Short-Term Intake of Euphorbia tirucalli Latex Modifies Kidney Function in Rats: Possible Role of Oxidative Stress and Inflammatory Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Preparation and Extraction
2.2. Experimental Animals and Study Design
2.3. MAP and Renal Function Measurements
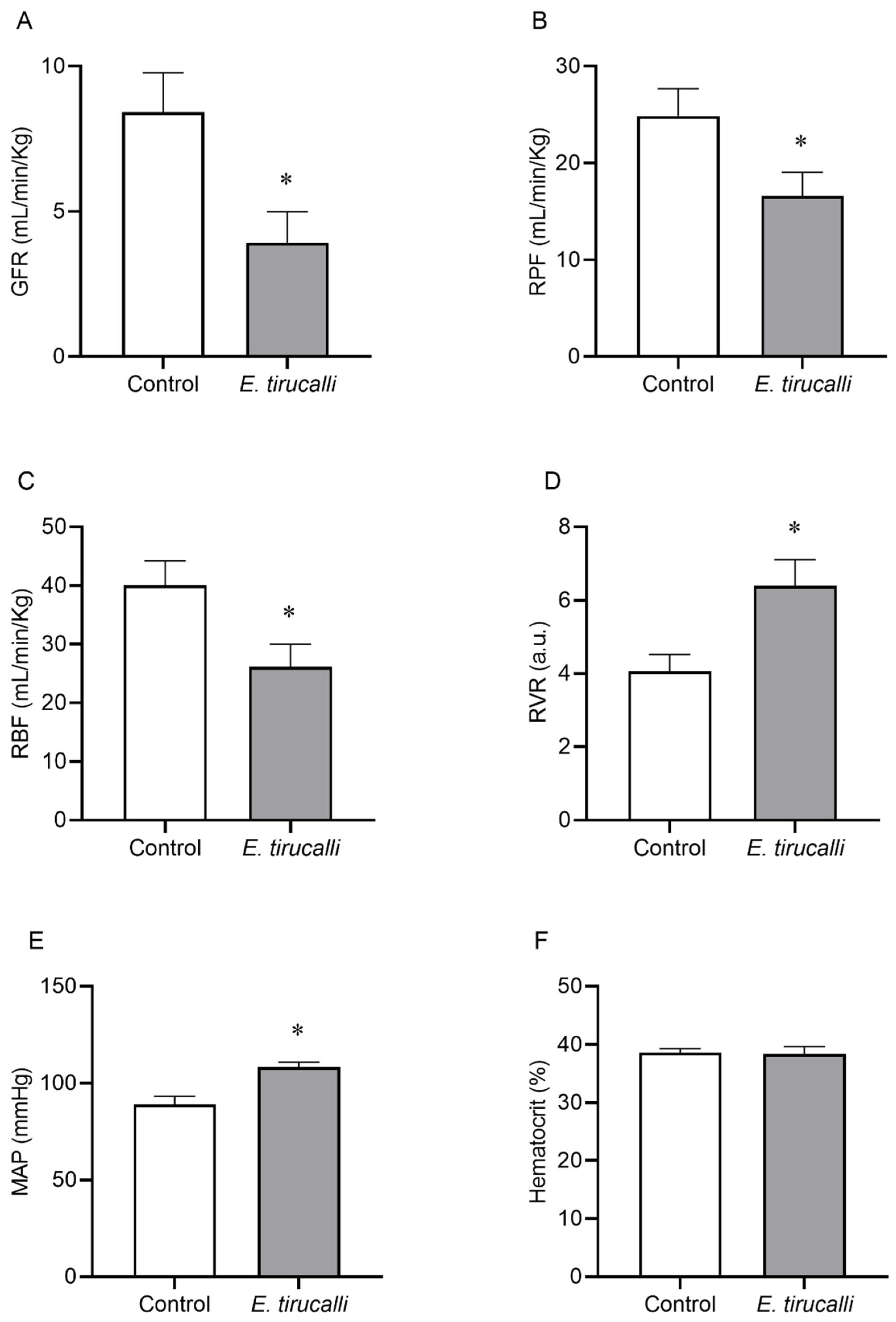
- RBF = renal blood flow (mL/min)
- RPF = renal plasma flow (mL/min)
- Hematocrit = fraction of blood volume occupied by red blood cells
- RVR = renal vascular resistance (mmHg·min/mL)
- MAP = mean arterial pressure (mmHg)
2.4. Kidney Morphometric Measurement
2.5. Kidney Oxidative Stress Analysis
2.6. Kidney Inflammatory Activity Measurement
2.7. Kidney Cell Isolation for ROS and RNS Evaluation
2.8. Quantification of Kidney Cells ROS and RNS Production
2.9. Data Analysis
3. Results
