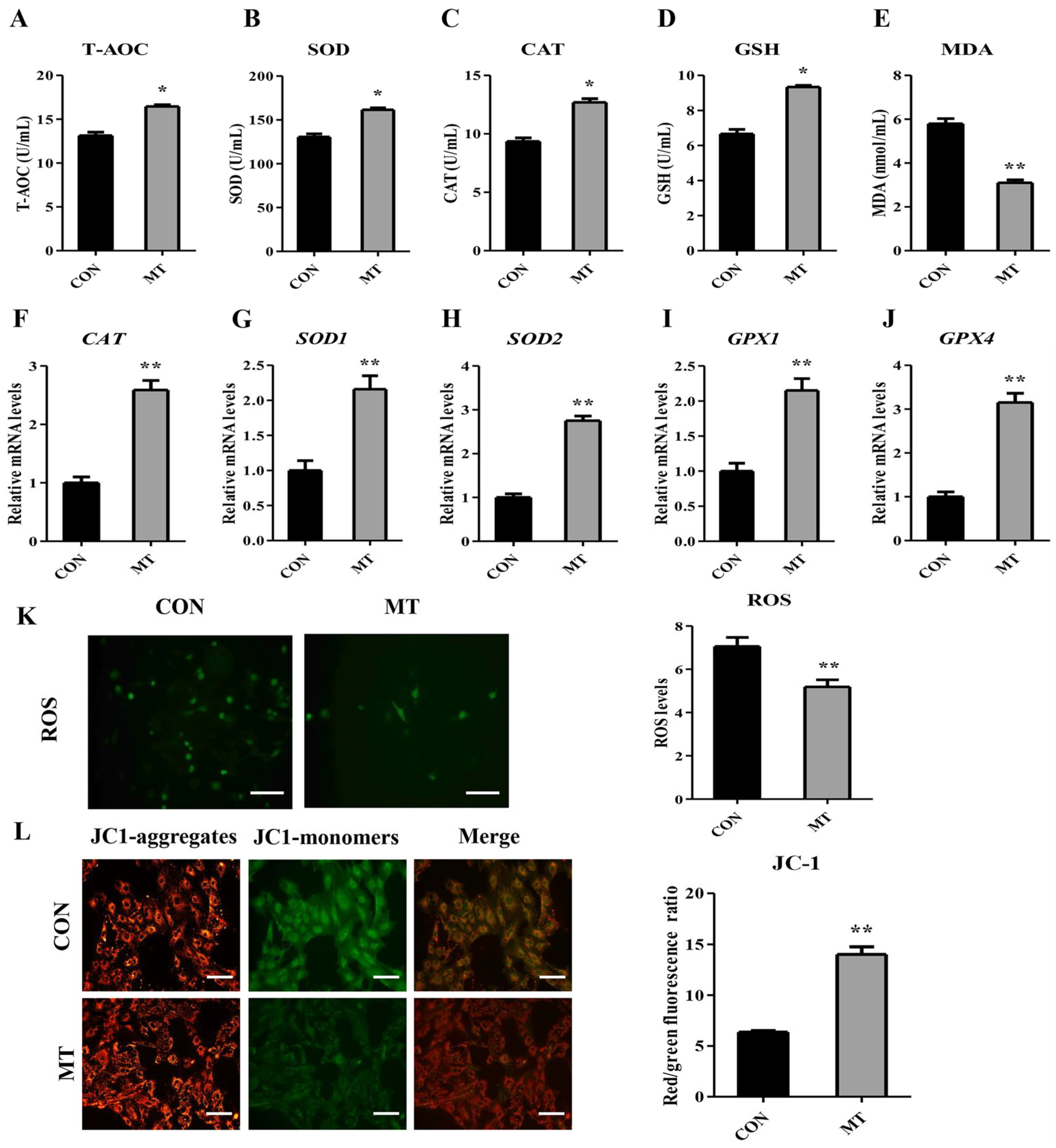
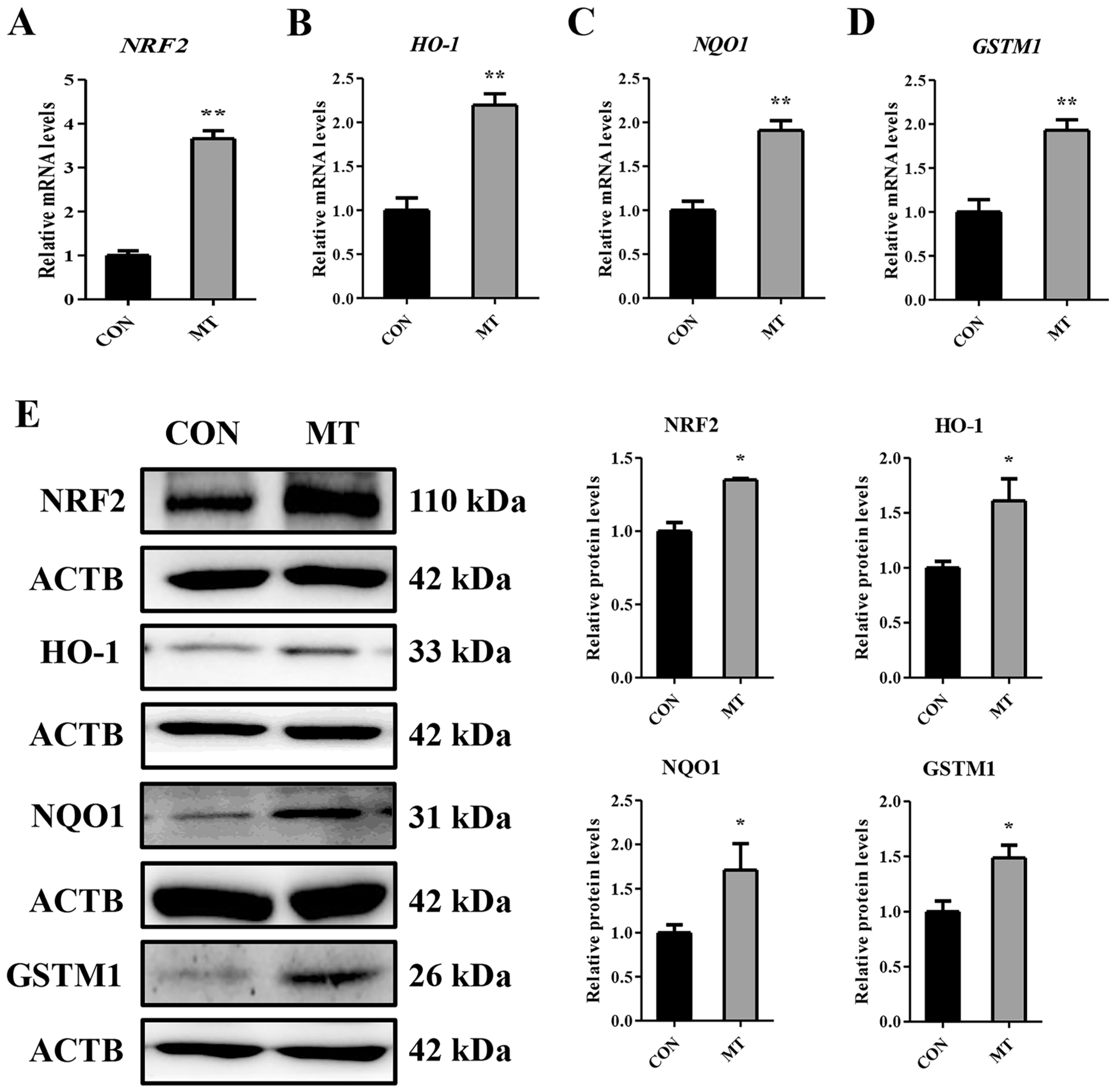
3.7. Melatonin Enhances the Antioxidant Capacity in Cultured Granulosa and Luteal Cells
3.8. Melatonin Enhances Steroidogenic Capacity in Cultured Granulosa and Luteal Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad Pampori, Z.; Ahmad Sheikh, A.; Aarif, O.; Hasin, D.; Ahmad Bhat, I. Physiology of reproductive seasonality in sheep—An update. Biol. Rhythm. Res. 2018, 51, 586–598. [Google Scholar] [CrossRef]
- Salarpoor, M.V.; Kadivar, A.; Davoodian, N.; Khosravian, P.; Esfandabadi, N.S.; Mohebbi, A.; Mehrban, H. Development and evaluation of an injectable slow-release progesterone formulation for estrus synchronization in ewes out of the breeding season. Reprod. Dom. Anim. 2023, 58, 935–945. [Google Scholar] [CrossRef]
- Ungerfeld, R.; Rubianes, E. Short term primings with different progestogen intravaginal devices (MAP, FGA and CIDR) for eCG-estrous induction in anestrus ewes. Small Rumin. Res. 2002, 46, 63–66. [Google Scholar] [CrossRef]
- Ozyurtlu, N.; Kucukaslan, I.; Cetin, Y. Characterization of oestrous induction response, oestrous duration, fecundity and fertility in Awassi ewes during the non-breeding season utilizing both CIDR and intravaginal sponge treatments. Reprod. Dom. Anim. 2010, 45, 464–467. [Google Scholar] [CrossRef] [PubMed]
- El-Mokadem, M.Y.; El-Din, A.; Ramadan, T.A.; Rashad, A.M.; Taha, T.A.; Samak, M.A.; Salem, M.H. Effectiveness of controlled internal drug release device treatment to alleviate reproductive seasonality in anestrus lactating or dry Barki and Rahmani ewes during non-breeding season. Reprod. Dom. Anim. 2018, 53, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Forcada, F.; González-Bulnes, A. Hormonal control of reproduction in small ruminants. Anim. Reprod. Sci. 2012, 130, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.B.; Zhang, F.H.; He, Q.Y.; Man, J.J.; Mu, Y.P.; Zhao, J.Q.; Zhu, L.; Loor, J.J.; Luo, J. ADCY5 Gene Affects Seasonal Reproduction in Dairy Goats by Regulating Ovarian Granulosa Cells Steroid Hormone Synthesis. Int. J. Mol. Sci. 2025, 26, 1622. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.L.; Hudgens, R.E.; Diekman, M.A.; Moss, G.E. Effect of melatonin on induction of estrous cycles in anestrous ewes. J. Anim. Sci. 1988, 66, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, B.; Udala, J.; Gaczarzewicz, D. Changes in estradiol, progesterone, melatonin, prolactin and thyroxine concentrations in blood plasma of goats following induced estrus in and outside the natural breeding season. Small Rumin. Res. 2004, 51, 209–219. [Google Scholar] [CrossRef]
- de Nicolo, G.; Morris, S.T.; Kenyon, P.R.; Morel, P.C.H.; Parkinson, T.J. Melatonin-improved reproductive performance in sheep bred out of season. Anim. Reprod. Sci. 2008, 109, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Yeates, N.T.M. The breeding season of the sheep with particular reference to its modification by artificial means using light. J. Agric. Sci. 1949, 39, 1–43. [Google Scholar] [CrossRef]
