Quercetin Can Alleviate ETECK88-Induced Oxidative Stress in Weaned Piglets by Inhibiting Quorum-Sensing Signal Molecule Autoinducer-2 Production in the Cecum
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials and Culture Conditions of Strains
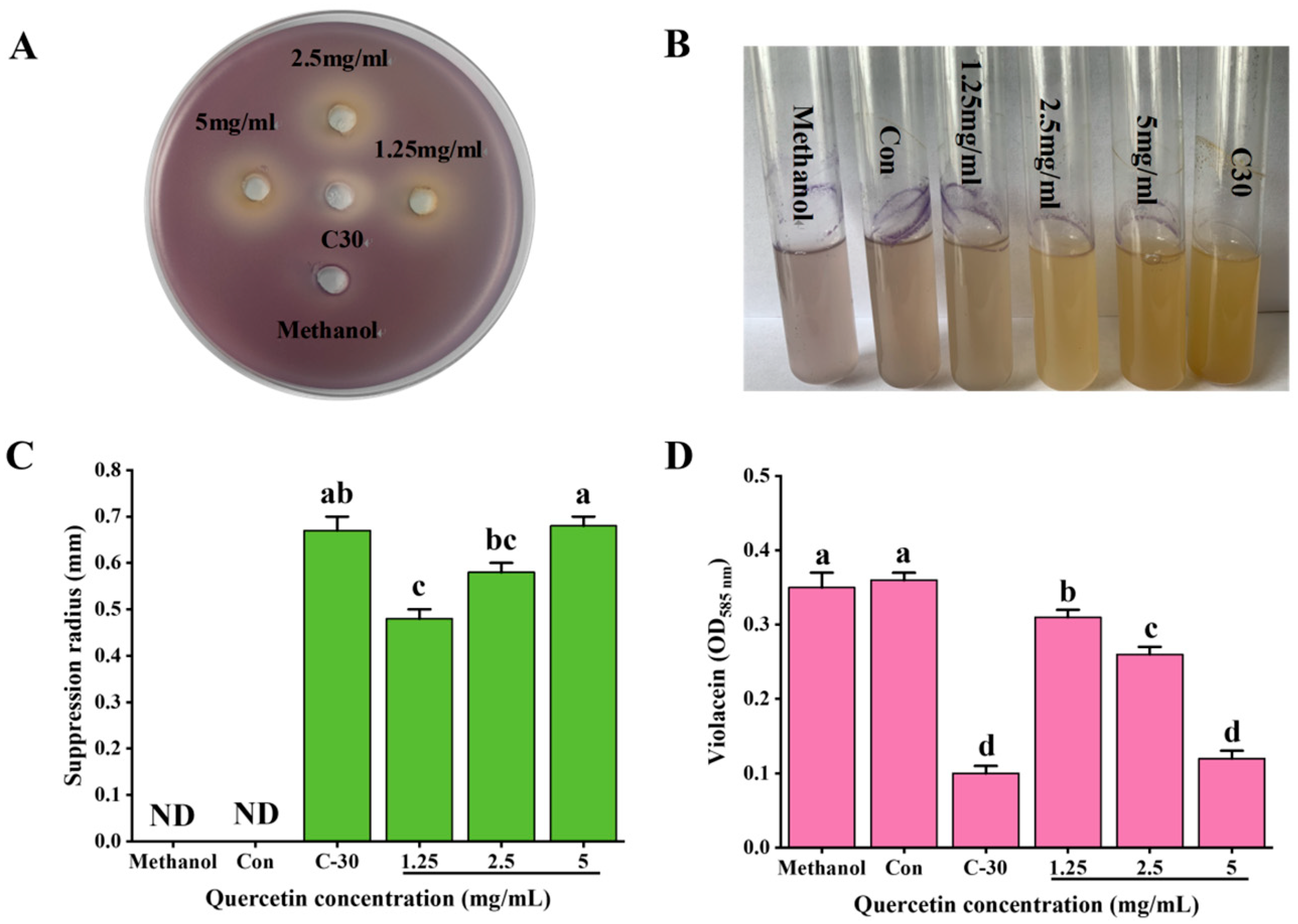
2.2. Evaluation of Quorum-Sensing Inhibitory Activity of Que
2.3. Quantitative Inhibition of Que on Violacein Production by C. violaceum ATCC12472
2.4. Experimental Design and Feed Management of Experimental Animals
2.5. Test Sample Collection
2.6. Determination of Antioxidant Immune Indexes in the Serum, Liver, Spleen, Ileum, and Colon Mucosa
2.7. Histopathological Examination
2.8. Real-Time qPCR Analysis of Liver and Spleen Tissues
2.9. Analysis of Short-Chain Fatty Acids in the Cecal Samples
2.10. Escherichia coli Plate Count Analysis of the Cecal Content Samples
2.11. Detection of AI-2 Signal Molecules in the Cecal Content Samples
2.12. Statistical Analysis
3. Results
3.1. Inhibitory Effect of Que on Quorum Sensing of C. violaceum ATCC12472
3.2. Effects of Que on Serum Antioxidant and Immune Indices of Weaned Piglets Challenged with K88
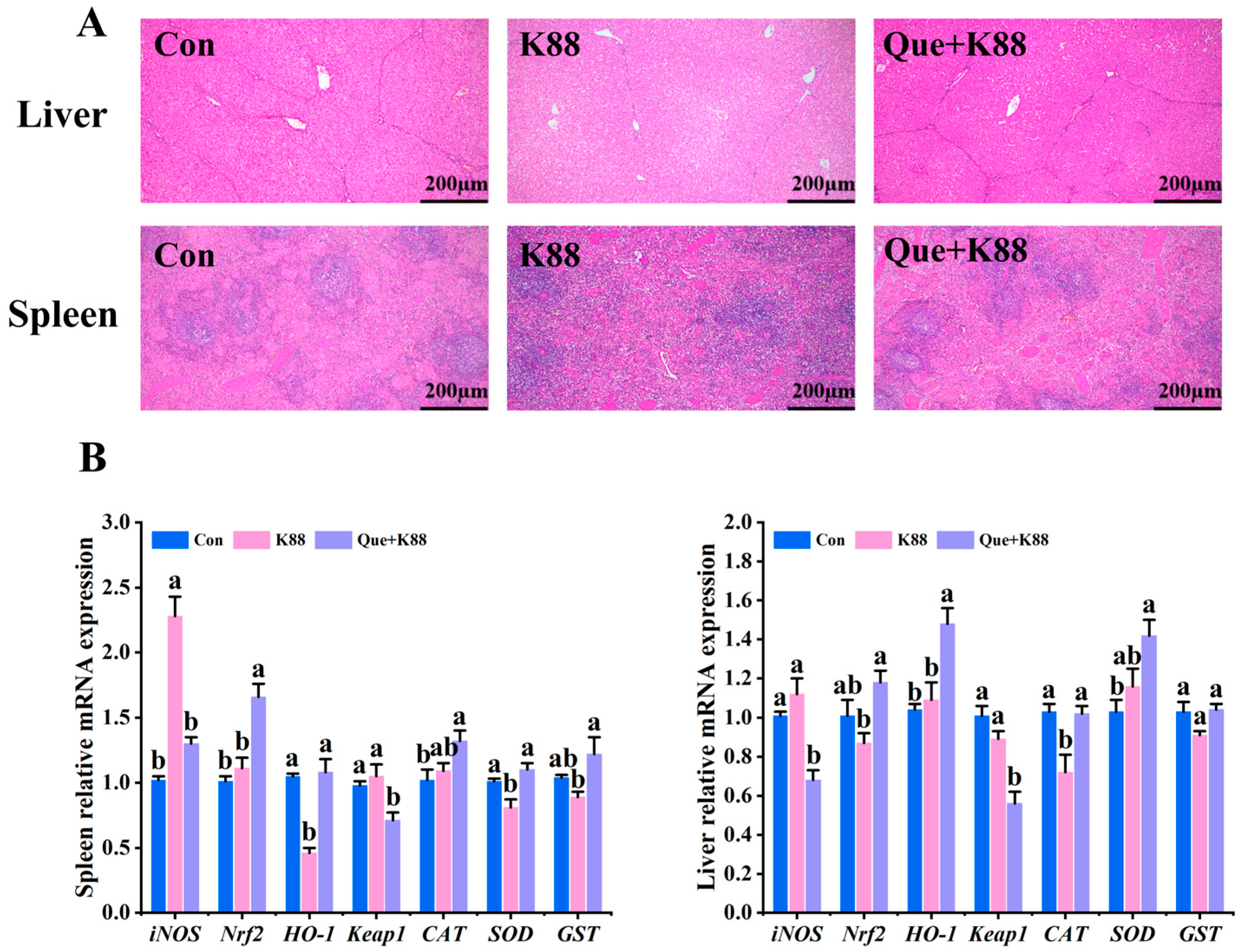
3.3. Effects of Que on Antioxidant and Immune Indexes in the Liver of Weaned Piglets Challenged with K88
3.4. Effects of Que on Antioxidant and Immune Indexes in the Spleen of Weaned Piglets Challenged with K88
3.5. Effects of K88 Challenge on Liver and Spleen Morphology and Antioxidant Gene Expression
3.6. Effects of Que on Antioxidant and Immune Indexes of the Ileum Mucosa in Weaned Piglets Challenged with K88
3.7. Effects of Que on Antioxidant and Immune Indexes of Colonic Mucosa in Weaned Piglets Challenged with K88
3.8. Effects of Que on Volatile Fatty Acids in the Cecal Contents of Weaned Piglets Challenged with K88
3.9. Effects of Que on the Number of Viable Escherichia coli and AI-2 Production in the Cecal Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, J.D.; Bagamian, K.H.; Muhib, F.; Amaya, M.P.; Laytner, L.A.; Wierzba, T.; Rheingans, R. Burden of enterotoxigenic Escherichia coli and shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: A modelling analysis. Lancet Glob. Health 2019, 7, e321–e330. [Google Scholar] [CrossRef] [PubMed]
- Bagamian, K.H.; Anderson, J.D.; Muhib, F.; Cumming, O.; Laytner, L.A.; Wierzba, T.; Rheingans, R.F. Heterogeneity in enterotoxigenic Escherichia coli and shigella infections in children under 5 years of age from 11 African countries: A subnational approach quantifying risk, mortality, morbidity, and stunting. Lancet Glob. Health 2020, 8, e101–e112. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.M.; Castro, J.; Araújo, D.; Campos, A.M.; Oliveira, R.; Silva, S.; Outor-Monteiro, D.; Almeida, C. Swine Colibacillosis: Global Epidemiologic and Antimicrobial Scenario. Antibiotics 2023, 12, 682. [Google Scholar] [CrossRef]
- Xia, P.; Wang, Y.; Zhu, C.; Zou, Y.; Yang, Y.; Liu, W.; Hardwidge, P.R.; Zhu, G. Porcine aminopeptidase N binds to F4+ enterotoxigenic Escherichia coli fimbriae. Vet. Res. 2016, 47, 24. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.; Zeng, X.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X.; et al. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef]