3.1. Effects of Aveloz Treatment on MAP Levels and Renal Function
3.2. Kidney/Tibia Ratio Analysis
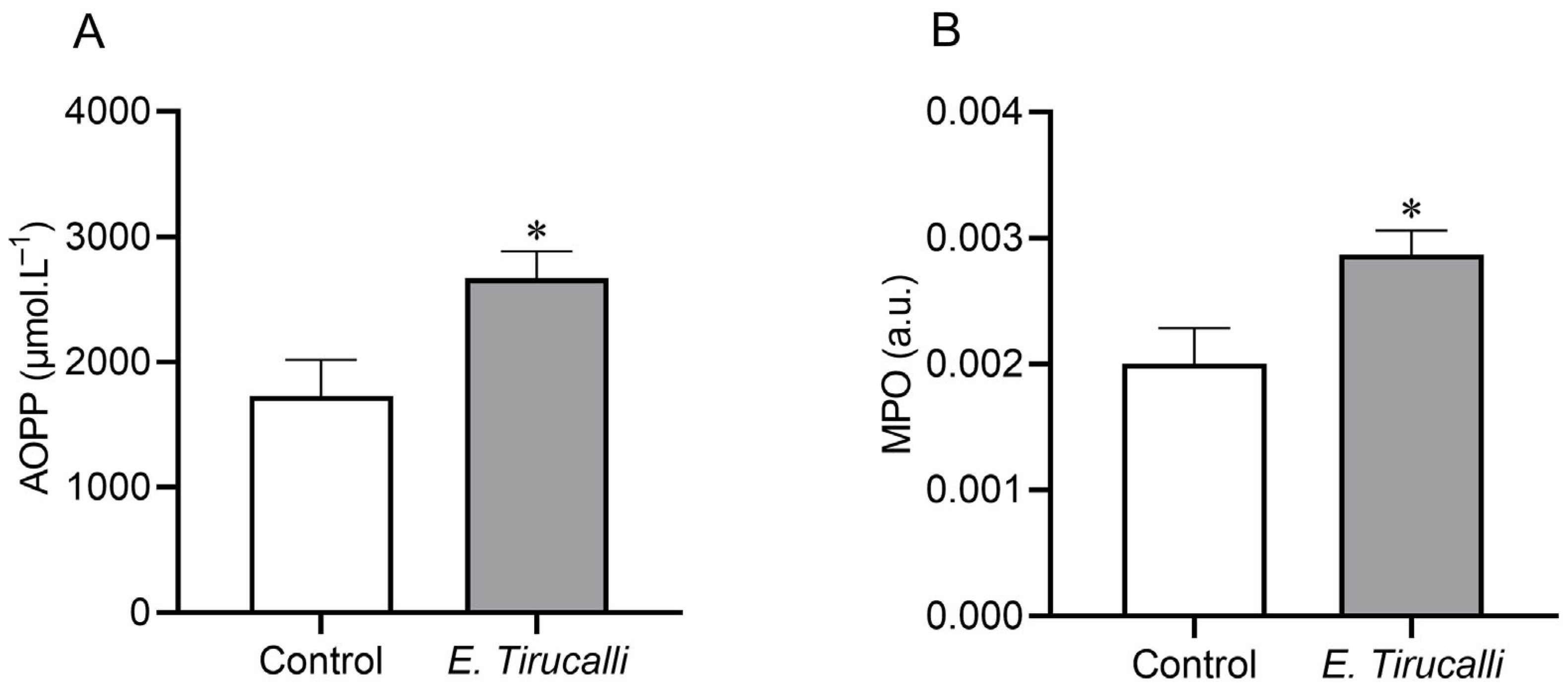
3.3. Renal Oxidative Stress and Inflammatory Response
3.4. Kidney Cells ROS and RNS Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonow, C.T.; Ceolin, T.; Lopes, C.V.; Graciela, J.; Zillmer, V.; Rosiely, N.; Vargas, C.; Heck, R. Plantas Medicinais Utilizadas na Auto atenção por Pessoas com Câncer em Cuidado Paliativo. Texto Contexto Enferm. 2020, 29, e20190329. [Google Scholar] [CrossRef]
- Schmelzer, G.H.; Gurib-Fakim, A.; Arroo, R.; Bosch, C.H.; de Ruijter, A.; Simmonds, M.S.J.; Lemmens, R.H.M.J.; Oyen, L.P.A. Medicinal plants 1 Plant Resources of Tropical Africa. 2008, 11. Available online: https://www.estantevirtual.com.br/livro/dicionario-das-plantas-uteis-do-brasil-1KP-0558-000 (accessed on 9 July 2025).
