Lemongrass Alleviates Primary Dysmenorrhea Symptoms by Reducing Oxidative Stress and Inflammation and Relaxing the Uterine Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CcE
2.2. Phytochemical Analysis
2.2.1. Total Phenolic Contents
2.2.2. Total Tannin Contents
2.2.3. Total Flavonoid Contents
2.2.4. Total Saponin Contents
2.3. HPLC Analysis
2.4. GC-MS Analysis
2.5. Antioxidant Assays
2.5.1. DPPH Assay
2.5.2. Nitric Oxide Scavenging Assay
2.5.3. CUPRAC Assay
2.5.4. H2O2 Scavenging Assay
2.5.5. FRAP Scavenging Assay
2.6. In Vitro Anti-Inflammatory Activity
2.6.1. Cyclooxygenase Inhibition
2.6.2. Lipoxygenase Inhibition
2.7. Animals
2.7.1. Acute Toxicity Study
2.7.2. Induction of PD and Treatments
2.7.3. Ex Vivo Uterine Tissue Relaxant Activity
2.7.4. Analgesic Activity
Hot-Plate Activity
Tail-Flick Activity
Acetic Acid-Induced Writhing
2.8. Statistical Analysis
3. Results
3.1. CcE Extraction and Phytochemical Analysis
3.2. Quantification of Phenolic Compounds
3.3. Phytochemical Analysis by GC-MS
3.4. C. citratus Inhibits COX and 5-LOX Enzymes
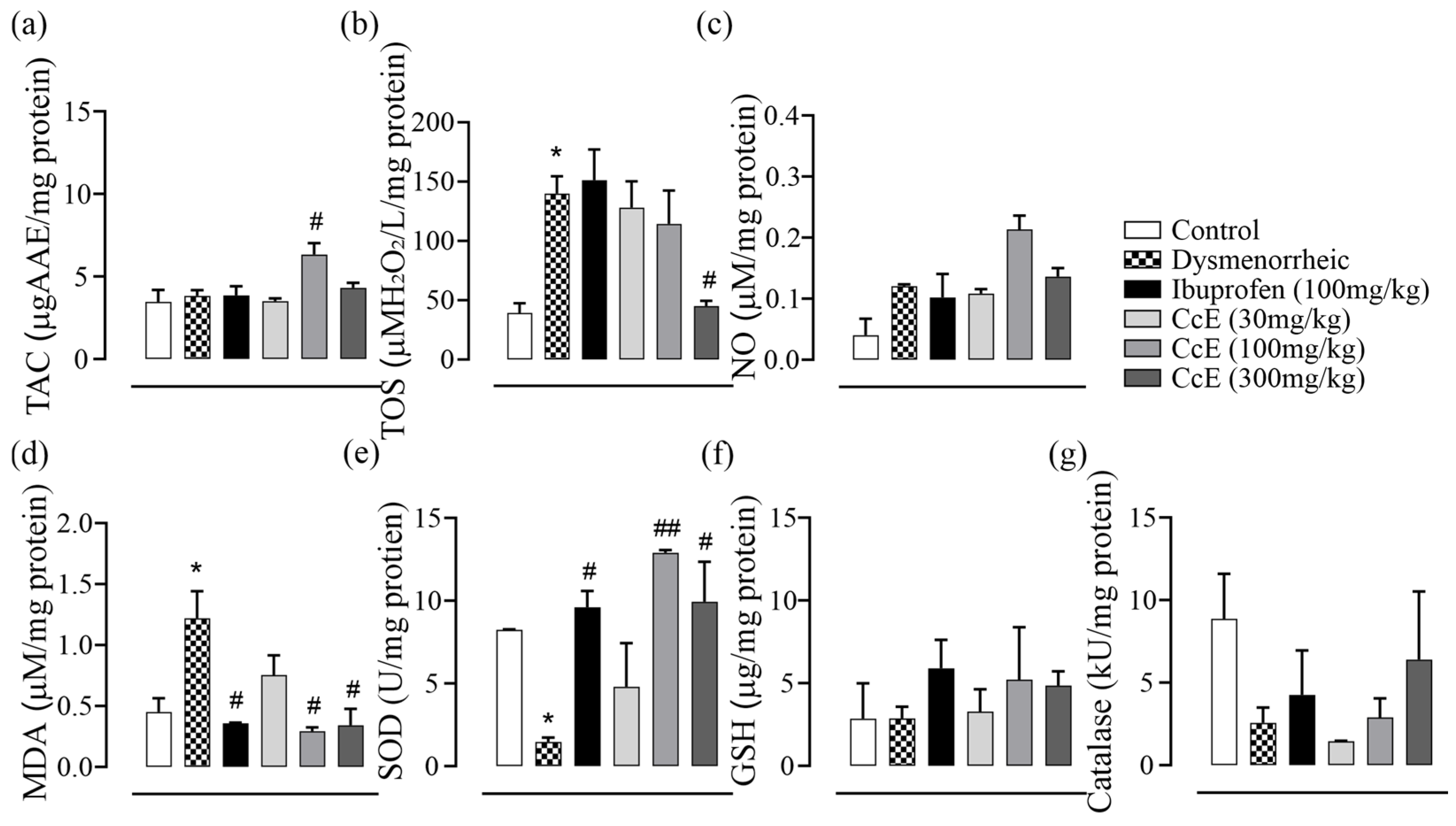
3.5. Antioxidant Activity
3.6. NOAEL CcE Dose
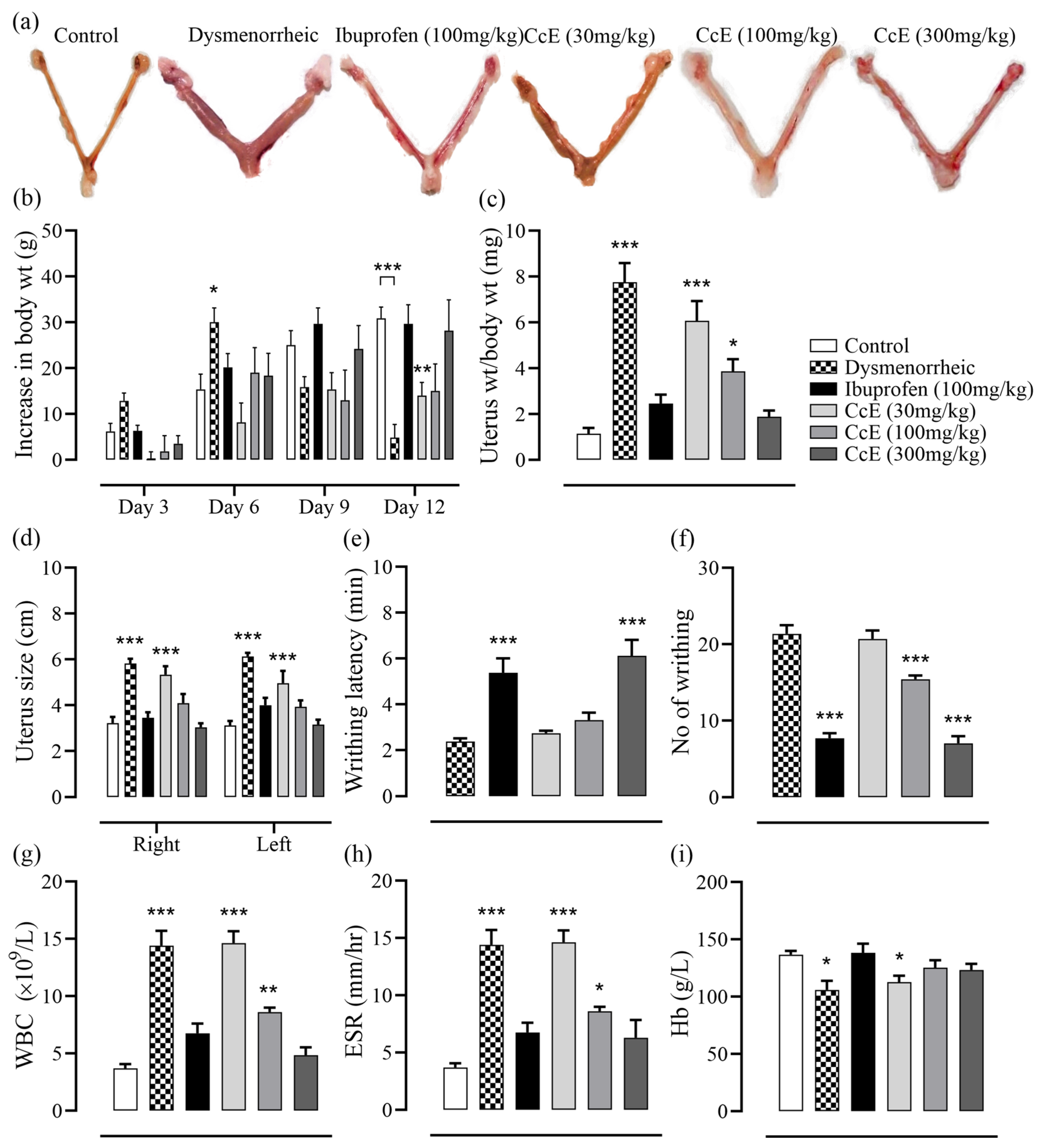
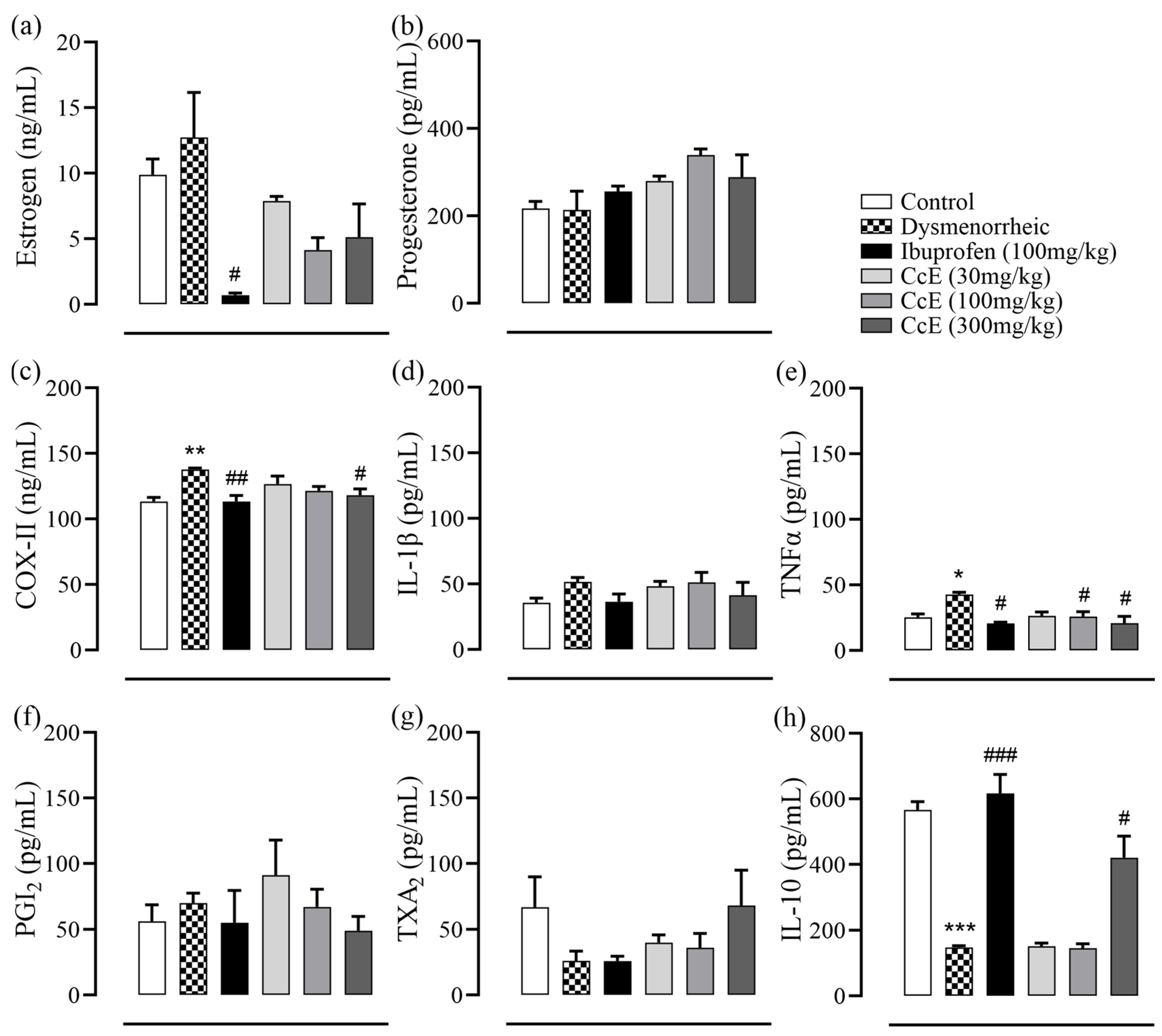
3.7. Amelioration of PD Symptoms
3.8. Ex Vivo Uterine Tissue Relaxant Effect
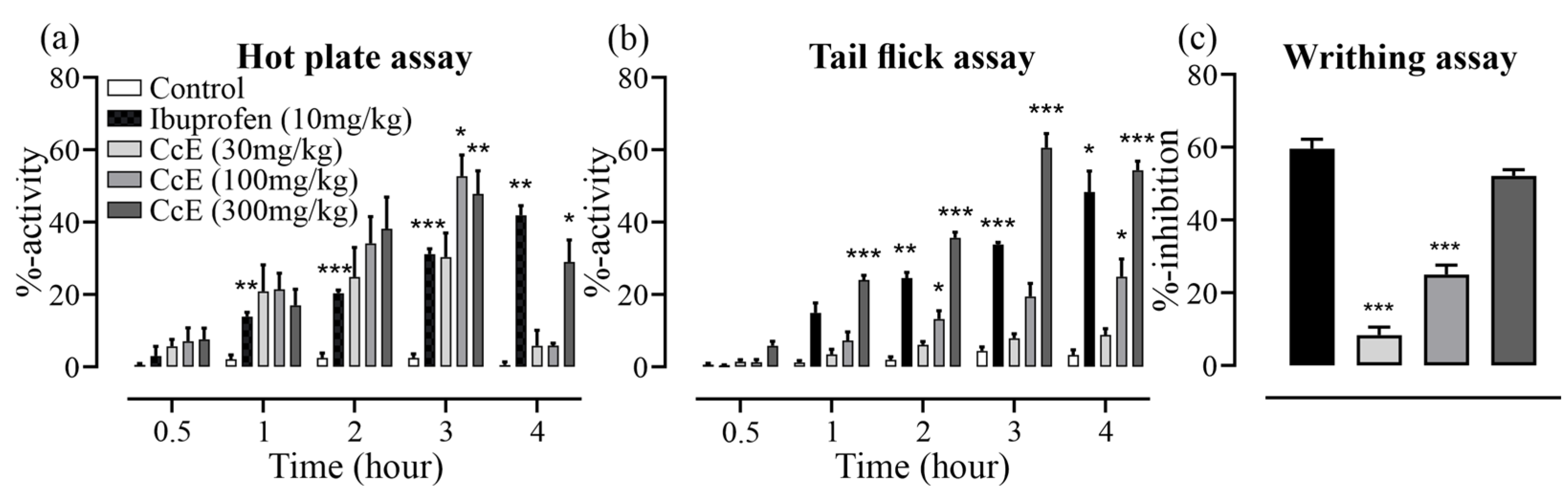
3.9. Analgesic Effects of CcE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mihm, M.; Gangooly, S.; Muttukrishna, S. The Normal Menstrual Cycle in Women. Anim. Reprod. Sci. 2011, 124, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Olena, L. Introductory Chapter: Regulation of Ovarian-Menstrual Cycle as a Systemic Problem of Physiology of Humans. In Menstrual Cycle; Olena Ivanivna, L., Ed.; IntechOpen: Rijeka, Croatia, 2019; p. Ch. 1. [Google Scholar]