- Bittman, E.L.; Dempsey, R.J.; Karsch, F.J. Pineal melatonin secretion drives the reproductive response to daylength in the ewe. Endocrinology 1983, 113, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, C.; Koumas, A.; Photiou, C. Initiation of the breeding season in ewe lambs and goat kids with melatonin implants. Small Rumin. Res. 2007, 73, 122–126. [Google Scholar] [CrossRef]
- Song, Y.; Yoon, M. Melatonin effects on animal behavior: Circadian rhythm, stress response, and modulation of behavioral patterns. J. Anim. Sci. Technol. 2025, 67, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From implantation to birth: Insight into molecular melatonin functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Zheng, T.H.; Chen, J.M.; Li, B.F.; Zhang, Q.Z.; Yang, S.W.; Shao, J.Y.; Guan, W.T.; Zhang, S.H. Exploring melatonin’s multifaceted role in female reproductive health: From follicular development to lactation and its therapeutic potential in obstetric syndromes. J. Adv. Res. 2025, 70, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Afzal, A. Melatonin as a multifunctional modulator: Emerging insights into its role in health, reproductive efficiency, and productive performance in livestock. Front. Physiol. 2024, 15, 1501334. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Grasselli, F. Role of melatonin in ovarian function. Animals 2024, 14, 644. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Sharma, R.; Chuffa, L.G.D.; Zuccari, D.A.; Amaral, F.G.; Cipolla-Neto, J. Melatonin-mediated actions and circadian functions that improve implantation, fetal health and pregnancy outcome. Reprod. Toxicol. 2024, 124, 108534. [Google Scholar] [CrossRef] [PubMed]
- Chemineau, P.; Normant, E.; Ravault, J.P.; Thimonier, J. Induction and persistence of pituitary and ovarian activity in the out-of-season lactating dairy goat after a treatment combining a skeleton photoperiod, melatonin and the male effect. J. Reprod. Fertil. 1986, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Zarazaga, L.A.; Gatica, M.C.; Celi, I.; Guzmán, J.L.; Malpaux, B. Effect of melatonin implants on sexual activity in Mediterranean goat females without separation from males. Theriogenology 2009, 72, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Purohit, G.N. Effect of a single subcutaneous injection of melatonin on estrous response and conception rate in goats. Small Rumin. Res. 2009, 82, 152–155. [Google Scholar] [CrossRef]