- Cruz, L.G. Dicionário das Plantas Úteis do Brasil; Bertrand Brasil: Rio de Janeiro, Brazil, 1964. [Google Scholar]
- Dantas, I.C. O Raizeiro, 1st ed.; EDUEP: Campina Grande, Brazil, 2007. [Google Scholar]
- Wal, A.; Wal, P.; Gupta, N.; Vishnoi, G.; Srivastava, R.S. Medicinal Value of Euphorbia tirucalli. Int. J. Pharm. Biol. Arch. 2013, 4, 31–40. [Google Scholar]
- Mali, P.Y.; Panchal, S.S. Euphorbia tirucalli L.: Review on morphology, medicinal uses, phytochemistry and pharmacological activities. Asian Pac. J. Trop. Biomed. 2017, 7, 603–613. [Google Scholar] [CrossRef]
- Dutra, R.C.; Souza, P.; Bento, A.F.; Marcon, R.; Bicca, M.A.; Pianowski, L.F.; Calixto, J.B. Euphol prevents experimental autoimmune encephalomyelitis in mice: Evidence for the underlying mechanisms. Biochem. Pharmacol. 2012, 83, 531–542. [Google Scholar] [CrossRef]
- Cataluña, P.; Rates, S.M.K. The traditional use of the latex from Euphorbia tirucalli Linnaeus (Euphorbiaceae) in the treatment of cancer in south Brazil. Acta Hortic. 1999, 501, 289–296. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S.; Francis, G.; Becker, K. Phorbol esters: Structure, biological activity, and toxicity in animals. Int. J. Toxicol. 2007, 26, 279–288. [Google Scholar] [CrossRef]
- Newton, A.C. Protein kinase C as a tumor suppressor. Semin. Cancer Biol. 2018, 48, 18–26. [Google Scholar] [CrossRef]
- Binckley, S.; Zahra, F. Euphorbia tirucalli Toxicity; StatPearls Publishing LLC.: Orlando, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK574526/ (accessed on 25 May 2025).
- Rodrigues, M.L.; Gomes, A.J.; Funez, M.I.; Marques, S.; Lunardi, C.N. Euphorbia tirucalli latex loaded polymer nanoparticles: Synthesis, characterization, in vitro release and in vivo antinociceptive action. PLoS ONE 2022, 17, e0274432. [Google Scholar] [CrossRef]
- Bessa, G.; Melo-Reis, P.; Araújo, L.; Mrué, F.; Freitas, G.; Brandão, M.; Silva Júnior, N. Angiogenic activity of latex from Euphorbia tirucalli Linnaeus 1753 (Plantae, Euphorbiaceae). Braz. J. Biol. 2015, 75, 752–758. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Hamed, M.B.; Salama, W.H.; Ali, M.M.; Fahmy, A.S.; Mohamed, S.A. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 2019, 20, 101199. [Google Scholar] [CrossRef]
- Martins, C.G.; Appel, M.H.; Coutinho, D.S.S.; Soares, I.P.; Fischer, S.; de Oliveira, B.C.; Fachi, M.M.; Pontarolo, R.; Bonatto, S.J.R.; Fernandes, L.C.; et al. Consumption of latex from Euphorbia tirucalli L. promotes a reduction of tumor growth and cachexia, and immunomodulation in Walker 256 tumor-bearing rats. J. Ethnopharmacol. 2020, 255, 112722. [Google Scholar] [CrossRef] [PubMed]
- Santana, S.S.; Gennari-Cardoso, M.L.; Carvalho, F.C.; Roque-Barreira, M.C.; Santiago, A.S.; Alvim, F.C.; Pirovani, C.P. Eutirucallin, a RIP-2 type lectin from the latex of Euphorbia tirucalli L. presents proinflammatory properties. PLoS ONE 2014, 9, e88422. [Google Scholar] [CrossRef] [PubMed]
- Avelar, B.A.; Lelis, F.J.N.; Avelar, R.S.; Weber, M.; Souza-Fagundes, E.M.; Lopes, M.T.P.; Martins-Filho, O.A.; Brito-Melo, G.E.A. The crude latex of Euphorbia tirucalli modulates the cytokine response of leukocytes, especially CD4+ T lymphocytes. Braz. J. Pharmacogn. 2011, 21, 662–667. [Google Scholar] [CrossRef]
