AZU1 as a DNA Methylation-Driven Gene: Promoting Oxidative Stress in High-Altitude Pulmonary Edema
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Whole-Blood DNA Extraction
2.3. Reduced-Representation Bisulfite Sequencing (RRBS)
2.4. Methylation EPIC 850k BeadChip Array
2.5. Validation of Methylation by Targeted Bisulfite Sequencing (TBS)
2.6. Immunohistochemical Analyses
2.7. Cell Culture
2.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.9. Protein Extraction and Western Blot (WB) Analysis
2.10. Lentivirus Transfection
2.11. Tube Formation Assay
2.12. Lactate Dehydrogenase Assay (LDH)
2.13. Cell Proliferation Assay
2.14. Assessment of Oxidative Stress Markers in HUVEC
2.14.1. Reactive Oxygen Species (ROS) Fluorescence Detection
2.14.2. Glutathione (GSH), Glutathione Oxydized (GSSG), Superoxide Dismutase (SOD), and Malondialdehyde (MDA)
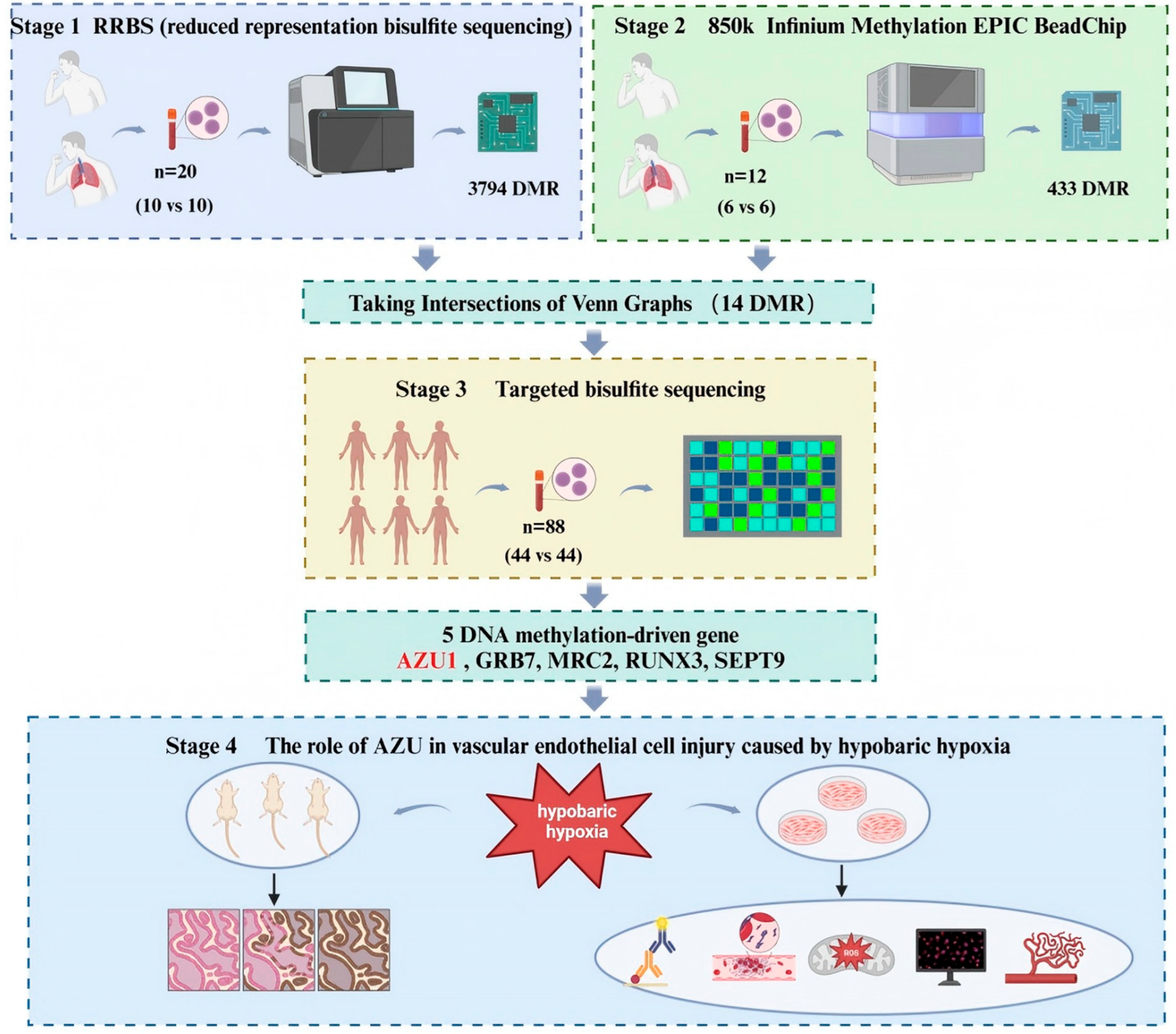
2.15. Bioinformatics Analysis
3. Results
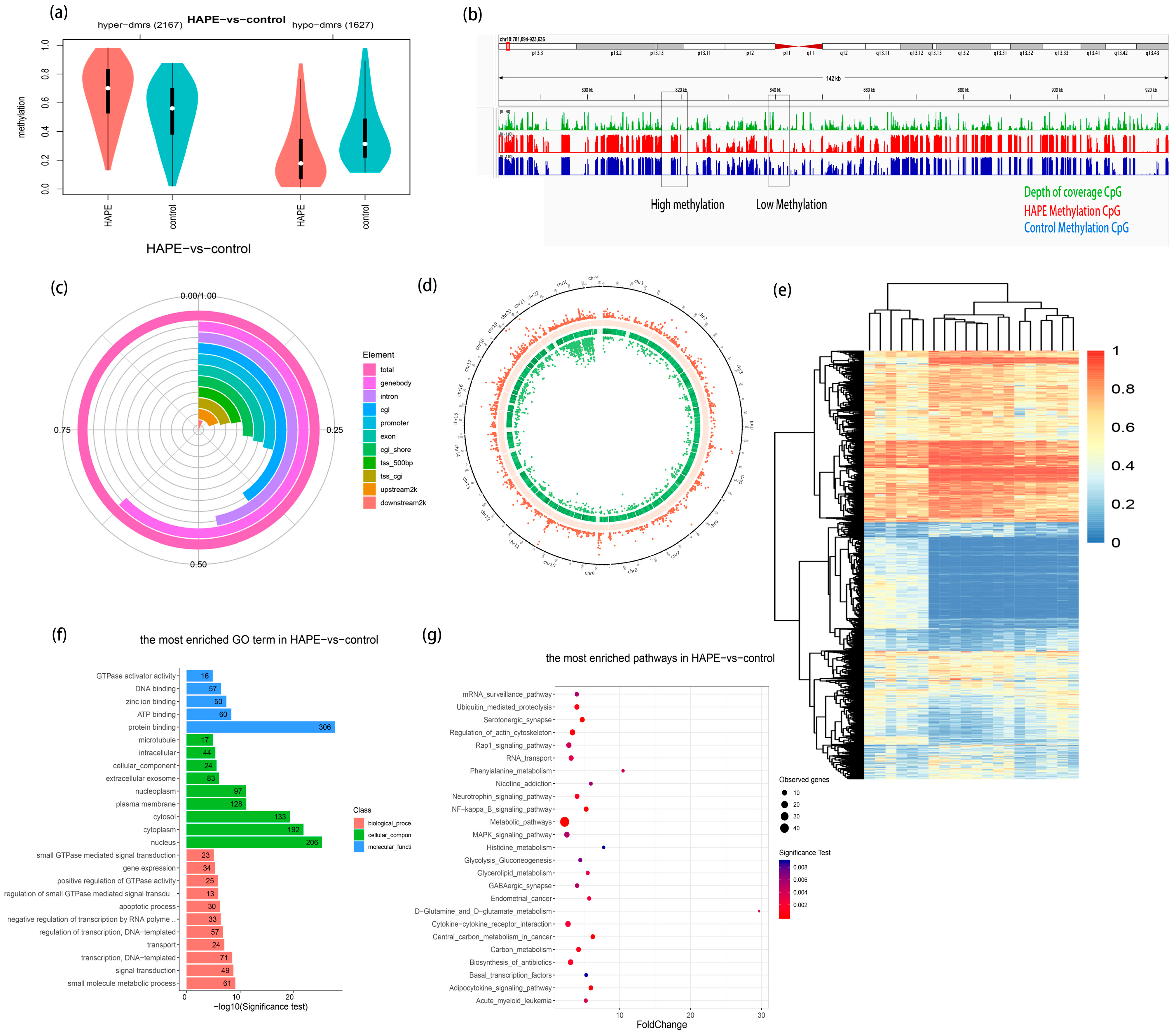
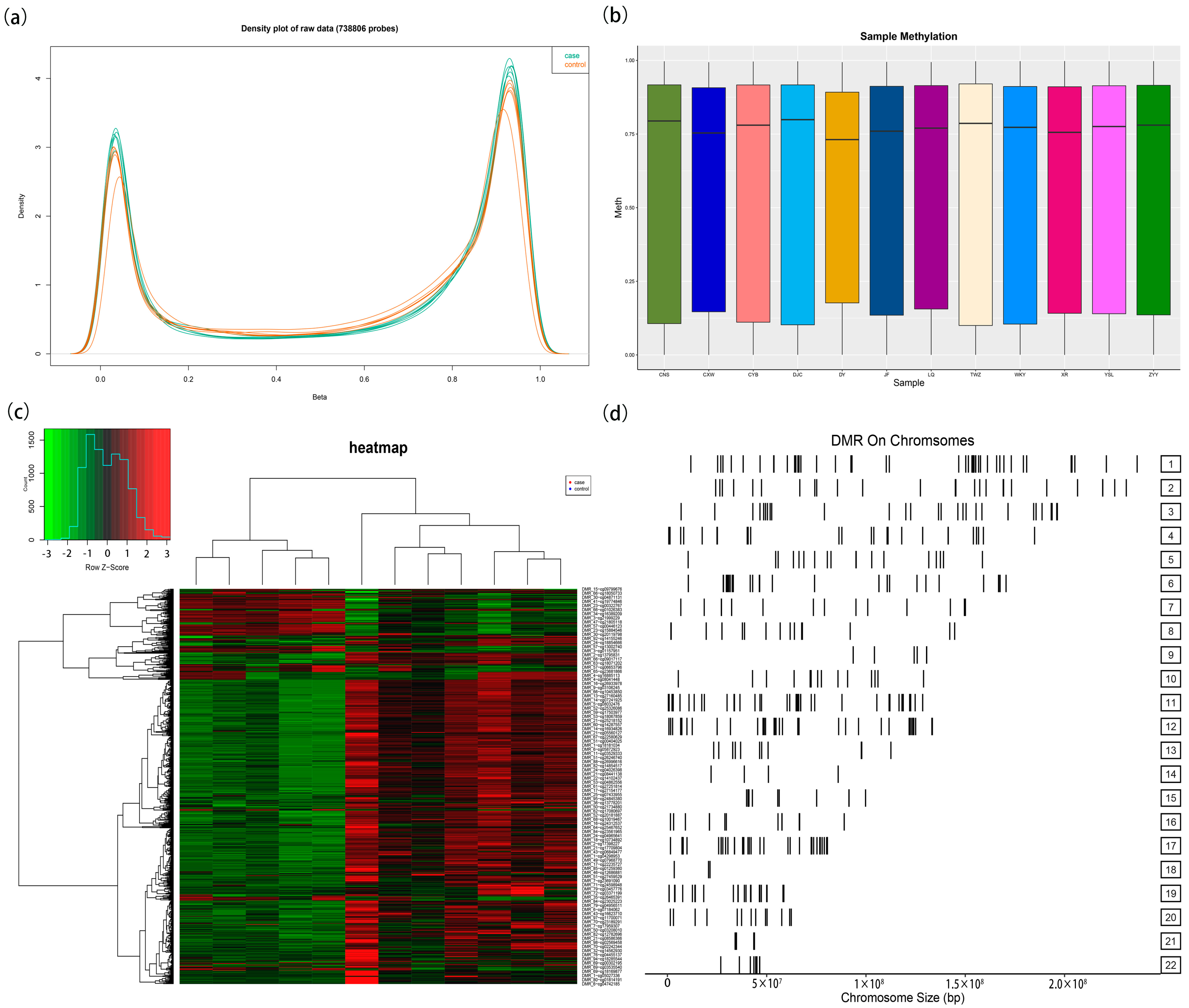
3.1. Comparison of Whole-Genome DNA Methylation Between HAPE Patients and Control Subjects
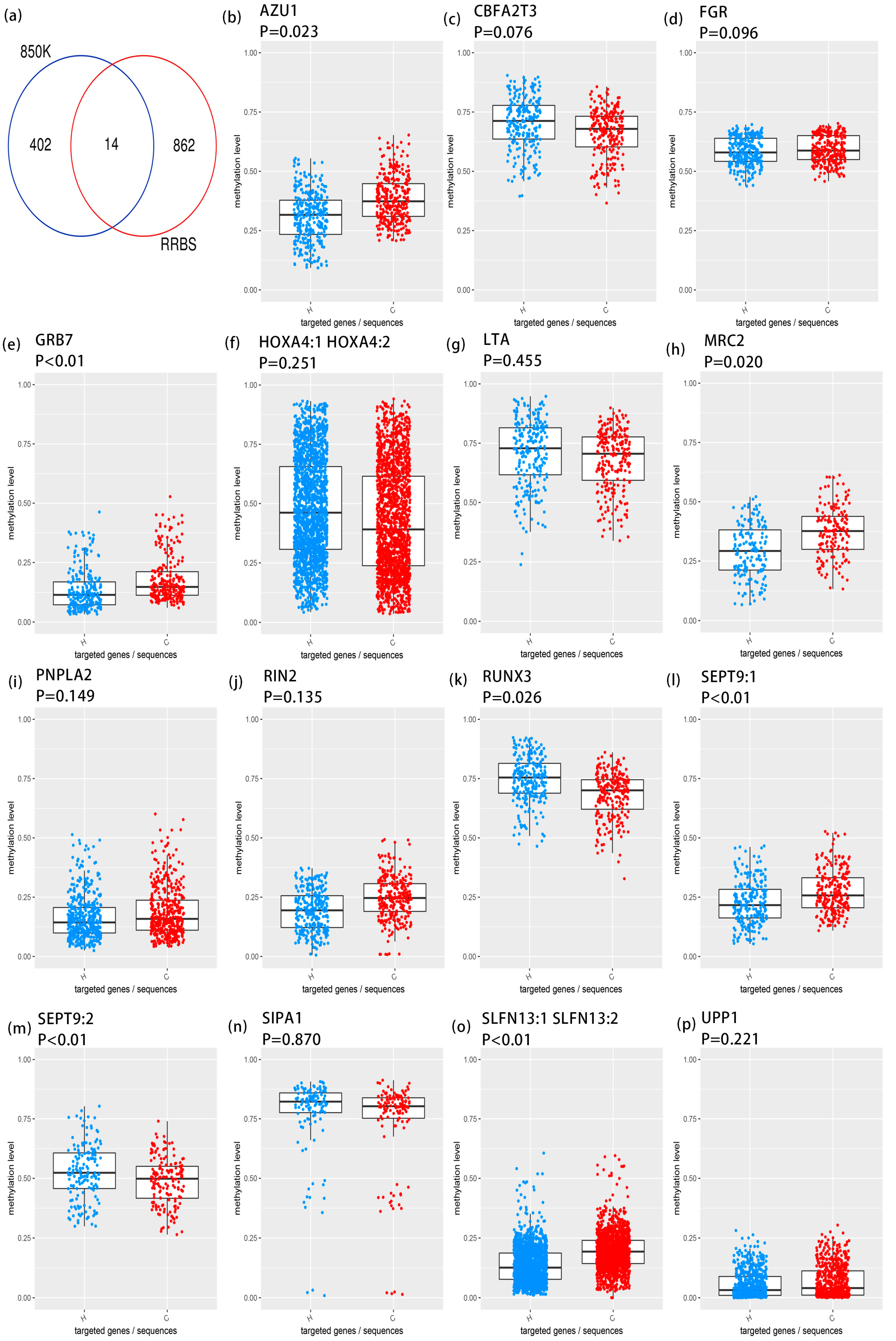
3.2. Targeted Bisulfite Sequencing Identifies Key Methylation Driver Genes
