Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Cell Culture
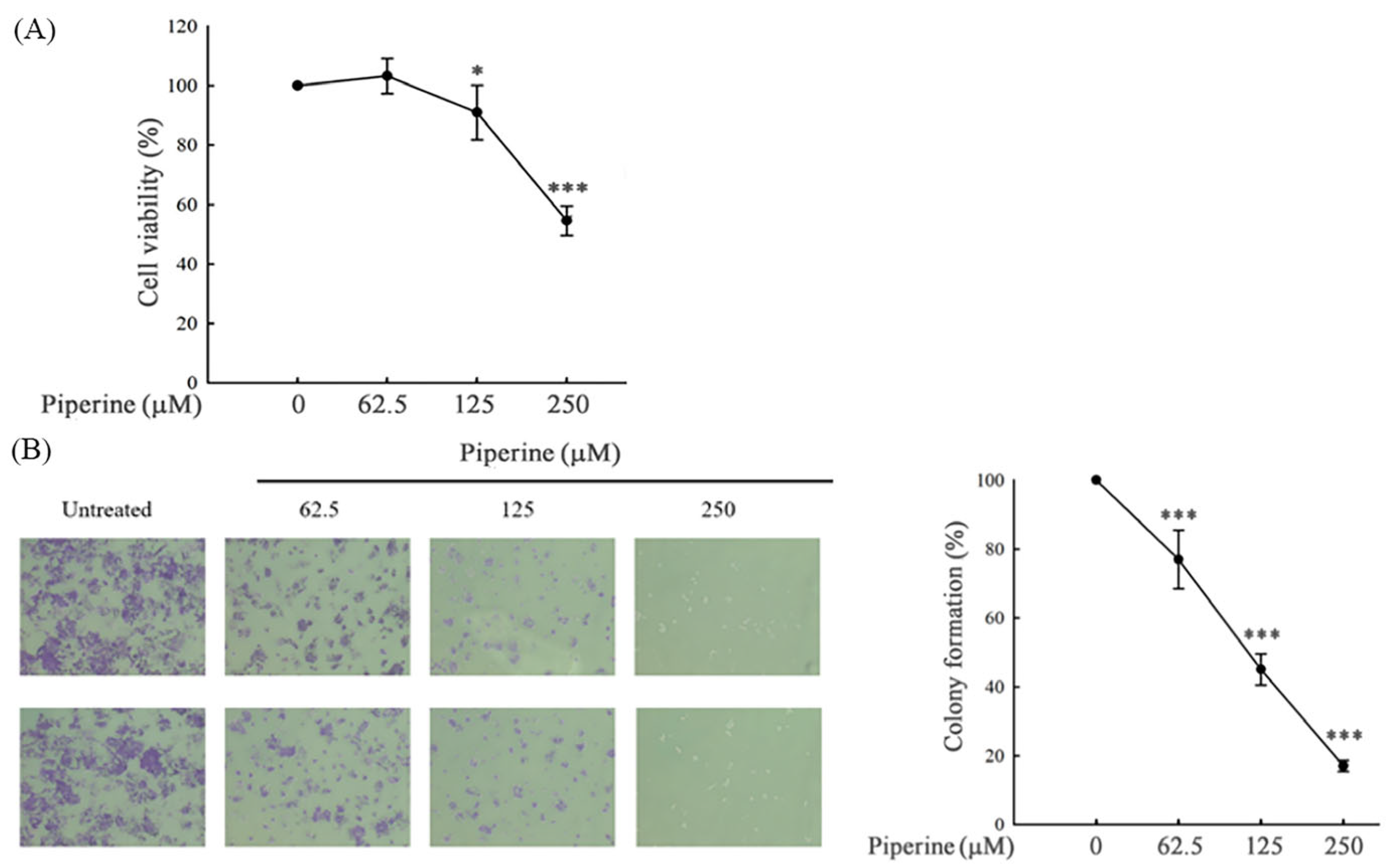
2.3. MTT Assay for Cell Viability
2.4. Colony Formation Assay
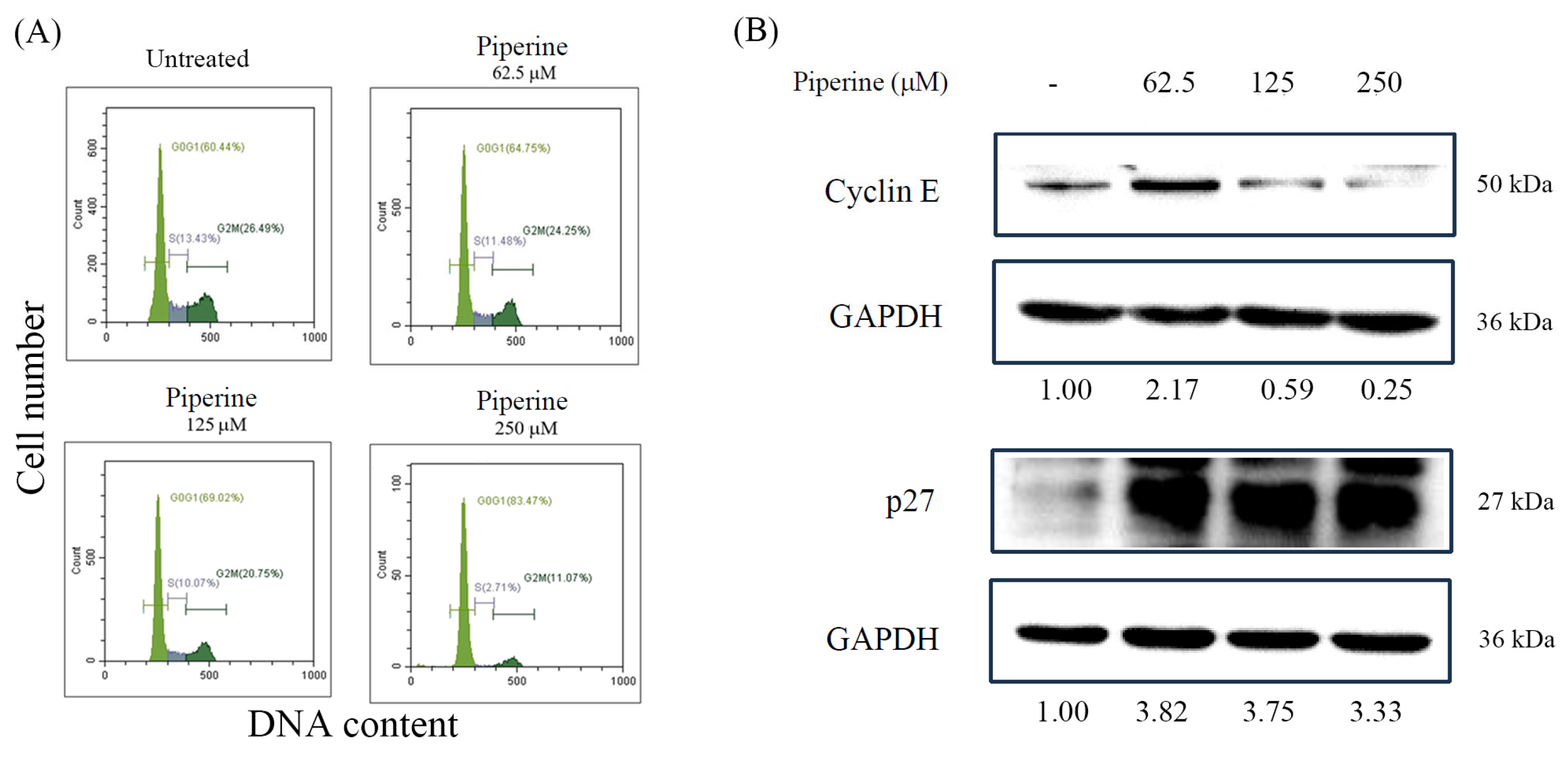
2.5. Cell Cycle Analysis
2.6. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Analysis
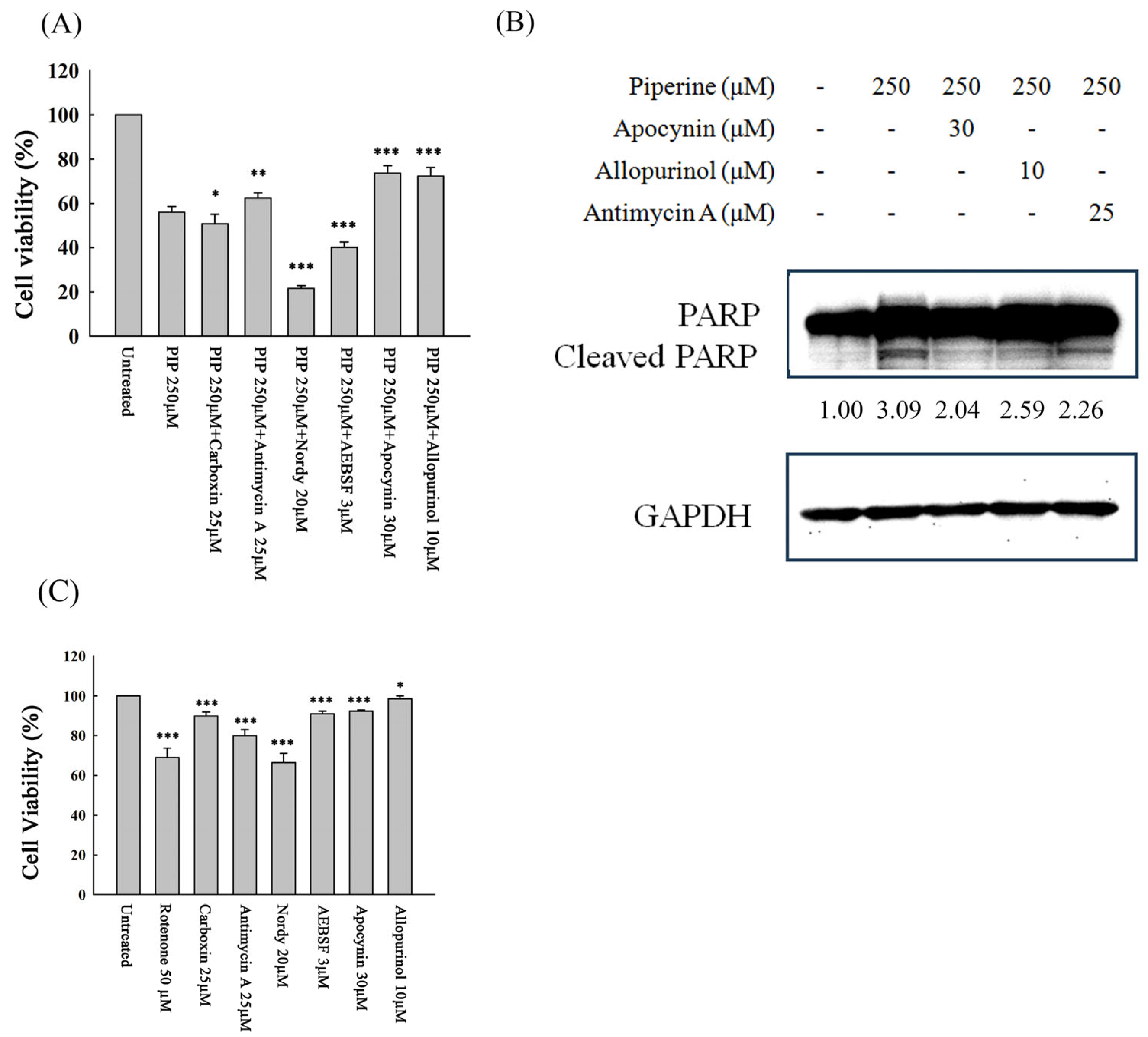
2.7. Western Blot
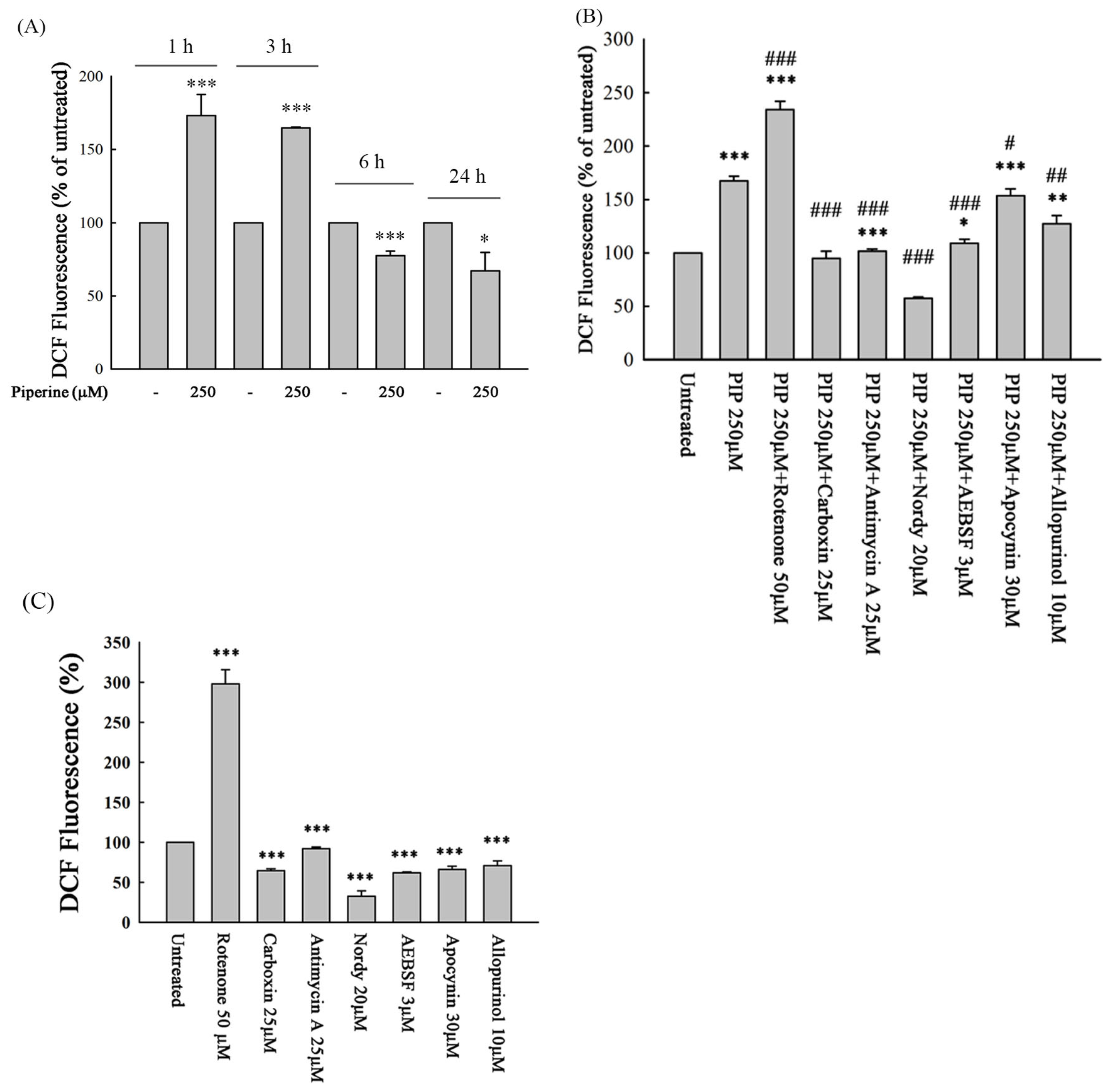
2.8. Determination of Intracellular ROS
2.9. Overexpression of Akt in Cancer Cells
2.10. Statistical Analysis
3. Results
3.1. Piperine Inhibits Cell Viability and Colony Formation in DLD-1 Cells
3.2. Piperine Induces Cell Cycle Arrest in DLD-1 Cells
3.3. Piperine Induces Apoptosis in DLD-1 Cells
3.4. Piperine Induces ROS in DLD-1 Cells
3.5. Piperine-Mediated ROS Induces Cell Death and Apoptosis in DLD-1 Cells
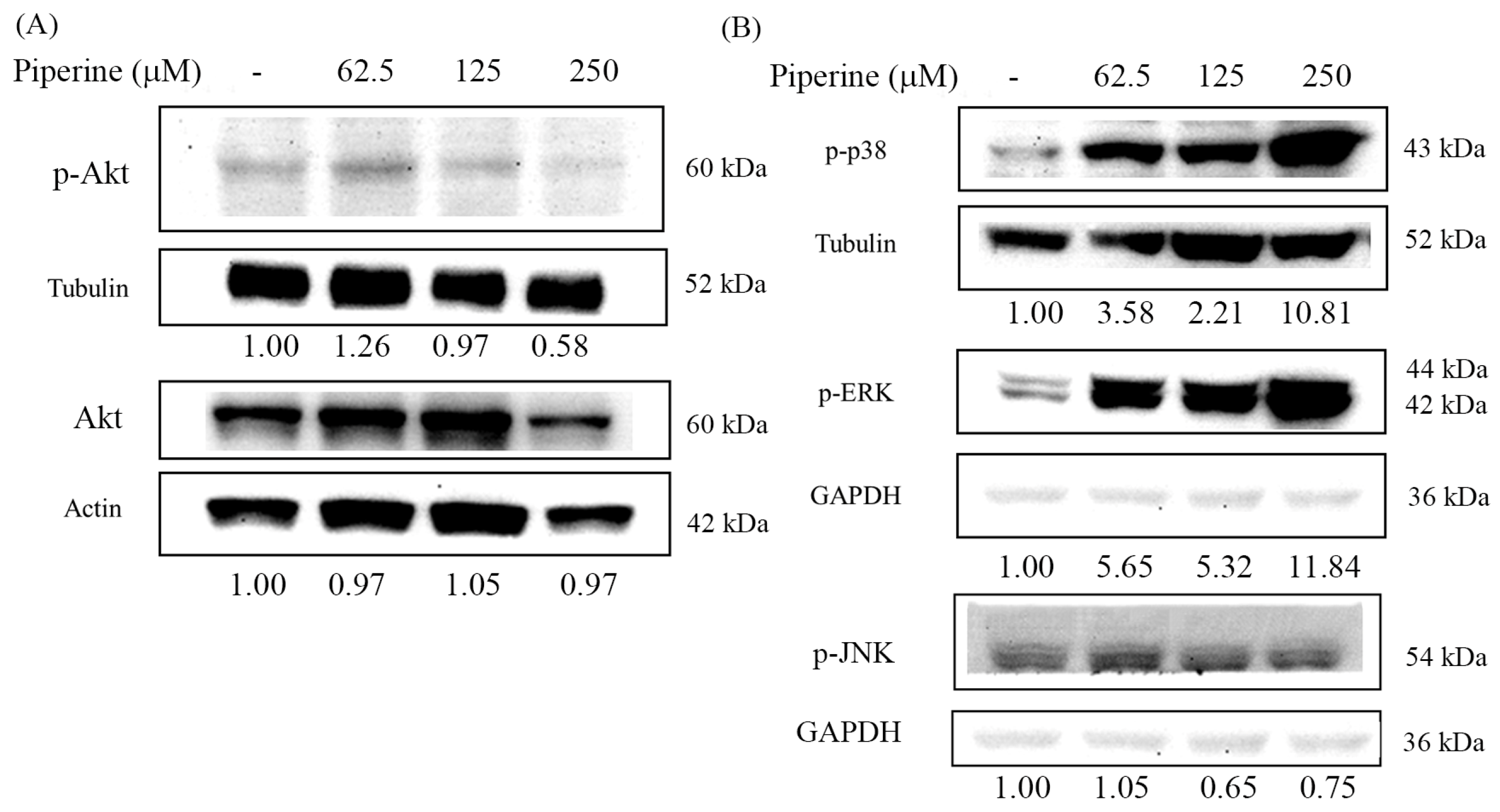
3.6. Piperine Inhibits p-Akt and Regulates MAPKs in DLD-1 Cells
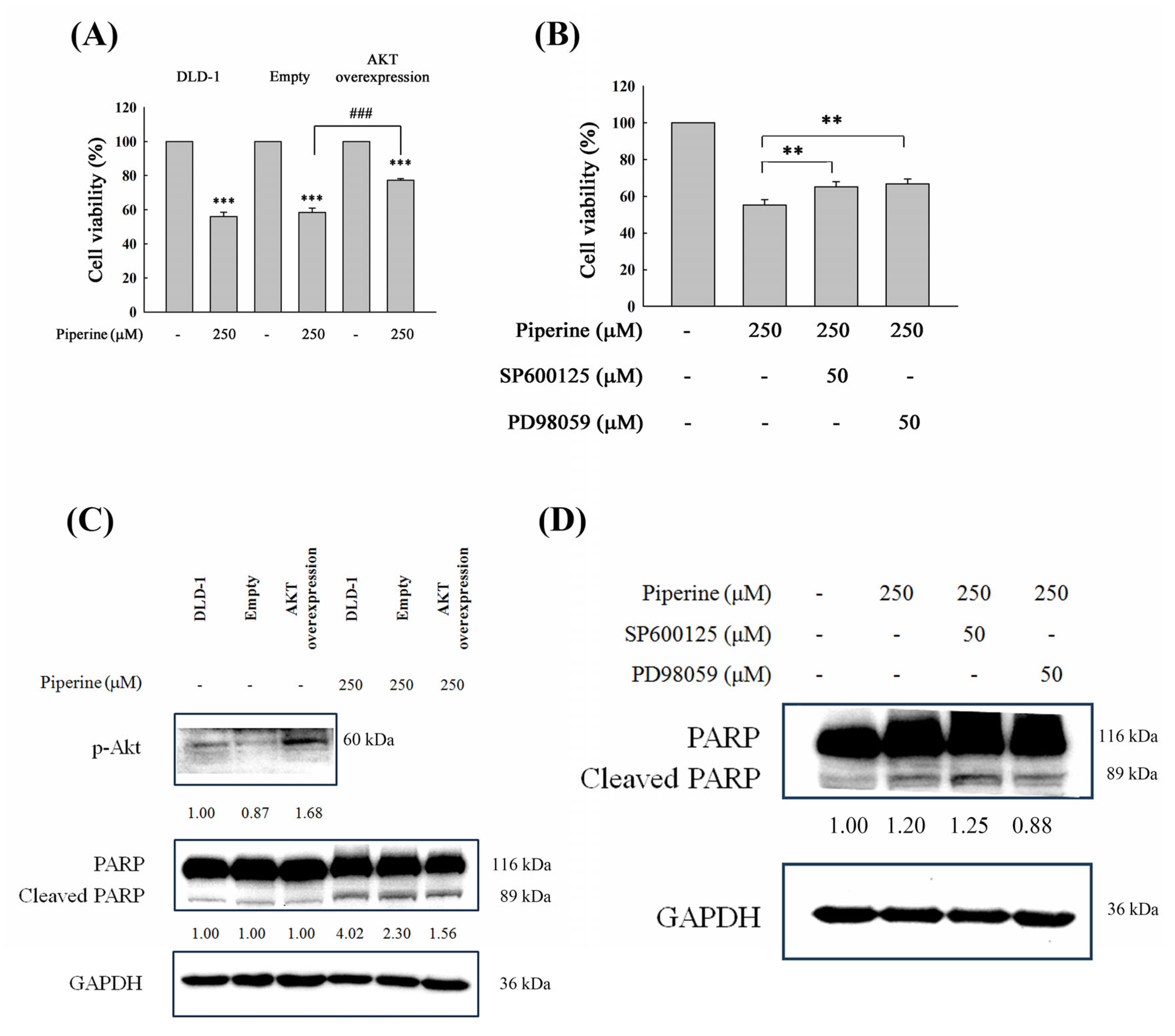
3.7. Piperine Induces Apoptosis Through Akt and ERK Signaling Regulation
3.8. Piperine Induces Cytotoxicity and Apoptosis in Multiple CRC Cell Lines
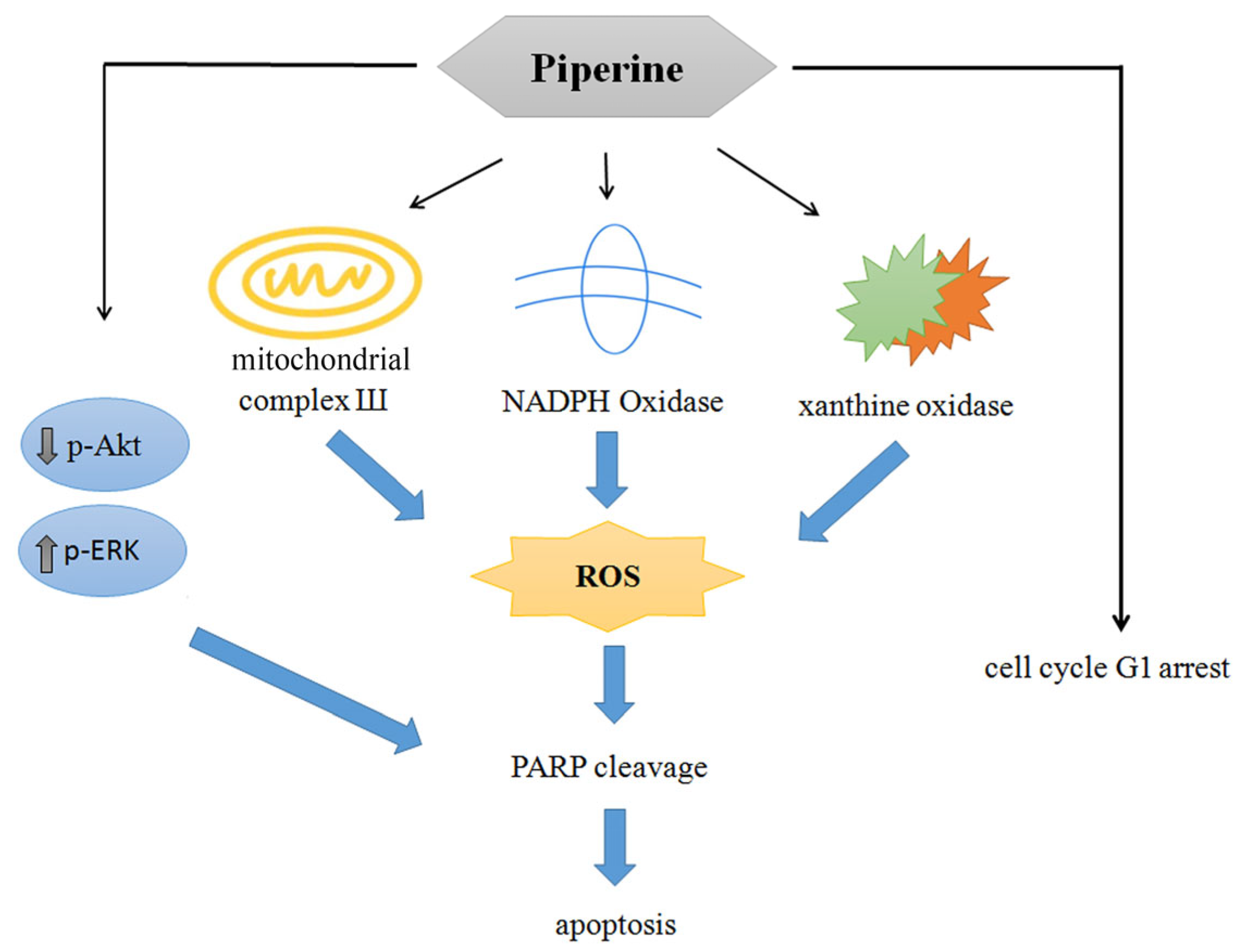
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Guo, P.; Yang, J.; Zhang, T.; Pan, K.; Wei, H. Piperine induces autophagy of colon cancer cells: Dual modulation of AKT/mTOR signaling pathway and ROS production. Biochem. Biophys. Res. Commun. 2024, 728, 150340. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Qian, Y.; Jiang, J.; Li, D.; Feng, L. Piperine inhibits the proliferation of colorectal adenocarcinoma by regulating ARL3- mediated endoplasmic reticulum stress. Biomol. Biomed. 2024, 6, 391. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, G.C.; Oliveira, L.F.S.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar] [CrossRef] [PubMed]
- Shaheer, K.; Somashekarappa, H.M.; Lakshmanan, M.D. Piperine sensitizes radiation-resistant cancer cells towards radiation and promotes intrinsic pathway of apoptosis. J. Food Sci. 2020, 85, 4070–4079. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjya, D.; Sivalingam, N. Mechanism of 5-fluorouracil induced resistance and role of piperine and curcumin as chemo-sensitizers in colon cancer. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 8445–8475. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Dewangan, J.; Mishra, S.; Divakar, A.; Chaturvedi, S.; Wahajuddin, M.; Kumar, S.; Rath, S.K. Piperine and Celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomedicine 2021, 84, 153484. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.M.; Banik, B.K.; Borah, P.; Jain, A. Reactive oxygen species (ROS): Key components in cancer therapies. Anticancer Agents Med. Chem. 2022, 22, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Peshavariya, H. NADPH oxidase-derived ROS signaling and therapeutic opportunities. Curr. Pharm. Des. 2015, 21, 5931–5932. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, C.; Mozziconacci, O.; Zhu, R.; Xu, Y.; Tang, Y.; Chen, R.; Huang, Y.; Holzbeierlein, J.M.; Schöneich, C.; et al. Xanthine oxidase-mediated oxidative stress promotes cancer cell-specific apoptosis. Free Radic. Biol. Med. 2019, 139, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Laws, K.; Eskandari, A.; Suntharalingam, K. A reactive oxygen species-generating, cyclooxygenase-2 inhibiting, cancer stem cell-potent tetranuclear copper (ii) cluster. Dalton Trans. 2017, 46, 12785–12789. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Hu, J.; Lv, Y.; Bai, B.; Shan, L.; Chen, K.; Dai, S.; Zhu, H. Pyrvinium pamoate inhibits cell proliferation through ROS-mediated AKT-dependent signaling pathway in colorectal cancer. Med. Oncol. 2021, 838, 21. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.T.; Lee, K.C.; Cheng, K.C.; Lee, K.F.; Yang, Y.L.; Chu, H.T.; Lin, T.W.; Chen, C.C.; Hsieh, M.C.; Huang, C.Y.; et al. Antrodin C isolated from Antrodia cinnamomea induced apoptosis through ROS/AKT/ERK/p38 signaling pathway and epigenetic histone acetylation of TNFα in colorectal cancer cells. Antioxidants 2023, 12, 764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhao, S.; Lu, X.; Shi, Z.; Liu, H.; Zhu, B. Metformin enhances the sensitivity of colorectal cancer cells to cisplatin through ROS-mediated PI3K/Akt signaling pathway. Gene 2020, 745, 144623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yuan, X.; Yu, T.; Huang, H.; Yang, C.; Zhang, L.; Yang, S.; Luo, X.; Luo, J. Lycorine inhibits cell proliferation, migration and invasion, and primarily exerts in vitro cytostatic effects in human colorectal cancer via activating the ROS/p38 and AKT signaling pathways. Oncol. Rep. 2021, 45, 19. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Lin, Z.; Shi, P.; Chen, B.; Wang, G.; Huang, J.; Sui, Y.; Liu, Q.; Li, S.; Lin, X.; et al. Delicaflavone induces ROS-mediated apoptosis and inhibits PI3K/AKT/mTOR and Ras/MEK/Erk signaling pathways in colorectal cancer cells. Biochem. Pharmacol. 