- Neiva, L. A cura do Câncer pelo Aveloz, 1st ed.; Arte Nova S.A.: Rio de Janeiro, Brazil, 1968. [Google Scholar]
- Costa, L.S. Estudo do uso do Aveloz (Euphorbia tirucalli) no Tratamento de Doenças Humanas: Uma Revisão; Trabalho de conclusão de curso; Universidade Estadual da Paraíba: Campina Grande, Brazil, 2011. [Google Scholar]
- FDA. Estimating the Safe Starting Dose in Clinical Trials for Therapeutics in Adult Healthy Volunteers; Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, U.S. Food and Drug Administration: Rockville, MD, USA, 2005; 30p. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Lima, I.L.B.; Bose, P.; Rodrigues, A.; Bergamaschi, C.T.; Campos, R.R.; Hirata, A.E.; Tufik, S.; Xylaras, B.P.; Visniauskas, B.; Chagas, J.R.; et al. Chronic sleep restriction during pregnancy—Repercussion on cardiovascular and renal functioning of male offspring. PLoS ONE 2014, 9, e113075. [Google Scholar] [CrossRef]
- Fuhr, J.; Kaczmarczyk, J.; Kruttgen, C.D. A simple colorimetric method of inulin determination in renal clearance studies on metabolically normal subjects and diabetics. Klin. Wochenschr. 1955, 33, 729–730. [Google Scholar] [CrossRef]
- Saud, A.; Luiz, R.; Paula, A.; Müller, C.R.; Visoná, I.; Reinecke, N.L.; Silva, W.H.; Aparecida, M.; Razvickas, C.V.; Casarini, D.E.; et al. Resistance exercise training ameliorates chronic kidney disease outcomes in a 5/6 nephrectomy model. Life Sci. 2021, 275, 119362. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ozenirler, S.; Erkan, G.; Degertekin, C.K.; Ercin, U.; Cengiz, M.; Bilgihan, A.; Yilmaz, G.; Akyol, G. The relationship between advanced oxidation protein products (AOPP) and biochemical and histopathological findings in patients with nonalcoholic steatohepatitis. J. Dig. Dis. 2014, 15, 131–136. [Google Scholar] [CrossRef]
- Bradley, P.P.; Christensen, R.D.; Rothstein, G. Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 1982, 60, 618–622. [Google Scholar] [CrossRef]
- Dias, A.T.; Rodrigues, B.P.; Porto, M.L.; Gava, A.L.; Balarini, C.M.; Freitas, F.P.S.; Palomino, Z.; Casarini, D.E.; Campagnaro, B.P.; Pereira, T.M.C.; et al. Sildenafil ameliorates oxidative stress and DNA damage in the stenotic kidneys in mice with renovascular hypertension. J. Transl. Med. 2014, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Folkmann, J.K.; Loft, S.; Moller, P. Oxidatively damaged DNA in aging dyslipidemic ApoE−/− and wild-type mice. Mutagenesis 2007, 22, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Campagnaro, B.; Louro, L.; Pereira, T.; Vasquez, E.; Meyrelles, S. Effects of aging and hypercholesterolemia on oxidative stress and DNA damage in bone marrow mononuclear cells in apolipoprotein E-deficient mice. Int. J. Mol. Sci. 2013, 14, 3325–3342. [Google Scholar] [CrossRef] [PubMed]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests; StatPearls Publishing LLC.: Orlando, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507821/#article-28359.s1 (accessed on 10 April 2023).
