Nitric Oxide Does Not Improve Liver Mitochondrial Function 48 Hours After Cecal Ligation and Perforation in Experimental Sepsis
Abstract
1. Introduction
2. Materials and Methods
2.1. Surgical Procedure
2.1.1. Animals
2.1.2. Cecal Ligation and Puncture (CLP)
2.2. Liver Mitochondrial Isolation
2.3. Mitochondrial Respiration and Cytochrome C Oxidase Activity
2.4. Mitochondrial Calcium Retention Capacity
2.5. Activation of Mitochondrial Nitric Oxide Synthase
2.6. Measurements of Nitric Oxide Metabolites Level in Mitochondrial Pellet
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
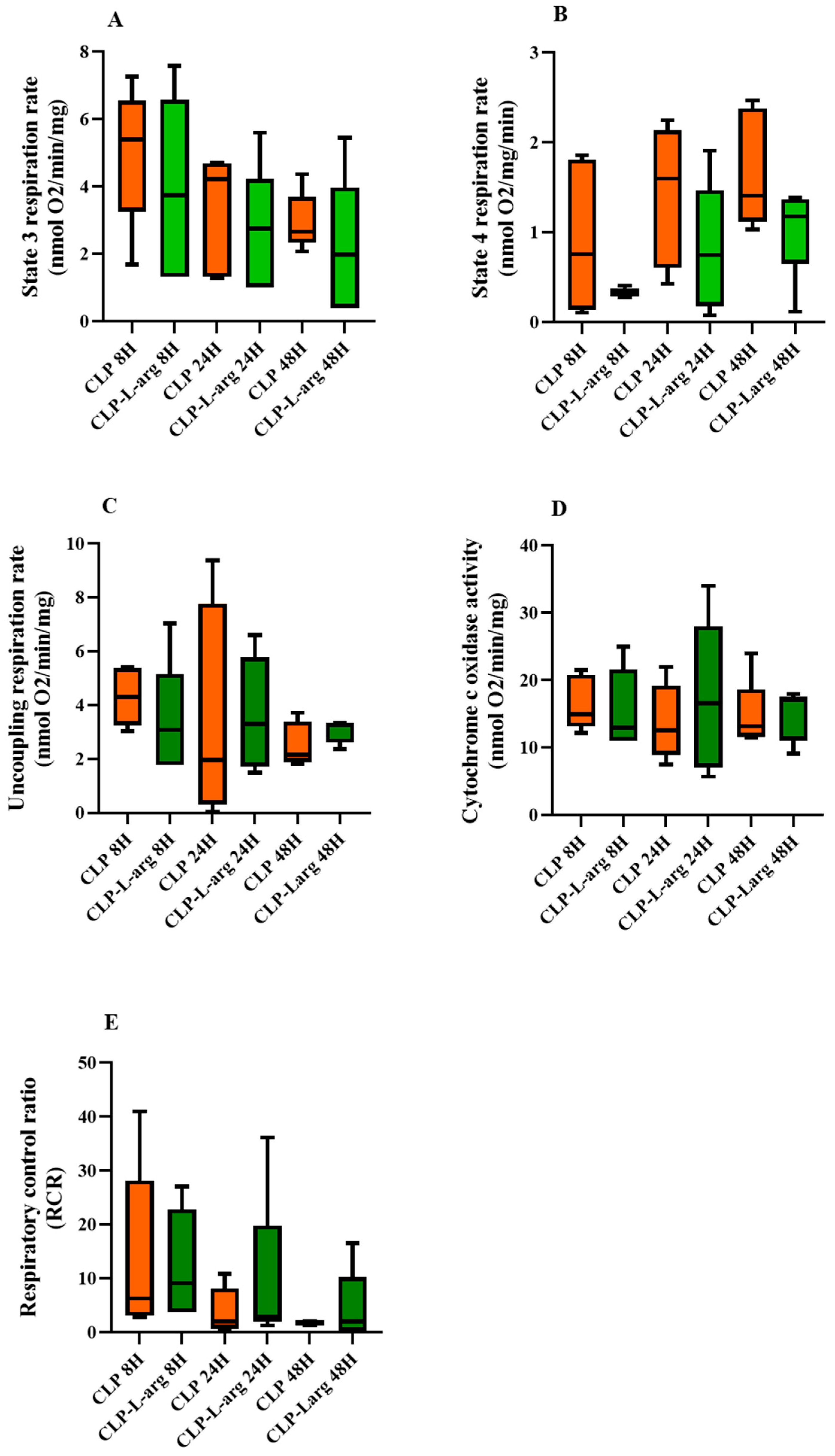
3.1. CLP Decreases Mitochondrial Active Respiration and Cytochrome C Oxidase Activity
3.2. CLP Decreases Mitochondrial Calcium Retention Capacity and Induces Mptp Opening
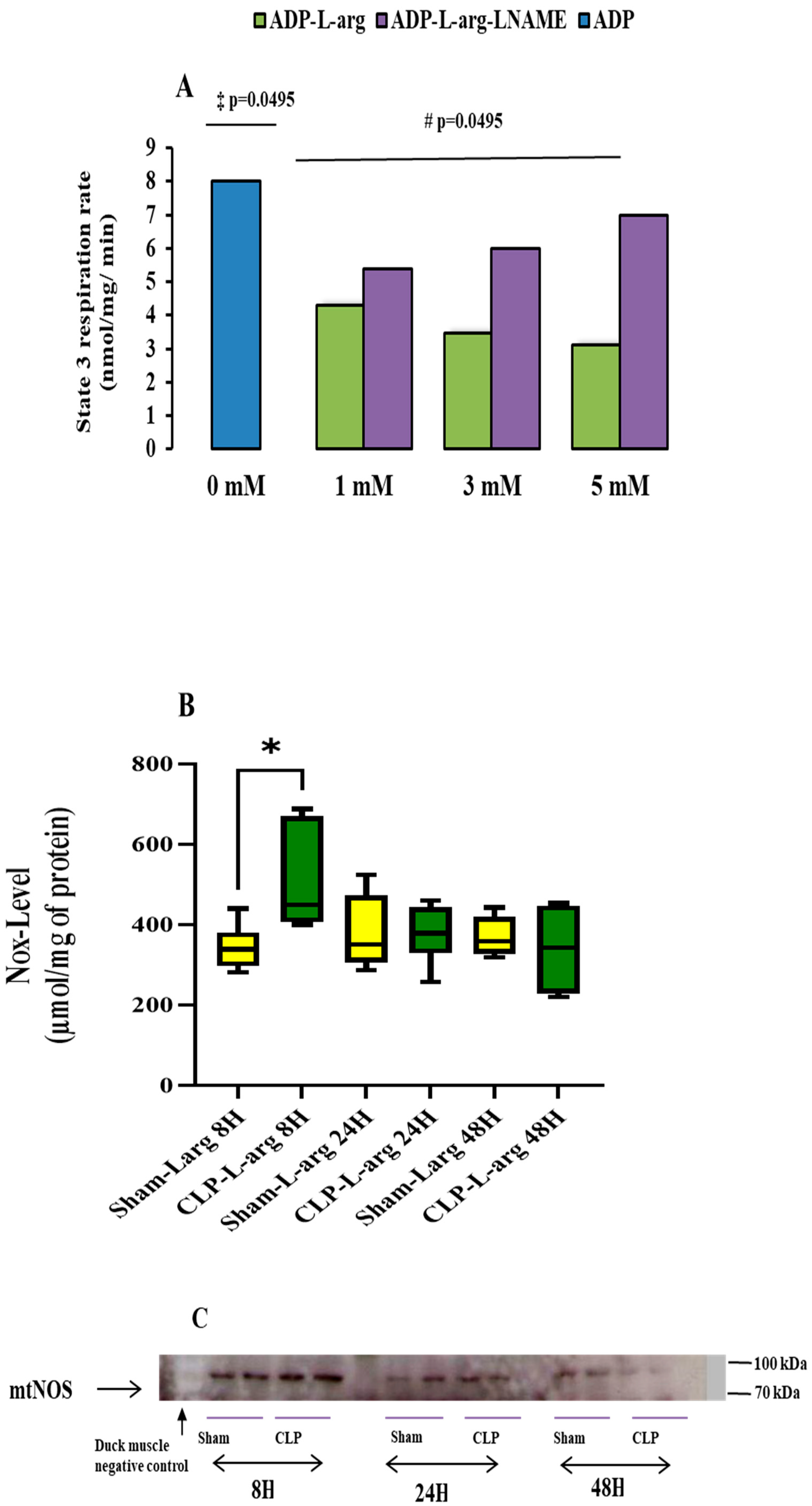
3.3. mtNOS Activation by L Arg Does Not Improve Mitochondrial Respiration or Prevent mPTP Opening
3.4. Effect of Sepsis on Mitochondrial mtNOS Content and Nitric Oxide Metabolites Level
4. Discussion
5. Limitations of Our Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Rivers, E.; Nguyen, B.; Havstad, S.; Ressler, J.; Muzzin, A.; Knoblich, E. Tomlanovich: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N. Engl. J. Med. 2001, 345, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Balk, R.A. Pathogenesis and management of multiple organ dysfunction or failure in severe sepsis and septic shock. Crit. Care Clin. 2000, 16, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Canabal, J.M.; Kramer, D.J. Management of sepsis in patients with liver failure. Curr. Opin. Crit. Care 2008, 14, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zang, K.; Shang, F.; Guo, S.; Gao, L.; Zhang, X. HMGB1 mediates acute liver injury in sepsis through pyroptosis of liver macrophages. Int. J. Burn. Trauma 2020, 10, 60–67. [Google Scholar] [PubMed]
- Eyenga, P.; Lhuillier, F.; Morel, J.; Roussel, D.; Sibille, B.; Letexier, D.; Cespuglio, R.; Duchamp, C.; Goudable, J.; Bricca, G.; et al. Time course of liver nitric oxide concentration in early septic shock by cecal ligation and puncture in rats. Nitric Oxide 2010, 23, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Eyenga, P.; Roussel, D.; Morel, J.; Rey, B.; Romestaing, C.; Teulier, L.; Sheu, S.S.; Goudable, J.; Négrier, C.; Viale, J.P. Early septic shock induces oxidative stress and loss of oxidative Phosphorylation yield plasticity in liver mitochondria. J. Physiol. Biochem. 