Abstract
Vanillin is a compound of great utility, and its production is, among others, based on using microorganisms such as Saccharomyces cerevisiae yeast. The effect of vanillin on cells is not fully understood. It has been demonstrated that vanillin induces oxidative stress; however, evidence also suggests its beneficial effects, including antioxidant and anti-inflammatory properties. For this reason, the present study was designed to elucidate the mechanism of vanillin’s action and to ascertain the extent to which its toxic effect is attributable to oxidative stress. The studies were conducted using wild-type and Δsod1 mutant strains. SOD1 deficiency results in cell hypersensitivity to oxidative factors, thus making the mutant strain a valuable model for investigating various aspects of oxidative stress. Based on an evaluation of cell vitality, Yap1p activation, ROS content, and glutathione and NADP(H) content, it can be concluded that oxidative stress is a secondary effect of metabolic and redox perturbations in cells rather than a direct consequence of vanillin reactivity. Furthermore, alterations observed in the redox couples GSH/GSSG and NADPH/NADP+ are one of the reasons for oxidative stress and suggest that vanillin may induce the utilization of NADPH for cellular needs other than antioxidant effects.
1. Introduction
Vanillin, an aromatic aldehyde (4-hydroxy-3-metoxybenzaldehyde), is a common flavor substance naturally occurring in Vanilla planifolia pods. Its usage in the food and cosmetics industries is approximately 20,000 tons per year [1]. Besides the natural origin and chemical synthesis, vanillin may be generated during lignocellulose degradation and biosynthesized using plant tissue culture, enzyme conversion, or microbial conversion [1,2,3,4]. Vanillin, as well as other by-products of lignocellulose degradation (furfural, 5-hydroxymethylfurfural, and several furanic and phenolic compounds), were found to be potent biomass fermentation inhibitors [5,6]. The need for improvement in the yield of biomass fermentation has prompted numerous efforts to enhance the tolerance of microbial strains used in biomass fermentation to inhibitory compounds, including detoxification and the development of new strains [7,8]. High demand for natural vanillin has led to a focus on genetic and metabolic engineering-assisted microbial synthesis, particularly using the yeast Saccharomyces cerevisiae. Engineered yeast cells that express enzymes of the shikimate pathway can produce vanillin from glucose [9]. The toxicity of the produced vanillin to host cells poses a significant challenge to achieving high yields in vanillin bioproduction. Conversely, an increasing amount of data is emerging from studies employing various models, which suggests that vanillin has antioxidant or protective effects against neurological diseases and oxidative damage [10,11].
The approaches to elucidate the mechanism of vanillin toxicity to yeast cells include vanillin uptake, vanillin biotransformation, removal of the vanillin metabolites, and the cellular response to stress generated by vanillin. Nguyen et al. have shown that vanillin is an oxidative stress-generating agent for yeast Saccharomyces cerevisiae cells [12], in a similar manner to another aromatic aldehyde, furfural [13]. They demonstrated that vanillin activated the Yap1 transcription factor, causing growth retardation in the Yap1-deficient strain and mitochondrial fragmentation, suggesting that vanillin induces oxidative stress in yeast cells [12]. The research presented in this work aimed to examine the role of oxidative stress in vanillin toxicity with greater breadth using the superoxide dismutase 1-deficient yeast mutant strain. Superoxide dismutases (SODs) catalyze the reaction of superoxide disproportionation to hydrogen peroxide and molecular oxygen and, thus, are crucial for oxidative stress protection. In yeast cells, two superoxide dismutase isoenzymes occur: cytosolic SOD1 (CuZnSOD) and mitochondrial SOD2 (MnSOD). A fraction of SOD1 is also distributed in the mitochondrial intermembrane space [14]. While the mitochondrial fraction of SOD1 and SOD2 is responsible for the removal of the respiratory chain-derived superoxide, cytosolic SOD1 accounts for peroxide signaling [15,16], including regulation of glycolysis and induction of NADPH production [17]. SOD1 was recently shown to prevent H2S cytotoxicity and the formation of other thiol-oxidizing reactive sulfur species [18]. SOD1 deficiency in yeast cells leads to hypersensitivity to several stress factors, including ROS-generating compounds such as menadione [19] and GSH-depleting compounds such as dithiopyridine [20] and aldehydes acrolein [21,22] and nonenal [23]. The lack of SOD1 protein results in the higher availability of pyridine nucleotide cofactors due to the upregulation of the pentose phosphate pathway, which implies higher activities of alcohol and aldehyde dehydrogenases [24]. Using the Δsod1 strain thus allows for examining the processes involved in maintaining redox balance and the impact of oxidative stress on the yeast cells after exposure to vanillin.
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
The following yeast strains were used: wild-type SP4 MATα leu1 arg4 [25] and Δsod1 mutant, isogenic to SP4, MATα leu1 arg4 sod1::natMX [26]. For Yap1p activation observations, isogenic WT and ∆sod1 strains expressing pRS cup1 cp-GFP-YAP1-kan plasmid encoding Yap1-GFP-tagged protein were used [27]. For Zwf1p location observations, the strain expressing Zwf1-GFP-tagged protein was used (MATα his3 leu2 met15 ura3 ZWF1-GFP::HIS3MX6, Invitrogen Yeast GFP Clone Collection).
The yeast was cultivated in a standard liquid YPD medium (comprising 1% Yeast Extract, 1% Yeast Bacto-Peptone, and 2% glucose) on a rotary shaker at 150 rpm, at 28 °C.
For the vanillin treatment, cells from the exponential phase culture (~16 h; density approximately 5 × 107 cells/mL) were centrifuged, washed twice, suspended to a final density of 108 cells/mL in 100 mM phosphate buffer, pH 7.0, containing 1 mM EDTA and 0.1% glucose, and incubated with 6 mM vanillin for 3 h at 28 °C with shaking. Vanillin (CAS number 121-33-5, 99%, Sigma-Aldrich, Poznan, Poland) was dissolved in DMSO to obtain a 2 M stock solution. The control cells were incubated with the addition of an equal volume of DMSO. Following incubation, the cells were centrifuged, washed twice, and used for further analysis.
2.2. Cell Growth Assays
For spotting tests, the cells were diluted to 107, 106, 105, or 104 cells/mL. Aliquots (5 µL) of each suspension were inoculated on a solid YPD medium with 0, 2, 4, or 6 mM vanillin. The growth of the cells was inspected after 48 h.
The growth of cells in liquid YPD medium containing various concentrations of vanillin (2–9 mM), was monitored automatically at OD 600 nm using the Bioscreen C (Oy Growth Curves Ab Ltd., Helsinki, Finland) for 24 h (every 1 h) at 28 °C. The starting density of the yeast suspension was of 5 × 106 cells/mL. The control cells were cultivated with an appropriate volume of DMSO (the same volume as the vanillin solution). The relative growth rate was calculated using the following equation: [(ln(OD)t2 − ln(OD)t1)/(t2 − t1)], where t1 is the earlier time point and t2 is the later time point of OD measurement during the logarithmic phase of growth.
The cell growth was also monitored by microscopic observation of the cells treated previously with 6 mM vanillin or DMSO as a control for 3 h and spread on solid YPD medium containing phloxine B (10 μg/mL; Sigma-Aldrich, Poznan, Poland). The growth of the cells was observed directly after inoculation and after 6 h using a Nikon Eclipse E200 microscope (Nikon, Tokyo, Japan) with a 20× lens and a DP10 digital camera.
2.3. Cell Viability and Vitality Assays
The cells after 3 h exposure to vanillin were stained with propidium iodide (PI, 5 μg/mL; Molecular Probes, Eugene, OR, USA) [28], incubated for 20 min, and observed using an Olympus BX-51 fluorescence microscope (Olympus, Hamburg, Germany). The number of dead cells, PI-positive, were expressed as a percentage of at least 200 cells in each of the three biological replicates.
The metabolic activity of the cells treated with vanillin was determined with FUN-1 stain (0.5 μM; Molecular Probes, Eugene, OR, USA) as previously described by [28]. The fluorescence of cell suspensions added with FUN-1 was quantified using a Tecan Infinite 200 microplate reader (Tecan Austria GmbH, Salzburg, Austria) with excitation and emission wavelengths of 480 and 500–650 nm, respectively. The metabolic activity was expressed as a red (λ = 575 nm) ratio to green (λ = 535 nm) fluorescence.
2.4. Determination of ROS Content
The generation of reactive oxygen species was assessed using dihydroethidine (DHET; Molecular Probes, Eugene, OR, USA). Following incubation with vanillin, cells were suspended to a density of 108 cells/mL in a 100 mM sodium phosphate buffer, pH 7.0, containing 1 mM EDTA and 0.1% glucose. The cell suspensions were added with DHET (8 µM final concentration; stock solution in DMSO). The kinetics of fluorescence increase, resulting from the oxidation of the fluorogenic probe, was measured immediately after the addition of the probe using a Tecan Infinite 200 microplate reader at λex = 518 nm, λem = 605 nm for DHET at a temperature of 28 °C. Additionally, cross-reactions between the probes and vanillin and DMSO in a blank (buffer without cells) were examined.
2.5. Determination of Glutathione Content
The total glutathione (sum of both GSH and GSSG) and separately GSSG levels were determined in the yeast cells with the GSH/GSSG-Glo Assay (Promega, Madison, WI, USA) according to the manufacturer’s protocol. Following vanillin treatment, the cells were centrifuged, washed twice with sterile water, suspended to a density of 5 × 105 cells/mL in PBS buffer, and then used for determination purposes. Luminescence was recorded after 15 min using a Tecan Infinite 200 microplate reader. Total glutathione and GSSG concentrations were determined based on standard curves, whereas the level of GSH was calculated by subtracting the GSSG from the total glutathione concentration.
2.6. Determination of NADP(H) Content
Following exposure to vanillin, the cells were centrifuged, washed twice with sterile water, suspended to a density of 2 × 106 cells/mL in a PBS buffer, and then used for the determination of cofactors with the NADP/NADPH-Glo Assay kit (Promega, Madison, WI, USA) according to the manufacturer’s protocol with own modifications [29]. The luminescence was recorded for 3 h using a Tecan Infinite 200 microplate reader. The results were presented as the content of individual pyridine cofactors in arbitrary units and the NADPH/NADP+ ratio.
2.7. Estimation of Gene Expression
Relative gene expression was estimated by real-time PCR with TaqMan chemistry. Following treatment with vanillin, the cells were centrifuged, washed twice with sterile water, and suspended to a density of 5 × 107 cells/mL in the spheroplast buffer (1 M sorbitol, 0.1 M EDTA, 0.1% β-mercaptoethanol) containing lyticase (250 U per sample) for 45 min at 30 °C. The RNA samples were obtained using the GeneMATRIX Universal RNA Purification Kit (EURx, Gdansk, Poland) following the manufacturer’s instructions. The RNA samples were stored at −80 °C, and each was thawed only once. A total of 500 ng of RNA was treated with DNase I (Roche, Mannheim, Germany) for 60 min at 25 °C (10 U per 1 µg RNA) and then used for reverse transcription. To synthesize cDNA, the smART First Strand cDNA Synthesis Kit (EURx, Gdansk, Poland) was applied following the manufacturer’s instructions, and the samples were stored at −50 °C until required. The cDNA sample was diluted and mixed with FastStart Essential DNA Probes Master (Roche, Mannheim, Germany) and TaqMan Gene Expression Assays (Applied Biosystems, Life Technologies, Pleasanton, CA, USA). Real-time PCR was run using the Roche LightCycler 96 system (Roche Life Science; Mannheim, Germany). The ACT1 gene was used as an internal control. The relative gene expression was calculated with the −ΔΔCT method to compare the expression of the tested gene after treatment with vanillin with that of the untreated control in the wild-type strain.
2.8. Enzyme Assays
For protein extraction, the cells after incubation with vanillin were centrifuged, washed twice with sterile water, and suspended in cold homogenization buffer (20 mM phosphate buffer, pH 6.8, containing 1 mM EDTA, 0.2% DTT, and 1 mM PMSF). Subsequently, the biomass was disrupted with 0.5 mm glass beads in 6 cycles of 30 s with intervals for cooling the sample on ice. Following this, the samples were centrifuged (14,000× g, 15 min, 4 °C). Protein extracts were immediately frozen at −80 °C. Protein concentration was determined using the Bradford method.