- Meltzer, J.S. Renal Physiology; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Johns, E.J.; Ahmeda, A.F. Renal circulation. Ref. Modul. Biomed. Sci. 2014, 3, 1–13. [Google Scholar] [CrossRef]
- Butterworth, J.F.; Mackey, D.C. Pharmacology of anesthetic agents. In Morgan & Mikhail’s Clinical Anesthesiology, 4th ed.; Butterworth, J.F., Mackey, D.C., Wasnick, J.D., Eds.; McGraw Hill: New York, NY, USA, 2001; pp. 167–190. [Google Scholar]
- Piper, S.N.; Suttner, S.W.; Maleck, W.H.; Boldt, J. Comparison of propofol and thiopental for induction of anesthesia in patients with coronary artery disease. J. Cardiothorac. Vasc. Anesth. 2004, 18, 304–308. [Google Scholar]
- Verbalis, J.G. Disorders of body water homeostasis. Best Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 329–346. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Khalil, R. Protein kinase C inhibitors as modulators of vascular function and their application in vascular disease. Pharmaceuticals 2013, 6, 407–439. [Google Scholar] [CrossRef]
- Ringvold, H.C.; Khalil, R.A. Protein kinase C as regulator of vascular smooth muscle function and potential target in vascular disorders. Adv. Pharmacol. 2017, 78, 203–301. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, K.; Ounissi, M.; Brahmi, N.; Goucha, R.; Hedri, H.; Abdellah, T.; El Younsi, F.; Maiz, H.; Kheder, A. Acute renal failure by ingestion of Euphorbia paralias. Saudi J. Kidney Dis. Transpl. 2013, 24, 571. [Google Scholar] [CrossRef] [PubMed]
- Al-Yousef, H.M.; Alqahtani, A.S.; Ghani, A.S.A.; El-Toumy, S.A.; El-Dougdoug, W.I.A.; Hassan, W.H.B.; Hassan, H.M. Nephroprotective, cytotoxic and antioxidant activities of Euphorbia paralias. Saudi J. Biol. Sci. 2020, 28, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.L. Kidney atrophy vs hypertrophy in diabetes: Which cells are involved? Cell Cycle 2018, 17, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shao, M.; Yang, H.; Chen, L.; Yu, L.; Xiao, J.; Tian, H.; Zhang, F.; Cheng, P.; Jin, L.; et al. Attenuation of hyperlipidemia- and diabetes-induced early-stage apoptosis and late-stage renal dysfunction via administration of fibroblast growth factor-21 is associated with suppression of renal inflammation. PLoS ONE 2013, 8, e82275. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, Ľ. Oxidative stress in the pathophysiology of kidney disease: Implications for noninvasive monitoring and identification of biomarkers. Oxid. Med. Cell. Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Meyrelles, S.S.; Peotta, V.A.; Pereira, T.M.; Vasquez, E.C. Endothelial dysfunction in the apolipoprotein E-deficient mouse: Insights into the influence of diet, gender and aging. Lipids Health Dis. 2011, 10, 211. [Google Scholar] [CrossRef]
- Basile, D.P.; Leonard, E.C.; Beal, A.G.; Schleuter, D.; Friedrich, J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am. J. Physiol. Renal Physiol. 2012, 302, F1494–F1502. [Google Scholar] [CrossRef]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Basu, P.; Hornung, R.S.; Averitt, D.L.; Maier, C. Euphorbia bicolor (Euphorbiaceae) latex extract reduces inflammatory cytokines and oxidative stress in a rat model of orofacial pain. Oxid. Med. Cell. Longev. 2019, 2019, 8594375. [Google Scholar] [CrossRef] [PubMed]
- Tsumbu, C.N.; Deby-Dupont, G.; Tits, M.; Angenot, L.; Frederich, M.; Kohnen, S.; Mouithys-Mickalad, A.; Serteyn, D.; Franck, T. Polyphenol content and modulatory activities of some tropical dietary plant extracts on the oxidant activities of neutrophils and myeloperoxidase. Int. J. Mol. Sci. 2012, 13, 628–650. [Google Scholar] [CrossRef] [PubMed]
- Ratliff, B.B.; Abdulmahdi, W.; Pawar, R.; Wolin, M.S. Oxidant mechanisms in renal injury and disease. Antioxid. Redox Signal. 2016, 25, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Superoxide anion chemistry—Its role at the core of the innate immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Kaushal, G.P.; Chandrashekar, K.; Juncos, L.A. Molecular interactions between reactive oxygen species and autophagy in kidney disease. Int. J. Mol. Sci. 2019, 20, 3791. [Google Scholar] [CrossRef]