- Poirel, L.; Madec, J.; Lupo, A.; Schink, A.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ben, Y.; Fu, C.; Hu, M.; Liu, L.; Wong, M.H.; Zheng, C. Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: A review. Environ. Res. 2019, 169, 483–493. [Google Scholar] [CrossRef]
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
- Patel, S.J.; Wellington, M.; Shah, R.M.; Ferreira, M.J. Antibiotic Stewardship in Food-producing Animals: Challenges, Progress, and Opportunities. Clin. Ther. 2020, 42, 1649–1658. [Google Scholar] [CrossRef]
- Millet, S.; Maertens, L. The European ban on antibiotic growth promoters in animal feed: From challenges to opportunities. Vet. J. 2011, 187, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, M.; Diggle, S.P.; Greenberg, E.P. Progress in and promise of bacterial quorum sensing research. Nature 2017, 551, 313–320. [Google Scholar] [CrossRef]
- Piewngam, P.; Chiou, J.; Chatterjee, P.; Otto, M. Alternative approaches to treat bacterial infections: Targeting quorum-sensing. Expert. Rev. Anti Infect. Ther. 2020, 18, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial Quorum Sensing. Cell 2018, 174, 1328.e1. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.; Meng, F.; Ma, X.; Song, X.; Liu, X.; Gao, W.; Dang, Y.; Meng, Y.; Cao, M.; Song, C. Regulation of bacteria population behaviors by AI-2 “consumer cells” and “supplier cells”. BMC Microbiol. 2017, 17, 198. [Google Scholar] [CrossRef]
- Song, S.; Wood, T.K. The Primary Physiological Roles of Autoinducer 2 in Escherichia coli Are Chemotaxis and Biofilm Formation. Microorganisms 2021, 9, 386. [Google Scholar] [CrossRef]
- Haque, S.; Ahmad, F.; Dar, S.A.; Jawed, A.; Mandal, R.K.; Wahid, M.; Lohani, M.; Khan, S.; Singh, V.; Akhter, N. Developments in strategies for Quorum Sensing virulence factor inhibition to combat bacterial drug resistance. Microb. Pathog. 2018, 121, 293–302. [Google Scholar] [CrossRef]
- Zhao, X.; Yu, Z.; Ding, T. Quorum-Sensing Regulation of Antimicrobial Resistance in Bacteria. Microorganisms 2020, 8, 425. [Google Scholar] [CrossRef]
- Brüssow, H. Adjuncts and alternatives in the time of antibiotic resistance and in-feed antibiotic bans. Microb. Biotechnol. 2017, 10, 674–677. [Google Scholar] [CrossRef]
- Biswas, S.; Ahn, J.M.; Kim, I.H. Assessing the potential of phytogenic feed additives: A comprehensive review on their effectiveness as a potent dietary enhancement for nonruminant in swine and poultry. J. Anim. Physiol. Anim. Nutr. 2024, 108, 711–723. [Google Scholar] [CrossRef]
- Alymanesh, M.R.; Solhjoo, A.; Pishgarm, E.; Akhlaghi, M. Falcaria vulgaris extract: A mixture of quorum sensing inhibitors for controlling Pectobacterium carotovorum subsp. carotovorum. Food Microbiol. 2024, 122, 104535. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.R.; Samir, R.; Jamil, M.; Abdel-Hafez, L.; Ramadan, M.A. Olive Leaf Extract Modulates Quorum Sensing Genes and Biofilm Formation in Multi-DrugResistant Pseudomonas aeruginosa. Antibiotics 2020, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.M.; Ahmad, I.; Al-Thubiani, A.S.; Abulreesh, H.H.; AlHazza, I.M.; Aqil, F. Leaf Extracts of Mangifera indica L. Inhibit Quorum Sensing—Regulated Production of Virulence Factors and Biofilm in Test Bacteria. Front. Microbiol. 