2020, 171, 113680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Jin, L.; Lei, K.; Liu, H.; Yang, Y. Fibulin-5 contributes to colorectal cancer cell apoptosis via the ROS/MAPK and Akt signal pathways by downregulating transient receptor potential cation channel subfamily V member 1. J. Cell Biochem. 2019, 120, 17838–17846. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Jia, L.; Huang, C.; Qi, Q.; Jahangir, A.; Xia, Y.; Liu, W.; Shi, R.; Tang, L.; Chen, Z. Polyphyllin I Promotes Autophagic Cell Death and Apoptosis of Colon Cancer Cells via the ROS-Inhibited AKT/mTOR Pathway. Int. J. Mol. Sci. 2022, 23, 9368. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.Q.; Wang, Y.; Luo, Y.H.; Piao, X.J.; Liu, C.; Wang, Y.; Zhang, Y.; Wang, J.R.; Wang, H.; Xu, W.T.; et al. Quinalizarin induces apoptosis through reactive oxygen species (ROS)-mediated mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3) signaling pathways in colorectal cancer cells. Med. Sci. Monit. 2018, 24, 3710–3719. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, X.; Ho, C.T.; Lin, X.; Zhang, Y.; Li, B.; Chen, Z. Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling pathway. Food Res. Int. 2020, 129, 108854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Xie, L.; Hu, J.; Li, L.; Yang, M.; Cheng, L.; Liu, B.; Qian, X. Antitumor effect of manumycin on colorectal cancer cells by increasing the reactive oxygen species production and blocking PI3K-AKT pathway. OncoTargets Ther. 2016, 9, 2885–2895. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Underwood, P.W.; Ruff, S.M.; Pawlik, T.M. Update on targeted therapy and immunotherapy for metastatic colorectal cancer. Cells 2024, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Vescio, G.; De Paola, G.; Sammarco, G. Therapeutic targets and tumor microenvironment in colorectal cancer. J. Clin. Med. 2021, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Galano, J.M.; Durand, T.; Le Guennec, J.Y.; Lee, J.C. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 2017, 31, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwa, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tafani, M.; Sansone, L.; Limana, F.; Arcangeli, T.; De Santis, E.; Polese, M.; Fini, M.; Russo, M.A. The Interplay of Reactive Oxygen Species, Hypoxia, Inflammation, and Sirtuins in Cancer Initiation and Progression. Oxid. Med. Cell. Longev. 