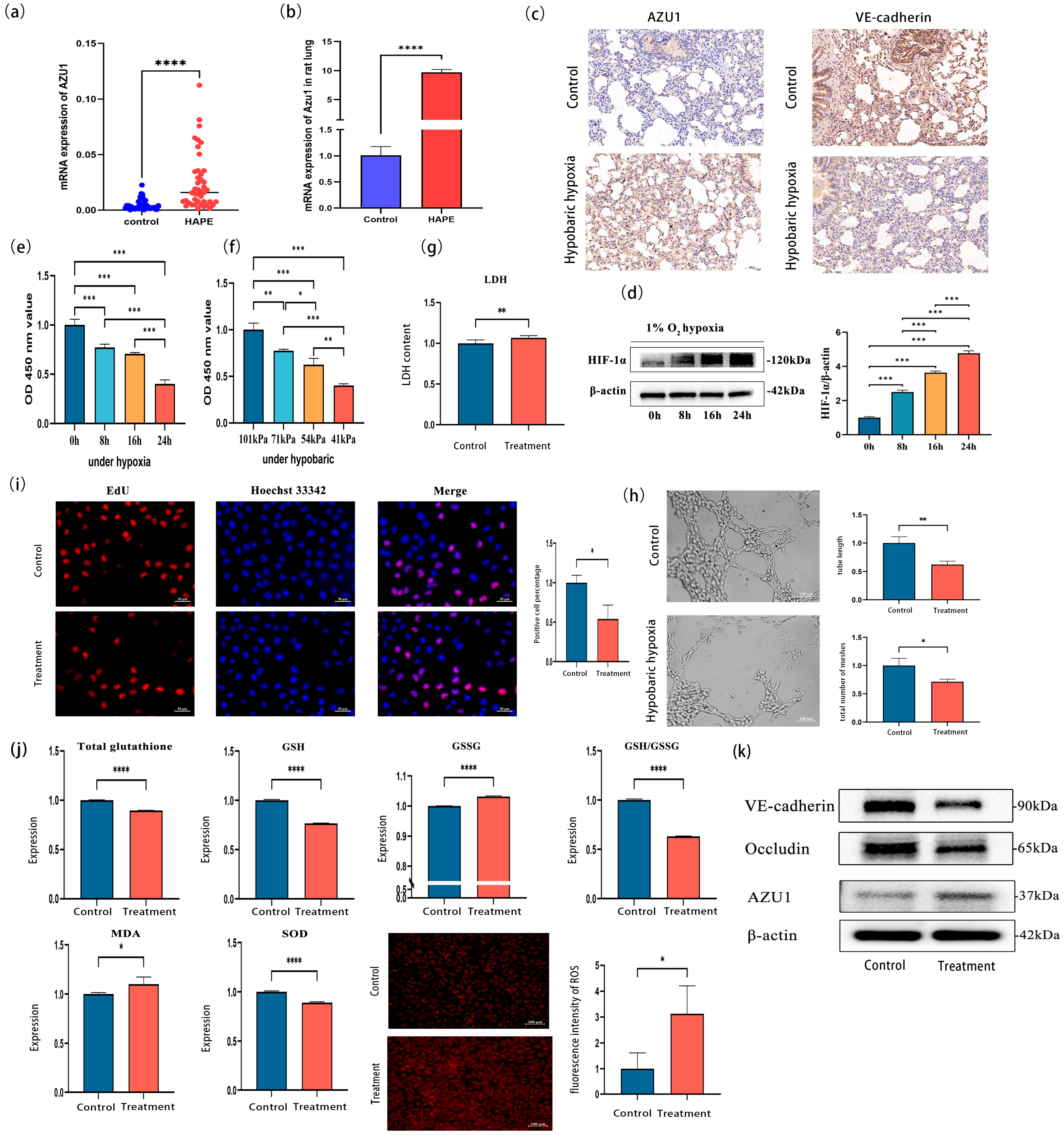
3.3. Verification of AZU1 Expression and Construction of an Acute Hypobaric Hypoxic Cell Injury Model
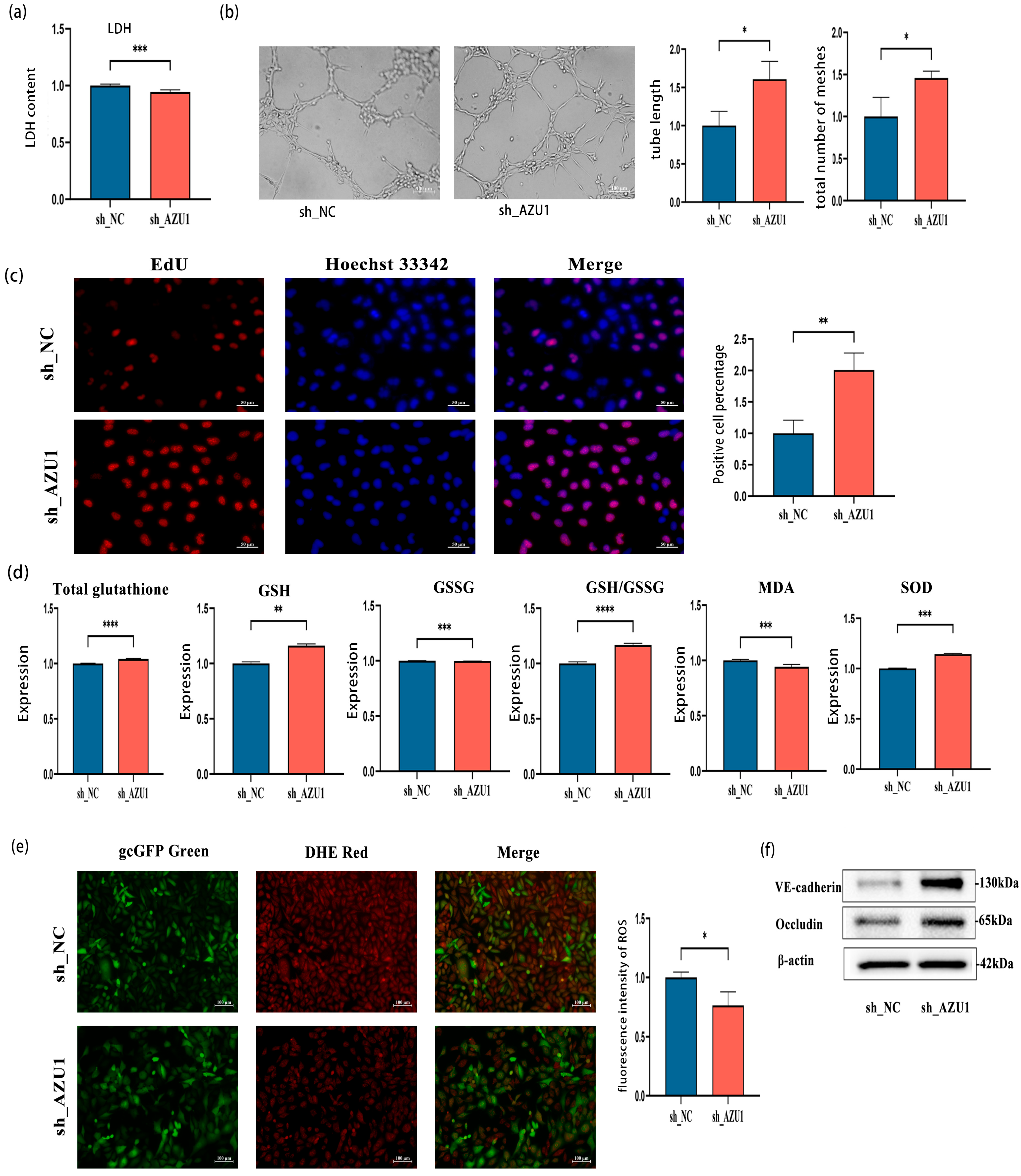
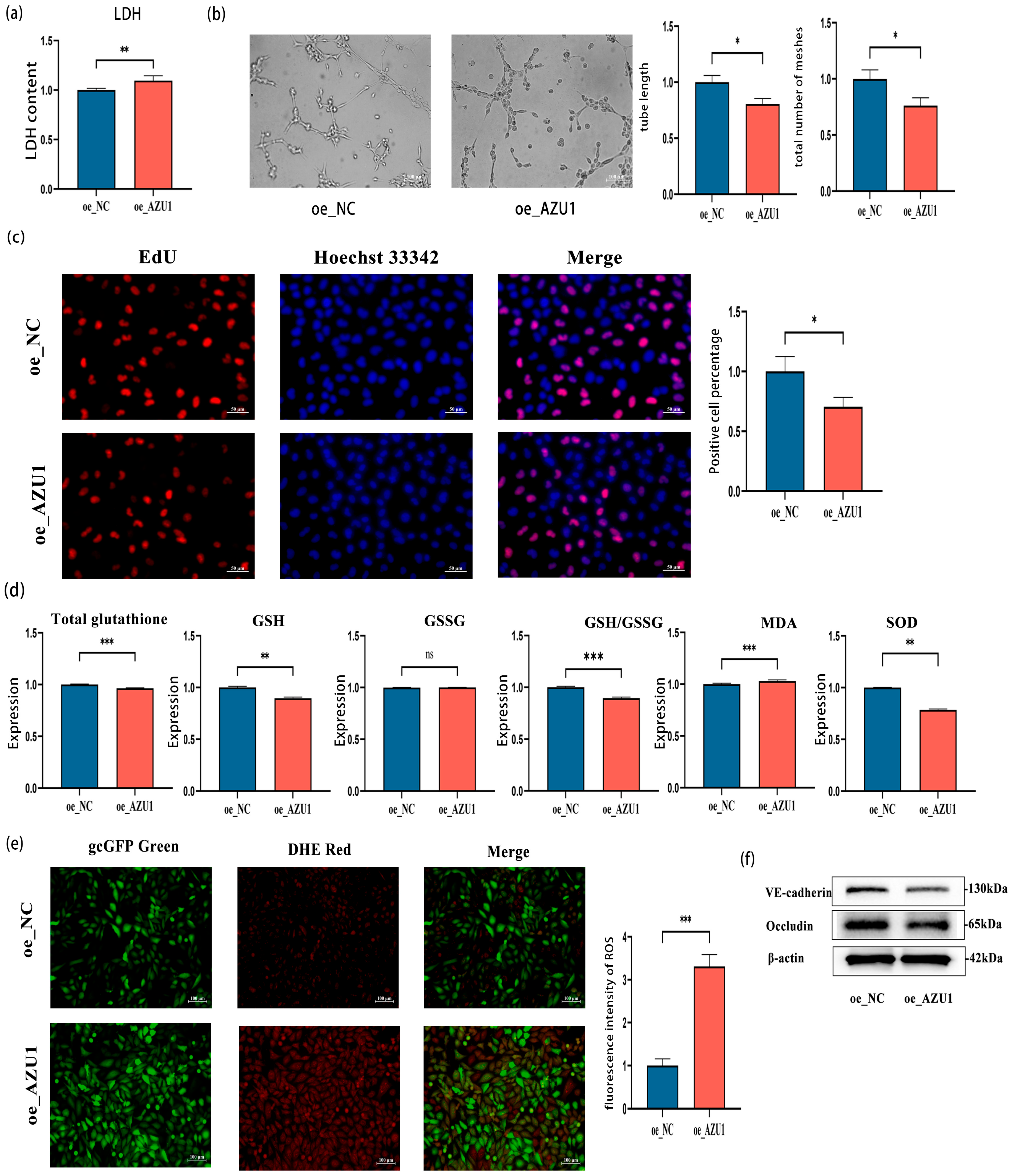
3.4. AZU1 Promotes HUVEC Injury Induced by Acute Hypobaric Hypoxic
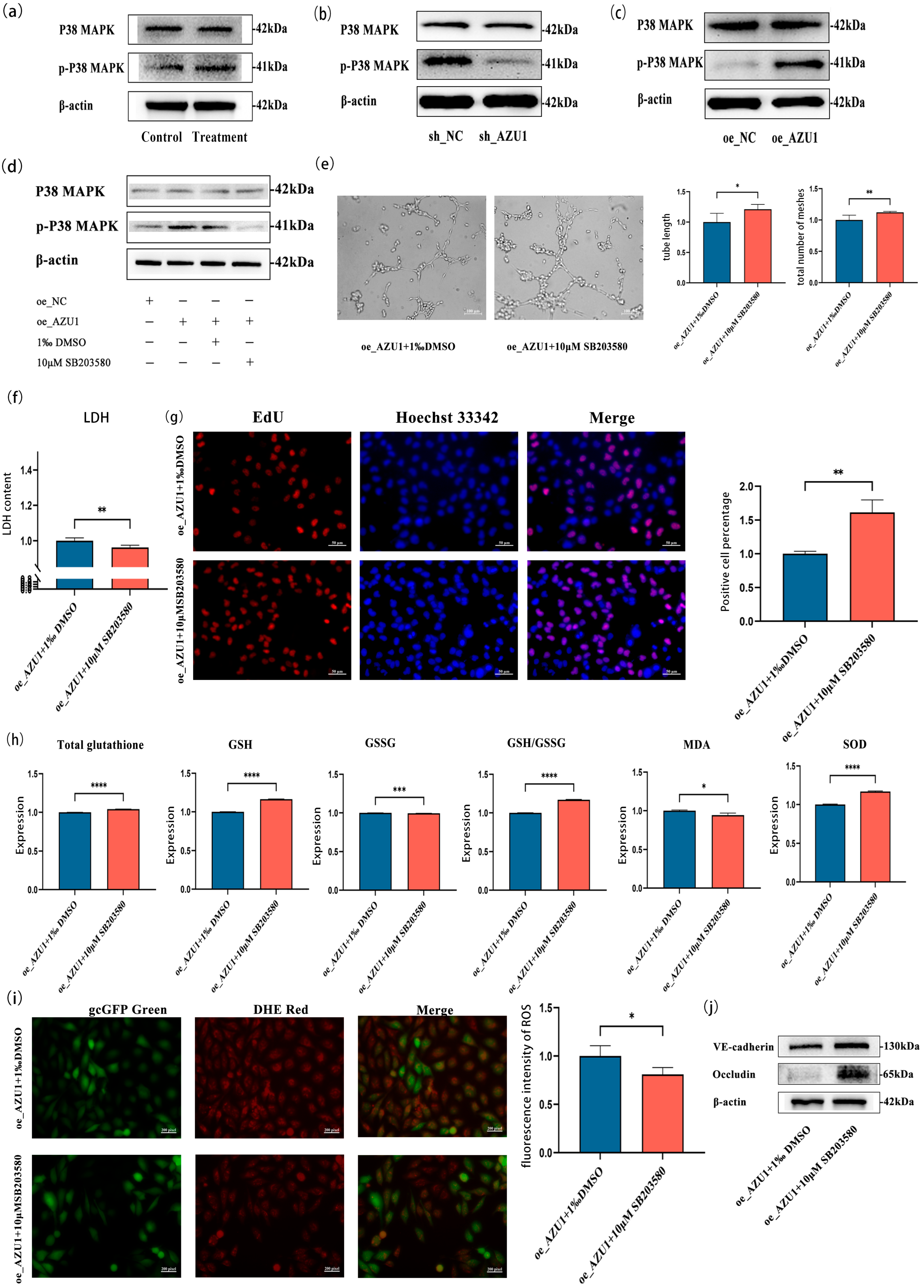
3.5. AZU1 Exacerbates HUVEC Injury Model Damage by Activating the P38 MAPK Signaling Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maggiorini, M.; Brunner-La Rocca, H.P.; Peth, S.; Fischler, M.; Bohm, T.; Bernheim, A.; Kiencke, S.; Bloch, K.E.; Dehnert, C.; Naeije, R.; et al. Both tadalafil and dexamethasone may reduce the incidence of high-altitude pulmonary edema: A randomized trial. Ann. Intern. Med. 2006, 145, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.; Strohl, K.; Faulhaber, M.; Gatterer, H.; Burtscher, M. Hypoxia-related altitude illnesses. J. Travel Med. 2013, 20, 247–255. [Google Scholar] [CrossRef]
- Lee, M.; Huan, T.; McCartney, D.L.; Chittoor, G.; de Vries, M.; Lahousse, L.; Nguyen, J.N.; Brody, J.A.; Castillo-Fernandez, J.; Terzikhan, N.; et al. Pulmonary Function and Blood DNA Methylation: A Multiancestry Epigenome-Wide Association Meta-analysis. Am. J. Respir. Crit. Care Med. 2022, 206, 321–336. [Google Scholar] [CrossRef]