- Forcada, F.; Zarazaga, L.; Abecia, J.A. Effect of exogenous melatonin and plane of nutrition after weaning on estrous activity, endocrine status and ovulation rate in Salz ewes lambing in the seasonal anestrus. Theriogenology 1995, 43, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Mura, M.C.; Luridiana, S.; Farci, F.; Di Stefano, M.V.; Daga, C.; Pulinas, L.; Staric, J.; Carcangiu, V. Melatonin treatment in winter and spring and reproductive recovery in Sarda breed sheep. Anim. Reprod. Sci. 2017, 185, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Luridiana, S.; Mura, M.C.; Daga, C.; Farci, F.; Di Stefano, M.V.; Zidda, F.; Carcangiu, V. Melatonin treatment in spring and reproductive recovery in sheep with different body condition score and age. Anim. Reprod. Sci. 2015, 160, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Staples, L.D.; McPhee, S.; Kennaway, D.J.; Williams, A.H. The influence of exogenous melatonin on the seasonal patterns of ovulation and estrus in sheep. Anim. Reprod. Sci. 1992, 30, 185–223. [Google Scholar] [CrossRef]
- Laliotis, V.; Vosniakou, A.; Zafrakas, A.; Lymberopoulos, A.; Alifakiotis, T. The effect of melatonin on lambing and litter size in milking ewes after advancing the breeding season with progestagen and PMSG followed by artificial insemination. Small Rumin. Res. 1998, 31, 79–81. [Google Scholar] [CrossRef]
- El-Mokadem, M.Y.; El-Din, A.; Ramadan, T.A.; Taha, T.A.; Samak, M.A.; Sharaby, M.A.; Rashad, A.M. Greater concentrations of IGF-1 are associated with increasing pregnancy rate in melatonin implanted anestrous Barki ewes. Anim. Reprod. Sci. 2020, 219, 106542. [Google Scholar] [CrossRef] [PubMed]
- El-Mokadem, M.Y.; El-Din, A.; Ramadan, T.A.; Rashad, A.M.A.; Taha, T.A.; Samak, M.A. Alleviation of reproductive seasonality in Barki ewes using CIDR-eCG with or without melatonin. Small Rumin. Res. 2019, 174, 170–178. [Google Scholar] [CrossRef]
- Hashem, N.M.; El-Hawy, A.S.; El-Bassiony, M.E.; Abd El-Hamid, I.S.; Gonzalez-Bulnes, A.; Martinez-Ros, P. Use of GnRH-encapsulated chitosan nanoparticles as an alternative to eCG for induction of estrus and ovulation during non-breeding season in sheep. Biology 2023, 12, 351. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin inhibits oxidative stress and apoptosis in cryopreserved ovarian tissues via Nrf2/HO-1 signaling pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE signaling in cellular protection: Mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Xiao, J.H. The Keap1-Nrf2 System: A Mediator between Oxidative Stress and Aging. Oxid. Med. Cell. Longev. 2021, 2021, 6635460. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, A.D.; Gallegos, A.M.; Okamura, Y.; Strauss, J.F.; Schroeder, F. Steroidogenic acute regulatory protein binds cholesterol and modulates mitochondrial membrane sterol domain dynamics. J. Biol. Chem. 2001, 276, 36970–36982. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.W.; Bartlewski, P.M.; Duggavathi, R.; Davies, K.L.; Huchkowsky, S.L.; Epp, T.; Rawlings, N.C. Synchronization of follicular wave emergence in the seasonally anestrous ewe: The effects of estradiol with or without medroxyprogesterone acetate. Theriogenology 2008, 69, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.T.; Schuch, V.; Hossack, D.J.; Chakraborty, R.; Johnson, E.L. Melatonin: The placental antioxidant and anti-inflammatory. Front. Immunol. 2024, 15, 1339304. [Google Scholar] [CrossRef] [PubMed]