2016, 2016, 3907147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.S.; Chang, J.H.; Hung, W.Y.; Yang, Y.C.; Chien, M.H. The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance. J. Exp. Clin. Cancer Res. 2018, 37, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, Y.; Wang, X.; Hu, D. Furanodienone induces G0/G1 arrest and causes apoptosis via the ROS/MAPKs-mediated caspase-dependent pathway in human colorectal cancer cells: A study in vitro and in vivo. Cell Death Dis. 2017, 8, e2815. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, L.; Laude, K.; Cai, H. Mitochondrial pathophysiology, reactive oxygen species, and cardiovascular diseases. Vet. Clin. North. Am. Small Anim. Pract. 2008, 38, 137–155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Li, L.; Lu, Y.; Shen, Y.; Zhang, M.; Ge, L.; Wang, M.; Yang, J.; Tian, Z.; Tang, X. Azoxystrobin Reduces Oral Carcinogenesis by Suppressing Mitochondrial Complex III Activity and Inducing Apoptosis. Cancer Manag. Res. 2020, 12, 11573–11583. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Yu, S.; Xing, X.; Qiao, J.; Yin, Y.; Wang, J.; Liu, M.; Zhang, W. Ginsenoside Rh2 stimulates the production of mitochondrial reactive oxygen species and induces apoptosis of cervical cancer cells by inhibiting mitochondrial electron transfer chain complex. Mol. Med. Rep. 2021, 24, 873. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchihara, Y.; Tago, K.; Taguchi, H.; Narukawa, Y.; Kiuchi, F.; Tamura, H.; Funakoshi-Tago, M. Taxodione induces apoptosis in BCR-ABL-positive cells through ROS generation. Biochem. Pharmacol. 2018, 154, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Jiang, L.; Xu, M.; Liu, Q.; Gao, N.; Li, P.; Liu, E.H. Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. Sci. Rep. 2016, 6, 20585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kari, S.; Kandhavelu, J.; Murugesan, A.; Thiyagarajan, R.; Kidambi, S.; Kandhavelu, M. Mitochondrial complex III bypass complex I to induce ROS in GPR17 signaling activation in GBM. Biomed. Pharmacother. 2023, 162, 114678. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, A.; Markel, S.; Gaur, S.; Liu, H.; Lu, J.; Jiang, G.; Wu, X.; Antony, S.; Wu, Y.; Melillo, G.; et al. NADPH oxidase 1 supports proliferation of colon cancer cells by modulating reactive oxygen species-dependent signal transduction. J. Biol. Chem. 2017, 292, 7866–7887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bánfi, B.; Clark, R.A.; Steger, K.; Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 2003, 278, 3510–3513. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.W.; Lim, J.W.; Kim, H. Lutein Induces Reactive Oxygen Species-Mediated Apoptosis in Gastric Cancer AGS Cells via NADPH Oxidase Activation. Molecules 2023, 28, 1178. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhavya, K.; Mantipally, M.; Roy, S.; Arora, L.; Badavath, V.N.; Gangireddy, M.; Dasgupta, S.; Gundla, R.; Pal, D. Novel imidazo[1, 2-a]pyridine derivatives induce apoptosis and cell cycle arrest in non-small cell lung cancer by activating NADPH oxidase mediated oxidative stress. Life Sci. 2022, 294, 120334. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, Y.; Wang, Y.; Li, X. Furanodienone induces apoptosis via regulating the PRDX1/MAPKs/p53/caspases signaling axis through NOX4-derived mitochondrial ROS in colorectal cancer cells. Biochem. Pharmacol. 2024, 227, 116456. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Chen, H.; Zhang, L.; Wu, M.; Zhang, F.; Yang, D.; Shen, J.; Chen, J. Glycyrrhetinic acid induces oxidative/nitrative stress and drives ferroptosis through activating NADPH oxidases and iNOS, and depriving glutathione in triple-negative breast cancer cells. Free Radic. Biol. Med. 2021, 173, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Heumüller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schröder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Diatchuk, V.; Lotan, O.; Koshkin, V.; Wikstroem, P.; Pick, E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J. Biol. Chem. 1997, 272, 13292–13301. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Lambeth, J.D. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J. Biol. Chem. 2004, 279, 4737–4742. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jiang, G.; Wu, Y.; Antony, S.; Meitzler, J.L.; Juhasz, A.; Liu, H.; Roy, K.; Makhlouf, H.; Chuaqui, R.; et al. NADPH oxidase 1 is highly expressed in human large and small bowel cancers. PLoS ONE 2020, 15, e0233208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Winston, G.W.; Moore, M.N.; Kirchin, M.A.; Soverchia, C. Production of reactive oxygen species by hemocytes from the marine mussel, Mytilus edulis: Lysosomal localization and effect of xenobiotics. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 113, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Lu, L.Q.; Jiang, Y.Q.; Li, N.S.; Luo, X.J.; Peng, J.W.; Peng, J. Allopurinol attenuates oxidative injury in rat hearts suffered ischemia/reperfusion via suppressing the xanthine oxidase/vascular peroxidase 1 pathway. Eur. J. Pharmacol. 