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet. Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Duman, N.B.; Yildirim, F.; Vural, G. Risk Factors for Primary Dysmenorrhea and the Effect of Complementary and Alternative Treatment Methods: Sample from Corum, Turkey. Int. J. Health Sci. 2022, 16, 35–43. [Google Scholar]
- Iacovides, S.; Avidon, I.; Baker, F.C. What We Know About Primary Dysmenorrhea Today: A Critical Review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef]
- Li, N.; Cui, X.; Ma, C.; Yu, Y.; Li, Z.; Zhao, L.; Xiong, H. Uncovering the Effects and Mechanism of Danggui Shaoyao San Intervention on Primary Dysmenorrhea by Serum Metabolomics Approach. J. Chromatogr. B 2022, 1209, 123434. [Google Scholar] [CrossRef]
- Campbell, M.A.; McGrath, P.J. Non-Pharmacologic Strategies Used by Adolescents for the Management of Menstrual Discomfort. Clin. J. Pain. 1999, 15, 313–320. [Google Scholar] [CrossRef]
- McKenna, K.A.; Fogleman, C.D. Dysmenorrhea. Am. Fam. Physician 2021, 104, 164–170. [Google Scholar]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.U.; Kubra, K.T.; Azam, M.; Zahid, H.F. Phytochemical Composition and Pharmacological Potential of Lemongrass (Cymbopogon) and Impact on Gut Microbiota. AppliedChem 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Zhao, J.; Fan, Y.; Cheng, Z.; Kennelly, E.J.; Long, C. Ethnobotanical Uses, Phytochemistry and Bioactivities of Cymbopogon Plants: A Review. J. Ethnopharmacol. 2024, 330, 118181. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. LC-MS/MS Characterization of Phenolic Metabolites and Their Antioxidant Activities from Australian Native Plants. Metabolites 2022, 12, 1016. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of Phytomedicine, Phytochemistry, Ethnopharmacology, Toxicology, and Pharmacological Activities of Cymbopogon Genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar] [CrossRef]
- Kiełtyka-Dadasiewicz, A.; Esteban, J.; Jabłońska-Trypuć, A. Antiviral, Antibacterial, Antifungal, and Anticancer Activity of Plant Materials Derived from Cymbopogon citratus (DC.) Stapf Species. Pharmaceuticals 2024, 17, 705. [Google Scholar] [CrossRef] [PubMed]
- Shah, G.; Shri, R.; Panchal, V.; Sharma, N.; Singh, B.; Mann, A.S. Scientific Basis for the Therapeutic Use of Cymbopogon citratus, Stapf (Lemon Grass). J. Adv. Pharm. Technol. Res. 2011, 2, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Barbara, R. Exploring the Anti-Hypertensive Potential of Lemongrass-A Comprehensive Review. Biology 2022, 11, 1382. [Google Scholar] [CrossRef]
- Anjum, F.; Touqeer, S.; Khan, M.Y.; Jamil, Q.; Rida, A.; Shirazi, J.H.; Ejaz, S.A.; Attaullah, H.M.; Sarwar, G.; Khan, Z.H.; et al. Pharmacognostic Evaluation, Chemical Characterization, and Antibacterial Activity of Bassia indica (Wight) A.J. Scott. Plants 2024, 13, 1753. [Google Scholar] [CrossRef]
- Masood, N.; Jamil, Q.; Aslam, M.I.; Masood, M.I.; Shirazi, J.H.; Jamil, Q.A.; Jan, M.S.; Alsuwayt, B.; Ahmad, A.; Abdullah Alnasser, S.M.; et al. Antioxidant, carbonic anhydrase inhibition and diuretic activity of Leptadenia pyrotechnica Forssk. Decne. Heliyon 2023, 9, e22485. [Google Scholar] [CrossRef]