2014, 70, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, A.J.; Levy, R.J.; Deutschman, C.S. Mitochondrial dysfunction and resuscitation in sepsis. Crit. Care Clin. 2010, 26, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Morel, J.; Li, J.Y.; Eyenga, P.; Meiller, A.; Gustin, M.P.; Bricca, G.; Molliex, S.; Viale, J.P. early adverse changes in liver microvascular circulation during experimental septic shock are not linked to an absolute nitric oxide deficit. Microvasc. Res. 2013, 90, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Goldman, D.; Lidington, D.; Ellis, C.G. Inhibiting nitric oxide overproduction during hypotensive sepsis increases local oxygen consumption in rat skeletal muscle. Crit. Care Med. 2008, 35, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.M.; Sharpe, M.D.; Ellis, C.G. Bench-to-bedside review: Microvascular dysfunction in sepsis-hemodynamics, oxygen transport, and nitric oxide. Crit. Care 2003, 7, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Bates, T.E.; Loesch, A.; Burnstock, G.; Clark, J.B. Immunolocytochemical evidence for mitochondrially located nitric oxide synthase in brain and liver. Biochem. Biophys. Res. Commun. 1995, 213, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Tatoyan, A.; Giulivi, C. Purification and characterization of nitric-oxide synthase from rat liver mitochondria. J. Biol. Chem. 1998, 273, 11044–11048. [Google Scholar] [CrossRef] [PubMed]
- Rey, B.; Roussel, D.; Teulier, L.; Eyenga, P.; Degletagne, C.; Belouze, M.; Duchamp, C. Functional argument for the existence of an avian nitric oxide synthase in muscle mitochondria: Effect of cold acclimation. FEBS Lett. 2011, 585, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.D.C.; Lores-Arnaiz, S.; Albertoni Borghese, F.; Balonga, S.; Lavagna, A.; Filipuzzi, A.L.; Cicerchia, D.; Majowicz, M.; Bustamante, J. Mitochondrial dysfunction in brain cortex mitochondria of STZ-diabetic rats: Effect of L-Arginine. Neurochem. Res. 2013, 38, 2570–2580. [Google Scholar] [CrossRef] [PubMed]