The total activity of the PP pathway dehydrogenases (the sum of the activities of both glucose-6-phosphate dehydrogenase (Zwf1p) and 6-phosphogluconate dehydrogenase (Gnd1p and Gnd2p)) and separately the 6-phosphogluconate dehydrogenase activity were determined spectrophotometrically by measuring the rate of NADP+ reduction at 340 nm following the methodology proposed by Tian et al. [30] with our own modifications [29]. Consequently, the activity of Zwf1p was calculated by subtracting the activity of Gnd1p and Gnd2p from the total enzyme activity. To obtain the total dehydrogenase activity, the following reaction substrates were used: 0.2 mM NADP+, 0.4 mM D-glucose-6-phosphate, and 0.4 mM 6-phosphogluconate. The substrates were added to a 100 mM Tris-HCl buffer, pH 8.0, containing 1 mM MgCl2. The addition of 5 µL cell extract (comprising 2 mg of protein per mL) initiated the reaction. Consequently, to obtain Gnd1p and Gnd2p activity, only 0.2 mM NADP+ and 0.4 mM 6-phosphogluconate were used as reaction substrates. The kinetics of the absorbance increase was recorded using a Tecan Infinite 200 microplate reader at λ = 340 nm. The activity was expressed in arbitrary units.
The activity of glutathione reductase (GR) was determined by measuring the decrease in NADPH absorbance at 340 nm using a Varian Cary 50 spectrophotometer (Varian Ltd., Palo Alto, CA, USA). The reaction mixture included 50 mM phosphate buffer at pH 7.0, 0.5 mM DTPA, and 80 µM NADPH. To exclude nonspecific NADPH oxidation, the reaction mixture was incubated with a protein extract sample for 1 min before adding 2 mM GSSG (final concentration), after which the absorbance was recorded. GR activity was calculated with an extinction coefficient of 6.22 mM−1 cm−1 and expressed as U per mg protein.
2.9. Observation of Yap1-GFP Activation and Zwf1-GFP Location
For observations of Yap1p activation, cells expressing Yap1-GFP from an exponential phase culture (~16 h) were washed and suspended in 100 mM phosphate buffer, pH 7.0, containing 1 mM EDTA and 0.1% glucose, then added with 6 mM vanillin and stained with 4′,6-diamidino-2-phenylindole (DAPI, 2 μg/mL; Molecular Probes, Eugene, OR, USA) to visualize cell nuclei and observed using fluorescence microscope Olympus BX-51 equipped with the DP-72 digital camera and Cell^D and also cellSens Dimension software 4.2.1. To calculate the percentage of activated cells, at least 100 cells were observed and counted as activated when the fluorescence signal was visible from the nuclei of the cells, predominantly or partitioning between the cytosol and nuclei, but the nucleus was noticeable. The microscopic analysis was repeated 3 times (independent biological replicates).
To observe the location and content of Zwf1p, the cells carrying GFP-protein Zwf1-GFP from the exponential phase culture were washed, suspended in 100 mM phosphate buffer, pH 7.0, containing 1 mM EDTA and 0.1% glucose, and incubated with 6 mM vanillin for 3 h. Then, the cells were washed, and Zwf1-GFP fluorescence was observed using a fluorescence microscope, APEXVIEW APX100 (Olympus, Hamburg, Germany), with a BP470-490 filter. The microscopic images present representative results obtained from the duplicate experiment. Concurrently, the GFP fluorescence of the cell suspension was quantified using a Tecan Infinite 200 microplate reader, as previously described by [31].
2.10. Data Analysis
The data are presented as the mean ± SD from three biological replicates. The statistical analysis was performed using STATISTICA 13.3 software (StatSoft, Inc., Krakow, Poland). The statistical significance of the differences between the means of the samples treated with 6 mM vanillin and control samples added with DMSO only and between the tested strains was estimated with a t-test. The statistical significance of differences between means of samples treated with various concentrations of vanillin compared to a control group treated with the same volume of DMSO was estimated using one-way ANOVA and the Dunnett post hoc test. The homogeneity of variances was checked with the Brown–Forsythe test. Statistical significance was assigned to values with a p-value less than 0.05.
Ternary plot analysis, generated using Excel software, indicated the changes in ROS content, NADPH/NADP+, and GSH/GSSG ratios after vanillin treatment in triangular coordinates, which were normalized to restrict the range of values between 0 and 1.
3. Results
3.1. Vanillin Inhibits Cell Proliferation by Diminishing Cell Vitality
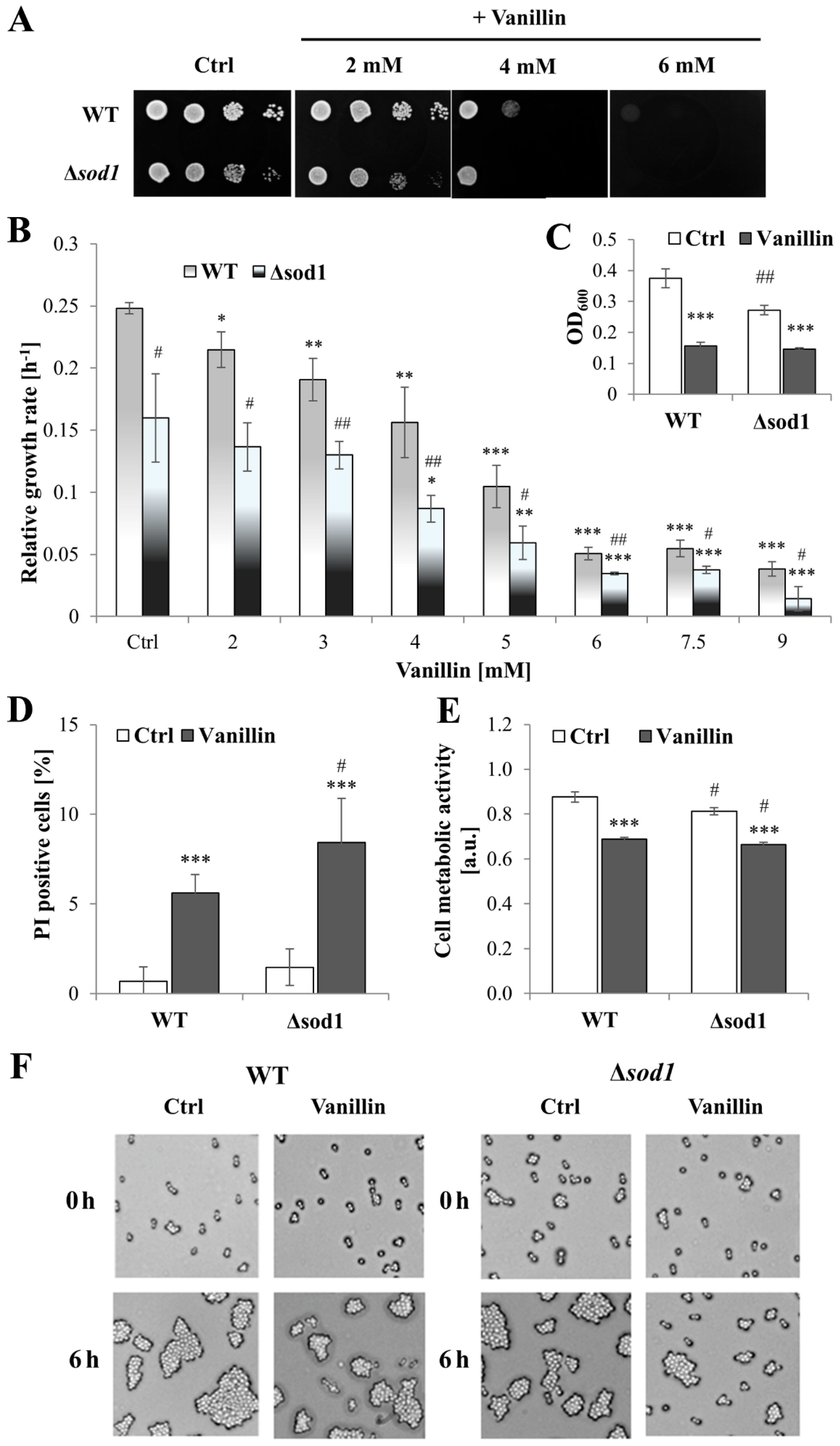
To gain further insight into the physiological effects of vanillin on yeast cells and the impact of oxidative stress on toxicity, a yeast strain was used that is highly sensitive to oxidative agents due to the lack of superoxide dismutase 1 (SOD1, CuZnSOD), a primary cytosolic enzyme involved in the protection against extracellular oxidative agents [32]. The inhibitory effect of vanillin on the growth rate was confirmed to be dose-dependent (Figure 1B) and prevents growth on a solid medium (Figure 1A). In the spotting test, when vanillin was present constantly in the growth medium and the growth inspection is after 48 h, the toxicity of vanillin was visible at the concentration of 2 mM for ∆sod1 strain and of 4 mM for both strains. The addition of vanillin at a concentration of 6 mM resulted in the complete inhibition of growth for both strains (Figure 1A). The relative growth rate reduction in the presence of vanillin (2–9 mM) in the liquid medium was not notably greater in the ∆sod1 strain than in the wild-type strain. This was even though, in control cells without stress conditions, the growth rate of the ∆sod1 strain was significantly reduced in comparison to the wild-type strain (Figure 1B, [26]). The observed reduction in cell growth on solid and liquid media after vanillin treatment may be attributed to either cell death and/or cell cycle arrest. Therefore, the number of dead cells was examined using propidium iodide fluorescence staining (Figure S1) after treatment of cells with 6 mM vanillin for 3 h in a buffer (3 h in the presence of vanillin in rich medium results in significant growth reduction, Figure 1C). It was found that after treatment with 6 mM vanillin, the number of dead cells increased but did not exceed 6% for the wild-type and 8% for ∆sod1 strain (Figure 1D and Figure S1). Furthermore, observations of the cells treated with vanillin and subsequently re-inoculated into a rich solid medium revealed that, after 6 h, the cells of both strains had formed budding cell clusters, indicating that they were alive and had resumed budding. The presence of the vital dye Phloxine B in the medium confirmed that cells that did not form colonies after 6 h of re-inoculation were not necessarily dead, but their ability to reproduce was blocked. Moreover, the data for the ∆sod1 mutant indicated slower growth of these cells but also a more extended time required to return to budding (Figure 1F). The assessment of cell vitality with fluorescent stain FUN-1 demonstrated that metabolic activity was considerably diminished in cells treated with vanillin of SOD1-deficient strain as well as of wild-type strain (Figure 1E). The impaired metabolic activity correlated with the reduced capacity of the cells to proliferate, as evidenced by the lower degree of colony development that formed after vanillin treatment compared to the control cells, which indicated that the cells undergo proliferation but with a delay (Figure 1F).
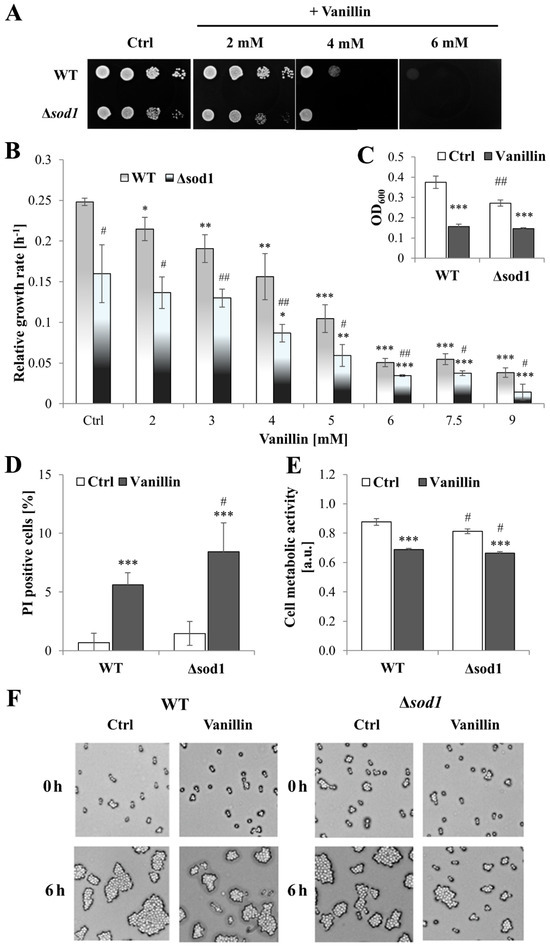
Figure 1.