2017, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, I.; Abbas, H.A.; Ashour, M.L.; Yasri, A.; El-Shazly, A.M.; Wink, M.; Sobeh, M. Polyphenols from Salix tetrasperma Impair Virulence and Inhibit Quorum Sensing of Pseudomonas aeruginosa. Molecules 2020, 25, 1341. [Google Scholar] [CrossRef]
- Rivera, M.L.C.; Hassimotto, N.M.A.; Bueris, V.; de Sircili, M.P.; Almeida, F.A.; Pinto, U.M. Effect of Capsicum Frutescens Extract, Capsaicin, and Luteolin on Quorum Sensing Regulated Phenotypes. J. Food Sci. 2019, 84, 1477–1486. [Google Scholar] [CrossRef]
- Quecan, B.X.V.; Santos, J.T.C.; Rivera, M.L.C.; Hassimotto, N.M.A.; Almeida, F.A.; Pinto, U.M. Effect of quercetin Rich Onion Extracts on Bacterial Quorum Sensing. Front. Microbiol. 2019, 10, 867. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Gao, L. Mechanism of antioxidant properties of quercetin and quercetin-DNA complex. J. Mol. Model. 2020, 26, 133. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lei, X.J.; Park, J.W.; Baek, D.H.; Kim, I.H. Effects of mixture of organic acids and medium chain fatty acids on growth performance, diarrhea incidence, and fecal microbial flora in weaning pigs orally challenged with enterotoxigenic Escherichia coli K88. J. Anim. Sci. 2024, 95, 107–108. [Google Scholar] [CrossRef]
- Vangroenweghe, F.A. Vaccination with an E. coli F4/F18 vaccine for the prevention of F4-ETEC post-weaning diarrhea resulted in reduced post-weaning mortality and antibiotic use. J. Anim. Sci. 2021, 99 (Suppl. S1), 140. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, X.; Han, L.; Wang, M.; Lian, S.; Wang, K.; Li, C. Effects on the intestinal morphology, inflammatory response and microflora in piglets challenged with enterotoxigenic Escherichia coli K88. Res. Vet. Sci. 2023, 157, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Xu, H.; Yang, C.O.K. Regulation of oxidative stress in the intestine of piglets after enterotoxigenic Escherichia coli (ETEC) infection. Biochim. Biophys. Acta Mol. Cell Res. 2024, 871, 119711. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.P.; Zeng, H.; Wan, C.X.; Zhou, Z.B. Amicoumacins from a desert bacterium: Quorum sensing inhibitor against Chromobacterium violaceum. Nat. Prod. Res. 2021, 35, 5508–5512. [Google Scholar] [CrossRef] [PubMed]
- Skogman, M.E.; Kanerva, S.; Manner, S.; Vuorela, P.M.; Fallarero, A. Flavones as Quorum Sensing Inhibitors Identified by a Newly Optimized Screening Platform Using Chromobacterium violaceum as Reporter Bacteria. Molecules 2016, 21, 1211. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine; The National Academic Press: Washington, DC, USA, 2012.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 6436-2018; Determination of Calcium in Feeds. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- GB/T 6437-2018; Determination of Phosphorus in Feeds—Spectrophotometry. Standardization Administration of the People’s Republic of China: Beijing, China, 2018.