- Dynnik, V.V.; Grishina, E.V.; Fedeotcheva, N.I. The mitochondrial NO-synthase/guanylate cyclase/protein kinase G signaling system underpins the dual effects of nitric oxide on mitochondrial respiration and opening of the permeability transition pore. FEBS J. 2020, 287, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Clerc, P.; Rigoulet, M.; Leverve, X.; Fontaine, E. Nitric oxide increases oxidative phosphorylation efficiency. J. Bioenerg. Biomembr. 2007, 39, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Azzone, G.F.; Zoratti, M.; Petronilli, V.; Pietrobon, D. The stoichiometry of H+ pumping in cytochrome oxidase and the mechanism of uncoupling. J. Inorg. Biochem. 1985, 23, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Eyenga, P.; Roussel, D.; Morel, J.; Rey, B.; Romestaing, C.; Gueguen-Chaignon, V.; Sheu Ss Viale, J.P. Time course of liver mitochondrial function and intrinsic changes in oxidative phosphorylation in a rat model of sepsis. Intensive Care Med. Exp. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Walter, L.; Avéret, N.; Fontaine, E.; Rigoulet, M.; Leverve, X.M. Thyroid status is a key regulator of both flux and efficiency of oxidative phosphorylation in rat hepatocytes. J. Bioenerg. Biomembr. 2002, 34, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Piquet, M.A.; Nogueira, V.; Devin, A.; Sibille, B.; Filippi, C.; Fontaine, E.; Roulet, M.; Rigoulet, M.; Leverve, X.M. Chronic ethanol ingestion increases efficiency of oxidative phosphorylation in rat liver mitochondria. FEBS Lett. 2000, 468, 239–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eyenga, P.; Rey, B.; Eyenga, L.; Sheu, S.S. Regulation of oxidative phosphorylation of liver mitochondria in sepsis. Cells 2022, 11, 1598. [Google Scholar] [CrossRef] [PubMed]
- Singer, M. Mitochondrial function in sepsis: Acute phase versus multiple organ failure. Crit. Care Med. 2007, 35, S441–S448. [Google Scholar] [CrossRef] [PubMed]
- Valdez, L.B.; Zaobornyj, T.; Boveris, A. Functional activity of mitochondrial nitric oxide synthase. Methods Enzymol. 2005, 396, 445–455. [Google Scholar]
- Giulivi, C. functional implication of nitric oxide produced by mitochondria in mitochondrial metabolism. Biochem. J. 1998, 332, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wang, N.; Bisetto SYi, D.; Sheu, S.S. Downregulation of adenine nucleotide translocator 1 exacerbates tumor necrosis factor-α-mediated cardiac inflammatory responses. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, H39–H48. [Google Scholar] [CrossRef] [PubMed]