- El-Mokadem, M.Y.; El-Din, A.; Ramadan, T.A.; Rashad, A.M.A.; Taha, T.A.; Samak, M.A. Manipulation of reproductive seasonality using melatonin implantation in Anglo-Nubian does treated with controlled internal drug release and equine chorionic gonadotropin during the nonbreeding season. J. Dairy Sci. 2017, 100, 5028–5039. [Google Scholar] [CrossRef] [PubMed]
- Barberino, R.S.; Menezes, V.G.; Ribeiro, A.; Palheta, R.C., Jr.; Jiang, X.; Smitz, J.E.J.; Matos, M.H.T. Melatonin protects against cisplatin-induced ovarian damage in mice via the MT1 receptor and antioxidant activity. Biol. Reprod. 2017, 96, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Peng, W.; Yin, S.; Zhao, J.; Fu, B.; Zhang, J.; Mao, T.; Wu, H.; Zhang, Y. Melatonin improves age-induced fertility decline and attenuates ovarian mitochondrial oxidative stress in mice. Sci. Rep. 2016, 6, 35165. [Google Scholar] [CrossRef] [PubMed]
- Kavita; Phogat, J.B.; Pandey, A.K.; Balhara, A.K.; Ghuman, S.S.; Gunwant, P. Effects of melatonin supplementation prior to Ovsynch protocol on ovarian activity and conception rates in anestrous Murrah buffalo heifers during out of breeding season. Reprod. Biol. 2018, 18, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Samir, H.; Samir, M.; Radwan, F.; Mandour, A.S.; El-Sherbiny, H.R.; Ahmed, A.E.; Al Syaad, K.M.; Al-Saeed, F.A.; Watanabe, G. Effect of pre-treatment of melatonin on superovulation response, circulatory hormones, and miRNAs in goats during environmental heat stress conditions. Vet. Res. Commun. 2024, 48, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Viola, I.; Sosa, C.; Accornero, P.; Manenti, I.; Canto, F.; Miretti, S.; Abecia, J.A.; Toschi, P. Exogenous melatonin ameliorates embryo-maternal cross-talk in early pregnancy in sheep. Reproduction 2024, 168, e240172. [Google Scholar] [CrossRef] [PubMed]
- Khan-Dawood, F.S.; Gargiulo, A.R.; Dawood, M.Y. In vitro microdialysis of the ovine corpus luteum of pregnancy: Effects of insulin-like growth factor on progesterone secretion. Biol. Reprod. 1994, 51, 1299–1306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devesa, J.; Caicedo, D. The role of growth hormone on ovarian functioning and ovarian angiogenesis. Front. Endocrinol. 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Berisha, B. Angiogenic factors (VEGF, FGF and IGF) in the bovine corpus luteum. J. Reprod. Dev. 2002, 48, 233–242. [Google Scholar] [CrossRef][Green Version]
- Sauerwein, H.; Miyamoto, A.; Gunther, J.; Meyer, H.H.D.; Schams, D. Binding and action of insulin-like growth-factors and insulin in bovine luteal tissue during the estrous-cycle. J. Reprod. Fertil. 1992, 96, 103–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zarkawi, M.; Al-Merestani, M.R.; Wardeh, M.F. Induction of synchronized oestrous and early pregnancy diagnosis in Syrian Awassi ewes, outside the breeding season. Small Rumin. Res. 1999, 33, 99–102. [Google Scholar] [CrossRef]