2021, 908, 174368. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.; Katiyar, S.K. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol. Appl. Pharmacol. 2008, 229, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, F.; Zhang, G.; Yang, X.; Wang, M.; Wang, R.; Wan, M.; Wang, J.; Wu, B.; Yan, T.; Jia, Y. A network pharmacology approach and experimental validation to investigate the anticancer mechanism and potential active targets of ethanol extract of Wei-Tong-Xin against colorectal cancer through induction of apoptosis via PI3K/AKT signaling pathway. J. Ethnopharmacol. 2023, 303, 115933. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fan, D. Ginsenoside Rg5 induces G2/M phase arrest, apoptosis and autophagy via regulating ROS-mediated MAPK pathways against human gastric cancer. Biochem. Pharmacol. 2019, 168, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, J.; Ye, X.; Zhu, J.; Hu, X.; Shen, M.; Ma, Y.; Mao, Z.; Song, H.; Chen, F. β-Thujaplicin induces autophagic cell death, apoptosis, and cell cycle arrest through ROS-mediated Akt and p38/ERK MAPK signaling in human hepatocellular carcinoma. Cell Death Dis. 2019, 10, 255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.J.; Lee, Y.C.; Huang, C.H.; Shi, Y.J.; Chen, Y.J.; Pei, S.N.; Chou, Y.W.; Chang, L.S. Non-mitotic effect of albendazole triggers apoptosis of human leukemia cells via SIRT3/ROS/p38 MAPK/TTP axis-mediated TNF-α upregulation. Biochem. Pharmacol. 2019, 162, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ren, D.; Wang, J.; Liu, X.; Zhang, H.; Wu, M.; Yang, G. Bruceine D induces lung cancer cell apoptosis and autophagy via the ROS/MAPK signaling pathway in vitro and in vivo. Cell Death Dis. 2020, 11, 126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osone, S.; Hosoi, H.; Kuwahara, Y.; Matsumoto, Y.; Iehara, T.; Sugimoto, T. Fenretinide induces sustained-activation of JNK/p38 MAPK and apoptosis in a reactive oxygen species-dependent manner in neuroblastoma cells. Int. J. Cancer 2004, 112, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jiang, D.; Liu, J.; Ye, S.; Xiao, S.; Wang, W.; Sun, Z.; Xie, Y.; Wang, J. Compound K induces apoptosis of bladder cancer T24 cells via reactive oxygen species-mediated p38 MAPK pathway. Cancer Biother. Radiopharm. 