- Rittirsch, D.; Huber-Lang, M.S.; Flierl, M.A.; Ward, P.A. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009, 4, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hermann, I.K.; Castellon, M.; Schwartz, D.E.; Hasler, M.; Urner, M.; Hu, G.; Minshall, R.D.; Beck-Schimmer, B. Volatile anesthetics improve survival after cecal ligation and puncture. Anesthesiology 2013, 119, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Eriksson, O.; Ichas, F.; Bernardi, P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation by electron flow through the respiratory chain complex I. J. Biol. Chem. 1998, 273, 12662–12668. [Google Scholar] [CrossRef] [PubMed]
- Batandier, C.; Poulet, L.; Hininger, I.; Couturier, K.; Fontaine, E.; Roussel, A.M.; Canini, F. Acute stress delays brain mitochondrial permeability transition pore. J. Neurochem. 2014, 131, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Clement, P.; Sarda, N.; Cespuglio, R.; Gharib, A. Changes occurring in cortical NO release and brain NO-synthases during a paradoxical sleep deprivation and subsequent recovery in the rats. J. Neurochem. 2004, 90, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Carreras, M.C.; Peralta, J.G.; Converso, D.P.; Finocchietto, P.V.; Rebagliati, I.; Zaninovich, A.A.; Pederoso, J.J. Modulation of liver mitochondrial NOS is implicated in thyroid-dependent regulation of O2 uptake. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, 2282–2288. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yi, C.; Wang, H.; Bruce, I.C.; Xia, Q. Mitochondrial Nitric Oxide Synthase Participates in Septic Shock Myocardial Depression by Nitric Oxide Overproduction and Mitochondrial Permeability Transition Pore Opening. Shock 2012, 37, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Alverdy, J.C.; Krezalek, M.A. Collapse of microbiome, emergence of pathobiome, and immunopathology of sepsis. Crit. Care Med. 2017, 45, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Portella, V.G.; Silva-Filho, J.L.; Landgraf, S.S.; Baldez de Rico, T.; Viera, M.A.R.; Takiya, C.M.; Souza, M.C.; Henriques, M.G.; Canetti, C.; Pinheiro, A.A.S.; et al. Sepsis-Surviving mice are more susceptible to a secondary kidney insult. Crit. Care Med. 2013, 41, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Skulachev, V.P. Reactive oxygen species, mitochondria, apoptosis, aging. Mol. Cell Biochem. 1997, 174, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Marcia, F.; Natali, A.J.; Da Silva, E.; Gomes, G.J.; Teodoro, B.G.; Cunha, D.N.Q.; Drummond, L.R.; Drummond, F.R.; Moura, A.G.; Belfort, F.G.; et al. Attenuation of Ca2+ homeostasis, oxidative stress, and mitochondrial dysfunctions in diabetic rats heart: Insulin therapy or aerobic exercise? J. Appl. Physiol. 2015, 119, 148–156. [Google Scholar] [CrossRef]