Effects of vanillin on the growth, viability, and vitality of the ∆sod1 mutant. (A) The growth on solid YPD medium was examined after 48 h. The drops contain 50,000, 5000, 500, and 50 cells, respectively. (B) Relative growth rate in the logarithmic phase of growth. (C) OD600 measurements after 3 h of the culture with 6 mM vanillin. (D) The viability of the cells was estimated with propidium iodide fluorescence staining after 3 h of treatment with 6 mM vanillin. (E) The cell metabolic activity of yeast cells determined with FUN-1 after 3 h of treatment with 6 mM vanillin. (F) The viability and budding ability of yeast cells examined by microscopic analysis of cells growing on YPD agar plates with phloxine B. The pictures show representative views after the indicated time. * denotes p < 0.05, ** p < 0.01, *** p < 0.001 assessed t-test for the cells treated with vanillin vs. untreated control cells, and # denotes p < 0.05, ## p < 0.01 for the comparison between WT and ∆sod1 strains.
3.2. Vanillin Increases ROS Content and Activates Yap1p
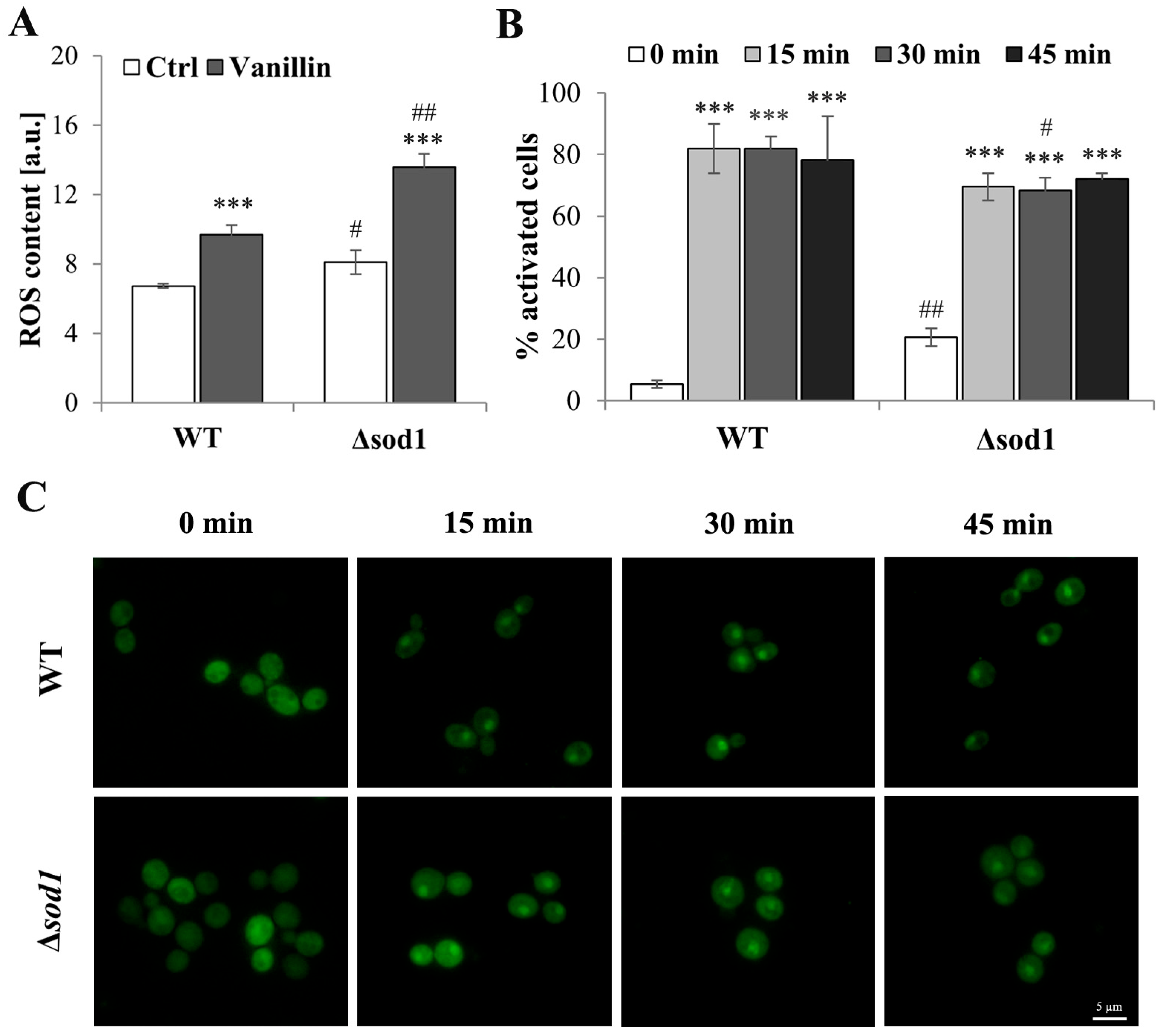
To assess the occurrence of oxidative stress markers in vanillin-treated cells, the intracellular ROS content was measured with a fluorescent stain. As shown in Figure 2A, control cells from the ∆sod1 strain showed an increased content of ROS compared to wild-type control cells. This more oxidative steady state of ∆sod1 cells implicates greater sensitivity to oxidative stress-generating agents. Intracellular ROS levels were increased in both wild-type and mutant cells after treatment with vanillin, but to a significantly greater extent in ∆sod1 cells (Figure 2A). These data confirmed that the disturbance of cellular homeostasis caused by vanillin had features of oxidative stress.
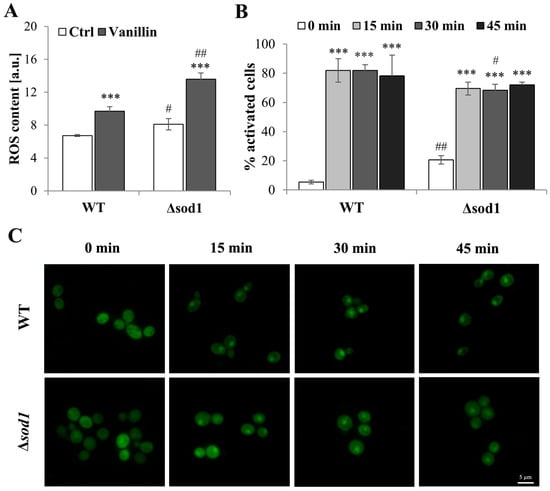
Figure 2.
ROS content and Yap1p localization in cells treated with vanillin. (A) ROS content in cells treated for 3 h with 6 mM vanillin was estimated with dihydroethidine. The kinetics of fluorescence increase was measured directly after the addition of the probe. The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed and *** denotes p < 0.001 and # denotes p < 0.05, ## p < 0.01 for the comparison between WT and ∆sod1 strains. (B) The number of cells with activated Yap1p in response to 6 mM vanillin calculated at indicated time points. The cells of WT and ∆sod1 strains expressing Yap1-GFP were observed for the indicated time and counted as activated cells when purely nuclear localization of Yap1-GFP was visible or Yap1-GFP localization was nuclear and cytosolic as well (partial activation). The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed and *** denotes p < 0.001 and # denotes p < 0.05, ## p < 0.01 for the comparison between WT and ∆sod1 strains. (C) Yap1p localization after treatment with 6 mM vanillin. The cells of WT and ∆sod1 strains expressing Yap1-GFP were treated with vanillin and photographed for the indicated time.
The state of oxidative stress is expected to induce a cellular stress response. Most oxidative stress signaling in Saccharomyces cerevisiae is mediated by the Yap1 transcription factor [33,34]. Induction of the Yap1-mediated oxidative stress response by vanillin was shown by Nguyen et al. [12]. For that reason, Yap1p activation after vanillin treatment in wild-type and Δsod1 mutant strains was analyzed (Figure 2B,C). The localization of the Yap1-GFP fusion protein was examined within 1 h of vanillin addition, and increased activation was observed with time (Figure 2C). The majority of the cells treated with vanillin revealed Yap1p activation (Figure 2B); in 70–80% of the cells, partial or complete localization of Yap1-GFP was noticed (Figure 2B; the percentage of activated cells was calculated, including all cells at least partially activated; Figure S2B). Moreover, in comparison to hydrogen peroxide, which causes complete activation (lack of cytosolic signal from Yap1-GFP, Figure S2A), 6 mM vanillin did not cause complete activation, and the response of the cells was diversified, from almost complete activation to the lack of activation (Figure 2C and Figure S2B). These results suggested that vanillin was not a strong oxidant for yeast cells. No significant differences were observed in the kinetics of Yap1p activation or duration between wild-type and ∆sod1 strains (Figure 2C).
3.3. Vanillin Alters Cellular Redox State
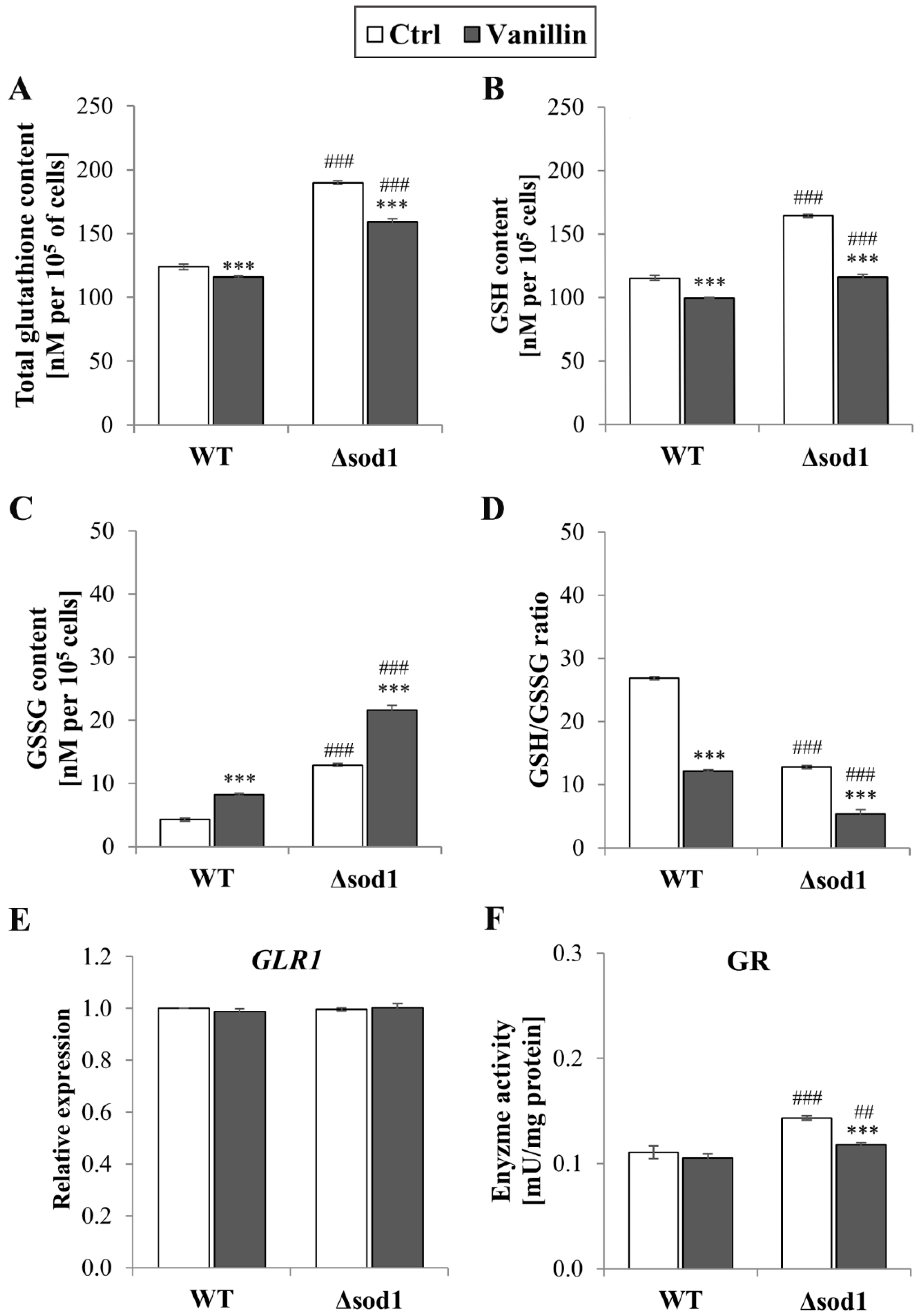
The maintenance of intracellular redox homeostasis depends on the glutathione system and the ability of the cells to preserve their buffering potential under stressful conditions. Therefore, the impact of vanillin treatment on the glutathione content was examined. Figure 3A–D present significant changes in total glutathione (A), reduced (B), and oxidized glutathione (C) content and the GSH/GSSG ratio (D) in both yeast strains. The depletion of reduced GSH caused by vanillin was more pronounced in ∆sod1 than in the wild-type strain because the initial level of reduced GSH in control cells was significantly higher (about 30% compared to wild-type, Figure 3B). The decrease in reduced GSH content in vanillin-treated cells was accompanied by a significant increase in GSSG content (Figure 3C). The intracellular content of GSSG was again affected in the ∆sod1 strain compared to the wild-type. In addition, an increase in the amount of GSSG in the ∆sod1 strain caused by vanillin resulted in a disturbance in redox potential, expressed as the ratio of GSH/GSSG, which was significantly reduced (Figure 3D).