- Attwood, J.T.; Yung, R.L.; Richardson, B.C. DNA methylation and the regulation of gene transcription. Cell Mol. Life Sci. 2002, 59, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, Y.; Yu, G.; Teng, H.; Wang, B.; Yang, Y.; Li, Q.; Sun, Z.; Xu, S.; Wang, W.; et al. Genome-wide DNA methylation landscape of four Chinese populations and epigenetic variation linked to Tibetan high-altitude adaptation. Sci. China Life Sci. 2023, 66, 2354–2369. [Google Scholar] [CrossRef]
- Sharma, K.; Mishra, A.; Singh, H.; Thinlas, T.; Pasha, M.A.Q. Differential methylation in EGLN1 associates with blood oxygen saturation and plasma protein levels in high-altitude pulmonary edema. Clin. Epigenet. 2022, 14, 123. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, M.; Song, K.; Wuren, T.; Yan, J.; Ge, R.L.; Ji, L.; Cui, S. VHL gene methylation contributes to excessive erythrocytosis in chronic mountain sickness rat model by upregulating the HIF-2alpha/EPO pathway. Life Sci. 2021, 266, 118873. [Google Scholar] [CrossRef]
- Mishra, A.; Kohli, S.; Dua, S.; Thinlas, T.; Mohammad, G.; Pasha, M.A. Genetic differences and aberrant methylation in the apelin system predict the risk of high-altitude pulmonary edema. Proc. Natl. Acad. Sci. USA 2015, 112, 6134–6139. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Q.; Shen, X. AZU1 (HBP/CAP37) and PRKCG (PKC-gamma) may be candidate genes affecting the severity of acute mountain sickness. BMC Med. Genom. 2023, 16, 28. [Google Scholar] [CrossRef]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, A.G.; Pham, C.T.; Herwald, H.; Bidzhekov, K.; Rottenberg, M.E.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Cherepanova, O.A.; Gomez, D.; Shankman, L.S.; Swiatlowska, P.; Williams, J.; Sarmento, O.F.; Alencar, G.F.; Hess, D.L.; Bevard, M.H.; Greene, E.S.; et al. Activation of the pluripotency factor OCT4 in smooth muscle cells is atheroprotective. Nat. Med. 2016, 22, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Linder, A. Heparin-binding protein: A key player in the pathophysiology of organ dysfunction in sepsis. J. Intern. Med. 2017, 281, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Hemmann, P.; Kloppenburg, L.; Breinbauer, R.; Ehnert, S.; Blumenstock, G.; Reumann, M.K.; Erne, F.; Jazewitsch, J.; Schwarz, T.; Baumgartner, H.; et al. AZU1: A new promising marker for infection in orthopedic and trauma patients? EXCLI J. 2024, 23, 53–61. [Google Scholar]