- Ayaz, A.; Jamil, Q.; Hussain, M.; Anjum, F.; Sarfraz, A.; Alqahtani, T.; Hussain, N.; Gahtani, R.M.; Dera, A.A.; Alharbi, H.M.; et al. Antioxidant and Gastroprotective Activity of Suaeda fruticosa Forssk. Ex J.F.Gmel. Molecules 2022, 27, 4368. [Google Scholar] [CrossRef]
- Kusar, S.; Saddiqe, Z.; Ali, F.; Bashir, S.; Zubairi, T. GCMS and HPLC Profiling, Antioxidant and Anti-Inflammatory Activities of Crotalaria medicaginea Lamk. S. Afr. J. Bot. 2024, 168, 196–208. [Google Scholar] [CrossRef]
- Abdul, Q.; Raja Adil, S.; Aisha, A.; Shazia, A. Phenolic Composition and Biological (Anti Diabetic and Antioxidant) Activities of Different Solvent Extracts of an Endemic Plant (Heliotropium strigosum). J. Chil. Chem. Soc. 2016, 61, 2903–2906. [Google Scholar]
- Aslam, M.I.; Touqeer, S.; Jamil, Q.; Masood, M.I.; Sarfraz, A.; Khan, S.Y.; Jan, M.S.; Alnasser, S.M.A.; Ahmad, A.; Aslam, F.; et al. Cenchrus ciliaris L. Ameliorates Cigarette-Smoke Induced Acute Lung Injury by Reducing Inflammation and Oxidative Stress. S. Afr. J. Bot. 2024, 171, 216–227. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. A Novel Hydrogen Peroxide Scavenging Assay of Phenolics and Flavonoids Using Cupric Reducing Antioxidant Capacity (CUPRAC) Methodology. J. Food Compos. Anal. 2010, 23, 689–698. [Google Scholar] [CrossRef]
- Debnath, T.; Park, P.-J.; Deb Nath, N.C.; Samad, N.B.; Park, H.W.; Lim, B.O. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem. 2011, 128, 697–703. [Google Scholar] [CrossRef]
- Nawaz, I.; Tahir, A.; Iqbal, S.M.; Anjum, F.; Naseem, M.; Aslam, M.I.; Hussain, M.; Jamil, Q.A.; Shirazi, J.H.; Jamil, Q. Anti-Inflammatory, Anti-Nociceptive and Anti-Pyretic Activities of Cenchrus ciliaris L. J. Ethnopharmacol. 2023, 309, 116332. [Google Scholar] [CrossRef] [PubMed]