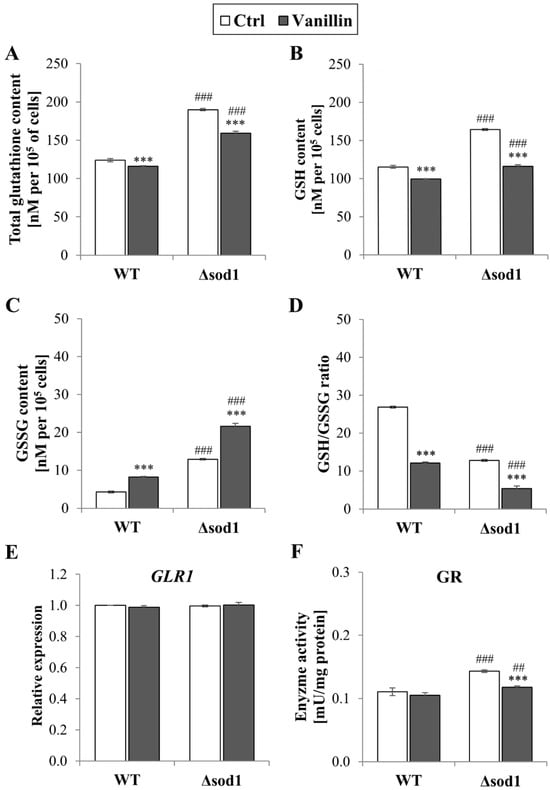
Figure 3.
The glutathione pool in cells treated with 6 mM vanillin for 3 h: (A) the total glutathione content, (B) the reduced, (C) the oxidized glutathione content, and (D) the ratio of [GSH]/[GSSG]. (E) The relative expression of the GLR1 gene was calculated using the −∆∆CT method with respect to untreated wild-type cells. (F) The activity of glutathione reductase in whole-cell protein extracts. The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed and *** denotes p < 0.001 and ## denotes p < 0.01, ### p < 0.001 for the comparison between WT and ∆sod1 strains.
The imbalance in glutathione redox potential in cells exposed to vanillin was also significant in the wild-type strain. The total glutathione content, as the sum of all individual glutathione molecules, reduced and derived from glutathione disulfide, was also affected by vanillin in both strains. This suggests that glutathione oxidation to disulfide is not the only way its consumption is caused by vanillin (Figure 3A). Again, the total glutathione pool was significantly enhanced in Δsod1 control cells (Figure 3A). It was investigated whether the more oxidizing redox state caused by vanillin implied the induction of glutathione reductase gene expression and activity to restore the initial redox state. Glutathione reductase (GR) catalyzes the reduction of GSSG to GSH with NADPH as a cofactor. GLR1 gene expression was unaffected after 3 h of vanillin treatment and GR activity (Figure 3E). The effect of vanillin was apparent in the case of glutathione reductase activity in the ∆sod1 strain (Figure 3F). Again, GR activity was also affected in control ∆sod1 cells compared to wild-type and lowered after treatment with vanillin to a level comparable with wild-type cells (Figure 3F).
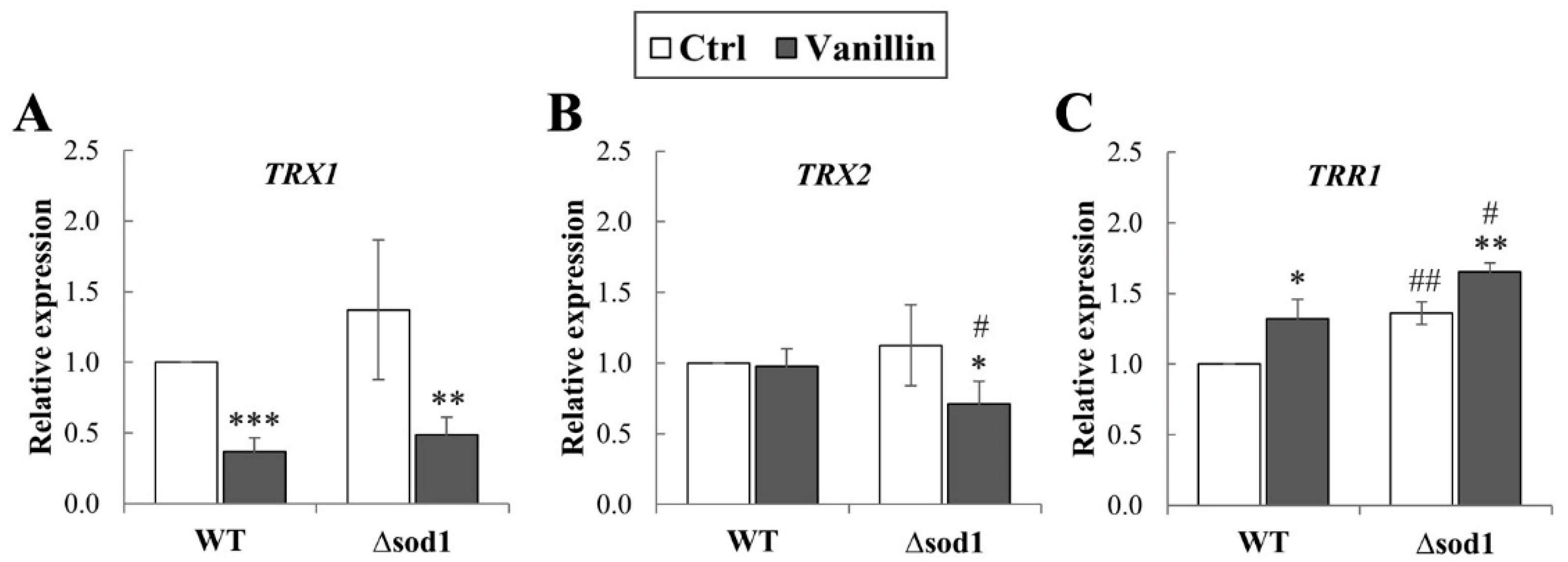
Maintaining the cell redox balance requires a strict and multimodal control of the redox state of sulfhydryl groups, occurring both in glutathione (the main cellular non-enzymatic, endogenous antioxidant), but also in proteins’ Cys residues. The vital role in this thiol redox control system is played by the thioredoxin system, which consists of thioredoxins (TRXs), thioredoxin reductases (TRRs), and NADPH used as a donor. A complete cytoplasmic thioredoxin system comprises the two thioredoxins (Trx1p and Trx2p) with one thioredoxin reductase, Trr1p. Hence, it was analyzed whether the vanillin-induced alterations in redox balance are connected with changes in expression of cytoplasmic thioredoxin system genes (TRX1, TRX2, and TRR1). It has been found that expression of the TRX1 gene significantly decreased in both strains after vanillin exposure (Figure 4A); the expression of TRX2 was only reduced in the case of the Δsod1 mutant after vanillin exposure (Figure 4B). In contrast, the level of both genes was comparable in the control cells regardless of the strain (Figure 4A,B). The expression of the TRR1 gene was significantly up-regulated in control ∆sod1 cells compared to wild-type cells (Figure 4C). Furthermore, the expression of the TRR1 gene increased similarly in both strains after exposure to vanillin (Figure 4C).
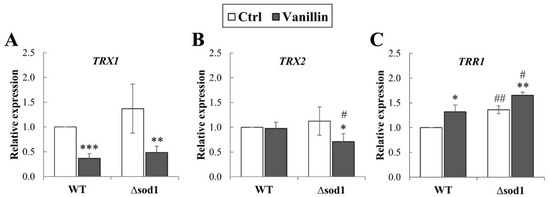
Figure 4.
Expression of cytoplasmic thioredoxin system genes in cells treated with 6 mM vanillin for 3 h. Expression of TRX1 (A), TRX2 (B), and TRR1 (C) genes was calculated using the −∆∆CT method compared to untreated wild-type cells. The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed and * denotes p < 0.05, ** p < 0.01, *** p < 0.001 and # denotes p < 0.05, ## p < 0.01 for the comparison between WT and ∆sod1 strains.