- Kuru, M.; Boga Kuru, B.; Sogukpinar, O.; Cebi Sen, C.; Oral, H.; Kirmizibayrak, T. Oestrus synchronisation with progesterone-containing sponge and equine chorionic gonadotropin in Pirlak ewes during the non-breeding season: Can Toryum improve fertility parameters? J. Vet. Res. 2020, 64, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Santos-Jimenez, Z.; Martinez-Herrero, C.; Encinas, T.; Martinez-Ros, P.; Gonzalez-Bulnes, A. Comparative efficiency of oestrus synchronization in sheep with progesterone/eCG and progesterone/GnRH during breeding and non-breeding season. Reprod. Dom. Anim. 2020, 55, 882–884. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Safdarian, M.; Kafi, M. Estrous response to synchronization of estrus using different progesterone treatments outside the natural breeding season in ewes. Small Rumin. Res. 2006, 65, 279–283. [Google Scholar] [CrossRef]
- Barros, V.R.P.; Monte, A.P.O.; Santos, J.M.S.; Lins, T.; Cavalcante, A.Y.P.; Gouveia, B.B.; Müller, M.C.; Oliveira, J.L.; Barberino, R.S.; Donfack, N.J.; et al. Effects of melatonin on the in vitro growth of early antral follicles and maturation of ovine oocytes. Domest. Anim. Endocrinol. 2020, 71, 106386. [Google Scholar] [CrossRef] [PubMed]
- Barros, V.R.P.; Monte, A.P.O.; Santos, J.M.S.; Lins, T.; Cavalcante, A.Y.P.; Gouveia, B.B.; Müller, M.C.; Oliveira, J.L., Jr.; Donfack, N.J.; Araújo, V.R.; et al. Melatonin improves development, mitochondrial function and promotes the meiotic resumption of sheep oocytes from in vitro grown secondary follicles. Theriogenology 2020, 144, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Wang, F.; Zhang, L.; He, C.J.; Ji, P.Y.; Wang, J.; Zhang, Z.Z.; Lv, D.Y.; Abulizi, W.; Wang, X.G.; et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ajofrín, I.; Martín-Maestro, A.; Medina-Chávez, D.A.; Laborda-Gomariz, J.; Peris-Frau, P.; Garde, J.J.; Soler, A.J. Melatonin rescues the development and quality of oocytes and cumulus cells after prolonged ovary preservation: An ovine in vitro model. Theriogenology 2022, 186, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Nandi, S.; Gupta, P.S.P.; Mondal, S. Antioxidants supplementation improves the quality of in vitro produced ovine embryos with amendments in key development gene expressions. Theriogenology 2023, 201, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.E.; Manunta, M.L.; Spezzigu, A.; Torres-Rovira, L.; Gonzalez-Bulnes, A.; Pasciu, V.; Piu, P.; Leoni, G.G.; Succu, S.; Chesneau, D.; et al. Melatonin deprival modifies follicular and corpus luteal growth dynamics in a sheep model. Reproduction 2014, 147, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Abecia, J.A.; Forcada, F.; Zúñiga, O. The effect of melatonin on the secretion of progesterone in sheep and on the development of ovine embryos in vitro. Vet. Res. Commun. 