- Gutman, G.; Nunez, A.T.; Fisher, M. Dysmenorrhea in Adolescents. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101186. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Kolar, M.J.; Konduri, S.; Chang, T.; Wang, H.; McNerlin, C.; Ohlsson, L.; Harrod, M.; Siegel, D.; Saghatelian, A. Linoleic Acid Esters of Hydroxy Linoleic Acids are Anti-Inflammatory Lipids Found in Plants and Mammals. J. Biol. Chem. 2019, 294, 10698–10707. [Google Scholar] [CrossRef]
- Pomposiello, S.I.; Alva, M.; Wilde, D.W.; Carretero, O.A. Linoleic Acid Induces Relaxation and Hyperpolarization of the Pig Coronary Artery. Hypertension 1998, 31, 615–620. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on Anti-Inflammatory Molecular Mechanisms Induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Al-Abbasi, F.A.; AlGhamdi, S.A.; Alghamdi, A.M.; Alzarea, S.I.; Almalki, W.H.; AlGhamdi, A.S.; Alkinani, K.B.; Sayyed, N. Review of the Potential Pharmacological Role of Erucic Acid: A Monounsaturated Omega-9 Fatty Acid. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 3663–3674. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Uraku, A.J.; Onuoha, S.C.; Edwin, N.; Ezeani, N.; Ogbanshi, M.E.; Ezeali, C.; Nwali, B.U.; Ominyi, M.C. Nutritional and anti-nutritional quantification assessment of Cymbopopgon citratus leaf. Pharmacol. Pharm. 2015, 6, 401–410. [Google Scholar] [CrossRef]



| Compound | Retention Time (min) | Area (%) | Quantification (ppm) |
|---|---|---|---|
| Quercetin | 2.64 | 0.5 | 1.41 |
| Gallic acid | 4.46 | 19.1 | 36.82 |
| Caffeic acid | 12.43 | 2.2 | 5.69 |
| Vanillic acid | 13.36 | 2.0 | 6.58 |
| Benzoic acid | 14.70 | 2.3 | 13.12 |
| Syringic acid | 16.86 | 1.9 | 2.52 |