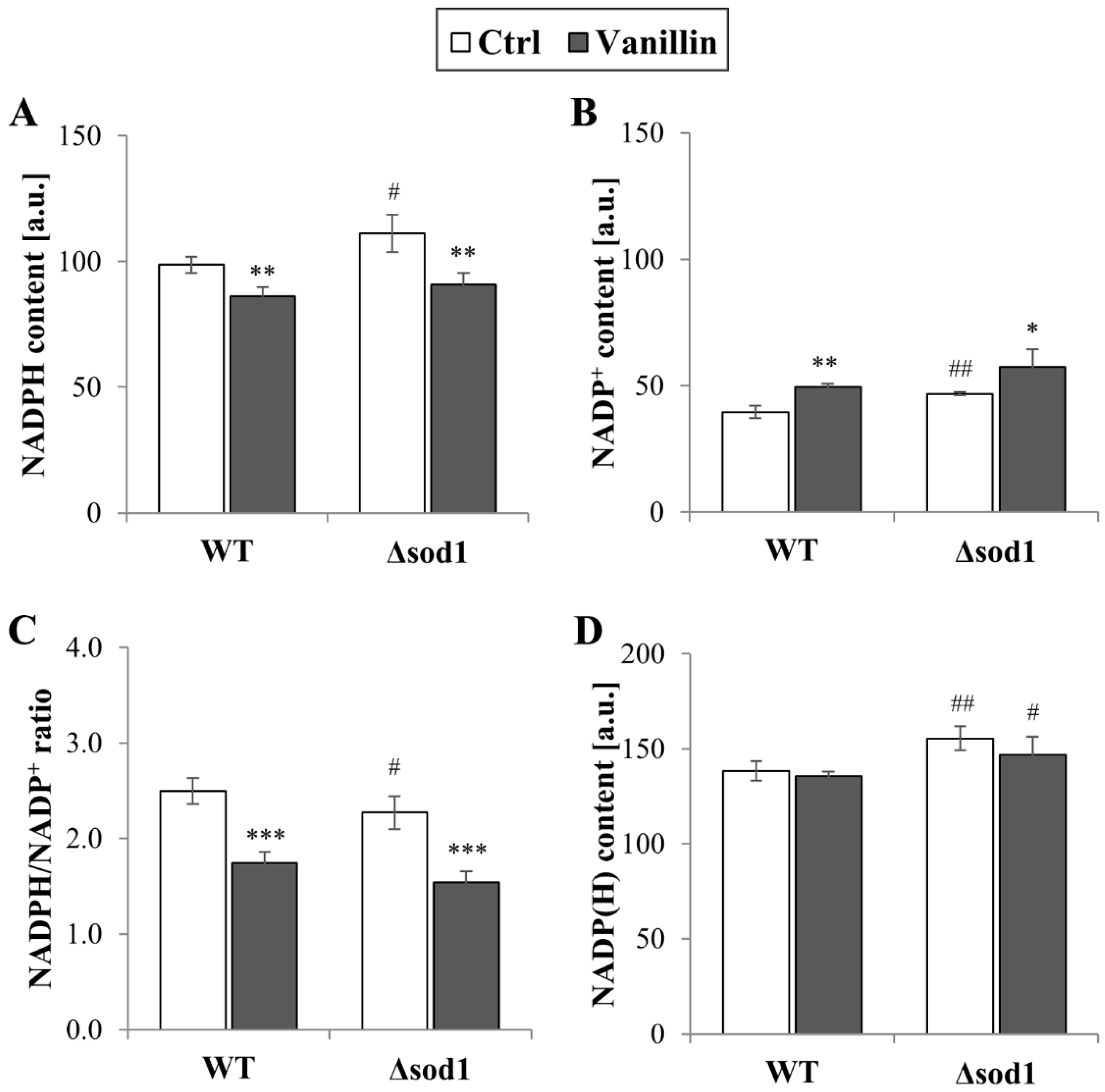
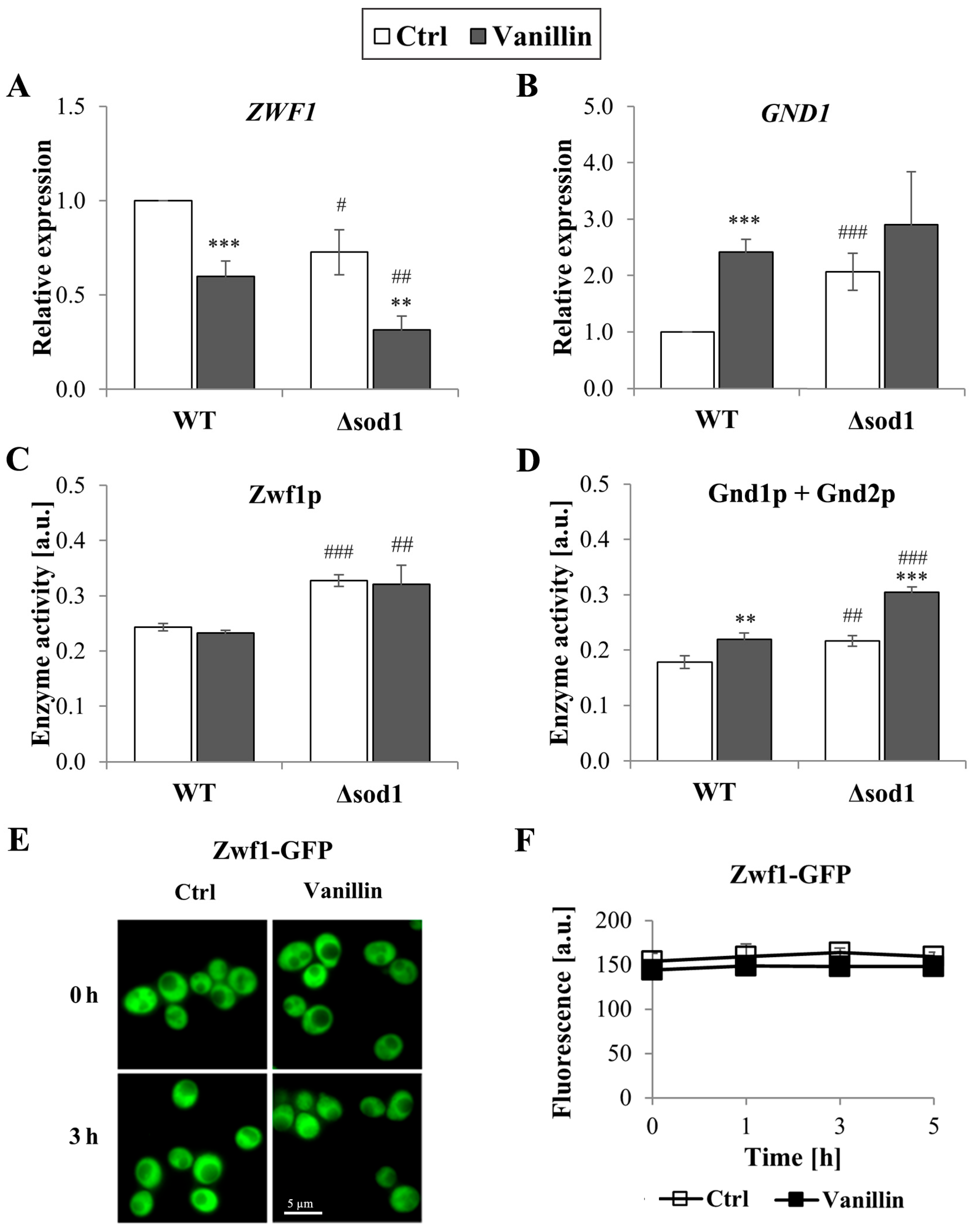
The redox homeostasis of cells is maintained by the glutathione system, supported by the thioredoxin system, both of which are dependent on the reducing power of NADPH. The levels of total and individual forms of NADP(H), as well as the ratio of reduced to oxidized forms of this cofactor, were therefore investigated. It was found that the level of NADPH decreased in cells exposed to vanillin for 3 h (a decrease of 10–20% NADPH, Figure 5A) with a concomitant increase in the level of NADP+ (Figure 5B). The NADPH/NADP+ ratio reflected these disturbances and was found appreciably diminished in vanillin-treated cells (Figure 5C). The ratio of NADPH/NADP+ and the content of the individual forms of the cofactor were altered again in control cells of the Δsod1 mutant strain (Figure 5, [24]). The total content of NADP(H) was not affected by vanillin (Figure 5D). These alterations in the redox state of the NADP(H) cofactor triggered the alterations in the expression of genes encoding dehydrogenases of the PP pathway, which is the primary source of cellular NADPH in yeast fermentative metabolism. The expression of the ZWF1 gene encoding glucose-6-phosphate dehydrogenase was significantly reduced in both strains after exposure to vanillin (Figure 6A). In contrast, the GND1 gene encoding 6-phosphogluconate dehydrogenase was up-regulated in both strains (Figure 6B). Determination of the activity of the respective proteins revealed that the activity of Zwf1p was not affected (Figure 6C), but the activity of Gnd1p and Gnd2p was enhanced in vanillin-treated cells (Figure 6D). The unchanged activity of Zwf1p after 3 h of vanillin treatment was confirmed by microscopic observations and quantitative measurement of Zwf1-GFP-tagged protein fluorescence (Figure 6E,F).
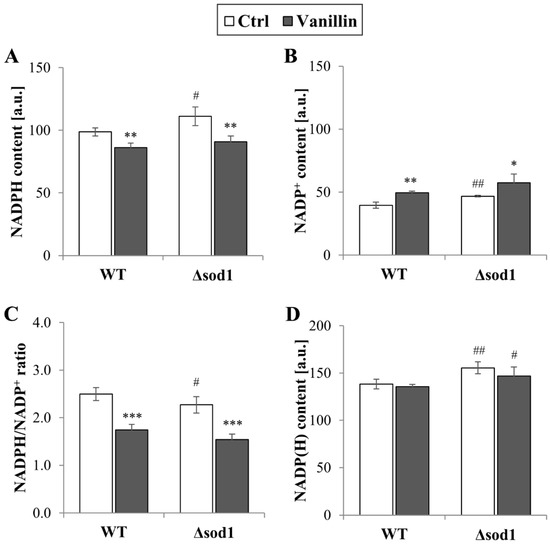
Figure 5.
Content of NADP(H) cofactors in cells treated with 6 mM vanillin for 3 h: (A) NADPH, (B) NADP+, (C) ratio of NADPH/NADP+, (D) total pool of NADP(H) (sum of NADPH and NADP+). The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed, and * denotes p < 0.05, ** p < 0.01, *** p < 0.001 and # denotes p < 0.05, ## p < 0.01 for the comparison between WT and ∆sod1 strains.
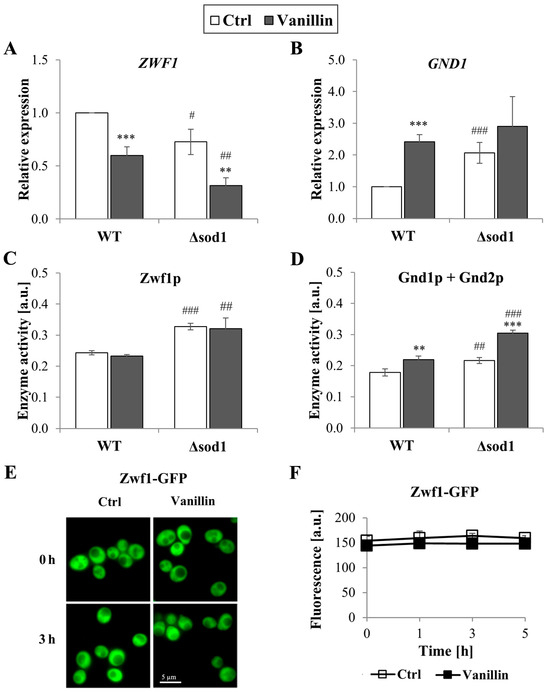
Figure 6.
Expression and activity of pentose phosphate pathway enzymes in cells treated with 6 mM vanillin for 3 h. Expression of ZWF1 (A) and GND1 (B) genes was calculated using the −∆∆CT method to untreated wild-type cells. Activity of PP pathway dehydrogenases (C) Zwf1p and (D) Gnd1p and Gnd2p was estimated in whole-cell extracts by measuring the increase in NADPH absorbance. Activity and location of Zwf1p in cells treated with vanillin were also assessed by observing the fluorescence of Zwf1-GFP-tagged protein (E) with a fluorescence microscope or by fluorescence measurements at the indicated times (F). The significance of the differences between vanillin-treated samples and control cells incubated with DMSO was analyzed and ** denotes p < 0.01, *** p < 0.001 and # denotes p < 0.05, ## p < 0.01, ### p < 0.001 for the comparison between WT and ∆sod1 strains.
4. Discussion
The toxicity of biologically active aldehydes to yeast cells depends on their chemical structure, particularly the presence of unsaturated bonds, and can result in either growth inhibition or cell death. Molecular mechanisms of aldehyde toxicity include protein damage, formation of protein and glutathione adducts, DNA damage, depletion of the glutathione pool, secondary induction of ROS generation, and stress response [35].
Vanillin, as a phenolic aldehyde, has been shown to repress translation, form P-bodies and stress granules, and disrupt membrane integrity [36]. Many efforts have been made to find ways to alleviate the toxic effects of vanillin and other yeast fermentation inhibitors, including the overexpression of genes related to redox homeostasis [37,38] and the identification of new genes responsible for vanillin tolerance [39,40]. Our objective was to conduct a thorough investigation into the role of oxidative stress in the toxicity of vanillin in yeast cells. The involvement of oxidative stress in vanillin toxicity for yeast cells was first demonstrated by Nguyen et al. [12]. They showed that vanillin induces an oxidative stress response through activation of the Yap1 transcription factor and fragmentation of mitochondria [12]. In these studies, the oxidative stress-hypersensitive yeast strain, the Δsod1 mutant, was used. Lack of superoxide dismutase 1 results in increased intracellular ROS content, disturbance in glutathione homeostasis, and thus increased sensitivity to oxidative stress-generating agents. The most apparent feature of oxidative stress is the enhancement of ROS content. It was shown that treatment of cells with 6 mM vanillin considerably increases ROS content in wild-type and Δsod1 mutant cells (Figure 2A). This increase in ROS content, which was even more pronounced in the Δsod1 mutant, is an undeniable feature of oxidative stress. The increase in ROS content was observed after 3 h of incubating the cells with vanillin; in turn, shorter treatment did not lead to a significant rise in ROS levels (Figure S3). This means that the increased ROS generation is a secondary effect of cellular metabolic or redox perturbations rather than a primary effect of vanillin reactivity.