- Dong, L.; Li, H.M.; Wang, S.N.; Wang, T.L.; Yu, L.H.; Wang, H.R. Meishan neonatal piglets tend to have higher intestinal barrier function than crossbred neonatal piglets. Animal 2021, 15, 100037. [Google Scholar] [CrossRef]
- Pang, X.; Wei, X.; Wu, Y.; Nan, S.; Feng, J.; Wang, F.; Yao, M.; Nie, C. Capsaicin Modulates Hepatic and Intestinal Inflammation and Oxidative Stress by Regulating the Colon Microbiota. Antioxidants 2024, 13, 942. [Google Scholar] [CrossRef] [PubMed]
- Ramić, D.; Klančnik, A.; Možina, S.S.; Dogsa, I. Elucidation of the AI-2 communication system in the food-borne pathogen Campylobacter jejuni by whole-cell-based biosensor quantification. Biosens. Bioelectron. 2022, 212, 114439. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Brackman, G.; Coenye, T. Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 2013, 21, 619–624. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Alcalde-Rico, M.; Gil-Gil, T.; Valverde, J.R.; Martínez, J.L. Naringenin Inhibition of the Pseudomonas aeruginosa Quorum Sensing Response Is Based on Its Time-Dependent Competition With N-(3-Oxo-dodecanoyl)-L-homoserine Lactone for LasR Binding. Front. Mol. Biosci. 2020, 7, 25. [Google Scholar] [CrossRef]
- Paczkowski, J.E.; Mukherjee, S.; McCready, A.R.; Cong, J.P.; Aquino, C.J.; Kim, H.; Henke, B.R.; Smith, C.D.; Bassler, B.L. Flavonoids Suppress Pseudomonas aeruginosa Virulence through Allosteric Inhibition of Quorum-sensing Receptors. J. Biol. Chem. 2017, 292, 4064–4076. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Wu, S.; Jia, H.; Si, X.; Song, Z.; Zhai, Z.; Bai, J.; Li, J.; Yang, Y.; Wu, Z. Resveratrol alleviates enterotoxigenic Escherichia coli K88-induced damage by regulating SIRT-1 signaling in intestinal porcine epithelial cells. Food Funct. 2022, 13, 7346–7360. [Google Scholar] [CrossRef] [PubMed]
- Tokuhara, D.; Kurashima, Y.; Kamioka, M.; Nakayama, T.; Ernst, P.; Kiyono, H.A. comprehensive understanding of the gut mucosal immune system in allergic inflammation. Allergol. Int. 2019, 68, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Maintenance of gut homeostasis by the mucosal immune system. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 423–435. [Google Scholar] [CrossRef]
- Chen, K.; Magri, G.; Grasset, E.K.; Cerutti, A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2016, 20, 427–441. [Google Scholar] [CrossRef]
- Čipak Gašparović, A.; Milković, L.; Rodrigues, C.; Mlinarić, M.; Soveral, G. Peroxiporins Are Induced upon Oxidative Stress Insult and Are Associated with Oxidative Stress Resistance in Colon Cancer Cell Lines. Antioxidants 2021, 10, 1856. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Wu, Z.; Ji, Y. Heat Stress-Induced Intestinal Barrier Impairment: Current Insights into the Aspects of Oxidative Stress and Endoplasmic Reticulum Stress. J. Agric. Food Chem. 2023, 71, 5438–5449. [Google Scholar] [CrossRef]
- Ramachandran, A.; Jaeschke, H. Oxidative stress and acute hepatic injury. Curr. Opin. Toxicol. 2018, 7, 17–21. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxid. Med. Cell Longev. 2016, 2016, 5987183. [Google Scholar] [CrossRef]
- Fentoğlu, Ö.; Kırzıoğlu, F.Y.; Bulut, M.T.; Kumbul Doğuç, D.; Kulaç, E.; Önder, C.; Günhan, M. Evaluation of lipid peroxidation and oxidative DNA damage in patients with periodontitis and hyperlipidemia. J. Periodontol. 2015, 86, 682–688. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.W.L.; Machado, K.D.C.; Machado, K.D.C.; Figueiredo, D.D.R.; David, J.M.; Islam, M.T.; Uddin, S.J.; Shilpi, J.A.; Costa, J.P. In vitro antioxidant properties of the biflavonoid agathisflavone. Chem. Cent. J. 2018, 12, 75. [Google Scholar] [CrossRef]
- Zeng, Y.; Song, J.; Zhang, M.; Wang, H.; Zhang, Y.; Suo, H. Comparison of In Vitro and In Vivo Antioxidant Activities of Six Flavonoids with Similar Structures. Antioxidants 2020, 9, 732. [Google Scholar] [CrossRef]
- Sun, J.; Liu, H.; Yan, Y.; Fang, F. Quercetin prevents sarcopenia by reversing oxidative stress and mitochondrial damage. J. Mol. Histol. 2025, 56, 133. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Zhang, S.; Wang, X.; Ran, G.; Zhang, X.; Xi, J.; Gao, Z.; Lei, Y.; Pan, J.; Liu, Y.; et al. Inflammation Intensifies Monocrotaline-Induced Liver Injury. J. Agric. Food Chem. 2023, 71, 3433–3443. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, J.; Zou, P.; Zhou, Y.; Wang, B.; Yu, D.; Li, W.; Zhan, X. Effects of Dietary Supplementation of Humic Acid Sodium and Zinc Oxide on Growth Performance, Immune Status and Antioxidant Capacity of Weaned Piglets. Animals 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Fong, F.L.Y.; El-Nezami, H.; Mykkänen, O.; Kirjavainen, P.V. The Effects of Single Strains and Mixtures of Probiotic Bacteria on Immune Profile in Liver, Spleen, and Peripheral Blood. Front. Nutr. 2022, 9, 773298. [Google Scholar] [CrossRef]