2013, 28, 607–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.O.; Kwak, A.W.; Lee, M.H.; Seo, J.H.; Cho, S.S.; Yoon, G.; Chae, J.I.; Joo, S.H.; Shim, J.H. Picropodophyllotoxin induces G1 cell cycle arrest and apoptosis in human colorectal cancer cells via ROS generation and activation of p38 MAPK signaling pathway. J. Microbiol. Biotechnol. 2021, 31, 1615–1623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Darling, N.J.; Cook, S.J. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 2014, 1843, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Penninger, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Kim, S.K.; Kwon, S.H.; Seo, J.Y.; Lee, B.R.; Kim, Y.J.; Hur, K.H.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. 7, 8, 4′-Trihydroxyisoflavone, a Metabolized Product of Daidzein, Attenuates 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells. Biomol. Ther. 2019, 27, 363–372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kulisz, A.; Chen, N.; Chandel, N.S.; Shao, Z.; Schumacker, P.T. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L1324–L1329. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.J.; Der, C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007, 26, 3291–3310. [Google Scholar] [CrossRef] [PubMed]
- Bastola, T.; An, R.B.; Kim, Y.C.; Kim, J.; Seo, J. Cearoin Induces Autophagy, ERK Activation and Apoptosis via ROS Generation in SH-SY5Y Neuroblastoma Cells. Molecules 2017, 22, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cagnol, S.; Chambard, J.C. ERK and cell death: Mechanisms of ERK-induced cell death—Apoptosis, autophagy and senescence. FEBS J. 2010, 277, 2–21. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Lee, N.G.; Lee, K.H.; Seo, J.T.; Choi, K.Y. The ERK pathway involves positive and negative regulations of HT-29 colorectal cancer cell growth by extracellular zinc. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1181–G1188. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Peng, L.; Deng, Q.; Liang, Y.; Qing, H.; Jiang, B. CD24-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Cancer Sci. 2010, 101, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.Y.; Fan, C.W.; Maa, M.C.; Leu, T.H. Lipopolysaccharide-promoted proliferation of Caco-2 cells is mediated by c-Src induction and ERK activation. Biomedicine 2015, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Li, W.; Jia, Z.; Peng, Y.; Hou, J.; Li, N.; Meng, R.; Fu, W.; Feng, Y.; Wu, L.; et al. Synaptotagmin 1 suppresses colorectal cancer metastasis by inhibiting ERK/MAPK signaling-mediated tumor cell pseudopodial formation and migration. Cancers 2023, 15, 5282. [Google Scholar] [CrossRef] [PubMed]