In yeast cells, the oxidative stress response is mainly mediated by transcription factor Yap1, which can be activated indirectly by modification of its reactive cysteines by Gpx3p or by direct reactive cysteines oxidation. Indirect activation is typical for peroxides, and direct activation is typical for electrophilic compounds [41], including reactive aldehydes like acrolein [21]. The activation of Yap1p in the Δsod1 mutant after vanillin treatment was examined (Figure 2B,C). The activation of Yap1p was observed as Yap1-GFP fluorescence accumulated in cell nuclei within 15–45 min (Figure 2C), long before the ROS increase could be detected. No difference was observed in the kinetics of Yap1p activation between the wild-type and mutant strains. The response of the cells to vanillin was very diverse, ranging from complete activation to partial to no activation (Figure 2B); even very high concentrations of vanillin did not cause Yap1p activation in each cell.
A similar effect—enhancement of ROS content after prolonged exposure (8 h) and upregulation of Yap1-dependent genes within 30–60 min—has also been shown for other aldehydes derived from lignocellulose biotransformation, furfural and 5-hydroxymethylfurfural [42]. The kinetics of Yap1p activation appear to be similar to those of menadione [41] or the thiol-reactive aldehyde, acrolein [21], in contrast to hydrogen peroxide or other peroxides, which activate Yap1p rapidly and relatively shortly [43]. This allows for the assumption that vanillin-mediated Yap1p activation is a direct reaction with Yap1p reactive cysteines rather than enzymatically mediated, as in the case of H2O2, similar to furfural and 5-hydroxymethylfurfural [42].
Cellular redox homeostasis and coping with oxidative stress require a balance between the reduced and oxidized forms of glutathione, the GSH/GSSG and NADP(H) redox couples. The connection and equilibration of the GSH/GSSG and NADPH/NADP+ systems have been demonstrated by several recent studies [44,45,46,47]. Glutathione is considered the main cellular source of reducing equivalents, and limiting its oxidation and GSSG formation is important. Cells possess several cooperating systems to prevent the accumulation of GSSG, but one of the most important seems to be the reduction of GSSG by glutathione reductase (GR). The GSSG reduction is accompanied by the oxidation of NADPH to NADP+, and glutathione reductase is an important consumer of NADPH [45]. Our results demonstrate the redox changes of the glutathione pool induced by vanillin (Figure 3). Treatment of cells for 3 h induced a significant decrease in reduced glutathione and a concomitant increase in oxidized glutathione content, affecting the glutathione redox potential expressed as the GSH/GSSG ratio (Figure 3B–D). Taking into account the total content of the two forms of glutathione, it was shown that it was significantly decreased in both strains (Figure 3A), indicating that a small amount in wild-type and about 15% of glutathione in the ∆sod1 mutant could form adducts with vanillin, its metabolites, or with proteins modified by vanillin. This observation confirms that vanillin induces electrophilic stress like other aldehydes, but the range of this effect is rather small when compared to very reactive aldehydes, e.g., acrolein [21]. No significant alteration in the glutathione pool was observed with shorter exposure to vanillin (Figure S3). Analyzing the changes in the redox systems between the wild-type and ∆sod1 strains under control conditions, it can be postulated that maintaining an appropriate balance between the GSH/GSSG and NADPH/NADP+ systems is crucial for cellular redox homeostasis. The deletion of the SOD1 gene results in a significantly increased level of GSSG (Figure 3C). To counteract this, the following occurred: (i) glutathione reductase activity increased, even though the GLR1 gene expression was maintained (Figure 3E,F); (ii) thioredoxin reductase (TRR1) gene expression is up-regulated (Figure 4C); (iii) NADPH production increased and simultaneously the level of NADP+ increased, indicating that the emerging NADPH pool is being consumed and can be used by GSSG-reducing systems and thioredoxin reductase (Figure 4 and Figure 5); (iv) the total glutathione and reduced glutathione levels increased (Figure 3A,B). While these activities work well enough under control conditions, the situation is entirely different when cells are exposed to vanillin. A significant increase in GSSG content was not accompanied by higher GLR1 gene expression or higher glutathione reductase activity (Figure 3E,F). Moreover, the expression of cytoplasmic thioredoxin (TRX1 and TRX2) genes decreased (Figure 4A,B), which, in light of the latest results presenting a connection between thioredoxin, Tsa1p (cytoplasmic 2-Cys peroxiredoxin), and GSSG production [48], could suggest their involvement in the development of oxidative stress. The relationship and overlap in function between the cytoplasmic thioredoxin and the glutathione/glutaredoxin system have been considered for a long time [49], although the work of Zimmmerman et al. shows directly that (i) Tsa1p is an essential source for cytosolic GSSG; (ii) Tsa1p is efficiently reduced by yeast Trx1p but not by Grx2p, and in a rate-limiting manner in a nonenzymatic way by GSH. Hence, as was suggested, the intracellular Tsa1-dependent GSSG production would require depletion or oxidation of the thioredoxin pool [48]. Related to this, a previous observation also noted that protein levels of glutathione peroxidase (Gpx2p) and cytoplasmic thioredoxin (Trx2p) did not increase in response to severe vanillin stress [12,50]. It should also be considered that the higher level of the oxidized form of glutathione in the ∆sod1 mutant may be related not only to the presence of GSSG itself, but also to polysulfides [18,51]. This is supported by recently published data indicating that SOD1 regulates H2S and reactive sulfur species (RSS) such as polysulfides. Therefore, the absence of SOD1 in mutant cells may increase polysulfide levels [18]. The changes in the GSH/GSSG system are not counteracted by NADPH/NADP+ system changes. However, with a relatively constant pool of cofactors, the NADPH level decreases, and the NADP+ level increases, directly indicating its consumption (Figure 5). As the increase in GLR1 gene expression or higher activity of glutathione reductase after vanillin exposure was not observed (Figure 3E,F), thus higher consumption of NADPH for maintaining redox balance might be connected with increased needs of thioredoxin reductase, as TRR1 gene expression was significantly up-regulated after vanillin exposure (Figure 4C). The inadequate compensation between the GSH/GSSG and NADPH/NADP+ redox couples is one reason for the oxidative stress observed following vanillin treatment. Conversely, it suggests that vanillin prompts the utilization of NADPH for cellular functions beyond antioxidant activities. The decrease in NADPH levels cannot be explained by its use for anabolic needs, since the growth and proliferation of yeast cells are inhibited in the presence of vanillin (Figure 1). The observed inhibition of cell growth and proliferation is undoubtedly related to the decrease in protein synthesis caused by the repression of bulk translation activity, serving as downregulation of genes involved in ribosome biogenesis and rRNA processing [52,53]. However, inhibiting cell proliferation is not entirely a negative effect. The oxidative stress response mechanism comprises several mutually complementary pathways, whose activation incurs a high cost and consequently affects the cell’s proliferative activity. Moreover, the delay in cell cycle progression allows cells to activate an adequate repair system or break down/metabolize the xenobiotic [34]. Therefore, the use of NADPH in the cells treated with vanillin appears to be also related to the participation of NADPH in the metabolism/detoxification of this aldehyde. The main pathway of vanillin bioconversion is its reduction to the less toxic form, vanillyl alcohol, and the ratio of this conversion observed in yeast can be almost 1:1 (tested in the presence of 1 mM vanillin) [54]. As is shown by the literature data, the reduction of vanillin in yeast cells can be catalyzed by several enzymes, but the NADPH-dependent medium-chain alcohol dehydrogenases Adh6p and Adh7p play a pivotal role. Expression of Adh6p is constitutive and gradually decreases under vanillin stress, whereas expression of Adh7p is inducible and occurs under severe vanillin stress, despite the repression of bulk translational activity [52,55]. Expression of the ADH7 gene can be activated by several transcription factors, one of which is Yap1 [52,56]. Enhanced vanillin tolerance may also be due to the effect of Gcy1p and Ypr1p, which exhibit NADPH-dependent and NAD(P)H-dependent vanillin reductase activity, respectively [39]. Additional functions in vanillin detoxification can also serve NADH-dependent aldehyde reductases (YLL056C and YNL134C), aldo-keto reductase (YJR096W), and the putative NADH-dependent alcohol dehydrogenase Bdh2p [55,56,57]. The connection between vanillin detoxification and NADPH content is supported by data investigating the deletion of the transcription factor Yrr1 or deficiency of glucose-6-phosphate dehydrogenase (Zwf1p) [50,53]. It has been shown that cells lacking Yrr1p exhibit both increased expression of the ADH7 gene and higher NADPH-dependent vanillin reductase activity, which is associated with increased vanillin tolerance [40,53,58]. In contrast, cells lacking Zwf1p show delayed growth and inefficient reduction of vanillin in the culture [50]. Thus, faster conversion of vanillin to vanillyl alcohol and increased tolerance to vanillin in yeast cells appear to be strictly dependent on the NADPH-based detoxification system. Similarly, the crucial role of NADPH in furfural tolerance has been shown by Liu et al. [38].
Therefore, part of the cellular response to vanillin is to alter the systems that provide the reducing power of NADPH. Although yeast cells possess several cellular strategies for producing NADPH [46], the pentose phosphate pathway is still the primary source of NADPH during fermentative metabolism. The obtained results show that the protein level and activity of Zwf1p are maintained in cells treated with vanillin, even though ZWF1 gene expression is down-regulated (Figure 6 and Figure S4). Conversely, the GND1 gene expression and Gnd1p, Gnd2p activity were significantly up-regulated (Figure 5 and Figure S4). The obtained results are consistent with previous reports highlighting the importance of Zwf1p and Gnd1p in vanillin or furfural tolerance [38,50,58,59]. Moreover, the results showed that modulation of the expression and activity of Gnd1p seems to be an essential cellular action triggered in response to phenolic fermentation inhibitors, including vanillin (this work, [58]) and furfural [59]. Another way to supply NADPH for vanillin reduction is through the activity of aldehyde dehydrogenase 6 (Ald6p, the product of the ALD6 gene). Our preliminary findings showed increased expression of the ALD6 gene in cells treated with vanillin, consistent with previous reports indicating that overexpression of the ALD6 gene enhances tolerance to vanillin, furfural, and HMF [55,56,57].