- Duan, G.; Huang, P.; Zheng, C.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; Li, F.; Guo, Q.; Yin, Y.; et al. Development and Recovery of Liver Injury in Piglets by Incremental Injection of LPS. Antioxidants 2023, 12, 1143. [Google Scholar] [CrossRef]
- Santos-Zas, I.; Lemarié, J.; Zlatanova, I.; Cachanado, M.; Seghezzi, J.C.; Benamer, H.; Goube, P.; Vandestienne, M.; Cohen, R.; Ezzo, M.; et al. Cytotoxic CD8+ T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat. Commun. 2021, 12, 1483. [Google Scholar] [CrossRef]
- Verso, L.L.; Matte, J.J.; Lapointe, J.; Talbot, G.; Bissonnette, N.; Blais, M.; Guay, F.; Lessard, M. Impact of birth weight and neonatal nutritional interventions with micronutrients and bovine colostrum on the development of piglet immune response during the peri-weaning period. Vet. Immunol. Immunopathol. 2020, 226, 110072. [Google Scholar] [CrossRef]
- Ferrara, F.; Tedin, L.; Pieper, R.; Meyer, W.; Zentek, J. Influence of medium-chain fatty acids and short-chain organic acids on jejunal morphology and intra-epithelial immune cells in weaned piglets. J. Anim. Physiol. Anim. Nutr. 2017, 101, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Jimbo, I.; Oda, M.; Noguchi, M.; Kawasaki, K.; Osada-Oka, M.; Tsukahara, T.; Inoue, R. Effect of Porcine Colostral Exosomes on T Cells in the Peripheral Blood of Suckling Piglets. Animals 2022, 12, 2172. [Google Scholar] [CrossRef]
- Hosseinzade, A.; Sadeghi, O.; Naghdipour Biregani, A.; Soukhtehzari, S.; Brandt, G.S.; Esmaillzadeh, A. Immunomodulatory Effects of Flavonoids: Possible Induction of T CD4+ Regulatory Cells Through Suppression of mTOR Pathway Signaling Activity. Front. Immunol. 2019, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Guo, J.; Li, X.; Wang, Y.; Wang, D.; Xiao, K.; Zhu, H.; Wang, X.; Hu, C.A.; Zhang, G.; et al. Necroptosis Underlies Hepatic Damage in a Piglet Model of Lipopolysaccharide-Induced Sepsis. Front. Immunol. 2021, 12, 633830. [Google Scholar] [CrossRef]
- Machado-Junior, P.A.; Araújo, N.P.S.; Souza, A.B.F.; Castro, T.F.; Oliveira, M.; Costa, G.P.; Matos, N.A.; Vieira, P.M.A.; Talvani, A.; Bezerra, F.S.; et al. Protective Effects of Quercetin on Livers from Mice Exposed to Long-Term Cigarette Smoke. Biomed. Res. Int. 2020, 2020, 2196207. [Google Scholar] [CrossRef]
- Shukla, P.; Sahu, N.K.; Kumar, R.; Dhalla, D.K.; Rakshit, S.; Bhadauria, M.; Agrawal, N.D.; Shrivastava, S.; Shukla, S.; Nirala, S.K. Quercetin Ameliorates Acute Acrylamide Induced Spleen Injury. Biotech. Histochem. 2023, 98, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxid. Med. Cell Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef]
- Li, B.; Nasser, M.I.; Masood, M.; Adlat, S.; Huang, Y.; Yang, B.; Luo, C.; Jiang, N. Efficiency of Traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed. Pharmacother. 2020, 126, 110074. [Google Scholar] [CrossRef]
- He, J.; Niu, Y.; Wang, F.; Wang, C.; Cui, T.; Bai, K.; Zhang, J.; Zhong, X.; Zhang, L.; Wang, T. Dietary curcumin supplementation attenuates inflammation, hepatic injury and oxidative damage in a rat model of intra-uterine growth retardation. Br. J. Nutr. 2018, 120, 537–548. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, L.; Zhang, L.; Ying, Z.; Su, W.; Wang, T. Curcumin attenuates D-galactosamine/lipopolysaccharide-induced liver injury and mitochondrial dysfunction in mice. J. Nutr. 2014, 144, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wang, Q.; Wang, S.; Diao, Q.; Zhang, N. The Facilitating Effect of Tartary Buckwheat Flavonoids and Lactobacillus plantarum on the Growth Performance, Nutrient Digestibility, Antioxidant Capacity, and Fecal Microbiota of Weaned Piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef]
- Li, R.; Tan, B.; Jiang, Q.; Chen, F.; Liu, K.; Liao, P. Eucommia ulmoides flavonoids alleviate intestinal oxidative stress damage in weaned piglets by regulating the Nrf2/Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2024, 288, 117373. [Google Scholar] [CrossRef] [PubMed]
- Romero-Durán, M.A.; Silva-García, O.; Perez-Aguilar, J.M.; Baizabal-Aguirre, V.M. Mechanisms of Keap1/Nrf2 modulation in bacterial infections: Implications in persistence and clearance. Front. Immunol. 2024, 15, 1508787. [Google Scholar] [CrossRef]
- Kong, Y.X.; Tian, J.X.; Niu, X.T.; Li, M.; Kong, Y.D.; Li, R.M.; Chen, X.M.; Wang, G.Q. Effects of dietary Quercetin on growth, antioxidant capacity, immune response and immune-related gene expression in snakehead fish, Channa argus. Aquacult. Rep. 2022, 26, 101314. [Google Scholar] [CrossRef]