2002, 26, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.D.; Vergani, G.B.; Rodrigues, J.N.D.; Dias, J.H.; Pereira, V.S.D.; Garcia, A.R.; Esteves, S.N.; da Fonseca, J.F.; Oliveira, M.E.F. Luteal tissue characteristics of Morada Nova ewes with hCG application 7.5 days after the end of estrus synchronization protocol in the breeding season. Anim. Reprod. Sci. 2024, 261, 107396. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Bruno-Galarraga, M.M.; Soto, A.T.; de la Sota, R.L.; Cueto, M.I.; Lacau, I.M.; Gibbons, A.E. Hormonal therapeutic strategy on the induction of accessory corpora lutea in relation to follicle size and on the increase of progesterone in sheep. Theriogenology 2018, 105, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.N.D.; Guimaraes, J.D.; Oliveira, M.E.F.; Dias, J.H.; Arrais, A.M.; de Sousa, M.A.P.; Bastos, R.; Ahmadi, B.; Bartlewski, P.M.; Fonseca, J.F. Human chorionic gonadotropin affects original (ovulatory) and induced (accessory) corpora lutea, progesterone concentrations, and pregnancy rates in anestrous dairy goats. Reprod. Biol. 2022, 22, 100591. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Forte, G.; Oliva, M.M.; Laganà, A.S.; Unfer, V. Melatonin and myo-inositol: Supporting reproduction from the oocyte to birth. Int. J. Mol. Sci. 2021, 22, 8433. [Google Scholar] [CrossRef] [PubMed]
- Chuffa, L.G.D.; Lupi, L.A.; Cucielo, M.S.; Silveira, H.S.; Reiter, R.J.; Seiva, F.R.F. Melatonin promotes uterine and placental health: Potential molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 300. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Sun, J.M.; Ouyang, Y.Q.; Shi, D.S.; Lu, F.H. Hypoxia enhances steroidogenic competence of buffalo (Bubalus bubalis) granulosa cells. Theriogenology 2023, 210, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chen, J.H.; Liu, M.Z.; Zhang, C.L.; Chao, X.H.; Yang, H.; Wang, T.S.; Bi, H.W.; Ding, Y.; Wang, Z.M.; et al. miR-107 suppresses porcine granulosa cell proliferation and estradiol synthesis while promoting apoptosis via targeting PTGS2. Theriogenology 2025, 238, 117367. [Google Scholar] [CrossRef] [PubMed]
- Min, T.H.; Lee, S.H.; Lee, S. Angiogenesis and apoptosis: Data comparison of similar microenvironments in the corpus luteum and tumors. Animals 2024, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Liu, L.; Yang, H.; Wan, Y.J.; Zhang, Y.L.; Wang, F. Melatonin regulates the antioxidant capacity of sheep granulosa cells through a novel uORF-Nrf2aa mediated Nrf2/KEAP1 pathway. Life Sci. 2024, 349, 122693. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.J.; Zhang, L.J.; Huang, G.L.; Hao, X.M.; Liu, Z.Z.; Huo, S.D. MEL regulates miR-21 and let-7b through the STAT3 cascade in the follicular granulosa cells of Tibetan sheep. Theriogenology 2023, 205, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Liu, Z.Y.; Li, W.T.; Wang, P.; Wang, X.Y.; Di, R.; He, X.Y.; Chu, M.X. Effect of melatonin on ATG2B-mediated autophagy regulation in sheep granulosa cells with different FecB genotypes. J. Pineal Res. 2023, 75, e12890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Zhang, Z.L.; Peng, J.; Yang, S.T.; Chen, J.X.; Wang, C.X.; Tong, D.W. Expression of arylalkylamine n-acetyltransferase (AANAT) and acetylserotonin o-methyltransferase (ASMT) in the corpus luteum of pregnant sows and synthesis of melatonin in luteal cells. Cell Tissue Res. 2022, 388, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Wang, Z.Y.; Zhang, L.; Zhang, Z.L.; Chen, J.X.; Chen, W.C.; Tong, D.W. Melatonin stimulates the secretion of progesterone along with the expression of cholesterol side-chain cleavage enzyme (P450scc) and steroidogenic acute regulatory protein (StAR) in corpus luteum of pregnant sows. Theriogenology 2018, 108, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Li, X.; Zhao, Z.; Cao, Y.; Liu, X.; Liu, Z.; Ma, H.; Lu, W. Melatonin protects the apoptosis of sheep granulosa cells by suppressing oxidative stress via MAP3K8 and FOS pathway. Genes 2023, 14, 1067. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.T.; Mu, X.Y.; Ding, Y.B.; Tan, Q.M.; Liu, X.Q.; He, J.L.; Gao, R.F.; Li, N.Y.; Geng, Y.Q.; Wang, Y.X.; et al. Melatonin alleviates benzo(a)pyrene-induced ovarian corpus luteum dysfunction by suppressing excessive oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 2021, 207, 111561. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Dong, Y.; Wang, Z.; Ding, H.; Wang, J.; Zhao, J.; Liu, H.; Lv, W. Melatonin attenuates oxidative stress-induced apoptosis of bovine ovarian granulosa cells by promoting mitophagy via SIRT1/FoxO1 signaling pathway. Int. J. Mol. Sci. 2023, 24, 12854. [Google Scholar] [CrossRef] [PubMed]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8, S40–S42. [Google Scholar] [CrossRef] [PubMed]
- Al-Gubory, K.H.; Garrel, C.; Faure, P.; Sugino, N. Roles of antioxidant enzymes in corpus luteum rescue from reactive oxygen species-induced oxidative stress. Reprod. Biomed. Online 2012, 25, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, K.; Shiroma, M.E.; Damous, L.L.; Simoes, M.D.; Simoes, R.D.; Cipolla-Neto, J.; Baracat, E.C.; Soares-Jr, J.M. Antioxidant actions of melatonin: A systematic review of animal studies. Antioxidants 2024, 13, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.Y.; Guo, J.; Wang, S.M.; Min, C.G.; Wang, J.; Liu, H.Y.; Fang, Y.; Ding, H.; Zhao, J.; Ma, X.; et al. Melatonin protects bovine oocyte from βHB-induced oxidative stress through the Nrf2 pathway. Theriogenology 2025, 234, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Y.; Zhang, X.; Si, L.N.; Shu, W.H.; Jiang, S.N.; Ding, P.J.; Cheng, L.Y.; Sun, T.C.; Yang, S.H. Melatonin activates Nrf2/HO-1 signalling pathway to antagonizes oxidative stress-induced injury via melatonin receptor 1 (MT1) in cryopreserved mice ovarian tissue. Reprod. Dom. Anim. 2024, 59, e14598. [Google Scholar] [CrossRef] [PubMed]