- Medico, E.; Russo, M.; Picco, G.; Cancelliere, C.; Valtorta, E.; Corti, G.; Buscarino, M.; Isella, C.; Lamba, S.; Martinoglio, B.; et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat. Commun. 2015, 6, 7002. [Google Scholar] [CrossRef] [PubMed]
- Gusson-Zanetoni, J.P.; Cardoso, L.P.; de Sousa, S.O.; de Melo Moreira Silva, L.L.; de Oliveira Martinho, J.; Henrique, T.; Tajara, E.H.; Oliani, S.M.; Rodrigues-Lisoni, F.C. Molecular aspects of piperine in signaling pathways associated with inflammation in head and neck cancer. Int. J. Mol. Sci. 2024, 25, 5762. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Meghwal, M.; Goswami, T.K. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef] [PubMed]
- Atal, C.K.; Dubey, R.K.; Singh, J. Biochemical basis of enhanced drug bioavailability by piperine: Evidence that piperine is a potent inhibitor of drug metabolism. J. Pharmacol. Exp. Ther. 1985, 232, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Yakubu, J.; Natsaridis, E.; du Toit, T.; Barata, I.S.; Tagit, O.; Pandey, A.V. Nanoparticles with curcumin and piperine modulate steroid biosynthesis in prostate cancer. Sci. Rep. 2025, 15, 13613. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, H.Y.; Back, S.Y.; Han, H.K. Piperine-mediated drug interactions and formulation strategy for piperine: Recent advances and future perspectives. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 43–57. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-L.; Peng, J.-Y.; Hong, C.-L.; Li, P.-C.; Chye, S.M.; Lu, F.-J.; Lin, H.-Y.; Chen, C.-H. Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants 2025, 14, 892. https://doi.org/10.3390/antiox14070892
Chang W-L, Peng J-Y, Hong C-L, Li P-C, Chye SM, Lu F-J, Lin H-Y, Chen C-H. Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants. 2025; 14(7):892. https://doi.org/10.3390/antiox14070892
Chicago/Turabian StyleChang, Wan-Ling, Jyun-Yu Peng, Chain-Lang Hong, Pei-Ching Li, Soi Moi Chye, Fung-Jou Lu, Huei-Yu Lin, and Ching-Hsein Chen. 2025. "Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells" Antioxidants 14, no. 7: 892. https://doi.org/10.3390/antiox14070892
APA StyleChang, W.-L., Peng, J.-Y., Hong, C.-L., Li, P.-C., Chye, S. M., Lu, F.-J., Lin, H.-Y., & Chen, C.-H. (2025). Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants, 14(7), 892. https://doi.org/10.3390/antiox14070892