- Lin, Q.; Shen, J.; Shen, L.; Zhang, Z.; Fu, F. Increased plasma levels of heparin-binding protein in patients with acute respiratory distress syndrome. Crit. Care 2013, 17, R155. [Google Scholar] [CrossRef]
- Swanson, C.M.; Shea, S.A.; Stone, K.L.; Cauley, J.A.; Rosen, C.J.; Redline, S.; Karsenty, G.; Orwoll, E.S. Obstructive sleep apnea and metabolic bone disease: Insights into the relationship between bone and sleep. J. Bone Miner. Res. 2015, 30, 199–211. [Google Scholar] [CrossRef]
- Dougherty, J.A.; Patel, N.; Kumar, N.; Rao, S.G.; Angelos, M.G.; Singh, H.; Cai, C.; Khan, M. Human Cardiac Progenitor Cells Enhance Exosome Release and Promote Angiogenesis Under Physoxia. Front. Cell Dev. Biol. 2020, 8, 130. [Google Scholar] [CrossRef]
- Coimbra-Costa, D.; Alva, N.; Duran, M.; Carbonell, T.; Rama, R. Oxidative stress and apoptosis after acute respiratory hypoxia and reoxygenation in rat brain. Redox Biol. 2017, 12, 216–225. [Google Scholar] [CrossRef]