- Wang, J.; Qian, X.; Gao, Q.; Lv, C.; Xu, J.; Jin, H.; Zhu, H. Quercetin increases the antioxidant capacity of the ovary in menopausal rats and in ovarian granulosa cell culture in vitro. J. Ovarian Res. 2018, 11, 51. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xion, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short chain fatty acids: Microbial metabolites for gut-brain axis signalling. Mol. Cell Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Zhang, D.; Jian, Y.P.; Zhang, Y.N.; Li, Y.; Gu, L.T.; Sun, H.H.; Liu, M.D.; Zhou, H.L.; Wang, Y.S.; Xu, Z.X. Short-chain fatty acids in diseases. Cell Commun. Signal. 2023, 21, 212. [Google Scholar] [CrossRef]
- Zhang, R.; Li, X.; Zhang, S. The Role of Bacteria in Central Nervous System Tumors: Opportunities and Challenges. Microorganisms 2024, 12, 1053. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Wang, P.; Huang, Y.; Wang, F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell Physiol. Biochem. 2018, 49, 190–205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, L.; Bhat, O.M.; Lohner, H.; Li, P.-L. Differential effects of short chain fatty acids on endothelial Nlrp3 inflammasome activation and neointima formation: Antioxidant action of butyrate. Redox Biol. 2018, 16, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Teng, Y.; Zhang, C.; Pan, Y.; Zhang, Q.; Zhu, X.; Liu, N.; Su, X.; Lin, J. The ketone body b-hydroxybutyrate alleviates CoCrMo alloy particles induced osteolysis by regulating NLRP3 inflammasome and osteoclast differentiation. J. Nanobiotechnol 2022, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Jiao, A.R.; Yu, B.; Mao, X.B.; Chen, D.W. Gastric infusion of short-chain fatty acids can improve intestinal barrier function in weaned piglets. Genes. Nutr. 2019, 14, 4. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zhou, S.; Fu, Y.; Yang, Q.; Li, Y. Effects of quercetin and daidzein on egg quality, lipid metabolism, and cecal short-chain fatty acids in layers. Front. Vet. Sci. 2023, 10, 1301542. [Google Scholar] [CrossRef]
- Feng, R.; Wang, Q.; Yu, T.; Hu, H.; Wu, G.; Duan, X.; Jiang, R.; Xu, Y.; Huang, Y. Quercetin ameliorates bone loss in OVX rats by modulating the intestinal flora-SCFAs-inflammatory signaling axis. Int. Immunopharmacol. 2024, 136, 112341. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, B.; Zhu, W. Pathogenic Escherichia coli-Specific Bacteriophages and Polyvalent Bacteriophages in Piglet Guts with Increasing Coliphage Numbers after Weaning. Appl. Environ. Microbiol. 2021, 87, e0096621. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, R.; Dong, Y.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Zhang, Y.; Hou, Y. Dietary Supplementation with Puerarin Improves Intestinal Function in Piglets Challenged with Escherichia coli K88. Animals 2023, 13, 1908. [Google Scholar] [CrossRef]
- López-Colom, P.; Castillejos, L.; Rodríguez-Sorrento, A.; Puyalto, M.; Mallo, J.J.; Martín-Orúe, S.M. Impact of in-feed sodium butyrate or sodium heptanoate protected with medium-chain fatty acids on gut health in weaned piglets challenged with Escherichia coli F4. Arch. Anim. Nutr. 2020, 74, 271–295. [Google Scholar] [CrossRef]
- Zuo, J.; Yin, H.; Hu, J.; Miao, J.; Chen, Z.; Qi, K.; Wang, Z.; Gong, J.; Phouthapane, V.; Jiang, W.; et al. Lsr operon is associated with AI-2 transfer and pathogenicity in avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 109. [Google Scholar] [CrossRef]
- Jani, S.; Seely, A.L.; Peabody, V.G.L.; Jayaraman, A.; Manson, M.D. Chemotaxis to self-generated AI-2 promotes biofilm formation in Escherichia coli. Microbiology 2017, 163, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, D.; Zhao, X.; Tan, W.; Zheng, X.; Zhang, Q.; Ji, X.; Wei, Y. Autoinducer-2-mediated quorum-sensing system resists T4 phage infection in Escherichia coli. J. Basic. Microbiol. 2021, 61, 1113–1123. [Google Scholar] [CrossRef]
- Cui, Z.Q.; Wu, Z.M.; Fu, Y.X.; Xu, D.X.; Guo, X.; Shen, H.Q.; Wei, X.B.; Yi, P.F.; Fu, B.D. Autoinducer-2 of quorum sensing is involved in cell damage caused by avian pathogenic Escherichia coli. Microb. Pathog. 2016, 99, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Jani, S.; Riordan, R.; Jayaraman, A. Serotonin Promotes Enterohemorrhagic Escherichia Coli Pathogenesis Through Altered AI-2 Production by Gut Microbiota. FASEB J. 2018, 32, 669. [Google Scholar] [CrossRef]
- Witsø, I.L.; Valen Rukke, H.; Benneche, T.; Aamdal Scheie, A. Thiophenone Attenuates Enteropathogenic Escherichia coli O103:H2 Virulence by Interfering with AI-2 Signaling. PLoS ONE 2016, 11, e0157334. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kathayat, D.; Deblais, L.; Srivastava, V.; Closs, G., Jr.; Tokarski, R.J., 2nd; Ayinde, O.; Fuchs, J.; Rajashekara, G. Evaluation of Novel Quorum Sensing Inhibitors Targeting Auto-Inducer 2 (AI-2) for the Control of Avian Pathogenic Escherichia coli Infections in Chickens. Microbiol. Spectr. 2022, 10, e0028622. [Google Scholar] [CrossRef]