- Lemasters, J.J.; Theruvath, T.P.; Zhong, Z. Mitochondrial calcium and the permeability transition in cell death. Biochim. Biophys. Acta 2009, 1787, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.G.; Todor, A.V.; Imai, M.; Sabbah, H.N. Inhibition of mitochondrial permeability transition pores by cyclosporine A improves cytochrome c oxidase function and increases rate of ATP synthesis in failing cardiomyocytes. Heart Fail. Rev. 2005, 10, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Yu, Y.M. The metabolic effect of thermal injury. World J. Surg. 1992, 16, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Cooper, C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994, 356, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Abe, M.; Shibata, K.; Shimizu, N.; Sakata, N.; Katsuragi, T.; Tanaka, K. Evaluating the role of inducible nitric oxide synthase using a novel and selective inducible nitric oxide synthase inhibitor in septic lung injury produced by cecal ligation and puncture. Am. J. Respir. Crit. Care Med. 2000, 162, 716–722. [Google Scholar] [CrossRef] [PubMed]
- Piantadosi, C.A. Regulation of mitochondrial processes by protein S-nitrosylation. Biochim. Biophys Acta 2012, 1820, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed]
- Brooke, P.S.; Juan PBolanos, J.P.; Heales, S.J.R. The assumption that nitric oxide inhibits mitochondrial ATP synthesis is correct. FEBS Lett. 1999, 446, 261–263. [Google Scholar] [CrossRef] [PubMed]
- West, M.B.; Rokosh, G.; Obal, D.; Velayutham, M.; Xuan, Y.T.; Hill, B.G.; Keith, R.J.; Schrader, J.; Guo, Y.; Conklin, D.J.; et al. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 2008, 118, 1970–1978. [Google Scholar] [CrossRef] [PubMed]

| 8 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| CLP | Sham | CLP | Sham | CLP | Sham | |
| State 3 Respiration Rate nmol O2/min/mg | 5.40 [3.26–6.55] | 6.53 [3.49–9.84] | 4.23 [1.33–4.70] * | 6.66 [5.13–10.02] | 2.66 [2.43–3.70] *⸸ | 6.24 [6.08–9.62] |
| State 4 Respiration Rate nmol O2/min/mg | 0.76 [0.14–1.81] | 1.07 [0.31–1.49] | 1.60 [0.60–2.13] | 0.53 [0.34–1.59] | 1.41 [1.12–2.38] * | 0.43 [0.16–0.50] |