| p-Coumaric acid | 17.92 | 2.0 | 1.39 |
| m-Coumaric acid | 19.78 | 2.4 | 1.55 |
| Ferulic acid | 22.43 | 1.0 | 3.79 |
| Cinnamic acid | 25.34 | 1.1 | 2.15 |
| Sinapic acid | 26.20 | 0.7 | 0.45 |
| Kaempferol | 9.1 | 69.9 | 16.5 |
| Peak No. | RT (min) | Area (%) | Compound | M.F. | M.W. g/mol | Qual |
|---|---|---|---|---|---|---|
| 1 | 17.2 | 1.3 | n-Hexadecanoic acid | C16H32O2 | 256.4 | 99 |
| 3 | 19.1 | 22.8 | 10E, 12Z-Octadecadienoic acid | C18H32O2 | 280.4 | 97 |
| 4 | 19.2 | 11.3 | Oleic acid | C18H34O2 | 282.5 | 99 |
| 5 | 19.3 | 2.0 | Octadecanoic acid | C18H36O2 | 284.5 | 99 |
| 6 | 19.4 | 1.3 | linoleic acid | C18H32O2 | 280.4 | 99 |
| 7 | 19.7 | 0.6 | Isolinoleic acid | C18H32O2 | 280.4 | 99 |
| 8 | 20.2 | 0.4 | 2-Octylcyclopropaneoctanal | C19H36O | 280.5 | 93 |
| 9 | 20.8 | 9.8 | cis-11-Eicosenoic acid | C20H38O2 | 310.5 | 99 |
| 10 | 21.0 | 0.9 | Eicosanoic acid | C20H40O2 | 312.5 | 95 |
| 11 | 21.2 | 0.1 | 9, 17-Octadecadienal, (Z)- | C18H32O | 264.4 | 96 |
| 12 | 21.3 | 0.2 | 1-cis-Vaccenoylglycerol | C21H40O4 | 356.0 | 99 |
| 13 | 21.6 | 1.6 | Linoelaidic acid | C18H32O2 | 280.4 | 92 |
| 14 | 21.7 | 3.6 | Glycidyl oleate | C21H38O3 | 338.5 | 99 |
| 15 | 21.9 | 0.9 | Gadoleic acid | C20H38O2 | 310.5 | 58 |
| 16 | 22.0 | 0.3 | 2-Hydroxycyclopentadecanone | C15H28O2 | 240.38 | 96 |
| 17 | 22.5 | 20.4 | Erucic acid | C22H42O2 | 338.6 | 99 |
| 18 | 22.6 | 1.2 | 18-Nonadecenoic acid | C19H36O2 | 296.5 | 97 |
| 19 | 22.9 | 0.2 | Z, E-2,13-Octadecadien-1-ol | C18H34O | 266.5 | 93 |
| 20 | 23.1 | 0.3 | Elaidic acid | C18H34O2 | 282.5 | 93 |
| 21 | 23.3 | 2.6 | Glyceryl monolinoleate | C21H38O4 | 354.5 | 96 |