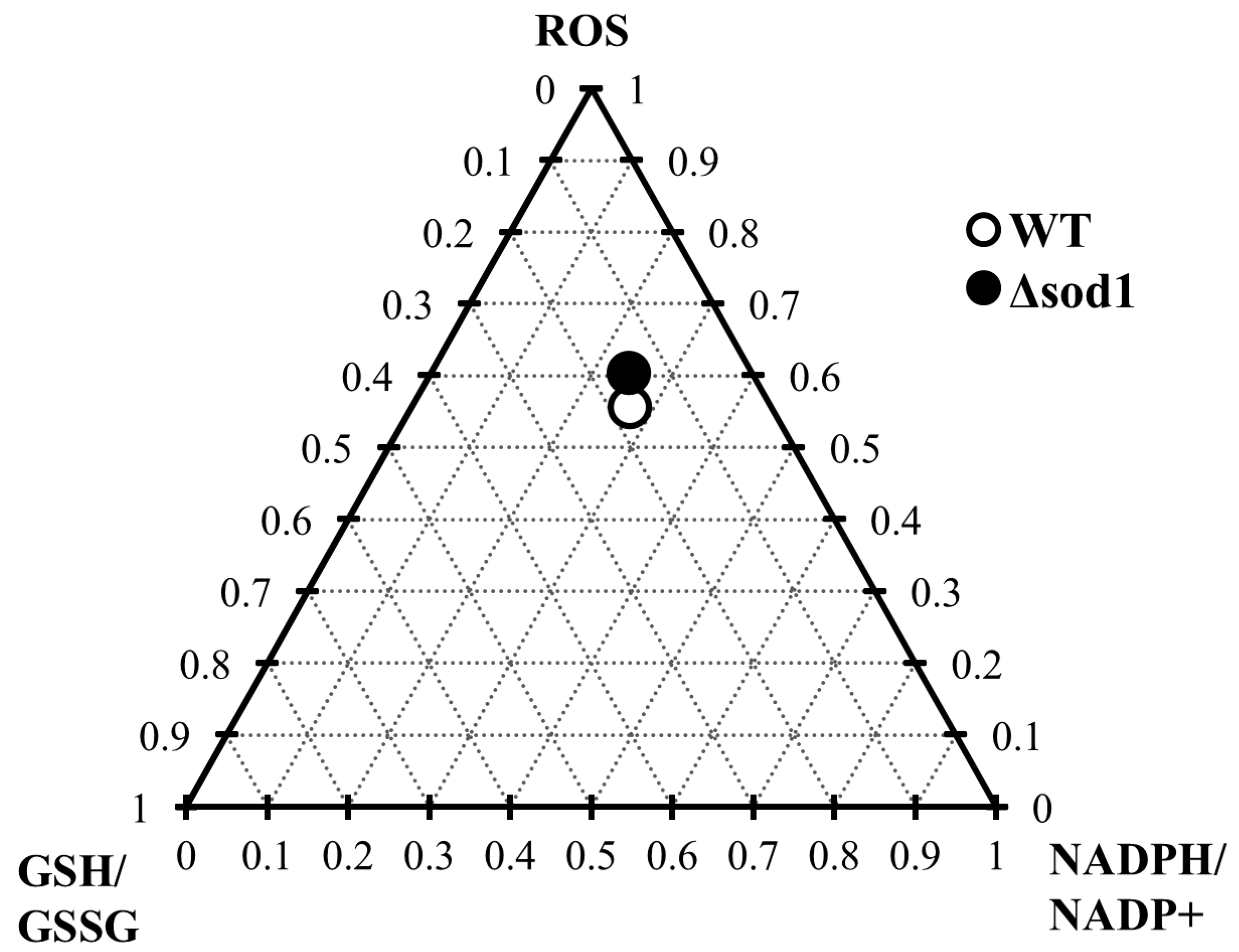
The depletion of the glutathione pool and the alteration in NADP(H) cofactor (Figure 3 and Figure 5) availability caused by vanillin seem to be related to diminished cellular metabolic activity (Figure 1E) and the observed inhibition of growth (Figure 1A,B), which may result from the fact that NADPH, apart from its importance for antioxidant defense systems, is also necessary for cellular biosynthetic processes. This cell vitality deterioration did not meaningfully imply cell death (Figure 1D,F), as is the case with other reactive aldehydes, e.g., acrolein [22]. This observation enables the assessment of vanillin stress as a mild electrophilic stress that leads to yeast cell cycle arrest, forcing cells to adapt to the stress conditions. To assess the impact of the SOD1 deficiency on the vanillin-induced redox perturbations, a ternary plot analysis was performed (Figure 7) with normalized ROS increase, and alterations in GSH/GSSG and NADPH/NADP+ ratios. The extent of vanillin-induced changes in the stress parameters is similar for wild-type and ∆sod1 strains (Figure 7). The lack of significant differences in the fold changes of main stress markers between wild-type and stress-sensitive ∆sod1 strains additionally supports the conclusion that oxidative stress is not the primary cause of vanillin toxicity. Our recently published data showed that deletion of the SOD1 gene causes several alterations in the cell proteome [60], and these changes may reflect in enrichment of the redox capabilities of ∆sod1 cells, like enhanced glutathione and pyridine cofactors content [24,61]. This redox compensation of the SOD1 deficiency may be partly responsible for the similar response of ∆sod1 cells to factors inducing secondary electrophilic stress, like vanillin.
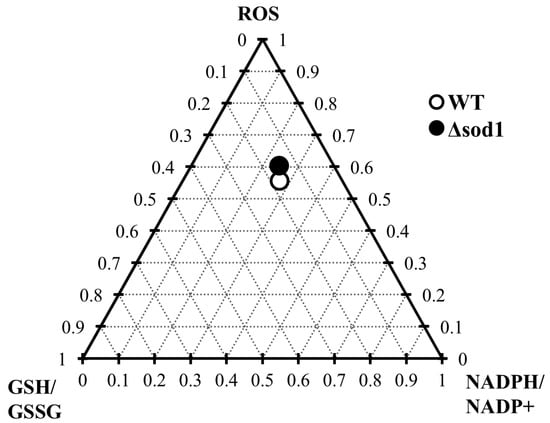
Figure 7.
Ternary plot analysis of the influence of the ∆sod1 mutation on the fold change in ROS content and GSH/GSSG and NADPH/NADP+ ratios induced by vanillin.
5. Conclusions
By analyzing the physiology of the cells exposed to vanillin, we can conclude that, although the markers of oxidative stress appear, their impact does not seem to be a critical factor explaining the inhibitory action of vanillin. The vanillin stress is mild and relatively a secondary electrophilic stress rather than a primary mode of vanillin’s action. We assume that alterations induced by vanillin force cells to use their available resources to detoxify this aldehyde at the expense of NADPH consumption. Although this may help reduce vanillin levels, it simultaneously reduces the pool of cellular NADPH, equivalent to the lowered abundance of overall reducing equivalents. Such alterations in the NADP(H) pool lead to a disturbance in redox homeostasis, manifested by disorders in glutathione homeostasis, lack of compensation between the GSH/GSSG and NADPH/NADP+ redox couples, and increased ROS generation. The results suggest that increasing the availability of NADPH cofactors appears promising in counteracting vanillin-induced inhibition of growth and fermentation in yeast cells, and it may be a direction for future research.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14070842/s1, Figure S1. Effect of vanillin on the viability of the cells. The viability of the cells was estimated with propidium iodide fluorescence staining after 3 h of treatment with 6 mM vanillin. The pictures show representative views after the indicated time. Dead cells show red fluorescence (PI-positive cells). Magnification × 400. BF—brightfield; PI—propidium iodide; Figure S2: (A) Complete activation of Yap1p in the cells exposed to hydrogen peroxide (H2O2) or 9 mM vanillin. The WT cells expressing Yap1-GFP were treated with 1 mM H2O2 or 9 mM vanillin, co-stained with DAPI to visualize cell nuclei, and observed under a fluorescent microscope after 10 min incubation. (B) Diversity of Yap1p localization in WT cells in response to vanillin; a—almost complete activation; b—partial activation; c—no response, cytosolic localization of Yap1-GFP; Figure S3: ROS and glutathione content in the cells treated with vanillin. ROS content in the cells treated with 6 mM vanillin for 1 and 3 h was estimated with dihydroethidine. The kinetics of fluorescence increase was directly measured after adding the probe. Reduced (GSH) and oxidized (GSSG) glutathione content were assessed after 1 and 3 h of treatment with 6 mM vanillin with GSH/GSSG-Glo Assay. Luminescence was recorded after 15 min. The results were presented as a % of WT control. The relevance of the differences of samples treated with vanillin for control cells incubated with DMSO was analyzed and *** denotes p < 0.001 and # denotes p < 0.05, ## p < 0.01, ### p < 0.001 for the comparison between WT and ∆sod1 strains; Figure S4: Expression of ZWF1 and GND1 genes in the cells treated with 6 mM vanillin for 1 and 3 h. The expression was calculated with the −ΔΔCT method with respect to WT untreated cells. The relevance of the differences of samples treated with vanillin for control cells incubated with DMSO was analyzed and ** denotes ** p < 0.01, *** p < 0.001 and # denotes p < 0.05, ## p < 0.01, ### p < 0.001 for the comparison between WT and ∆sod1 strains.
Author Contributions
Conceptualization, S.B. and M.K.-M.; methodology, S.B., M.K.-M., R.M. and R.Z.-T.; validation, S.B., M.K.-M., R.M. and R.Z.-T.; formal analysis, S.B., M.K.-M., R.M., D.G., G.Ś. and R.Z.-T.; investigation, S.B., M.K.-M., R.M., D.G., G.Ś. and R.Z.-T.; resources, S.B., M.K.-M., R.M. and R.Z.-T.; data curation, R.M.; writing—original draft preparation, S.B., M.K.-M., R.M. and R.Z.-T.; writing—review and editing, S.B., M.K.-M., R.M. and R.Z.-T.; visualization, S.B. and R.M.; project administration, S.B., M.K.-M. and R.Z.-T.; funding acquisition, R.Z.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Minister of Science of the Republic of Poland under the Program “Regional initiative of excellence”. Agreement No. RID/SP/0010/2024/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Venkataraman, S.; Athilakshmi, J.K.; Rajendran, D.S.; Bharathi, P.; Kumar, V.V. A comprehensive review of eclectic approaches to the biological synthesis of vanillin and their application towards the food sector. Food Sci. Biotechnol. 2024, 33, 1019–1036. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, P.; Rossato, L.A.M.; Strini, A.; Serra, S. From Waste to Value: Recent Insights into Producing Vanillin from Lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Feng, Y.; Sun, J.; Jiang, Y.; Zhang, W.; Xin, F.; Jiang, M. Current Status, Challenges, and Prospects for the Biological Production of Vanillin. Fermentation 2023, 9, 389. [Google Scholar] [CrossRef]
- Xu, L.; Liaqat, F.; Sun, J.; Khazi, M.I.; Xie, R.; Zhu, D. Advances in the vanillin synthesis and biotransformation: A review. Renew. Sustain. Energy Rev. 2024, 189, 113905. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial detoxification of lignocellulosic biomass hydrolysates: Biochemical and molecular aspects, challenges, exploits and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667. [Google Scholar] [CrossRef]
- Divate, N.R.; Huang, P.J.; Chen, G.H.; Chung, Y.C. Construction of Recombinant Saccharomyces cerevisiae with Ethanol and Aldehydes Tolerance via Overexpression of Aldehyde Reductase. Microorganisms 2022, 10, 850. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Brochado, A.R.; Matos, C.; Møller, B.L.; Hansen, J.; Mortensen, U.H.; Patil, K.R. Improved vanillin production in baker’s yeast through in silico design. Microb. Cell Fact. 2010, 9, 84. [Google Scholar] [CrossRef]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the Role of Vanillin in Neurodegenerative Diseases and Neuropathophysiological Conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Sefi, M.; Elwej, A.; Chaabane, M.; Bejaoui, S.; Marrekchi, R.; Jamoussi, K.; Gouiaa, N.; Boudawara-Sellemi, T.; El Cafsi, M.; Zeghal, N.; et al. Beneficial role of vanillin, a polyphenolic flavoring agent, on maneb-induced oxidative stress, DNA damage, and liver histological changes in Swiss albino mice. Hum. Exp. Toxicol. 2019, 38, 619–631. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Iwaki, A.; Ohya, Y.; Izawa, S. Vanillin causes the activation of Yap1 and mitochondrial fragmentation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2014, 117, 33–38. [Google Scholar] [CrossRef]
- Allen, S.A.; Clark, W.; McCaffery, J.M.; Cai, Z.; Lanctot, A.; Slininger, P.J.; Liu, Z.L.; Gorsich, S.W. Furfural induces reactive oxygen species accumulation and cellular damage in Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 2. [Google Scholar] [CrossRef]
- Sturtz, L.A.; Diekert, K.; Jensen, L.T.; Lill, R.; Culotta, V.C. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J. Biol. Chem. 2001, 276, 38084–38089. [Google Scholar] [CrossRef] [PubMed]
- Montllor-Albalate, C.; Colin, A.E.; Chandrasekharan, B.; Bolaji, N.; Andersen, J.L.; Wayne Outten, F.; Reddi, A.R. Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae. Redox Biol. 2019, 21, 101064. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar]
- Montllor-Albalate, C.; Kim, H.; Thompson, A.E.; Jonke, A.P.; Torres, M.P.; Reddi, A.R. Sod1 integrates oxygen availability to redox regulate NADPH production and the thiol redoxome. Proc. Natl. Acad. Sci. USA 2022, 119, e2023328119. [Google Scholar] [CrossRef]
- Switzer, C.H.; Kasamatsu, S.; Ihara, H.; Eaton, P. SOD1 is an essential H(2)S detoxifying enzyme. Proc. Natl. Acad. Sci. USA 2023, 120, e2205044120. [Google Scholar] [CrossRef]
- Gralla, E.B.; Valentine, J.S. Null mutants of Saccharomyces cerevisiae Cu, Zn superoxide dismutase: Characterization and spontaneous mutation rates. J. Bacteriol. 1991, 173, 5918–5920. [Google Scholar] [CrossRef]