- Pohler, K.; Geary, T.; Atkins, J.; Perry, G.; Jinks, E.; Smith, M. Follicular determinants of pregnancy establishment and maintenance. Cell Tissue Res. 2012, 349, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Karsch, F.J.; Bowen, J.M.; Caraty, A.; Evans, N.P.; Moenter, S.M. Gonadotropin-releasing hormone requirements for ovulation. Biol. Reprod. 1997, 56, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Caraty, A.; Franceschini, I.; Hoffman, G.E. Kisspeptin and the preovulatory gonadotrophin-releasing hormone/luteinising hormone surge in the ewe: Basic aspects and potential applications in the control of ovulation. J. Neuroendocrinol. 2010, 22, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Ciernia, L.A.; Perry, G.A.; Smith, M.F.; Rich, J.J.; Northrop, E.J.; Perkins, S.D.; Green, J.A.; Zezeski, A.L.; Geary, T.W. Effect of estradiol preceding and progesterone subsequent to ovulation on proportion of postpartum beef cows pregnant. Anim. Reprod. Sci. 2021, 227, 106723. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, N.A.; Whirledge, S.; Kallen, A.N. Updates on molecular and environmental determinants of luteal progesterone production. Mol. Cell. Endocrinol. 2020, 515, 110930. [Google Scholar] [CrossRef] [PubMed]





| Parameters | CON Group (n = 20) | MT Group (n = 20) | p Value |
|---|---|---|---|
| Estrus rate (%) | 95.00 (19/20) | 95.00 (19/20) | n.s. |
| Ovulation rate (n) | 1.00 ± 0.00 | 1.14 ± 0.14 | n.s. |
| Pregnancy rate (%) | 56.25 (9/16) | 86.00 (13/15) | n.s. |
| Lambing rate (%) | 56.25 (9/16) | 86.00 (13/15) | n.s. |
| Litter size (n) | 1.00 ± 0.00 (9/9) | 1.00 ± 0.00 (13/13) | n.s. |
| Parameters | CON Group (n = 85) | MT Group (n = 85) | p Value |
|---|---|---|---|
| Pregnancy rate (%) | 50.59 b (43/85) | 68.23 a (58/85) | p = 0.019 |
| Lambing rate (%) | 47.06 b (40/85) | 63.53 a (54/85) | p = 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, Z.; Liu, M.; Li, J.; Liu, K.; Qi, Q.; Yu, Z.; Samoo, H.A.; Wang, C.; Hou, J. Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells. Antioxidants 2025, 14, 895. https://doi.org/10.3390/antiox14070895
Duan Z, Liu M, Li J, Liu K, Qi Q, Yu Z, Samoo HA, Wang C, Hou J. Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells. Antioxidants. 2025; 14(7):895. https://doi.org/10.3390/antiox14070895
Chicago/Turabian StyleDuan, Zengyi, Menghao Liu, Junjin Li, Kexiong Liu, Qi Qi, Zhixuan Yu, Hadia Akber Samoo, Chunxin Wang, and Jian Hou. 2025. "Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells" Antioxidants 14, no. 7: 895. https://doi.org/10.3390/antiox14070895
APA StyleDuan, Z., Liu, M., Li, J., Liu, K., Qi, Q., Yu, Z., Samoo, H. A., Wang, C., & Hou, J. (2025). Melatonin Implantation Improves the Reproductive Performance of Estrus-Synchronized Ewes During Seasonal Anestrus and Enhances the Antioxidant and Steroidogenic Capacities of Granulosa and Luteal Cells. Antioxidants, 14(7), 895. https://doi.org/10.3390/antiox14070895