- Almasoud, A.; Hettiarachchy, N.; Rayaprolu, S.; Babu, D.; Kwon, Y.M.; Mauromoustakos, A. Inhibitory effects of lactic and malic organic acids on autoinducer type 2 (AI-2) quorum sensing of Escherichia coli O157:H7 and Salmonella Typhimurium. LWT-Food Sci. Technol. 2016, 66, 560–564. [Google Scholar] [CrossRef]
- Escobar-Muciño, E.; Arenas-Hernández, M.M.P.; Luna-Guevara, M.L. Mechanisms of Inhibition of Quorum Sensing as an Alternative for the Control of E. coli and Salmonella. Microorganisms 2022, 10, 884. [Google Scholar] [CrossRef]
- Long, J.; Guan, P.; Hu, X.; Yang, L.; He, L.; Lin, Q.; Luo, F.; Li, J.; He, X.; Du, Z.; et al. Natural Polyphenols as Targeted Modulators in Colon Cancer: Molecular Mechanisms and Applications. Front. Immunol. 2021, 12, 635484. [Google Scholar] [CrossRef]
- Yu, W.; Sun, S.; Fu, Q. The role of short-chain fatty acid in metabolic syndrome and its complications: Focusing on immunity and inflammation. Front. Immunol. 2025, 16, 1519925. [Google Scholar]
- Khan, H.; Ullah, H.; Aschner, M.; Cheang, W.S.; Akkol, E.K. Neuroprotective Effects of Quercetin in Alzheimer’s Disease. Biomolecules 2019, 10, 59. [Google Scholar] [CrossRef]
- Revankar, A.A.; Patil, A.S.; Karishetti, R.; Chougule, K.R.; Patil, P.; Salokhe, A. Enhanced bioavailability of Quercetin-loaded niosomal in situ gel for the management of Parkinson’s disease. Front. Pharmacol. 2025, 15, 1519649. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef]
- Ge, C.; Wang, S.; Wu, X.; Lei, L. Quercetin attenuates brain apoptosis in mice with chronic unpredictable mild stress-induced depression. Behav. Brain Res. 2024, 465, 114934. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.T.; Ding, W.Y.; Lu, X.X.; Zhang, Q.H.; Du, J.X.; Wang, L.J.; Yang, M.N.; Yin, Y.; Liu, F.J. Pharmacological and mechanistic aspects of quercetin in osteoporosis. Front. Pharmacol. 2024, 15, 1338951. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Yao, L.; Hu, Y.M.; Zhao, B.T. Effect of Quercetin-Loaded Mesoporous Silica Nanoparticles on Myocardial Ischemia-Reperfusion Injury in Rats and Its Mechanism. Int. J. Nanomedicine. 2021, 16, 741–752. [Google Scholar] [CrossRef]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61, 1700031. [Google Scholar] [CrossRef]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar]
- Lyu, Y.L.; Zhou, H.F.; Yang, J.; Wang, F.X.; Sun, F.; Li, J.Y. Biological Activities Underlying the Therapeutic Effect of Quercetin on Inflammatory Bowel Disease. Mediators Inflamm. 2022, 2022, 5665778. [Google Scholar] [CrossRef]
- Mao, Y.; Yang, Q.; Liu, J.; Fu, Y.; Zhou, S.; Liu, J.; Ying, L.; Li, Y. Quercetin Increases Growth Performance and Decreases Incidence of Diarrhea and Mechanism of Action in Weaned Piglets. Oxid. Med. Cell. Longev. 2024, 2024, 5632260. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ding, G.; Ren, S.; Xi, J.; Liu, K. The bioavailability, absorption, metabolism, and regulation of glucolipid metabolism disorders by quercetin and its important glycosides: A review. Food Chem. 2024, 458, 140262. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Contents | Nutrient Content (2) | Contents |
|---|---|---|---|
| Corn | 67.3 | DE, (kcal/kg) | 3559 |
| Soybean meal | 10 | CP | 20.15 |
| Full-fat soybeans | 5.5 | Ca | 0.73 |
| Fish meal | 13 | P | 0.65 |
| Whey powder | 1 | SID Lysine | 1.01 |
| Soybean oil | 1 | SID Methionine | 0.37 |
| Limestone | 0.3 | SID Threonine | 0.62 |
| Salt | 1 | SID Tryptophan | 0.18 |
| L-lysine HCl | 0.1 | ||
| Methionine | 0.1 | ||
| Threonine | 0.1 | ||
| Tryptophan | 0.1 | ||
| Vitamin and mineral premix (1) | 0.5 | ||
| Total | 100 |
| Genes | Primer Sequences (5′—3′) | Amplification Length/bp | Accession Number |
|---|---|---|---|
| GADPH | F: AAGGTCGGAGTGAACGGATTT | 248 | NM_001206359.1 |
| R: CATTTGATGTTGGCGGGAT | |||
| SOD1 | F: GGTCCTCACTTCAATCCTG | 218 | NM_001190422.1 |
| R: TCTTCATTTCCACCTCTGC | |||
| CAT | F: GGGAATCCGATAGGAGACA | 258 | NM_214301.2 |
| R: AGCAACGGTGGAGAAACGA | |||
| HO-1 | F: AGCACTCACAGCCCAACAG | 161 | NM_001004027.1 |
| R: GTACAAGGACGCCATCACC | |||
| Nrf2 | F: CATGAGCGTACCACGAAAT | 196 | NM_001114671.1 |
| R: GTAGAGCAGACGGTTGAGGA | |||
| Keap1 | F: GTGAGCAGCGGCGTTTCTA | 482 | XM_021076667.1 |
| R: CCCAATTCGATTTCGTGGT | |||
| GST | F: GGTTGAGATTGACGGGATG | 375 | NM_214389.2 |
| R: TTCAGCAGAGGGAAGTTGG | |||
| iNOS | F: CCGCCCAGATGAAGACCAC | 349 | NM_001143690.1 |
| R: GGGAAATACAGCACCAAAGAT |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| Con | K88 | Que + K88 | ||
| IgA (ng/mL) | 1401.04 ± 45.13 a | 784.05 ± 29.25 c | 1179.06 ± 49.38 b | 0.0001 |
| IgM (ng/mL) | 2573.28 ± 102.27 a | 1561.62 ± 79.06 b | 2330.06 ± 79.59 a | 0.0001 |