- Freitas, F.P.; Porto, M.L.; Tranhago, C.P.; Piontkowski, R.; Miguel, E.C.; Miguel, T.B.; Martins, J.L.; Nascimento, K.S.; Balarini, C.M.; Cavada, B.S.; et al. Dioclea violacea lectin ameliorates oxidative stress and renal dysfunction in an experimental model of acute kidney injury. Am. J. Transl. Res. 2015, 7, 2573–2588. [Google Scholar]
- Griner, E.M.; Kazanietz, M.G. Protein kinase C and other diacylglycerol effectors in cancer. Nat. Rev. Cancer 2007, 7, 281–294. [Google Scholar] [CrossRef]
- Wu-Zhang, A.X.; Newton, A.C. Protein kinase C pharmacology: Refining the toolbox. Biochem. J. 2013, 452, 195–209. [Google Scholar] [CrossRef]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative stress and hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Jaken, S. Protein kinase C signaling and oxidative stress. Free Radic. Biol. Med. 2000, 28, 1349–1361. [Google Scholar] [CrossRef]
- Roberts, A.C.; Porter, K.E. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diabetes Vasc. Dis. Res. 2013, 10, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-J.; Suo, P.; Wang, Y.-N.; Zou, L.; Nie, X.-L.; Zhao, Y.-Y.; Miao, H. Arachidonic acid metabolism as a therapeutic target in AKI-to-CKD transition. Front. Pharmacol. 2024, 15, 1365802. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gobe, G. Protein kinase C activation and its role in kidney disease (review article). Nephrology 2006, 11, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Nowak, G.; Bakajsova, D. Protein kinase C-α activation promotes recovery of mitochondrial function and cell survival following oxidant injury in renal cells. Am. J. Physiol. Renal Physiol. 2012, 303, F515–F526. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, D.-C.; Choi, B.-H.; Ha, H.; Kim, K.-T. Regulation of p53 by activated protein kinase C-δ during nitric oxide-induced dopaminergic cell death. J. Biol. Chem. 2006, 281, 2215–2224. [Google Scholar] [CrossRef]

| Group\Parameters | Kidney Weight (mg) | Tibia Length (cm) | Kidney/Tibia Ratio (mg/cm) |
|---|---|---|---|
| Control | 1353 ± 22.97 | 4.36 ± 0.02 | 310.2 ± 4.29 |
| E. tirucalli | 1313 ± 11.04 | 4.36 ± 0.02 | 301.2 ± 2.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kampke, E.H.; Barroso, M.E.S.; Escouto, L.d.S.; Covre, L.P.; Gava, Á.L.; Campagnaro, B.P.; Kuster, R.M.; Meyrelles, S.S. Short-Term Intake of Euphorbia tirucalli Latex Modifies Kidney Function in Rats: Possible Role of Oxidative Stress and Inflammatory Response. Antioxidants 2025, 14, 856. https://doi.org/10.3390/antiox14070856
Kampke EH, Barroso MES, Escouto LdS, Covre LP, Gava ÁL, Campagnaro BP, Kuster RM, Meyrelles SS. Short-Term Intake of Euphorbia tirucalli Latex Modifies Kidney Function in Rats: Possible Role of Oxidative Stress and Inflammatory Response. Antioxidants. 2025; 14(7):856. https://doi.org/10.3390/antiox14070856
Chicago/Turabian StyleKampke, Edgar Hell, Maria Eduarda Souza Barroso, Leonardo da Silva Escouto, Luciana Polaco Covre, Ágata Lages Gava, Bianca Prandi Campagnaro, Ricardo Machado Kuster, and Silvana Santos Meyrelles. 2025. "Short-Term Intake of Euphorbia tirucalli Latex Modifies Kidney Function in Rats: Possible Role of Oxidative Stress and Inflammatory Response" Antioxidants 14, no. 7: 856. https://doi.org/10.3390/antiox14070856
APA StyleKampke, E. H., Barroso, M. E. S., Escouto, L. d. S., Covre, L. P., Gava, Á. L., Campagnaro, B. P., Kuster, R. M., & Meyrelles, S. S. (2025). Short-Term Intake of Euphorbia tirucalli Latex Modifies Kidney Function in Rats: Possible Role of Oxidative Stress and Inflammatory Response. Antioxidants, 14(7), 856. https://doi.org/10.3390/antiox14070856