- Gunes, I.; Sungu, N.; Kilicarslan, A.; Şıvgın, V.; Alkan, M.; Küçük, A.; Boyunağa, H.; Ünal, Y.; Arslan, M. Effects of hydroxyethyl starch 130/0.4 on the kidney tissue of rats with ureteral obstruction. Drug Des. Devel. Ther. 2018, 12, 3061–3070. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Prada, C.; Lattarulo, C.; Fine, S.; Borrelli, L.A.; Betensky, R.; Greenberg, S.M.; Frosch, M.P.; Bacskai, B.J. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J. Neurochem. 2009, 109, 1636–1647. [Google Scholar] [CrossRef]
- Chertov, O.; Michiel, D.F.; Xu, L.; Wang, J.M.; Tani, K.; Murphy, W.J.; Longo, D.L.; Taub, D.D.; Oppenheim, J.J. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 1996, 271, 2935–2940. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, M.; Sun, Y.; Li, X.; Cao, L.; Ma, X. Transforming growth factor-beta receptor type 2 is required for heparin-binding protein-induced acute lung injury and vascular leakage for transforming growth factor-beta/Smad/Rho signaling pathway activation. FASEB J. 2022, 36, e22580. [Google Scholar] [CrossRef]
- Kuhn, M. Endothelial actions of atrial and B-type natriuretic peptides. Br. J. Pharmacol. 2012, 166, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.; Russell, J.A.; Bentzer, P.; Parsons, D.; Secchia, S.; Mörgelin, M.; Walley, K.R.; Boyd, J.H.; Linder, A. Heparin-Binding Protein (HBP): A