| 22 | 23.3 | 5.3 | Glyceryl monooleate | C21H40O4 | 356.5 | 95 |
| 23 | 23.5 | 0.2 | Glyceryl monostearate | C21H42O4 | 358.6 | 99 |
| 24 | 23.9 | 0.2 | bis(2-ethylhexyl) benzene-1, 4-dicarboxylate | C24H38O4 | 390.6 | 87 |
| 25 | 24.6 | 0.4 | Erucoyl chloride | C22H41ClO | 357 | 55 |
| 26 | 25.2 | 2.5 | 11-Eicosenoic acid, methyl ester | C21H40O2 | 324.5 | 46 |
| 29 | 28.2 | 5.8 | Monoerucin | C25H48O4 | 412.6 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidiq, S.S.; Jabeen, Q.; Jamil, Q.; Jan, M.S.; Iqbal, I.; Saqib, F.; Aufy, M.; Iqbal, S.M. Lemongrass Alleviates Primary Dysmenorrhea Symptoms by Reducing Oxidative Stress and Inflammation and Relaxing the Uterine Muscles. Antioxidants 2025, 14, 838. https://doi.org/10.3390/antiox14070838
Sidiq SS, Jabeen Q, Jamil Q, Jan MS, Iqbal I, Saqib F, Aufy M, Iqbal SM. Lemongrass Alleviates Primary Dysmenorrhea Symptoms by Reducing Oxidative Stress and Inflammation and Relaxing the Uterine Muscles. Antioxidants. 2025; 14(7):838. https://doi.org/10.3390/antiox14070838
Chicago/Turabian StyleSidiq, Sheikh Safeena, Qaiser Jabeen, QurratUlAin Jamil, Muhammad Saeed Jan, Iram Iqbal, Fatima Saqib, Mohammed Aufy, and Shahid Muhammad Iqbal. 2025. "Lemongrass Alleviates Primary Dysmenorrhea Symptoms by Reducing Oxidative Stress and Inflammation and Relaxing the Uterine Muscles" Antioxidants 14, no. 7: 838. https://doi.org/10.3390/antiox14070838
APA StyleSidiq, S. S., Jabeen, Q., Jamil, Q., Jan, M. S., Iqbal, I., Saqib, F., Aufy, M., & Iqbal, S. M. (2025). Lemongrass Alleviates Primary Dysmenorrhea Symptoms by Reducing Oxidative Stress and Inflammation and Relaxing the Uterine Muscles. Antioxidants, 14(7), 838. https://doi.org/10.3390/antiox14070838