- López-Mirabal, H.R.; Thorsen, M.; Kielland-Brandt, M.C.; Toledano, M.B.; Winther, J.R. Cytoplasmic glutathione redox status determines survival upon exposure to the thiol-oxidant 4,4′-dipyridyl disulfide. FEMS Yeast Res. 2007, 7, 391–403. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Bednarska, S.; Bartosz, G.; Bilinski, T. Acrolein toxicity involves oxidative stress caused by glutathione depletion in the yeast Saccharomyces cerevisiae. Cell Biol. Toxicol. 2009, 25, 363–378. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S.; Bartosz, G. Acrolein-Induced Oxidative Stress and Cell Death Exhibiting Features of Apoptosis in the Yeast Saccharomyces cerevisiae Deficient in SOD1. Cell Biochem. Biophys. 2015, 71, 1525–1536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bilinski, T.; Kwolek, M.; Sas, E.; Krynicka, M.; Koziol, S.; Owsiak-Teleon, A.; Krzepilko, A.; Bartosz, G. A novel test for identifying genes involved in aldehyde detoxification in the yeast. Increased sensitivity of superoxide-deficient yeast to aldehydes and their metabolic precursors. Biofactors 2005, 24, 59–65. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Bednarska, S.; Dubicka-Lisowska, A.; Maslanka, R.; Zadrag-Tecza, R.; Kaszycki, P. Unbalance between Pyridine Nucleotide Cofactors in The SOD1 Deficient Yeast Saccharomyces cerevisiae Causes Hypersensitivity to Alcohols and Aldehydes. Int. J. Mol. Sci. 2022, 24, 659. [Google Scholar] [CrossRef]
- Biliński, T.; Lukaszkiewicz, J.; Sledziewski, A. Demonstration of anaerobic catalase synthesis in the cz1 mutant of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1978, 83, 1225–1233. [Google Scholar] [CrossRef]
- Koziol, S.; Zagulski, M.; Bilinski, T.; Bartosz, G. Antioxidants protect the yeast Saccharomyces cerevisiae against hypertonic stress. Free Radic. Res. 2005, 39, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, S.; Leroy, P.; Zagulski, M.; Bartosz, G. Efficacy of antioxidants in the yeast Saccharomyces cerevisiae correlates with their effects on protein thiols. Biochimie 2008, 90, 1476–1485. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Maslanka, R.; Molon, M. Disorders in NADPH generation via pentose phosphate pathway influence the reproductive potential of the Saccharomyces cerevisiae yeast due to changes in redox status. J. Cell Biochem. 2019, 120, 8521–8533. [Google Scholar] [CrossRef]
- Tian, W.N.; Braunstein, L.D.; Pang, J.; Stuhlmeier, K.M.; Xi, Q.C.; Tian, X.; Stanton, R.C. Importance of glucose-6-phosphate dehydrogenase activity for cell growth. J. Biol. Chem. 1998, 273, 10609–10617. [Google Scholar] [CrossRef]
- Maslanka, R.; Bednarska, S.; Zadrag-Tecza, R. Virtually identical does not mean exactly identical: Discrepancy in energy metabolism between glucose and fructose fermentation influences the reproductive potential of yeast cells. Arch. Biochem. Biophys. 2024, 756, 110021. [Google Scholar] [CrossRef]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Moye-Rowley, W.S. Transcription factors regulating the response to oxidative stress in yeast. Antioxid. Redox Signal 2002, 4, 123–140. [Google Scholar] [CrossRef]
- Zadrag-Tecza, R.; Maslanka, R.; Bednarska, S.; Kwolek-Mirek, M. Response Mechanisms to Oxidative Stress in Yeast and Filamentous Fungi. In Stress Response Mechanisms in Fungi: Theoretical and Practical Aspects; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Jayakody, L.N.; Jin, Y.S. In-depth understanding of molecular mechanisms of aldehyde toxicity to engineer robust Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 2675–2692. [Google Scholar] [CrossRef]
- Iwaki, A.; Ohnuki, S.; Suga, Y.; Izawa, S.; Ohya, Y. Vanillin inhibits translation and induces messenger ribonucleoprotein (mRNP) granule formation in Saccharomyces cerevisiae: Application and validation of high-content, image-based profiling. PLoS ONE 2013, 8, e61748. [Google Scholar] [CrossRef]
- Ask, M.; Mapelli, V.; Höck, H.; Olsson, L.; Bettiga, M. Engineering glutathione biosynthesis of Saccharomyces cerevisiae increases robustness to inhibitors in pretreated lignocellulosic materials. Microb. Cell Factories 2013, 12, 87. [Google Scholar] [CrossRef]
- Liu, C.G.; Li, K.; Li, K.Y.; Sakdaronnarong, C.; Mehmood, M.A.; Zhao, X.Q.; Bai, F.W. Intracellular Redox Perturbation in Saccharomyces cerevisiae Improved Furfural Tolerance and Enhanced Cellulosic Bioethanol Production. Front. Bioeng. Biotechnol. 2020, 8, 615. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Wu, J.; Mu, S.; Wu, Z.; Jin, J.-M.; Tang, S.-Y. Dynamic control of toxic natural product biosynthesis by an artificial regulatory circuit. Metab. Eng. 2020, 57, 239–246. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, Y.; Hu, J.; Shi, C.; Ao, X.; Wang, S.; Lin, Y.; Sun, Z.; Han, S. Disruption of phosphate metabolism and sterol transport-related genes conferring yeast resistance to vanillin and rapid ethanol production. Bioresour. Technol. 2023, 369, 128489. [Google Scholar] [CrossRef]
- Azevedo, D.; Tacnet, F.; Delaunay, A.; Rodrigues-Pousada, C.; Toledano, M.B. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radic. Biol. Med. 2003, 35, 889–900. [Google Scholar] [CrossRef]
- Kim, D.; Hahn, J.S. Roles of the Yap1 transcription factor and antioxidants in Saccharomyces cerevisiae’s tolerance to furfural and 5-hydroxymethylfurfural, which function as thiol-reactive electrophiles generating oxidative stress. Appl. Environ. Microbiol. 2013, 79, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, A.; Pflieger, D.; Barrault, M.-B.; Vinh, J.; Toledano, M.B. A Thiol Peroxidase Is an H2O2 Receptor and Redox-Transducer in Gene Activation. Cell 2002, 111, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Gansemer, E.R.; McCommis, K.S.; Martino, M.; King-McAlpin, A.Q.; Potthoff, M.J.; Finck, B.N.; Taylor, E.B.; Rutkowski, D.T. NADPH and Glutathione Redox Link TCA Cycle Activity to Endoplasmic Reticulum Homeostasis. iScience 2020, 23, 101116. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, A.; Przywara, M.; Maslanka, R.; Ras, B.; Ziaja, K.; Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S. Redox perturbations in yeast cells lacking glutathione reductase. Fungal Genet. Biol. 2023, 167, 103810. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Maslanka, R.; Bednarska, S.; Przywara, M.; Kwolek, K.; Zadrag-Tecza, R. Strategies to Maintain Redox Homeostasis in Yeast Cells with Impaired Fermentation-Dependent NADPH Generation. Int. J. Mol. Sci. 2024, 25, 9296. [Google Scholar] [CrossRef]
- Masanta, S.; Wiesyk, A.; Panja, C.; Pilch, S.; Ciesla, J.; Sipko, M.; De, A.; Enkhbaatar, T.; Maslanka, R.; Skoneczna, A.; et al. Fmp40 ampylase regulates cell survival upon oxidative stress by controlling Prx1 and Trx3 oxidation. Redox Biol. 2024, 73, 103201. [Google Scholar] [CrossRef]
- Zimmermann, J.; Lang, L.; Calabrese, G.; Laporte, H.; Amponsah, P.S.; Michalk, C.; Sukmann, T.; Oestreicher, J.; Tursch, A.; Peker, E.; et al. Tsa1 is the dominant peroxide scavenger and a source of H(2)O(2)-dependent GSSG production in yeast. Free Radic. Biol. Med. 2025, 226, 408–420. [Google Scholar] [CrossRef]
- Draculic, T.; Dawes, I.W.; Grant, C.M. A single glutaredoxin or thioredoxin gene is essential for viability in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 2000, 36, 1167–1174. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Kitajima, S.; Izawa, S. Importance of glucose-6-phosphate dehydrogenase (G6PDH) for vanillin tolerance in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2014, 118, 263–269. [Google Scholar] [CrossRef]
- Huang, C.W.; Deed, R.C.; Parish-Virtue, K.; Pilkington, L.I.; Walker, M.E.; Jiranek, V.; Fedrizzi, B. Characterization of polysulfides in Saccharomyces cerevisiae cells and finished wine from a cysteine-supplemented model grape medium. Food Microbiol. 2023, 109, 104124. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Iwaki, A.; Izawa, S. The ADH7 Promoter of Saccharomyces cerevisiae is Vanillin-Inducible and Enables mRNA Translation Under Severe Vanillin Stress. Front. Microbiol. 2015, 6, 1390. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Z.; Hou, J.; Shen, Y.; Bao, X. The Absence of the Transcription Factor Yrr1p, Identified from Comparative Genome Profiling, Increased Vanillin Tolerance Due to Enhancements of ABC Transporters Expressing, rRNA Processing and Ribosome Biogenesis in Saccharomyces cerevisiae. Front. Microbiol. 2017, 8, 367. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Stratford, M.; Narbad, A. Analysis of the inhibition of food spoilage yeasts by vanillin. Int. J. Food Microbiol. 2003, 86, 113–122. [Google Scholar] [CrossRef]
- Wang, X.; Liang, Z.; Hou, J.; Bao, X.; Shen, Y. Identification and functional evaluation of the reductases and dehydrogenases from Saccharomyces cerevisiae involved in vanillin resistance. BMC Biotechnol. 2016, 16, 31. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Ishida, Y.; Kato, S.; Iwaki, A.; Izawa, S. The VFH1 (YLL056C) promoter is vanillin-inducible and enables mRNA translation despite pronounced translation repression caused by severe vanillin stress in Saccharomyces cerevisiae. Yeast 2018, 35, 465–475. [Google Scholar] [CrossRef]
- Ishida, Y.; Nguyen, T.T.M.; Izawa, S. The yeast ADH7 promoter enables gene expression under pronounced translation repression caused by the combined stress of vanillin, furfural, and 5-hydroxymethylfurfural. J. Biotechnol. 2017, 252, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Zhao, W.; Yang, B.; Wang, X.; Shen, Y.; Wei, T.; Qin, W.; Li, Z.; Bao, X. Proteomic analysis revealed the roles of YRR1 deletion in enhancing the vanillin resistance of Saccharomyces cerevisiae. Microb. Cell Fact. 2021, 20, 142. [Google Scholar] [CrossRef]
- Gorsich, S.W.; Dien, B.S.; Nichols, N.N.; Slininger, P.J.; Liu, Z.L.; Skory, C.D. Tolerance to furfural-induced stress is associated with pentose phosphate pathway genes ZWF1, GND1, RPE1, and TKL1 in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2006, 71, 339–349. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Dubicka-Lisowska, A.; Bednarska, S.; Zadrag-Tecza, R.; Kaszycki, P. Changes in a Protein Profile Can Account for the Altered Phenotype of the Yeast Saccharomyces cerevisiae Mutant Lacking the Copper-Zinc Superoxide Dismutase. Metabolites 2023, 13, 459. [Google Scholar] [CrossRef]
- Maslanka, R.; Zadrag-Tecza, R.; Kwolek-Mirek, M. Linkage between Carbon Metabolism, Redox Status and Cellular Physiology in the Yeast Saccharomyces cerevisiae Devoid of SOD1 or SOD2 Gene. Genes 2020, 11, 780. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).