Causative Marker and Potential Target for Heparin Treatment of Human Sepsis-Induced Acute Kidney Injury. Shock 2017, 48, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, F.; Yang, L.; Xian, J.; Zou, Q.; Jin, H.; Wang, L.; Zhang, L. Nucleophosmin Mutations Induce Chemosensitivity in THP-1 Leukemia Cells by Suppressing NF-kappaB Activity and Regulating Bax/Bcl-2 Expression. J. Cancer 2016, 7, 2270–2279. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jordan, S.E.; Saad, H.; Covarrubias, A.S.; Siemon, J.; Pearson, J.M.; Slomovitz, B.M.; Huang, M.; Pinto, A.; Schlumbrecht, M.; George, S.H. mRNA expression in low grade serous ovarian cancer: Results of a nanoString assay in a diverse population. Gynecol. Oncol. 2020, 159, 554–562. [Google Scholar] [CrossRef]
- Berggren, K.L.; Restrepo Cruz, S.; Hixon, M.D.; Cowan, A.T.; Keysar, S.B.; Craig, S.; James, J.; Barry, M.; Ozbun, M.A.; Jimeno, A.; et al. MAPKAPK2 (MK2) inhibition mediates radiation-induced inflammatory cytokine production and tumor growth in head and neck squamous cell carcinoma. Oncogene 2019, 38, 7329–7341. [Google Scholar] [CrossRef]