| IgG (ng/mL) | 24.83 ± 1.04 a | 15.52 ± 0.78 c | 20.50 ± 0.91 b | 0.0001 |
| GSH-Px (ng/L) | 158.52 ± 8.81 a | 104.01 ± 5.52 b | 140.26 ± 4.15 a | 0.0001 |
| CD3 (ng/mL) | 32.66 ± 2.81 b | 44.32 ± 1.40 a | 34.90 ± 2.19 b | 0.0029 |
| CD4 (ng/mL) | 70.57 ± 2.62 a | 52.52 ± 4.68 b | 55.05 ± 3.85 b | 0.0061 |
| CD8 (U/mL) | 121.67 ± 7.29 c | 205.60 ± 4.97 a | 180.72 ± 6.61 b | 0.0001 |
| MDA (nmol/L) | 3.54 ± 0.17 b | 4.38 ± 0.11 a | 3.76 ± 0.09 b | 0.0004 |
| CAT (ng/L) | 82.65 ± 3.08 a | 57.70 ± 2.39 c | 67.56 ± 1.74 b | 0.0001 |
| SOD (μmol/g) | 204.76 ± 6.55 a | 118.05 ± 7.83 c | 149.72 ± 5.79 b | 0.0001 |
| MPO (nmol/L) | 316.91 ± 15.05 b | 457.06 ± 11.64 a | 416.37 ± 19.26 a | 0.0001 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| Con | K88 | Que + K88 | ||
| CD3 (ng/mL) | 29.23 ± 2.92 b | 41.36 ± 1.41 a | 34.73 ± 1.37 ab | 0.0015 |
| CD4 (ng/mL) | 64.90 ± 3.01 a | 47.17 ± 4.62 b | 49.42 ± 3.53 b | 0.0063 |
| CD8 (U/mL) | 108.08 ± 6.42 c | 190.18 ± 4.84 a | 173.69 ± 4.14 b | 0.0001 |
| GSH-Px (ng/L) | 146.44 ± 8.36 a | 91.04 ± 5.76 b | 129.21 ± 4.80 a | 0.0001 |
| MDA (nmol/L) | 3.27 ± 0.15 b | 4.10 ± 0.13 a | 3.48 ± 0.11 b | 0.0008 |
| CAT (ng/L) | 77.47 ± 3.06 a | 52.75 ± 2.40 c | 62.19 ± 1.99 b | 0.0001 |
| SOD (μmol/g) | 189.32 ± 6.07 a | 104.61 ± 7.23 c | 136.41 ± 4.93 b | 0.0001 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| Con | K88 | Que + K88 | ||
| CD3 (ng/mL) | 29.07 ± 1.62 b | 39.87 ± 1.53 a | 35.34 ± 2.42 ab | 0.0024 |
| CD4 (ng/mL) | 69.24 ± 3.19 a | 47.25 ± 2.89 b | 52.32 ± 2.86 b | 0.0001 |
| CD8 (U/mL) | 130.45 ± 6.39 b | 186.56 ± 9.35 a | 166.74 ± 9.79 a | 0.0006 |
| GSH-Px (ng/L) | 152.11 ± 5.73 a | 94.94 ± 5.83 b | 109.03 ± 5.12 b | 0.0001 |
| MDA (nmol/L) | 3.00 ± 0.19 b | 4.04 ± 0.14 a | 3.75 ± 0.16 a | 0.0006 |
| CAT (ng/L) | 83.48 ± 2.72 a | 53.40 ± 3.11 c | 66.62 ± 2.52 b | 0.0001 |
| SOD (μmol/g) | 184.01 ± 7.50 a | 114.22 ± 8.77 c | 145.06 ± 9.68 b | 0.0001 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| Con | K88 | Que + K88 | ||
| IgA (ng/mL) | 1324.54 ± 41.52 a | 710.12 ± 29.94 c | 1108.91 ± 44.43 b | 0.0001 |
| IgM (ng/mL) | 2385.02 ± 86.14 a | 1390.91 ± 69.20 b | 2139.79 ± 77.80 a | 0.0001 |
| IgG (ng/mL) | 23.31 ± 0.99 a | 13.61 ± 0.65 c | 18.82 ± 0.91 b | 0.0001 |
| GSH-Px (ng/L) | 161.79 ± 6.38 a | 90.38 ± 7.84 b | 123.35 ± 8.83 a | 0.0001 |
| MDA (nmol/L) | 3.34 ± 0.14 b | 4.14 ± 0.16 a | 3.91 ± 0.13 a | 0.0021 |
| CAT (ng/L) | 82.02 ± 2.74 a | 56.19 ± 2.22 b | 65.78 ± 3.58 b | 0.0001 |
| SOD (μmol/g) | 175.14 ± 5.24 a | 114.91 ± 6.64 b | 154.45 ± 6.86 a | 0.0001 |
| MPO (nmol/L) | 286.33 ± 15.26 c | 439.74 ± 12.13 a | 384.04 ± 18.39 b | 0.0001 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| Con | K88 | Que + K88 | ||
| IgA (ng/mL) | 1279.94 ± 39.22 a | 790.78 ± 24.98 c | 1072.39 ± 46.84 b | 0.0001 |
| IgM (ng/mL) | 2268.28 ± 203.07 a | 1183.22 ± 77.64 b | 2049.85 ± 76.27 a | 0.0001 |
| IgG (ng/mL) | 23.10 ± 0.65 a | 12.47 ± 0.83 b | 20.32 ± 1.00 a | 0.0001 |
| GSH-Px (ng/L) | 162.01 ± 4.75 a | 97.97 ± 2.75 c | 125.31 ± 5.59 b | 0.0001 |
| MDA (nmol/L) | 3.24 ± 0.11 b | 4.10 ± 0.15 a | 3.73 ± 0.22 ab | 0.0053 |
| CAT (ng/L) | 81.67 ± 2.84 a | 53.00 ± 2.73 c | 66.29 ± 2.70 b | 0.0001 |
| SOD (μmol/g) | 177.46 ± 6.67 a | 102.26 ± 8.76 c | 133.74 ± 8.97 b | 0.0001 |
| MPO (nmol/L) | 308.79 ± 21.83 b | 399.83 ± 15.27 a | 404.57 ± 13.67 a | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yao, M.; Wang, D.; Geng, M.; Nan, S.; Peng, X.; Xue, Y.; Zhang, W.; Nie, C. Quercetin Can Alleviate ETECK88-Induced Oxidative Stress in Weaned Piglets by Inhibiting Quorum-Sensing Signal Molecule Autoinducer-2 Production in the Cecum. Antioxidants 2025, 14, 852. https://doi.org/10.3390/antiox14070852
Wang H, Yao M, Wang D, Geng M, Nan S, Peng X, Xue Y, Zhang W, Nie C. Quercetin Can Alleviate ETECK88-Induced Oxidative Stress in Weaned Piglets by Inhibiting Quorum-Sensing Signal Molecule Autoinducer-2 Production in the Cecum. Antioxidants. 2025; 14(7):852. https://doi.org/10.3390/antiox14070852
Chicago/Turabian StyleWang, Hailiang, Min Yao, Dan Wang, Mingyang Geng, Shanshan Nan, Xiangjian Peng, Yuyang Xue, Wenju Zhang, and Cunxi Nie. 2025. "Quercetin Can Alleviate ETECK88-Induced Oxidative Stress in Weaned Piglets by Inhibiting Quorum-Sensing Signal Molecule Autoinducer-2 Production in the Cecum" Antioxidants 14, no. 7: 852. https://doi.org/10.3390/antiox14070852
APA StyleWang, H., Yao, M., Wang, D., Geng, M., Nan, S., Peng, X., Xue, Y., Zhang, W., & Nie, C. (2025). Quercetin Can Alleviate ETECK88-Induced Oxidative Stress in Weaned Piglets by Inhibiting Quorum-Sensing Signal Molecule Autoinducer-2 Production in the Cecum. Antioxidants, 14(7), 852. https://doi.org/10.3390/antiox14070852