| Uncoupling Respiration Rate nmol O2/min/mg | 4.30 [3.25–5.37] | 4.67 [3.76–6.82] | 1.97 [0.32–7.78] | 4.89 [3.84–7.82] | 2.17 [1.89–3.39] * | 4.52 [4.23–5.18] |
| Cytochrome C oxidase activity nmol O2/min/mg | 15 [13.20–20.83] * | 33.86 [27.61–40.55] | 12.60 [8.95–19.21] * | 36.90 [26.74–42.80] | 13.21 [11.63–18.73] * | 41.22 [25.44–45.46] |
| Respiratory Control Ratio | 6.37 [3.11–28.19] | 6.54 [5.81–13.29] | 2.09 [0.74–8.18] | 9.38 [6.16–19.59] | 1.85 [1.54–2.10] *⸸ | 17.06 [13.91–48.45] |
| 8 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| CLP | Sham | CLP | Sham | CLP | Sham | |
| State 3 Respiration Rate nmol O2/min/mg | 3.75 [1.32–6.58] | 4.27 [0.945–8.29] | 2.76 [1.01–4.23] | 4.74 [3.30–5.78] | 1.99 [0.41–3.98] | 4.59 [2.20–7.23] |
| State 4 Respiration Rate nmol O2/min/mg | 1.41 [1.12–2.38] | 1.20 [0.44–2.20] | 0.75 [0.18–1.47] | 0.15 [0.06–0.061] | 1.18 [0.65–1.37] | 0.68 [0.46–1.08] |
| Uncoupling Respiration Rate nmol O2/min/mg | 3.09 [1.77–5.17] | 3.93 [2.02–8.50] | 3.30 [1.72–5.79] | 4.23 [2.21–4.72] | 3.29 [2.61–3.32] | 4.37 [2.33–5.31] |
| Cytochrome C oxidase activity nmol O2/min/mg | 13 [11–21.64] | 14.88 [12.72–27.85] | 16.6 [7.10–28] | 18.29 [17.32–23.45] | 17.1 [11.13–17.55] | 18.50 [14.97–26] |
| Respiratory Control Ratio | 9.41 [3.77–22.84] | 2.26 [1.30–12.51] | 2.93 [2.01–19.85] | 22 [14.19–74.06] | 2.11 [0.32–10.31] | 5.59 [4.32–9.32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyenga, P.; Sheu, S.-S. Nitric Oxide Does Not Improve Liver Mitochondrial Function 48 Hours After Cecal Ligation and Perforation in Experimental Sepsis. Antioxidants 2025, 14, 868. https://doi.org/10.3390/antiox14070868
Eyenga P, Sheu S-S. Nitric Oxide Does Not Improve Liver Mitochondrial Function 48 Hours After Cecal Ligation and Perforation in Experimental Sepsis. Antioxidants. 2025; 14(7):868. https://doi.org/10.3390/antiox14070868
Chicago/Turabian StyleEyenga, Pierre, and Shey-Shing Sheu. 2025. "Nitric Oxide Does Not Improve Liver Mitochondrial Function 48 Hours After Cecal Ligation and Perforation in Experimental Sepsis" Antioxidants 14, no. 7: 868. https://doi.org/10.3390/antiox14070868
APA StyleEyenga, P., & Sheu, S.-S. (2025). Nitric Oxide Does Not Improve Liver Mitochondrial Function 48 Hours After Cecal Ligation and Perforation in Experimental Sepsis. Antioxidants, 14(7), 868. https://doi.org/10.3390/antiox14070868