- Nakano, R.; Kitanaka, T.; Namba, S.; Kitanaka, N.; Suwabe, Y.; Konno, T.; Yamazaki, J.; Nakayama, T.; Sugiya, H. Non-Transcriptional and Translational Function of Canonical NF-kappaB Signaling in Activating ERK1/2 in IL-1beta-Induced COX-2 Expression in Synovial Fibroblasts. Front. Immunol. 2020, 11, 579266. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Xu, Z.; Gong, Q.; Chen, L.; Shen, X.; Chen, X. AZU1 as a DNA Methylation-Driven Gene: Promoting Oxidative Stress in High-Altitude Pulmonary Edema. Antioxidants 2025, 14, 835. https://doi.org/10.3390/antiox14070835
Li Q, Xu Z, Gong Q, Chen L, Shen X, Chen X. AZU1 as a DNA Methylation-Driven Gene: Promoting Oxidative Stress in High-Altitude Pulmonary Edema. Antioxidants. 2025; 14(7):835. https://doi.org/10.3390/antiox14070835
Chicago/Turabian StyleLi, Qiong, Zhichao Xu, Qianhui Gong, Liyang Chen, Xiaobing Shen, and Xiaowei Chen. 2025. "AZU1 as a DNA Methylation-Driven Gene: Promoting Oxidative Stress in High-Altitude Pulmonary Edema" Antioxidants 14, no. 7: 835. https://doi.org/10.3390/antiox14070835
APA StyleLi, Q., Xu, Z., Gong, Q., Chen, L., Shen, X., & Chen, X. (2025). AZU1 as a DNA Methylation-Driven Gene: Promoting Oxidative Stress in High-Altitude Pulmonary Edema. Antioxidants, 14(7), 835. https://doi.org/10.3390/antiox14070835






